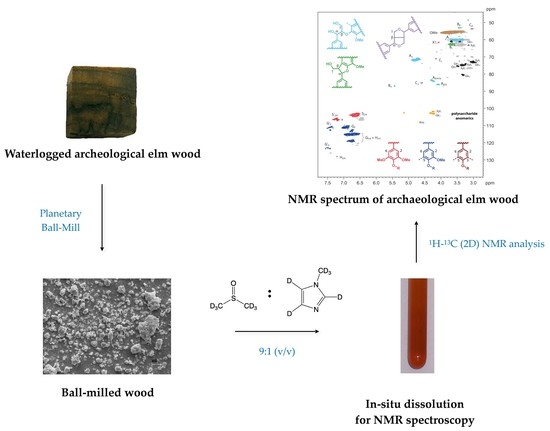
Reactivity of Waterlogged Archeological Elm Wood with Organosilicon Compounds Applied as Wood Consolidants: 2D 1H–13C Solution-State NMR Studies
Abstract
:1. Introduction
2. Results
2.1. Effectiveness of Organosilicon Compounds in Waterlogged Wood Stabilization
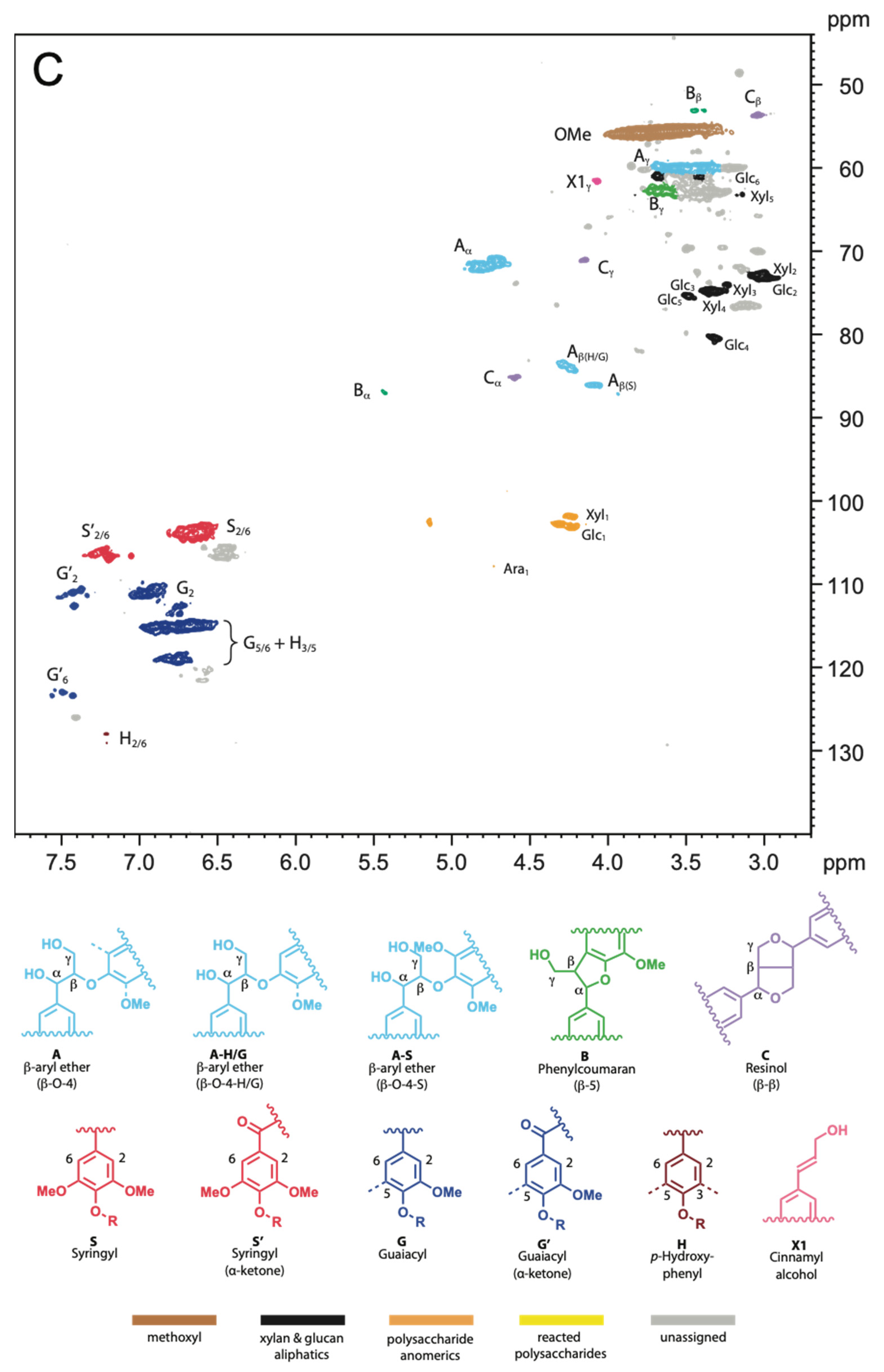
2.2. NMR Spectra
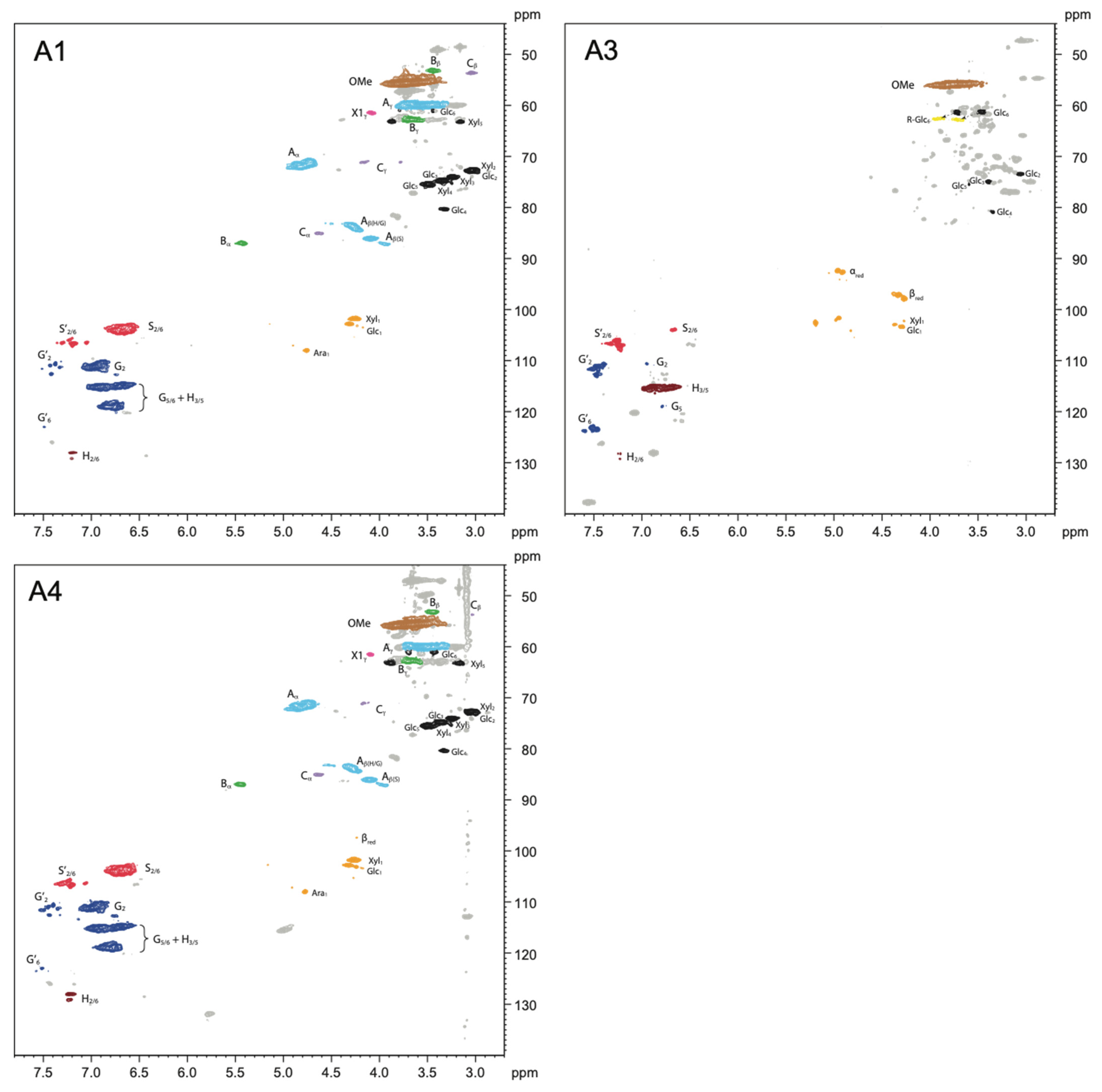
2.2.1. Alkoxysilane-Treated Wood
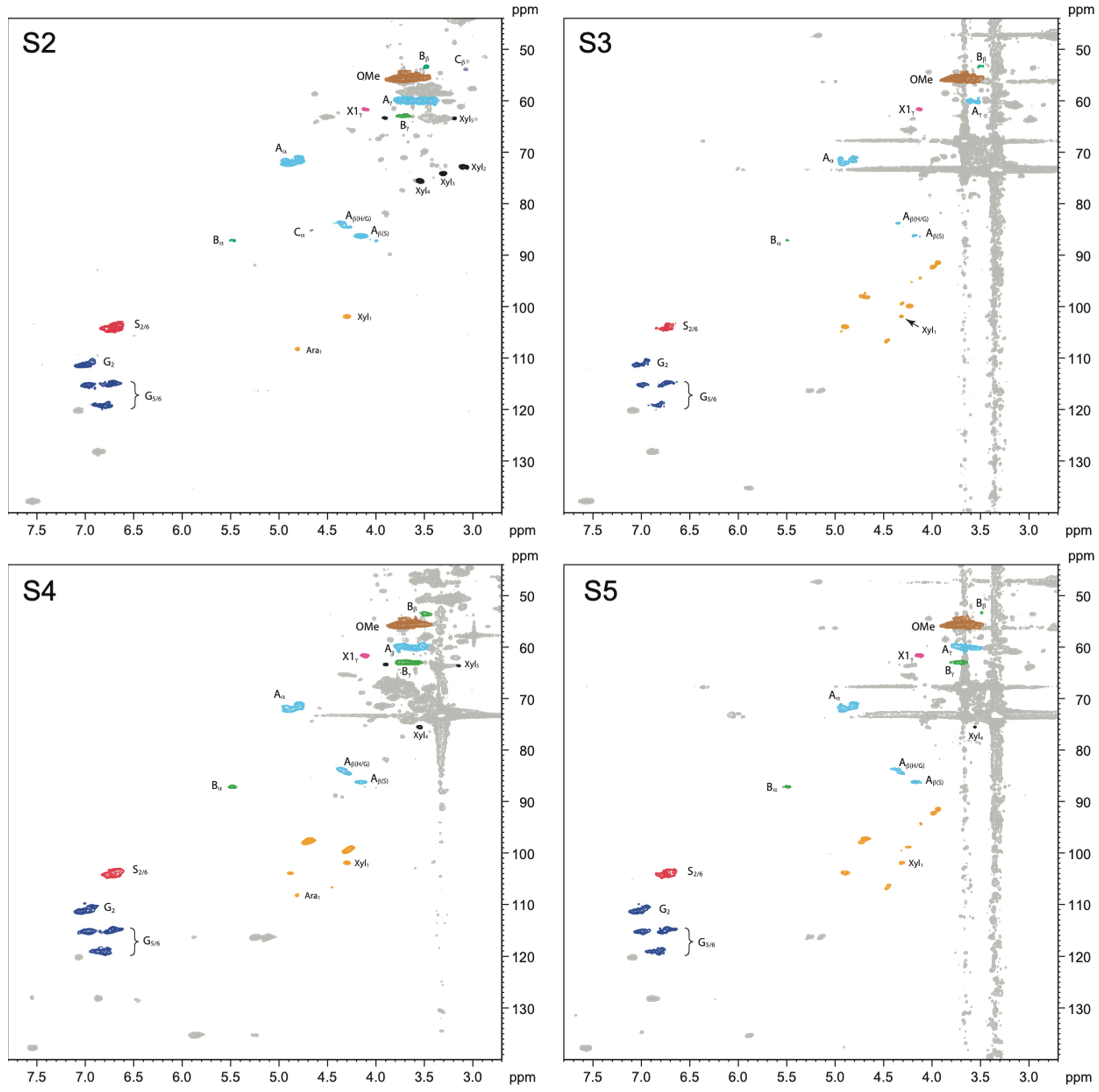
2.2.2. Siloxane-Treated Wood
3. Discussion
4. Materials and Methods
4.1. Materials
- -
- methyltrimethoxysilane CH3Si(OCH3)3 (A1);
- -
- 1-[3-(trimethoxysilyl)propyl]pyridinium chloride (C5H5NCl)C3H6Si(OCH3)3 (A2);
- -
- (3-mercaptopropyl)trimethoxysilane HS(CH2)3Si(OCH3)3 (A3);
- -
- (3-thiocyanatopropyl)trimethoxysilane NCS(CH2)3Si(OCH3)3 (A4);
- -
- 1,3-bis(3-glycidyloxypropyl)-1,1,3,3-tetramethyldisiloxane [CH2(O)CHCH2O(CH2)3Si(CH3)2]2O (S1);
- -
- 1,3-bis(3-aminopropyl)-1,1,3,3-tetramethyldisiloxane [H2N(CH2)3Si(CH3)2]2O (S2);
- -
- 1,3-bis-[(diethylamino)-3-(propoxy)propan-2-ol]-1,1,3,3-tetramethyldisiloxane [(C2H5)2NCH2CH(OH)CH2O(CH2)3Si(CH3)2]2O (S3);
- -
- 1,3-bis-[(ethylenodiamino)-3-(propoxy)propan-2-ol]-1,1,3,3-tetramethyldisiloxane [(NH2(CH2)2HNCH2CH(OH)CH2O(CH2)3Si(CH3)2]2O (S4);
- -
- 1,3,5,7-tetrakis(1-(diethylamino)-3-(propoxy)propan-2-ol)-1,3,5,7-tetramethylcyclotetrasiloxane [(C2H5)2NCH2CH(OH)CH2O(CH2)3Si(CH3)O]4 (S5);
- -
- 1,3,5,7-tetrakis(3-polyethoxypropyl)-1,3,5,7-tetramethyltetracyclosiloxane methoxy terminated [CH3O(CH2CH2O)7(CH2)3Si(CH3)O]4 (S6);
- -
- 3-[3-(hydroxy)(polyethoxypropyl)]1,1,1,3,5,5,5-heptamethyltrisiloxane HO(CH2CH2O)7(CH2)3Si(CH3)[OSi(CH3)3]2 (S7).
4.2. Methods
4.2.1. Waterlogged Wood Treatment
4.2.2. Wood Preparation for NMR
4.2.3. Wood Cell Wall Dissolution
4.2.4. NMR Analysis
5. Conclusions
- -
- New chemical bonds were formed between (3-mercaptopropyl)trimethoxysilane and cellulose in waterlogged wood. In the case of other organosilicons, it was difficult to assign unidentified peaks in NMR spectra to potential new bonds formed between them and wood polymers. This problem is planned to be solved in future research.
- -
- The active sites in wood polymers that interacted with organosilicons were C6 primary hydroxyls in cellulose (in the case of (3-mercaptopropyl)trimethoxysilane treatment), as well as methoxyl (in both types of organosilicon treatments) and α-carbonyl groups in aromatic lignin units (in the case of siloxane treatment).
- -
- In general, alkoxysilanes appear to preferentially react with lignin, while siloxanes can modify lignin and polysaccharides; only (3-mercaptopropyl)trimethoxysilane was confirmed to react also with cellulose.
- -
