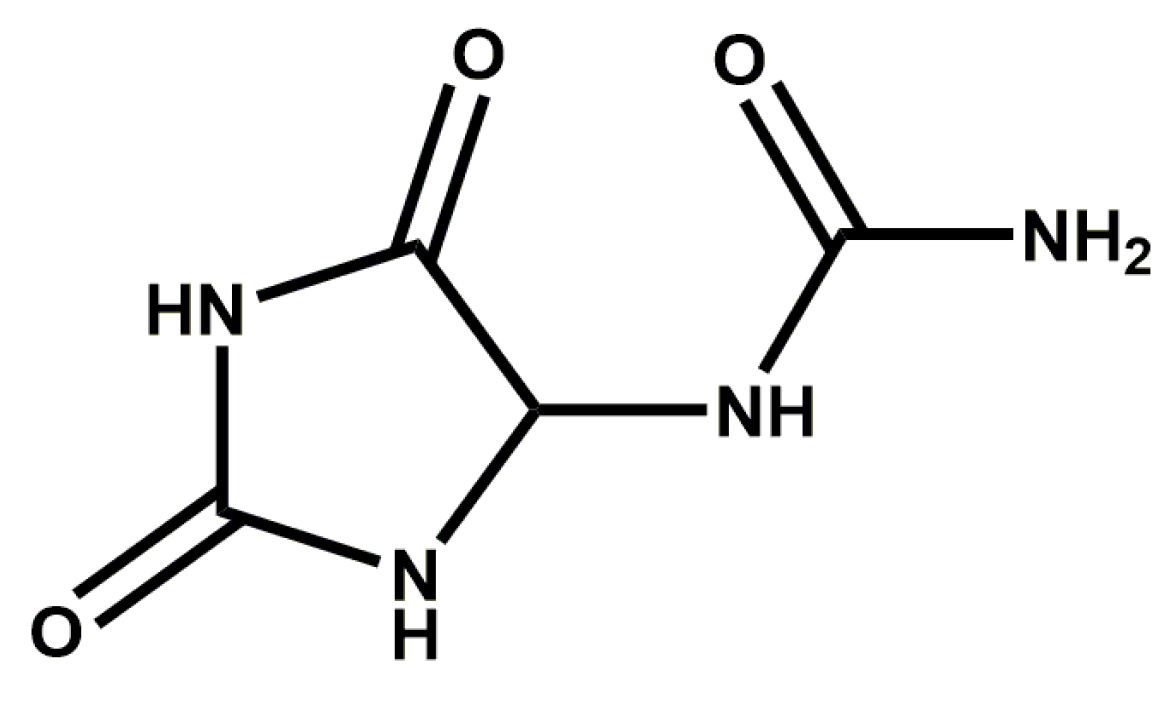
Allantoin Inhibits Compound 48/80-Induced Pseudoallergic Reactions In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
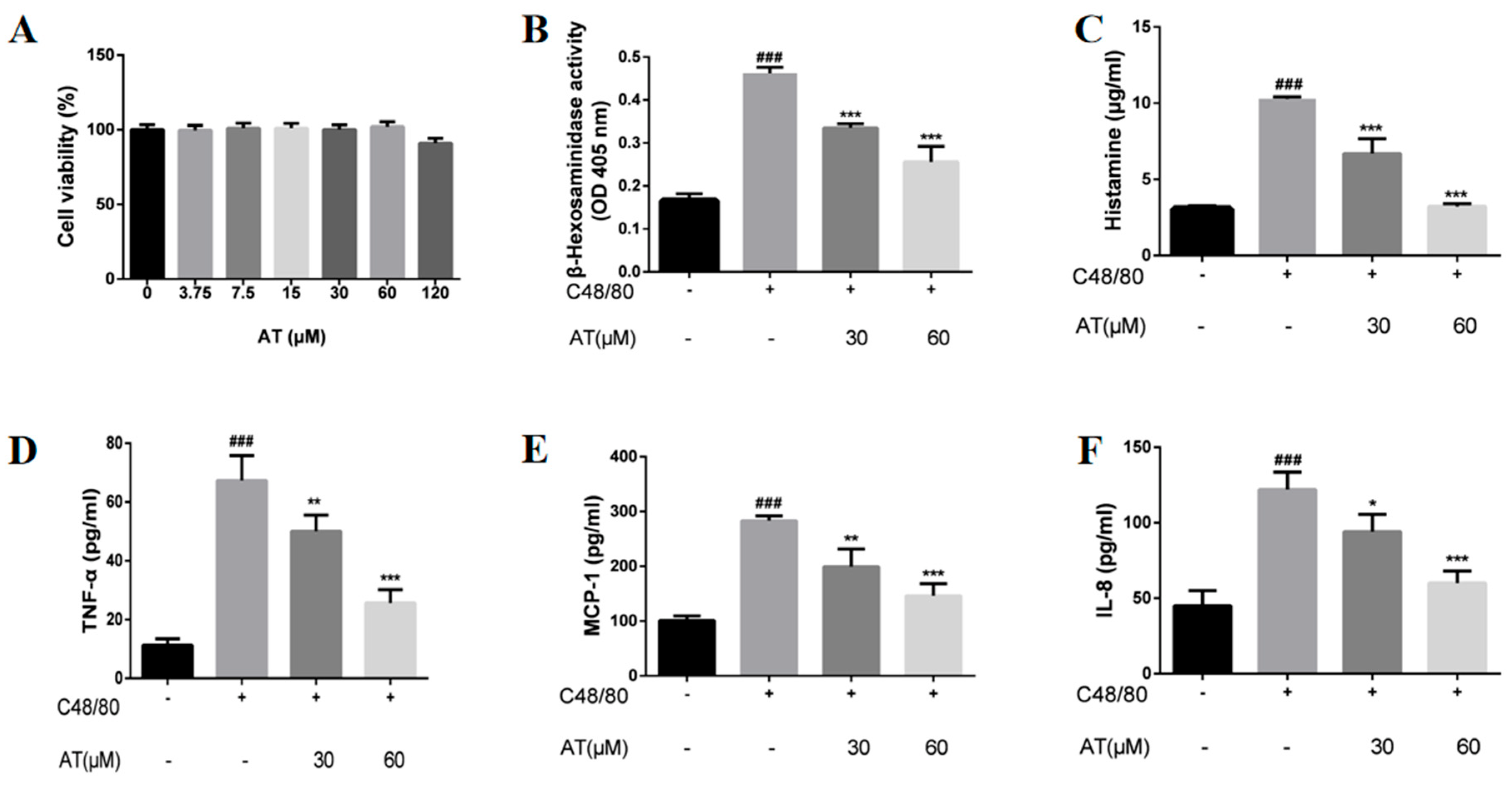
2.1. Effects of AT on the Release of β-Hex, HIS, and Cytokines by C48/80-Stimulated RBL-2H3 Cells
2.2. Effect of AT on the Release of Granules by C48/80-Stimulated RBL-2H3 Cells
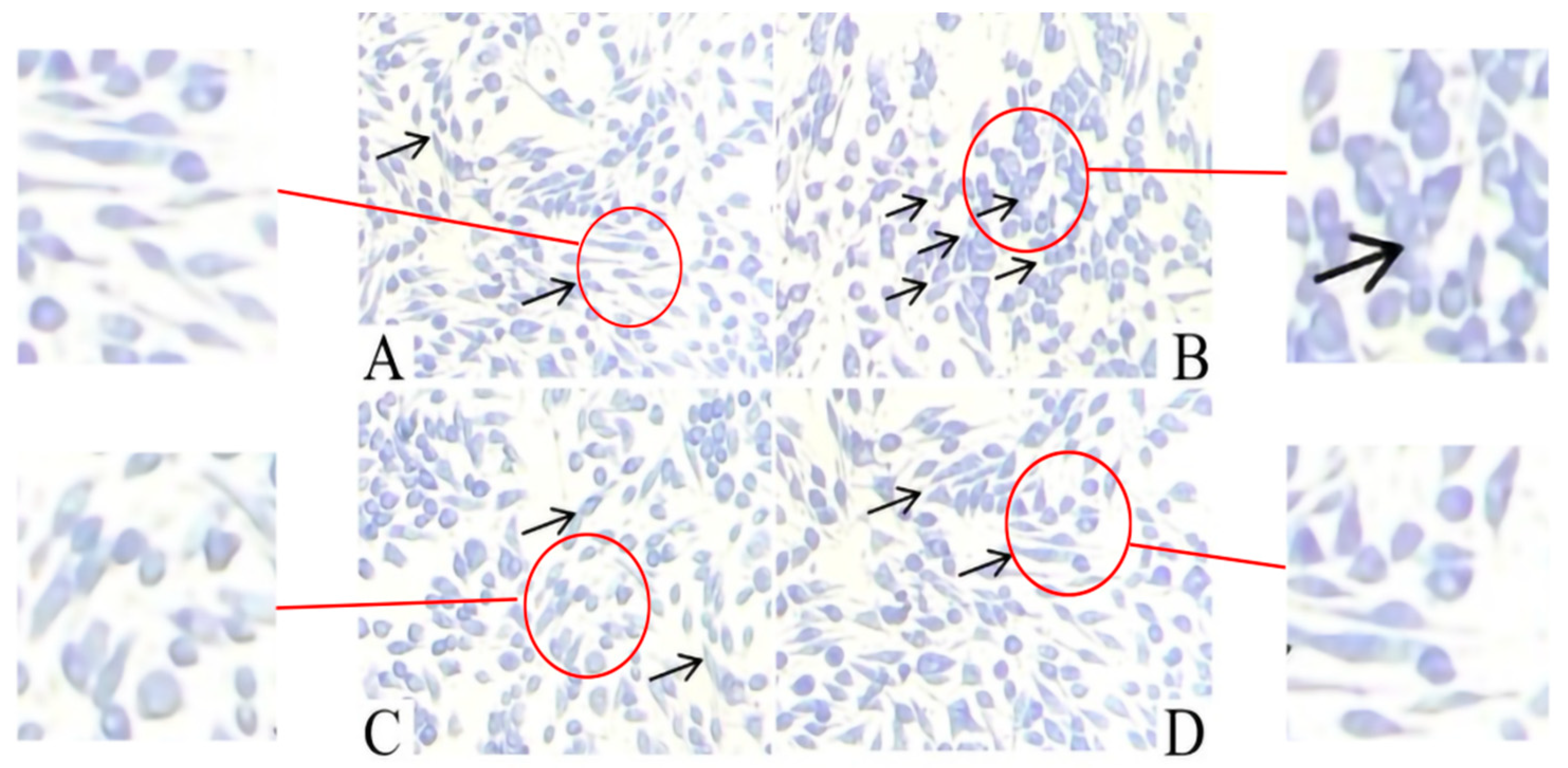
2.3. Effect of AT on Membrane Ruffling in C48/80-Stimulated RBL-2H3 Cells
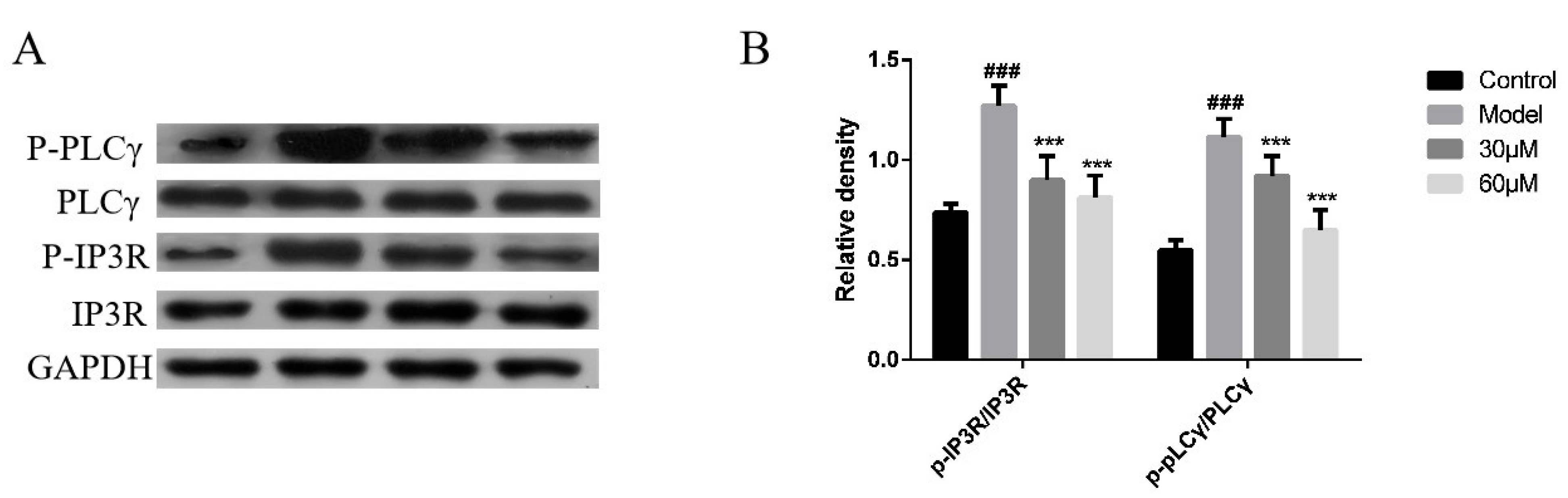
2.4. Effect of AT on the PLCγ/IP3R Signaling Pathway in C48/80-Stimulated RBL-2H3 Cells
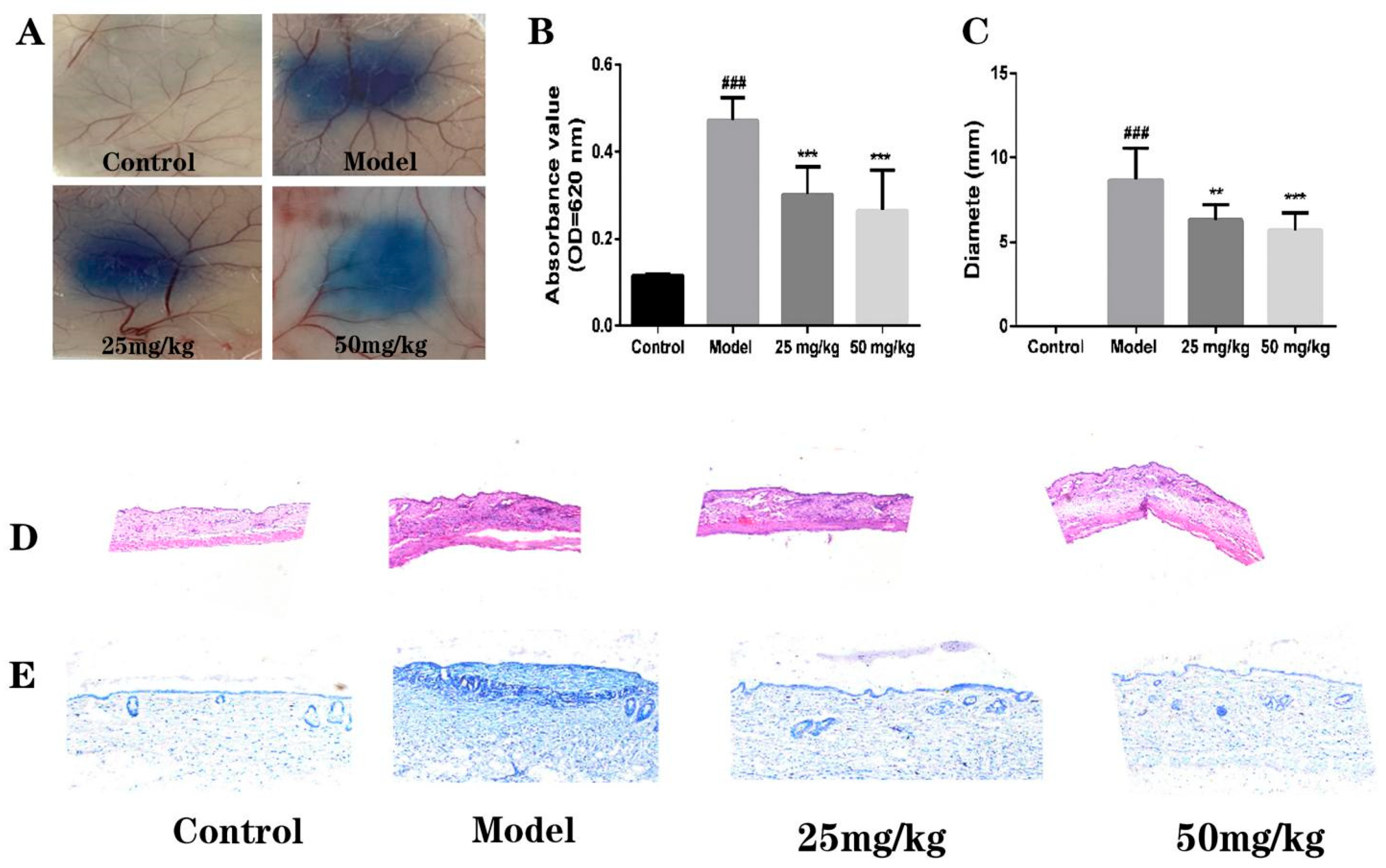
2.5. Effect of AT on C48/80-Induced Local Cutaneous Inflammation in PCA
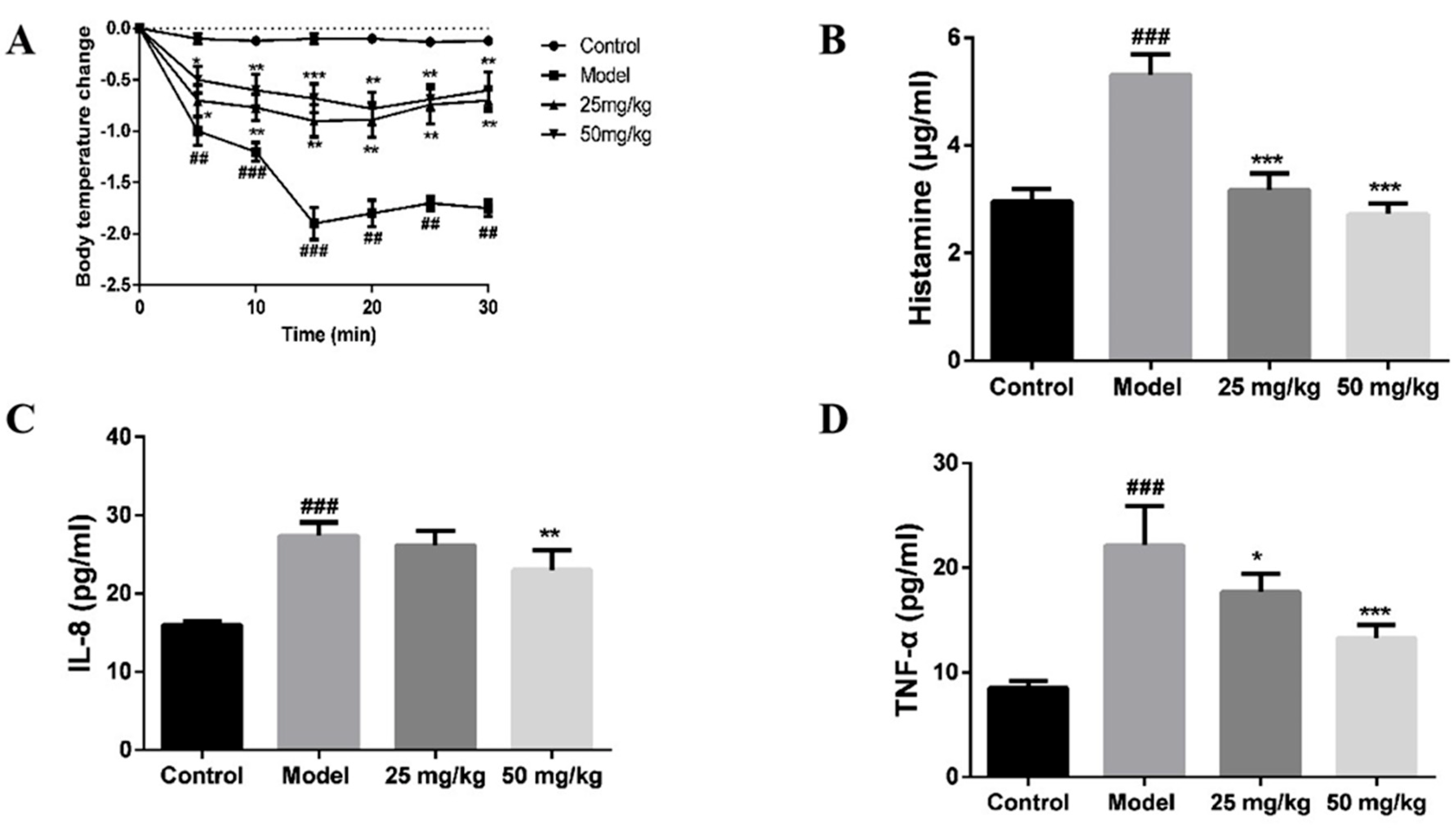
2.6. Effect of AT on C48/80-Induced Active Systemic Anaphylaxis
3. Discussion
4. Materials and Methods
4.1. Drugs and Reagents
4.2. Cell Culture
4.3. Animals
4.4. Cell Viability Assay
4.5. β-Hex and HIS Release Assay
4.6. Inflammatory Cytokine Assay
4.7. Toluidine Blue Staining
4.8. F-Actin Microfilament Staining
4.9. Western Blot
4.10. Passive Cutaneous Anaphylaxis
4.11. Histological Analysis
4.12. Active Systemic Anaphylaxis
4.13. Enzyme-Linked Immunosorbent Assay (ELISA)
