Investigation of the Formation of Squalene Oligomers Exposed to Ultraviolet Light and Changes in the Exposed Squalene as a Potential Skin Model
Abstract
:1. Introduction
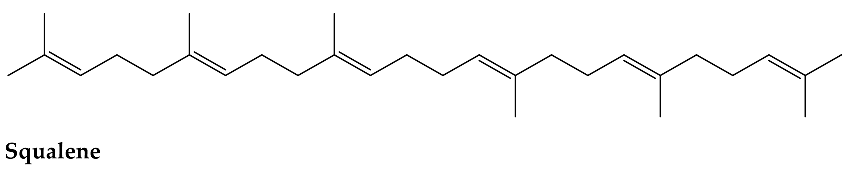
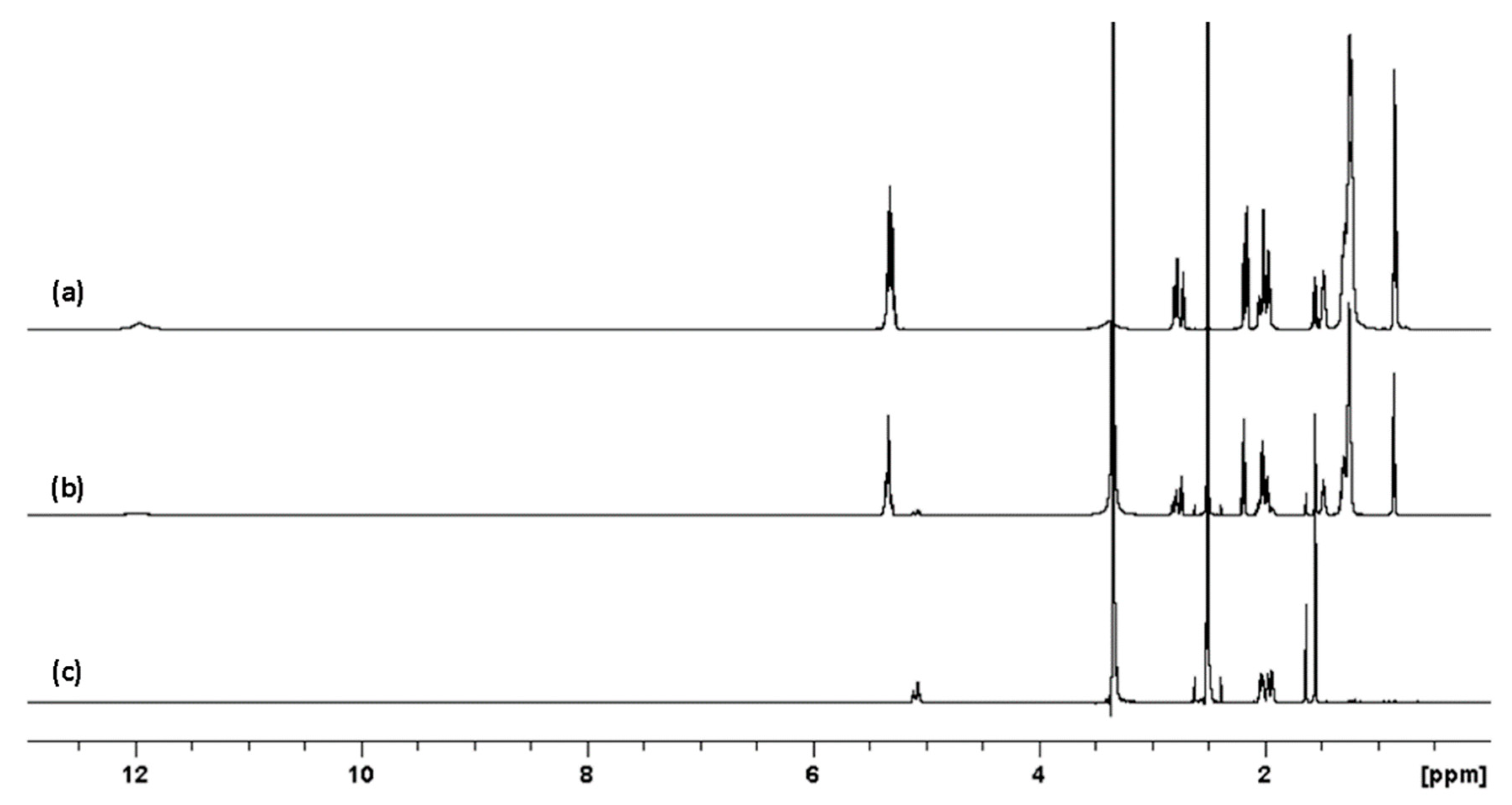
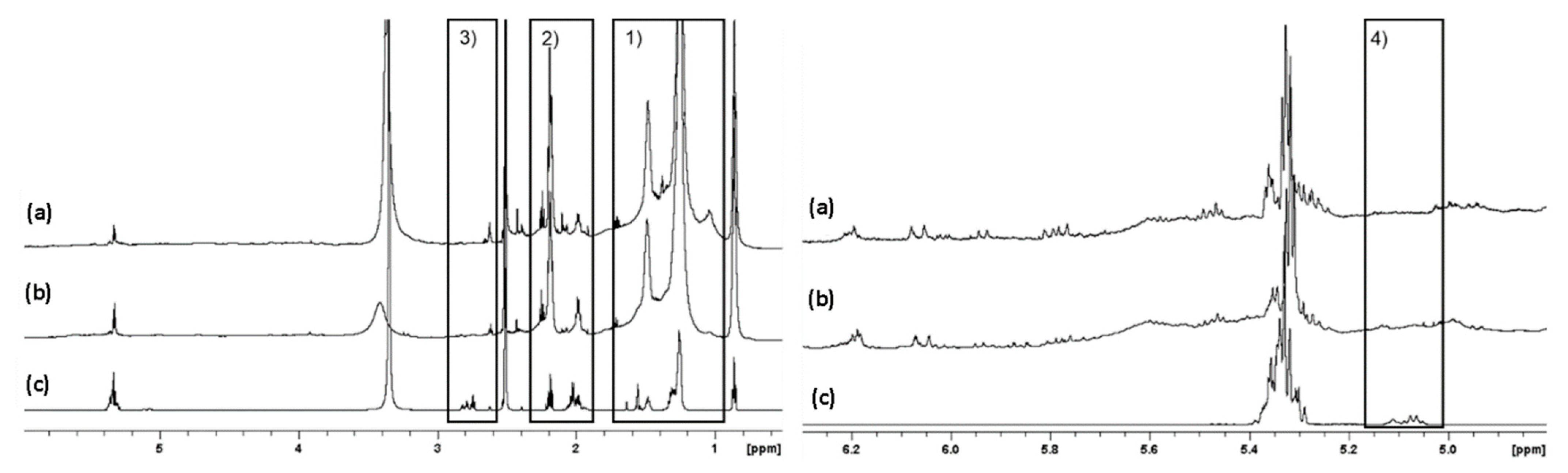
2. Results
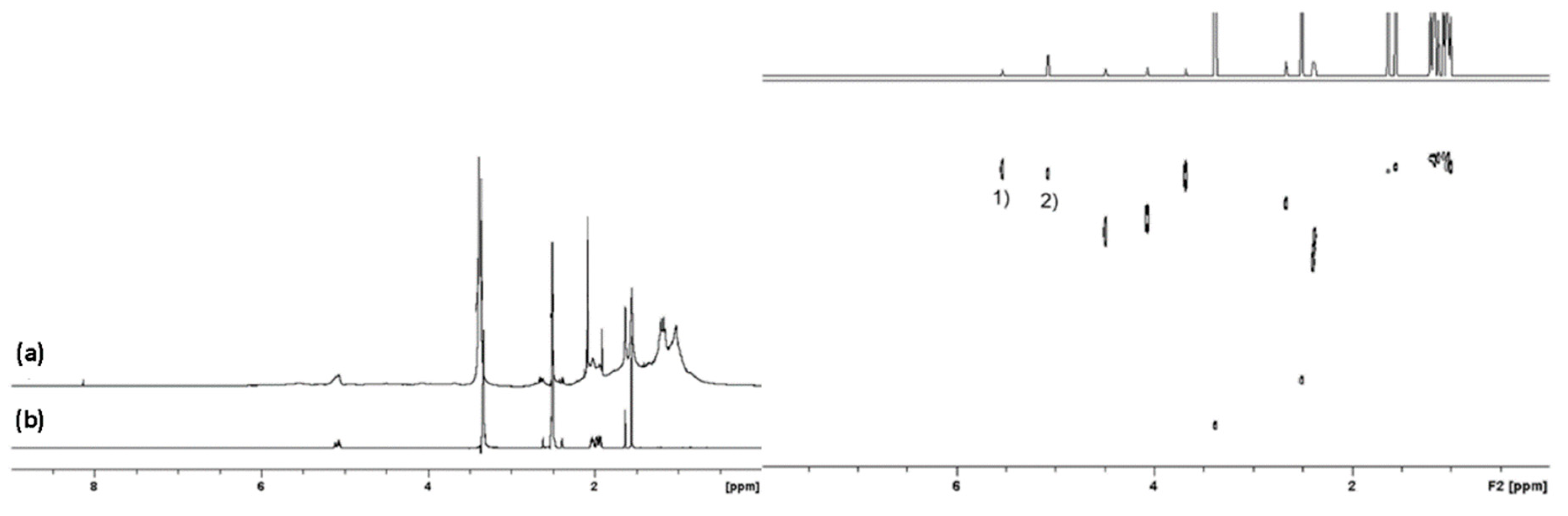
2.1. 1H and DOSY NMR
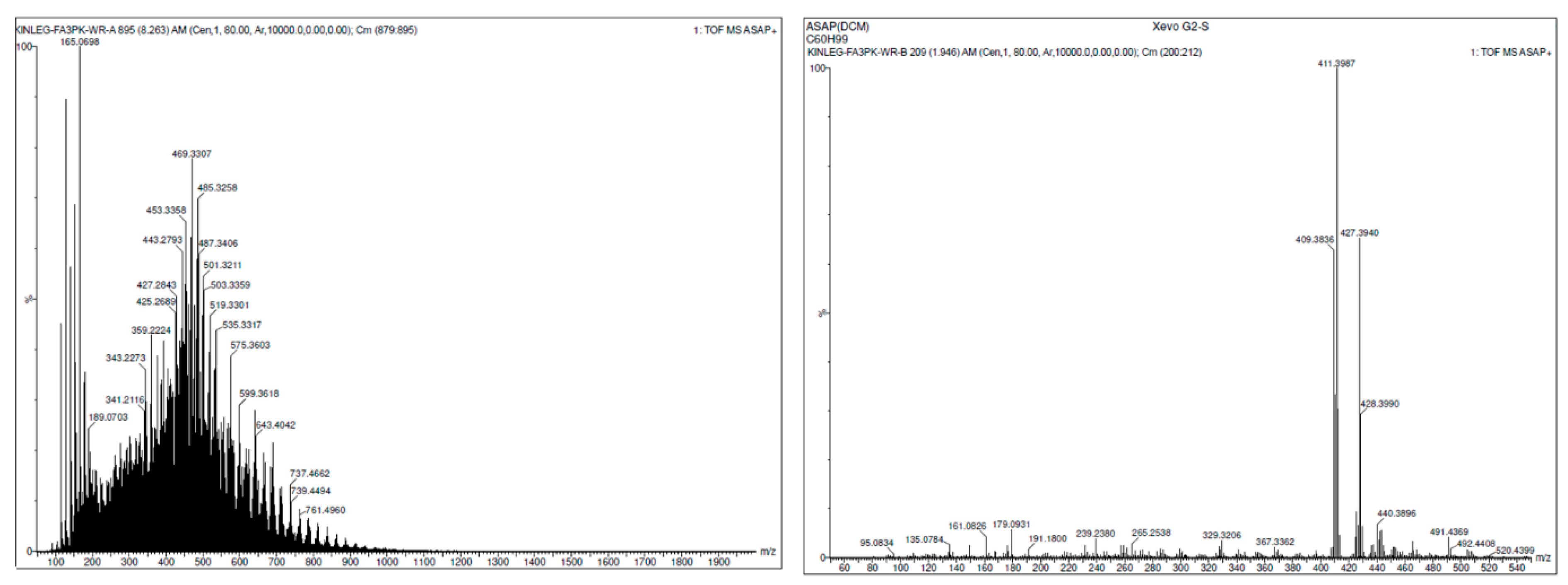
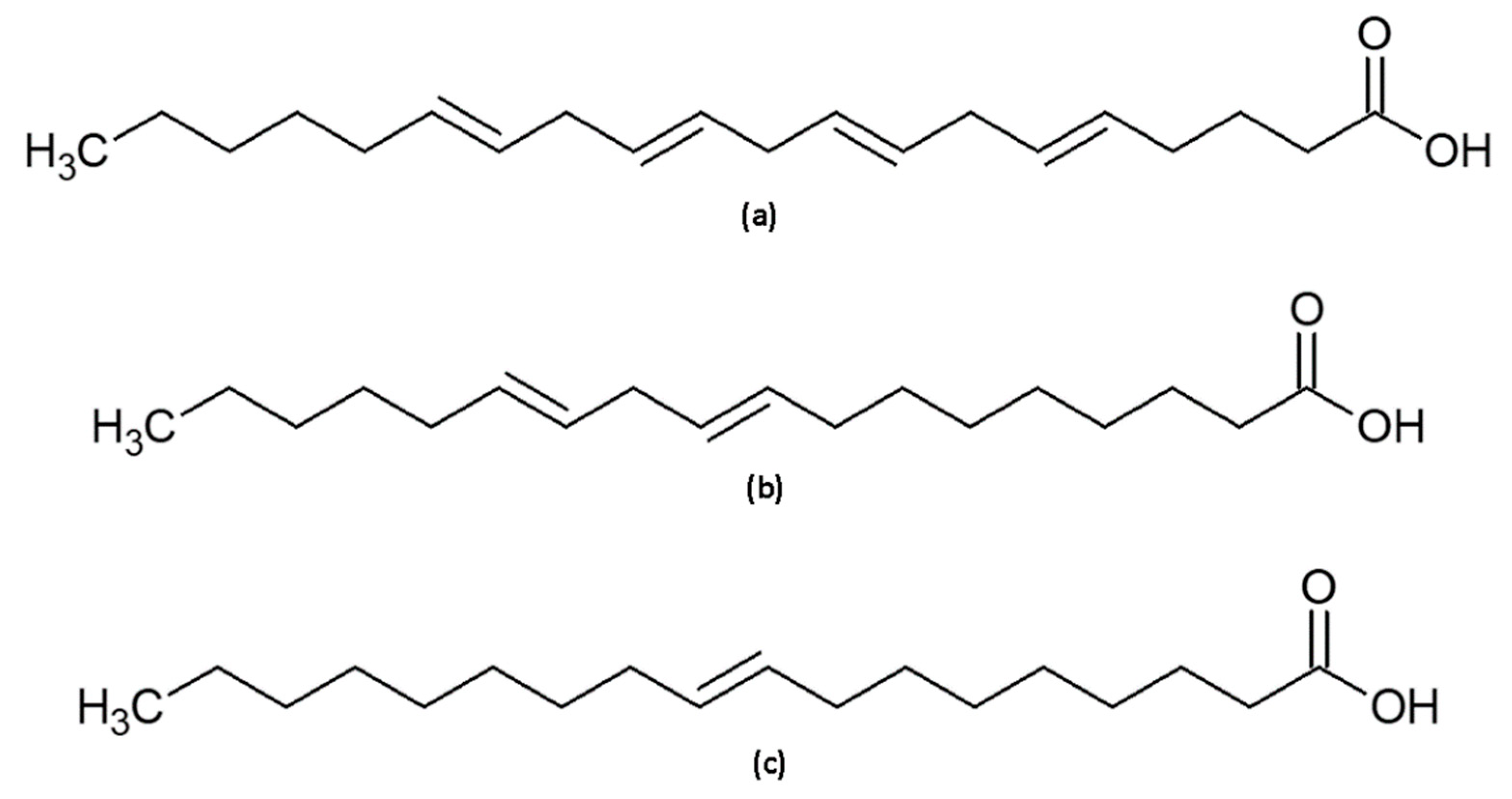
2.2. Irradiation of Squalene
2.3. Sebum Mimicking Squalene Preparation
Irradiation at 285 nm and 300 nm
2.4. Verification of Reaction as Opposed to Rheology
3. Discussion
4. Material and Methods
4.1. Materials
4.2. Irradiation Methods
4.3. NMR Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Li, X.; He, C.; Chen, Z.; Zhou, C.; Gan, Y.; Jia, Y. A Review of the Role of Sebum in the Mechanism of Acne Pathogenesis. J. Cosmet. Dermatol. 