- Since a similar modification of wood polymers was observed for both groups of organosilicons used in this study, but their effectiveness as wood stabilizers was different, we cannot state if lignin demethoxylation or modification of lignin aromatics by organosilicons plays a crucial role in the stabilizing mechanism; on the other hand, we can clearly state that the extensive chemical modification of 3-mercaptopropyl)trimethoxysilane (containing a reactive mercapto group) with wood polymers is crucial for the excellent stabilization of waterlogged wood dimensions during drying.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Braovac, S.; Kutzke, H. The Presence of Sulfuric Acid in Alum-Conserved Wood–Origin and Consequences. J. Cult. Herit. 2012, 13, S203–S208. [Google Scholar] [CrossRef]
- Braovac, S.; Tamburini, D.; Lucejko, J.J.; McQueen, C.; Kutzke, H.; Colombini, M.P. Chemical Analyses of Extremely Degraded Wood Using Analytical Pyrolysis and Inductively Coupled Plasma Atomic Emission Spectroscopy. Microchem. J. 2016, 124, 368–379. [Google Scholar] [CrossRef]
- Hoffmann, P. On the Stabilization of Waterlogged Oakwood with PEG. II. Designing a Two-Step Treatment for Multi-Quality Timbers. Stud. Conserv. 1986, 31, 103–113. [Google Scholar] [CrossRef]
- Hoffmann, P. On the Stabilization of Waterlogged Softwoods with Polyethylene Glycol (PEG). Four Species from China and Korea. Holzforschung 1990, 44, 87–93. [Google Scholar] [CrossRef]
- Hocker, E.; Almkvist, G.; Sahlstedt, M. The Vasa Experience with Polyethylene Glycol: A Conservator’s Perspective. J. Cult. Herit. 2012, 13, S175–S182. [Google Scholar] [CrossRef]
- Collett, H.; Bouville, F.; Giuliani, F.; Schofield, E. Structural Monitoring of a Large Archaeological Wooden Structure in Real Time, Post PEG Treatment. Forests 2021, 12, 1788. [Google Scholar] [CrossRef]
- Tahira, A.; Howard, W.; Pennington, E.R.; Kennedy, A. Mechanical Strength Studies on Degraded Waterlogged Wood Treated with Sugars. Stud. Conserv. 2017, 62, 223–228. [Google Scholar] [CrossRef]
- Morgós, A.; Imazu, S.; Ito, K. Sugar Conservation of Waterlogged Archaeological Finds in the Last 30 Years. In Proceedings of the 2015 Conservation and Digitalization Conference, London, UK, 19–22 May 2015; pp. 15–20. [Google Scholar]
- Hoffmann, P. To Be and to Continue Being a Cog: The Conservation of the Bremen Cog of 1380. Int. J. Naut. Archaeol. 2001, 30, 129–140. [Google Scholar] [CrossRef]
- Broda, M.; Hill, C.A.S. Conservation of Waterlogged Wood–Past, Present and Future Perspectives. Forests 2021, 12, 1193. [Google Scholar] [CrossRef]
- McQueen, C.M.; Tamburini, D.; Lucejko, J.J.; Braovac, S.; Gambineri, F.; Modugno, F.; Colombini, M.P.; Kutzke, H. New Insights into the Degradation Processes and Influence of the Conservation Treatment in Alum-Treated Wood from the Oseberg Collection. Microchem. J. 2017, 132, 119–129. [Google Scholar] [CrossRef] [Green Version]
- McQueen, C.M.A.; Mortensen, M.N.; Caruso, F.; Mantellato, S.; Braovac, S. Oxidative Degradation of Archaeological Wood and the Effect of Alum, Iron and Calcium Salts. Herit. Sci. 2020, 8, 32. [Google Scholar] [CrossRef]