4.14. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- da Silva, E.Z.; Jamur, M.C.; Oliver, C. Mast cell function: A new vision of an old cell. J. Histochem. Cytochem. 2014, 62, 698–738. [Google Scholar] [CrossRef] [PubMed]
- Castells, M. Diagnosis and management of anaphylaxis in precision medicine. J. Allergy Clin. Immunol. 2017, 140, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Bulfone-Paus, S.; Nilsson, G.; Draber, P.; Blank, U.; Levi-Schaffer, F. Positive and Negative Signals in Mast Cell Activation. Trends Immunol. 2017, 38, 657–667. [Google Scholar] [CrossRef] [Green Version]
- Niyonsaba, F.; Ushio, H.; Hara, M.; Yokoi, H.; Tominaga, M.; Takamori, K.; Kajiwara, N.; Saito, H.; Nagaoka, I.; Ogawa, H.; et al. Antimicrobial peptides human beta-defensins and cathelicidin LL-37 induce the secretion of a pruritogenic cytokine IL-31 by human mast cells. J. Immunol. 2010, 184, 3526–3534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNeil, B.D.; Pundir, P.; Meeker, S.; Han, L.; Undem, B.J.; Kulka, M.; Dong, X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015, 519, 237–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatemoto, K.; Nozaki, Y.; Tsuda, R.; Konno, S.; Tomura, K.; Furuno, M.; Ogasawara, H.; Edamura, K.; Takagi, H.; Iwamura, H.; et al. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem. Biophys. Res. Commun. 2006, 349, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, Y.; Hu, S.; Ding, Y.; Jia, Q.; Zhu, J.; An, H. Kaempferol ameliorates secretagogue-induced pseudo-allergic reactions via inhibiting intracellular calcium fluctuation. J. Pharm. Pharmacol. 2020, 72, 1221–1231. [Google Scholar] [CrossRef]
- Che, D.; Wang, J.; Ding, Y.; Liu, R.; Cao, J.; Zhang, Y.; Hou, Y.; An, H.; Gao, Z.; Zhang, T. Mivacurium induce mast cell activation and pseudo-allergic reactions via MAS-related G protein coupled receptor-X2. Cell. Immunol. 2018, 332, 121–128. [Google Scholar] [CrossRef]
- Liu, R.; Che, D.; Zhao, T.; Pundir, P.; Cao, J.; Lv, Y.; Wang, J.; Ma, P.; Fu, J.; Wang, N.; et al. MRGPRX2 is essential for sinomenine hydrochloride induced anaphylactoid reactions. Biochem. Pharmacol. 2017, 146, 214–223. [Google Scholar] [CrossRef]
- Wernersson, S.; Pejler, G. Mast cell secretory granules: Armed for battle. Nat. Rev. Immunol. 2014, 14, 478–494. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Wohrl, S.; Bielory, L. Mast Cell Biology at Molecular Level: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2020, 58, 342–365. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Khan, F.A.; Pandupuspitasari, N.; Hussain, S.; Khan, I.A.; Saeed, M.; Saud, S.; Hassan, S.; Adnan, M.; Arif, M.; et al. Suppressing photorespiration for the improvement in photosynthesis and crop yields: A review on the role of S-allantoin as a nitrogen source. J. Environ. Manage. 2019, 237, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Ud-Din, S.; McGeorge, D.; Bayat, A. Topical management of striae distensae (stretch marks): Prevention and therapy of striae rubrae and albae. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 211–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosse, M.A.; Silva, M.B.D.; Oliveira, N.; Araujo, M.A.; Rodrigues, C.; Azevedo, J.P.; Reis, A.R.D. Physiological impact of flavonoids on nodulation and ureide metabolism in legume plants. Plant Physiol. Biochem. 2021, 166, 512–521. [Google Scholar] [CrossRef]
- Lee, M.Y.; Lee, N.H.; Jung, D.; Lee, J.A.; Seo, C.S.; Lee, H.; Kim, J.H.; Shin, H.K. Protective effects of allantoin against ovalbumin (OVA)-induced lung inflammation in a murine model of asthma. Int. Immunopharmacol. 2010, 10, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Wei, L.; Du, Y.; Wang, L.; Han, J.; He, Q.; Chen, H.; Zou, X.; Wu, H.; Shang, J. Effect of the Tibetan Medicine Zuotai on Degranulation and Inflammatory Mediator Release in RBL-2H3 Cells. Chem. Pharm. Bull. 2018, 66, 818–821. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Hu, S.; Ge, S.; Jia, M.; Wang, N. Resveratrol inhibits MRGPRX2-mediated mast cell activation via Nrf2 pathway. Int. Immunopharmacol. 2021, 93, 107426. [Google Scholar] [CrossRef]
- Ding, Y.; Che, D.; Li, C.; Cao, J.; Wang, J.; Ma, P.; Zhao, T.; An, H.; Zhang, T. Quercetin inhibits Mrgprx2-induced pseudo-allergic reaction via PLCgamma-IP3R related Ca(2+) fluctuations. Int. Immunopharmacol. 2019, 66, 185–197. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Wang, J.; Liu, R.; Zhang, G.; Dong, K.; Zhang, T. Paeoniflorin inhibits MRGPRX2-mediated pseudo-allergic reaction via calcium signaling pathway. Phytother. Res. 2020, 34, 401–408. [Google Scholar] [CrossRef]
- Finkelman, F.D. Anaphylaxis: Lessons from mouse models. J. Allergy Clin. Immunol. 2007, 120, 506–515. [Google Scholar] [CrossRef]
- Amatya, B.; Nordlind, K.; Wahlgren, C.F. Responses to intradermal injections of substance P in psoriasis patients with pruritus. Ski. Pharmacol. Physiol. 2010, 23, 133–138. [Google Scholar] [CrossRef]
- Che, D.; Hou, Y.; Zeng, Y.; Li, C.; Zhang, Y.; Wei, D.; Hu, S.; Liu, R.; An, H.; Wang, Y.; et al. Dehydroandrographolide inhibits IgE-mediated anaphylactic reactions via calcium signaling pathway. Toxicol. Appl. Pharmacol. 