2017, 16, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Cunliffe, W.J.; Holland, D.B.; Jeremy, A. Comedone Formation: Etiology, Clinical Presentation, and Treatment. Clin. Dermatol. 2004, 22, 367–374. [Google Scholar] [CrossRef] [PubMed]
- DeLeo, V.; Scheide, S.; Meshulam, J.; Hanson, D.; Cardullo, A. Ultraviolet Radiation Alters Choline Phospholipid Metabolism in Human Keratinocytes. J. Investig. Dermatol. 1988, 91, 303–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evelson, P.; Ordóñez, C.P.; Llesuy, S.; Boveris, A. Oxidative Stress and in Vivo Chemiluminescence in Mouse Skin Exposed to UVA Radiation. J. Photochem. Photobiol. B Biol. 1997, 38, 215–219. [Google Scholar] [CrossRef]
- Ekanayake Mudiyanselage, S.; Hamburger, M.; Elsner, P.; Thiele, J.J. Ultraviolet a Induces Generation of Squalene Monohydroperoxide Isomers in Human Sebum and Skin Surface Lipids in Vitro and in Vivo. J. Investig. Dermatol. 2003, 120, 915–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottaviani, M.; Alestas, T.; Flori, E.; Mastrofrancesco, A.; Zouboulis, C.C.; Picardo, M. Peroxidated Squalene Induces the Production of Inflammatory Mediators in HaCaT Keratinocytes: A Possible Role in Acne vulgaris. J. Investig. Dermatol. 2006, 126, 2430–2437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zouboulis, C.C.; Nestoris, S.; Adler, Y.D.; Orth, M.; Orfanos, C.E.; Picardo, M.; Camera, E.; Cunliffe, W.J. A New Concept for Acne Therapy: A Pilot Study with Zileuton, an Oral 5-Lipoxygenase Inhibitor. Arch. Dermatol. 2003, 139, 668–670. [Google Scholar] [CrossRef] [PubMed]
- Wenhui, X.; Dean, D.N.; Stockmal, K.A.; Morgan, S.E.; Hansmann, U.H.E.; Rangachari, V. Large fatty acid-derived Aβ42 oligomers form ring-like assemblies. J. Chem. Phys. 2019, 150, 075101. [Google Scholar] [CrossRef]
- Kumar, A.; Paslay, L.C.; Lyons, D.; Morgan, S.E.; Correia, J.J.; Rangachari, V. Specific soluble oligomers of amyloid-β peptide undergo replication and form non-fibrillar aggregates in interfacial environments. J. Biol. Chem. 2012, 287, 