- Mortensen, M.N.; Egsgaard, H.; Hvilsted, S.; Shashoua, Y.; Glastrup, J. Characterisation of the Polyethylene Glycol Impregnation of the Swedish Warship Vasa and One of the Danish Skuldelev Viking Ships. J. Archaeol. Sci. 2007, 34, 1211–1218. [Google Scholar] [CrossRef]
- Mortensen, M.N.; Egsgaard, H.; Hvilsted, S.; Shashoua, Y.; Glastrup, J. Tetraethylene Glycol Thermooxidation and the Influence of Certain Compounds Relevant to Conserved Archaeological Wood. J. Archaeol. Sci. 2012, 39, 3341–3348. [Google Scholar] [CrossRef]
- Broda, M.; Dąbek, I.; Dutkiewicz, A.; Dutkiewicz, M.; Popescu, C.-M.; Mazela, B.; Maciejewski, H. Organosilicons of Different Molecular Size and Chemical Structure as Consolidants for Waterlogged Archaeological Wood–a New Reversible and Retreatable Method. Sci. Rep. 2020, 10, 2188. [Google Scholar] [CrossRef] [Green Version]
- Broda, M.; Mazela, B.; Dutkiewicz, A. Organosilicon Compounds with Various Active Groups as Consolidants for the Preservation of Waterlogged Archaeological Wood. J. Cult. Herit. 2019, 35, 123–128. [Google Scholar] [CrossRef]
- Kavvouras, P.K.; Kostarelou, C.; Zisi, A.; Petrou, M.; Moraitou, G. Use of Silanol-Terminated Polydimethylsiloxane in the Conservation of Waterlogged Archaeological Wood. Stud. Conserv. 2009, 54, 65–76. [Google Scholar] [CrossRef]
- Hidayat, B.J.; Felby, C.; Johansen, K.S.; Thygesen, L.G. Cellulose Is Not Just Cellulose: A Review of Dislocations as Reactive Sites in the Enzymatic Hydrolysis of Cellulose Microfibrils. Cellulose 2012, 19, 1481–1493. [Google Scholar] [CrossRef]
- Heinze, T. Cellulose: Structure and Properties. In Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Rojas, O.J., Ed.; Advances in Polymer Science; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–52. ISBN 978-3-319-26015-0. [Google Scholar]
- Peng, F.; Ren, J.L.; Xu, F.; Sun, R.-C. Chemicals from Hemicelluloses: A Review. In Sustainable Production of Fuels, Chemicals, and Fibers from Forest Biomass; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2011; Volume 1067, pp. 219–259. ISBN 978-0-8412-2643-2. [Google Scholar]
- Sun, R.; Sun, X.F.; Tomkinson, J. Hemicelluloses and Their Derivatives. ACS Symp. Ser. 2004, 864, 2–22. [Google Scholar] [CrossRef] [Green Version]
- Varila, T.; Romar, H.; Luukkonen, T.; Hilli, T.; Lassi, U. Characterization of Lignin Enforced Tannin/Furanic Foams. Heliyon 2020, 6, e03228. [Google Scholar] [CrossRef]
- Antonino, L.D.; Gouveia, J.R.; de Sousa Júnior, R.R.; Garcia, G.E.S.; Gobbo, L.C.; Tavares, L.B.; dos Santos, D.J. Reactivity of Aliphatic and Phenolic Hydroxyl Groups in Kraft Lignin towards 4,4′ MDI. Molecules 2021, 26, 2131. [Google Scholar] [CrossRef]
- Donath, S.; Militz, H.; Mai, C. Wood Modification with Alkoxysilanes. Wood Sci. Technol. 2004, 38, 555–566. [Google Scholar] [CrossRef]
- Tesoro, G. Yulong Wu Silane Coupling Agents: The Role of the Organofunctional Group. J. Adhes. Sci. Technol. 1991, 5, 771–784. [Google Scholar] [CrossRef]
- Tingaut, P.; Weigenand, O.; Mai, C.; Militz, H.; Sèbe, G. Chemical Reaction of Alkoxysilane Molecules in Wood Modified with Silanol Groups. Holzforschung 2006, 60, 271–277. [Google Scholar] [CrossRef]