2019, 366, 46–53. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, Z.; Mao, Z.; Zhang, Y.; Jin, M.; Chen, W.; Zhang, W.; Yu, B.; Zhang, W.; Alaster Lau, H.Y. Go is required for the release of IL-8 and TNF-alpha, but not degranulation in human mast cells. Eur. J. Pharmacol. 2016, 780, 115–121. [Google Scholar] [CrossRef]
- Kim, S.M.; Ryu, H.W.; Kwon, O.K.; Hwang, D.; Kim, M.G.; Min, J.H.; Zhang, Z.; Kim, S.Y.; Paik, J.H.; Oh, S.R.; et al. Callicarpa japonica Thunb. ameliorates allergic airway inflammation by suppressing NF-kappaB activation and upregulating HO-1 expression. J. Ethnopharmacol. 2021, 267, 113523. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Ni, S.; Wang, D.; Hong, T. Coptisine Suppresses Mast Cell Degranulation and Ovalbumin-Induced Allergic Rhinitis. Molecules 2018, 23, 3039. [Google Scholar] [CrossRef] [Green Version]
- Fu, M.; Fu, S.; Ni, S.; Wang, D.; Hong, T. Inhibitory effects of bisdemethoxycurcumin on mast cell-mediated allergic diseases. Int. Immunopharmacol. 2018, 65, 182–189. [Google Scholar] [CrossRef]
- Gilfillan, A.M.; Tkaczyk, C. Integrated signalling pathways for mast-cell activation. Nat. Rev. Immunol. 2006, 6, 218–230. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, T.; Che, D.; Cao, J.; Wang, J.; Lv, Y.; Ma, P.; Ding, Y.; Wang, N.; Wang, X.; et al. The anti-anaphylactoid effects of hydroxysafflor yellow A on the suppression of mast cell Ca(2+) influx and degranulation. Phytomedicine 2018, 48, 43–50. [Google Scholar] [CrossRef] [PubMed]
- El-Sibai, M.; Backer, J.M. Phospholipase C gamma negatively regulates Rac/Cdc42 activation in antigen-stimulated mast cells. Eur. J. Immunol. 2007, 37, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.L.; van Rossum, D.B.; Nikolaidis, N.; Gill, D.L.; Snyder, S.H. Phospholipase C-gamma: Diverse roles in receptor-mediated calcium signaling. Trends Biochem. Sci. 2005, 30, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, J.; Hou, Y.; Fu, J.; Wei, D.; Jia, Q.; Lv, Y.; Wang, C.; Han, S.; He, L. Isosalvianolic acid C-induced pseudo-allergic reactions via the mast cell specific receptor MRGPRX2. Int. Immunopharmacol. 2019, 71, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, H.; Gupta, K.; Ali, H. Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J. Allergy Clin. Immunol. 2016, 138, 700–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikelis, C.M.; Simaan, M.; Ando, K.; Fukuhara, S.; Sakurai, A.; Amornphimoltham, P.; Masedunskas, A.; Weigert, R.; Chavakis, T.; Adams, R.H.; et al. RhoA and ROCK mediate histamine-induced vascular leakage and anaphylactic shock. Nat. Commun. 