21253–21264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, R.; Dal Poggetto, G.; Nilsson, M.; Morris, G.A. Improving the Interpretation of Small Molecule Diffusion Coefficients. Anal. Chem. 2018, 90, 3987–3994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manchester NMR Group Web Resource. Available online: https://nmr.chemistry.manchester.ac.uk/?q=node/432 (accessed on 11 September 2019).
- Evans, R.; Deng, Z.; Rogerson, A.K.; McLachlan, A.S.; Richards, J.J.; Nilsson, M.; Morris, G.A. Quantitative Interpretation of Diffusion-Ordered NMR Spectra: Can We Rationalize Small Molecule Diffusion Coefficients? Angew. Chem. Int. Ed. 2013, 52, 3199–3202. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zecchini, M.; Lucas, R.A.; Robertson, C.; Coban, T.; Thatti, R.; Le Gresley, A. Investigation of the Formation of Squalene Oligomers Exposed to Ultraviolet Light and Changes in the Exposed Squalene as a Potential Skin Model. Molecules 2022, 27, 3481. https://doi.org/10.3390/molecules27113481
Zecchini M, Lucas RA, Robertson C, Coban T, Thatti R, Le Gresley A. Investigation of the Formation of Squalene Oligomers Exposed to Ultraviolet Light and Changes in the Exposed Squalene as a Potential Skin Model. Molecules. 2022; 27(11):3481. https://doi.org/10.3390/molecules27113481
Chicago/Turabian StyleZecchini, Matteo, Robert A. Lucas, Cameron Robertson, Tomris Coban, Ravtej Thatti, and Adam Le Gresley. 2022. "Investigation of the Formation of Squalene Oligomers Exposed to Ultraviolet Light and Changes in the Exposed Squalene as a Potential Skin Model" Molecules 27, no. 11: 3481. https://doi.org/10.3390/molecules27113481
APA StyleZecchini, M., Lucas, R. A., Robertson, C., Coban, T., Thatti, R., & Le Gresley, A. (2022). Investigation of the Formation of Squalene Oligomers Exposed to Ultraviolet Light and Changes in the Exposed Squalene as a Potential Skin Model. Molecules, 27(11), 3481. https://doi.org/10.3390/molecules27113481