- Brochier Salon, M.-C.; Abdelmouleh, M.; Boufi, S.; Belgacem, M.N.; Gandini, A. Silane Adsorption onto Cellulose Fibers: Hydrolysis and Condensation Reactions. J. Colloid Interface Sci. 2005, 289, 249–261. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Taipina, M.; Ferrarezi, M.M.F.; Yoshida, I.V.P.; Gonçalves, M.D.C. Surface Modification of Cotton Nanocrystals with a Silane Agent. Cellulose 2013, 20, 217–226. [Google Scholar] [CrossRef]
- Nakatani, H.; Iwakura, K.; Miyazaki, K.; Okazaki, N.; Terano, M. Effect of Chemical Structure of Silane Coupling Agent on Interface Adhesion Properties of Syndiotactic Polypropylene/Cellulose Composite. J. Appl. Polym. Sci. 2011, 119, 1732–1741. [Google Scholar] [CrossRef]
- Salon, M.-C.B.; Gerbaud, G.; Abdelmouleh, M.; Bruzzese, C.; Boufi, S.; Belgacem, M.N. Studies of Interactions between Silane Coupling Agents and Cellulose Fibers with Liquid and Solid-State NMR. Magn. Reson. Chem. 2007, 45, 473–483. [Google Scholar] [CrossRef]
- Robles, E.; Csóka, L.; Labidi, J. Effect of Reaction Conditions on the Surface Modification of Cellulose Nanofibrils with Aminopropyl Triethoxysilane. Coatings 2018, 8, 139. [Google Scholar] [CrossRef] [Green Version]
- Neves, R.M.; Ornaghi, H.L.; Zattera, A.J.; Amico, S.C. The Influence of Silane Surface Modification on Microcrystalline Cellulose Characteristics. Carbohydr. Polym. 2020, 230, 115595. [Google Scholar] [CrossRef]
- Siuda, J.; Perdoch, W.; Mazela, B.; Zborowska, M. Catalyzed Reaction of Cellulose and Lignin with Methyltrimethoxysilane—FT-IR, 13C NMR and 29Si NMR Studies. Materials 2019, 12, 2006. [Google Scholar] [CrossRef] [Green Version]
- Prasetyo, E.N.; Kudanga, T.; Fischer, R.; Eichinger, R.; Nyanhongo, G.S.; Guebitz, G.M. Enzymatic Synthesis of Lignin–Siloxane Hybrid Functional Polymers. Biotechnol. J. 2012, 7, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.M.; Wang, H.; Lau, K.T.; Cardona, F. Effects of Chemical Treatments on Hemp Fibre Structure. Appl. Surf. Sci. 2013, 276, 13–23. [Google Scholar] [CrossRef]
- Li, H.; Bunrit, A.; Li, N.; Wang, F. Heteroatom-Participated Lignin Cleavage to Functionalized Aromatics. Chem. Soc. Rev. 2020, 49, 3748–3763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Y.; Brook, M.A. Reductive Degradation of Lignin and Model Compounds by Hydrosilanes. ACS Sustain. Chem. Eng. 2014, 2, 1983–1991. [Google Scholar] [CrossRef]
- Zhu, J.; Xue, L.; Wei, W.; Mu, C.; Jiang, M.; Zhou, Z. Modification of Lignin with Silane Coupling Agent to Improve the Interface of Poly(L-Lactic) Acid/Lignin Composites. BioResources 2015, 10, 4315–4325. [Google Scholar] [CrossRef] [Green Version]
- Baur, S.I.; Easteal, A.J. Improved Photoprotection of Wood by Chemical Modification with Silanes: NMR and ESR Studies. Polym. Adv. Technol. 2013, 24, 97–103. [Google Scholar] [CrossRef]