2015, 6, 6725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, M.; Cato, A.C.B.; Ainooson, G.K.; Freichel, M.; Tsvilovskyy, V.; Jessberger, R.; Riedlinger, E.; Sommerhoff, C.P.; Bischoff, S.C. Regulation of the pleiotropic effects of tissue-resident mast cells. J. Allergy Clin. Immunol. 2019, 144, S31–S45. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Wang, Y.; Zhang, J.; Hong, T. Allantoin Inhibits Compound 48/80-Induced Pseudoallergic Reactions In Vitro and In Vivo. Molecules 2022, 27, 3473. https://doi.org/10.3390/molecules27113473
Zhang P, Wang Y, Zhang J, Hong T. Allantoin Inhibits Compound 48/80-Induced Pseudoallergic Reactions In Vitro and In Vivo. Molecules. 2022; 27(11):3473. https://doi.org/10.3390/molecules27113473
Chicago/Turabian StyleZhang, Ping, Yanjie Wang, Jingyu Zhang, and Tie Hong. 2022. "Allantoin Inhibits Compound 48/80-Induced Pseudoallergic Reactions In Vitro and In Vivo" Molecules 27, no. 11: 3473. https://doi.org/10.3390/molecules27113473
APA StyleZhang, P., Wang, Y., Zhang, J., & Hong, T. (2022). Allantoin Inhibits Compound 48/80-Induced Pseudoallergic Reactions In Vitro and In Vivo. Molecules, 27(11), 3473. https://doi.org/10.3390/molecules27113473