- Wang, Q.; Xiao, Z.; Wang, W.; Xie, Y. Coupling Pattern and Efficacy of Organofunctional Silanes in Wood Flour-Filled Polypropylene or Polyethylene Composites. J. Compos. Mater. 2015, 49, 677–684. [Google Scholar] [CrossRef]
- Sèbe, G.; Tingaut, P.; Safou-Tchiama, R.; Pétraud, M.; Grelier, S.; Jéso, B.D. Chemical Reaction of Maritime Pine Sapwood (Pinus Pinaster Soland) with Alkoxysilane Molecules: A Study of Chemical Pathways. Holzforschung 2004, 58, 511–518. [Google Scholar] [CrossRef]
- Grubbström, G.; Holmgren, A.; Oksman, K. Silane-Crosslinking of Recycled Low-Density Polyethylene/Wood Composites. Compos. Part A Appl. Sci. 2010, 41, 678–683. [Google Scholar] [CrossRef]
- Popescu, C.-M.; Broda, M. Interactions between Different Organosilicons and Archaeological Waterlogged Wood Evaluated by Infrared Spectroscopy. Forests 2021, 12, 268. [Google Scholar] [CrossRef]
- Yelle, D.J.; Ralph, J. Characterizing Phenol–Formaldehyde Adhesive Cure Chemistry within the Wood Cell Wall. Int. J. Adhes. Adhes. 2016, 70, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Namyslo, J.C.; Drafz, M.H.H.; Kaufmann, D.E. Durable Modification of Wood by Benzoylation—Proof of Covalent Bonding by Solution State NMR and DOSY NMR Quick-Test. Polymers 2021, 13, 2164. [Google Scholar] [CrossRef] [PubMed]
- Yelle, D.J.; Ralph, J.; Frihart, C.R. Delineating PMDI Model Reactions with Loblolly Pine via Solution-State NMR Spectroscopy. Part 2. Non-Catalyzed Reactions with the Wood Cell Wall. Holzforschung 2011, 65, 145–154. [Google Scholar] [CrossRef]
- Lu, F.; Ralph, J. Non-Degradative Dissolution and Acetylation of Ball-Milled Plant Cell Walls: High-Resolution Solution-State NMR. Plant J. 2003, 35, 535–544. [Google Scholar] [CrossRef] [Green Version]
- Yelle, D.J. Multifaceted Approach for Determining the Absolute Values for Lignin Subunits in Lignocellulosic Materials; Res. Note FPL-RN-0384; US Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 2020; p. 384. [Google Scholar]
- Miyagawa, Y.; Tobimatsu, Y.; Lam, P.Y.; Mizukami, T.; Sakurai, S.; Kamitakahara, H.; Takano, T. Possible Mechanisms for the Generation of Phenyl Glycoside-Type Lignin–Carbohydrate Linkages in Lignification with Monolignol Glucosides. Plant J. 2020, 104, 156–170. [Google Scholar] [CrossRef]
- Yelle, D.J.; Ralph, J.; Lu, F.; Hammel, K.E. Evidence for Cleavage of Lignin by a Brown Rot Basidiomycete. Environ. Microbiol. 2008, 10, 1844–1849. [Google Scholar] [CrossRef]
- Yelle, D.J.; Kapich, A.N.; Houtman, C.J.; Lu, F.; Timokhin, V.I.; Fort Jr, R.C.; Ralph, J.; Hammel, K.E. A Highly Diastereoselective Oxidant Contributes to Ligninolysis by the White Rot Basidiomycete Ceriporiopsis Subvermispora. Appl. Environ. Microbiol. 2014, 80, 7536–7544. [Google Scholar] [CrossRef] [Green Version]
- Yelle, D.J.; Kaparaju, P.; Hunt, C.G.; Hirth, K.; Kim, H.; Ralph, J.; Felby, C. Two-Dimensional NMR Evidence for Cleavage of Lignin and Xylan Substituents in Wheat Straw Through Hydrothermal Pretreatment and Enzymatic Hydrolysis. Bioenerg. Res. 2013, 6, 211–221. [Google Scholar] [CrossRef]
- Giachi, G.; Capretti, C.; Macchioni, N.; Pizzo, B.; Donato, I.D. A Methodological Approach in the Evaluation of the Efficacy of Treatments for the Dimensional Stabilisation of Waterlogged Archaeological Wood. J. Cult. Herit. 2010, 11, 91–101. [Google Scholar] [CrossRef]
- Grattan, D.W. 3-Waterlogged Wood. In Conservation of Marine Archaeological Objects; Pearson, C., Ed.; Butterworth-Heinemann: Oxford, UK, 1987; pp. 55–67. ISBN 978-0-408-10668-9. [Google Scholar]
- Broda, M.; Majka, J.; Olek, W.; Mazela, B. Dimensional Stability and Hygroscopic Properties of Waterlogged Archaeological Wood Treated with Alkoxysilanes. Int. Biodeter. Biodegr. 2018, 133, 34–41. [Google Scholar] [CrossRef]
- Broda, M.; Curling, S.F.; Spear, M.J.; Hill, C.A. Effect of Methyltrimethoxysilane Impregnation on the Cell Wall Porosity and Water Vapour Sorption of Archaeological Waterlogged Oak. Wood Sci. Technol. 2019, 53, 703–726. [Google Scholar] [CrossRef] [Green Version]
- Van Opdenbosch, D.; Dörrstein, J.; Klaithong, S.; Kornprobst, T.; Plank, J.; Hietala, S.; Zollfrank, C. Chemistry and Water-Repelling Properties of Phenyl-Incorporating Wood Composites. Holzforschung 2013, 67, 931–940. [Google Scholar] [CrossRef]
- Hill, C.A.S.; Farahani, M.R.M.; Hale, M.D.C. The Use of Organo Alkoxysilane Coupling Agents for Wood Preservation. Holzforschung 2004, 58, 316–325. [Google Scholar] [CrossRef]
- Pesti, J.; Larson, G.L. Tetramethyldisiloxane: A Practical Organosilane Reducing Agent. Org. Process Res. Dev. 2016, 20, 1164–1181. [Google Scholar] [CrossRef] [Green Version]
- Larson, G.L.; Fry, J.L. Ionic and Organometallic-Catalyzed Organosilane Reductions. In Organic Reactions; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 1–737. ISBN 978-0-471-26418-7. [Google Scholar]
- Marciniec, B. Hydrosilylation: A Comprehensive Review on Recent Advances. In Advances in Silicon Science; Springer: Dordrecht, Germany, 2009. [Google Scholar]
- Hossain, M.A.; Rahaman, M.S.; Yelle, D.; Shang, H.; Sun, Z.; Renneckar, S.; Dong, J.; Tulaphol, S.; Sathitsuksanoh, N. Effects of Polyol-Based Deep Eutectic Solvents on the Efficiency of Rice Straw Enzymatic Hydrolysis. Ind. Crops. Prod. 2021, 167, 113480. [Google Scholar] [CrossRef]
- Yelle, D.J.; Ralph, J.; Frihart, C.R. Characterization of Nonderivatized Plant Cell Walls Using High-Resolution Solution-State NMR Spectroscopy. Magn. Reson. Chem. 2008, 46, 508–517. [Google Scholar] [CrossRef]
- Methyltrimethoxysilane(1185-55-3) 13C NMR. Available online: https://www.chemicalbook.com/SpectrumEN_1185-55-3_13CNMR.htm (accessed on 29 April 2022).
- Safaei-Ghomi, J.; Nazemzadeh, S.H. Ionic Liquid-Attached Colloidal Silica Nanoparticles as a New Class of Silica Nanoparticles for the Preparation of Propargylamines. Catal. Lett. 2017, 147, 1696–1703. [Google Scholar] [CrossRef]
- 1-(Aminoformylmethyl)Pyridinium Chloride(41220-29-5) 13C NMR. Available online: https://www.chemicalbook.com/SpectrumEN_41220-29-5_13CNMR.htm (accessed on 29 April 2022).
- Wieszczycka, K.; Filipowiak, K.; Wojciechowska, I.; Buchwald, T.; Siwińska-Ciesielczyk, K.; Strzemiecka, B.; Jesionowski, T.; Voelkel, A. Novel Highly Efficient Ionic Liquid-Functionalized Silica for Toxic Metals Removal. Sep. Purif. Technol. 2021, 265, 118483. [Google Scholar] [CrossRef]
- Scott, A.F.; Gray-Munro, J.E.; Shepherd, J.L. Influence of Coating Bath Chemistry on the Deposition of 3-Mercaptopropyl Trimethoxysilane Films Deposited on Magnesium Alloy. J. Colloid Interface Sci. 2010, 343, 474–483. [Google Scholar] [CrossRef]
- The NMR of (3-Mercaptopropyl)Trimethoxysilane. Available online: http://www.hanhonggroup.com/nmr/nmr_en/MR040137.html (accessed on 29 April 2022).
- Wipfelder, E.; Höhn, K. Epoxysiloxane Resins by the Condensation of 3-Glycidyloxypropyltrimethoxysilane with Diphenylsilanediol. Angew. Makromolek. Chem. 1994, 218, 111–126. [Google Scholar] [CrossRef]
- 3-Glycidoxypropyltrimethoxysilane(2530-83-8) 13C NMR. Available online: https://www.chemicalbook.com/SpectrumEN_2530-83-8_13CNMR.htm (accessed on 29 April 2022).
- Li, S.; Kong, X.; Feng, S. Preparation of Uniform Poly(Urea–Siloxane) Microspheres through Precipitation Polymerization. RSC Adv. 2015, 5, 90313–90320. [Google Scholar] [CrossRef]
- Karasiewicz, J.; Krawczyk, J. Thermodynamic Analysis of Trisiloxane Surfactant Adsorption and Aggregation Processes. Molecules 2020, 25, 5669. [Google Scholar] [CrossRef] [PubMed]


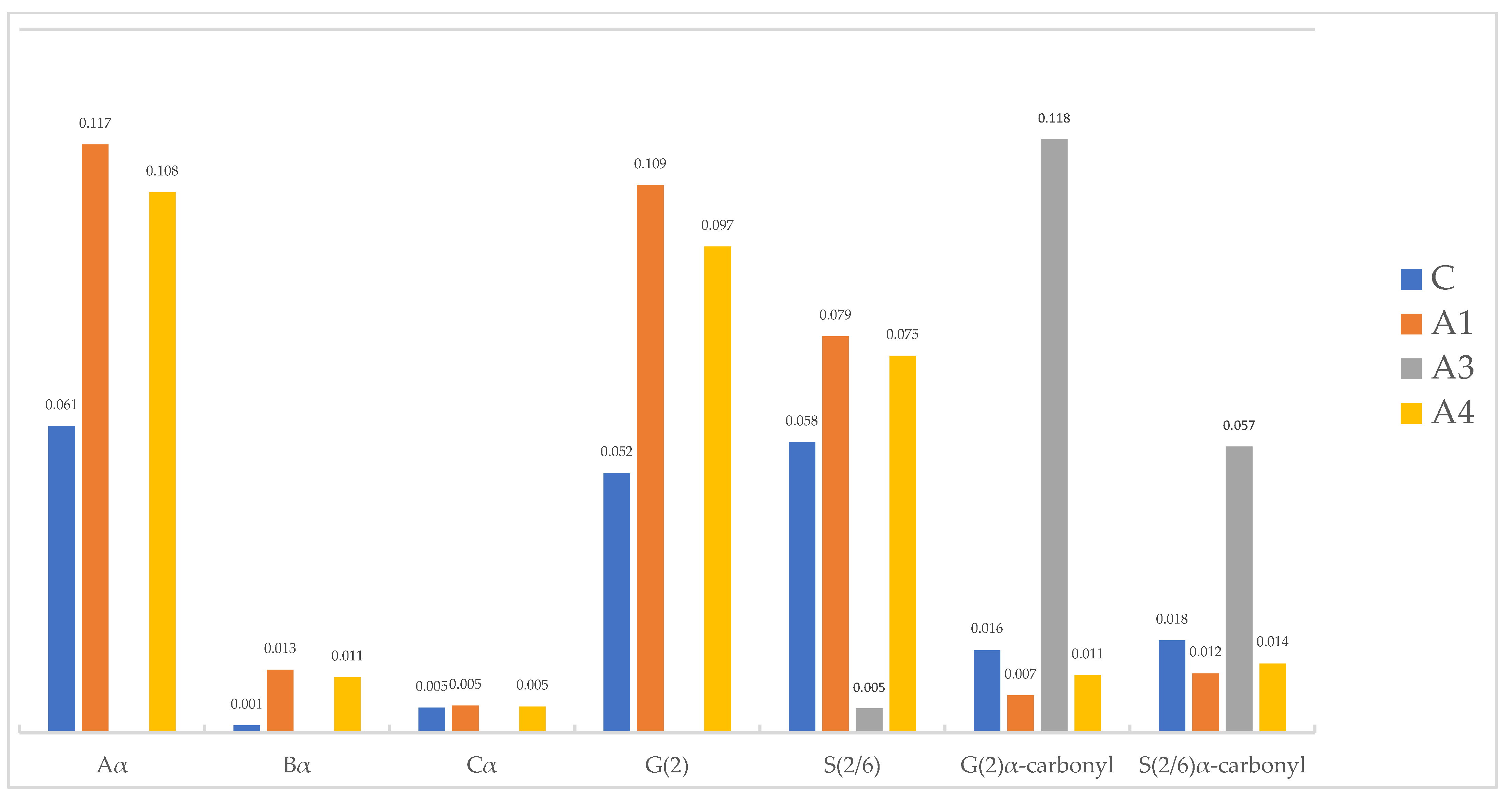
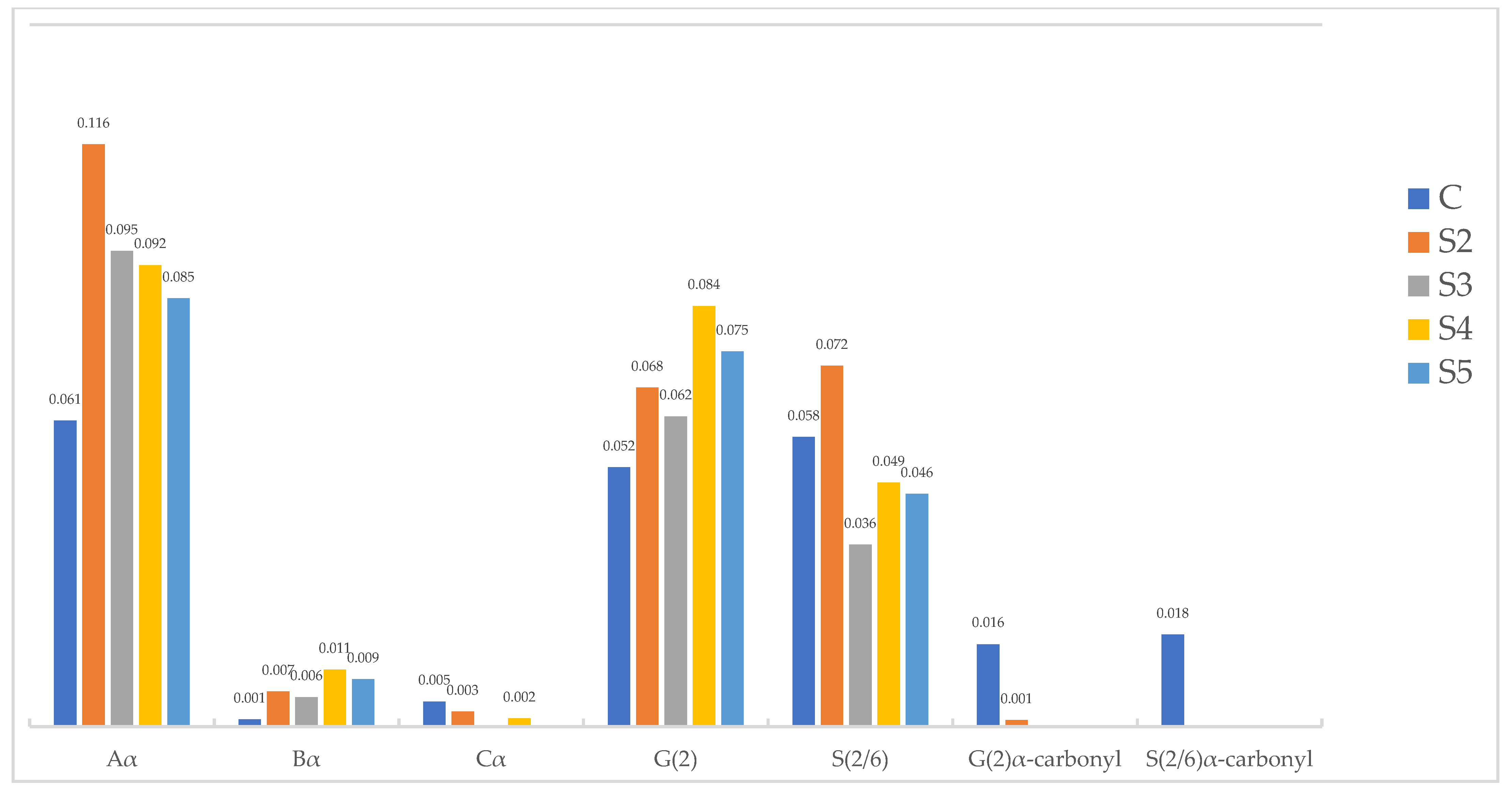
| Organosilicon Applied | Sv [%] | ASEv [%] | MW [g/mol] | WPG [%] |
|---|---|---|---|---|
| C | 55.1 ± 4.9 | - | - | - |
| A1 | 9.7 ± 1.3 | 82.4 | 136.22 | 231.9 ± 6.8 |
| A2 | 29.2 ± 7.3 | 47.0 | 277.82 | 328.8 ± 1.3 |
| A3 | 0.7 ± 0.5 | 98.7 | 196.34 | 136.9 ± 9.4 |
| A4 | 15.9 ± 3.5 | 71.1 | 221.35 | 212.5 ± 1.9 |
| S1 | 24.9 ± 1.7 | 54.8 | 362.61 | 227.3 ± 0.5 |
| S2 | 4.5 ± 1.4 | 91.8 | 248.51 | 236.2 ± 1.9 |
| S3 | 4.5 ± 1.4 | 91.8 | 508.88 | 219.8 ± 5.9 |
| S4 | 29.7 ± 2.5 | 46.1 | 482.80 | 234.1 ± 2.2 |
| S5 | 15.0 ± 0.9 | 72.8 | 989.62 | 231.8 ± 1.3 |
| S6 | 26.3 ± 1.1 | 52.3 | 1762.40 | 270.8 ± 2.1 |
| S7 | 5.3 ± 2.5 | 90.4 | 588.95 | 227.3 ± 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broda, M.; Yelle, D.J. Reactivity of Waterlogged Archeological Elm Wood with Organosilicon Compounds Applied as Wood Consolidants: 2D 1H–13C Solution-State NMR Studies. Molecules 2022, 27, 3407. https://doi.org/10.3390/molecules27113407
Broda M, Yelle DJ. Reactivity of Waterlogged Archeological Elm Wood with Organosilicon Compounds Applied as Wood Consolidants: 2D 1H–13C Solution-State NMR Studies. Molecules. 2022; 27(11):3407. https://doi.org/10.3390/molecules27113407
Chicago/Turabian StyleBroda, Magdalena, and Daniel J. Yelle. 2022. "Reactivity of Waterlogged Archeological Elm Wood with Organosilicon Compounds Applied as Wood Consolidants: 2D 1H–13C Solution-State NMR Studies" Molecules 27, no. 11: 3407. https://doi.org/10.3390/molecules27113407
APA StyleBroda, M., & Yelle, D. J. (2022). Reactivity of Waterlogged Archeological Elm Wood with Organosilicon Compounds Applied as Wood Consolidants: 2D 1H–13C Solution-State NMR Studies. Molecules, 27(11), 3407. https://doi.org/10.3390/molecules27113407