N,N-Diethyl-3-toluamide Formulation Based on Ethanol Containing 0.1% 2-Hydroxypropyl-β-cyclodextrin Attenuates the Drug’s Skin Penetration and Prolongs the Repellent Effect without Stickiness
Abstract
1. Introduction
2. Results
2.1. Changes in Characteristics of DEET/EtOH Formulations by the Addition of CDs
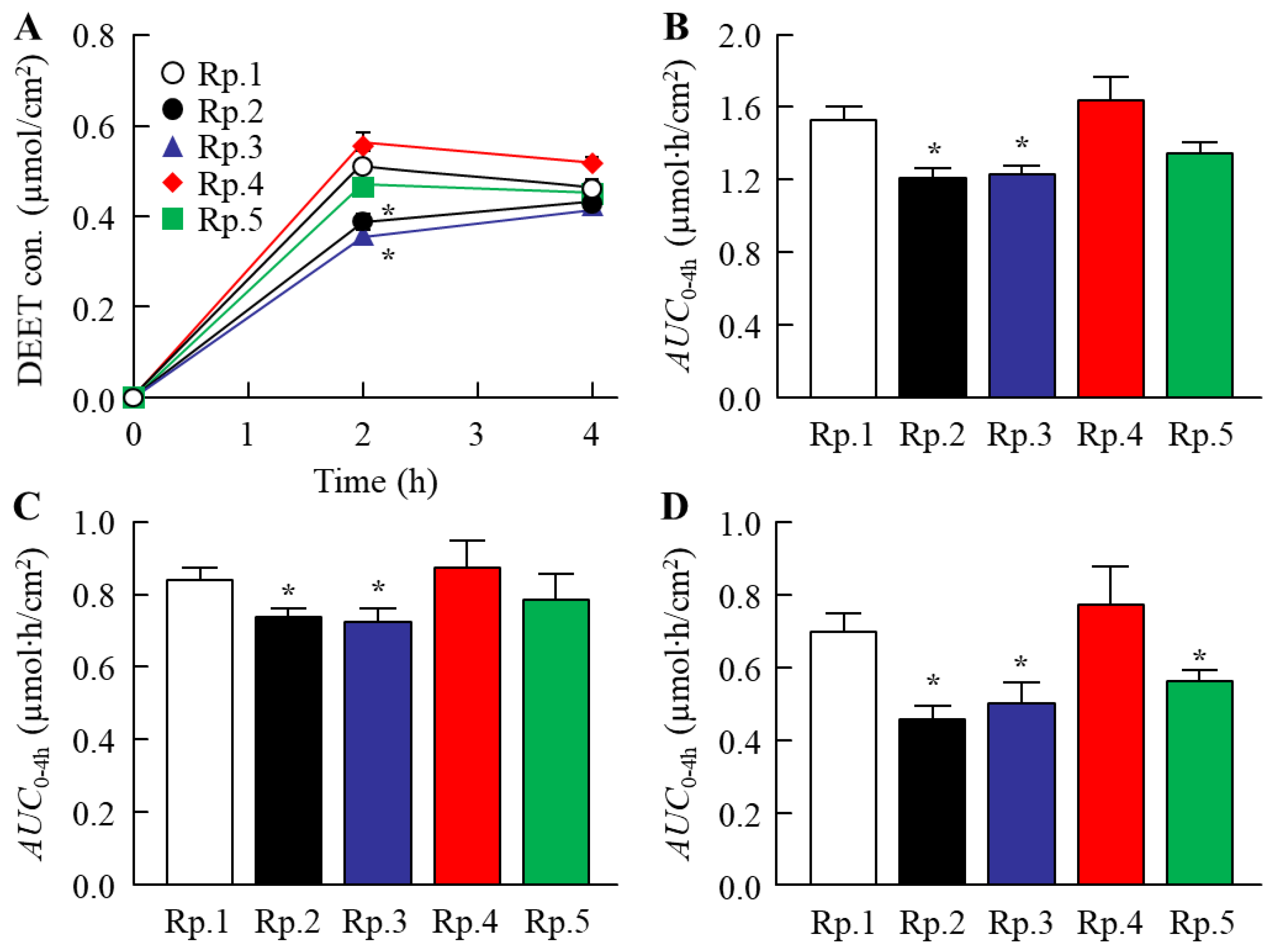
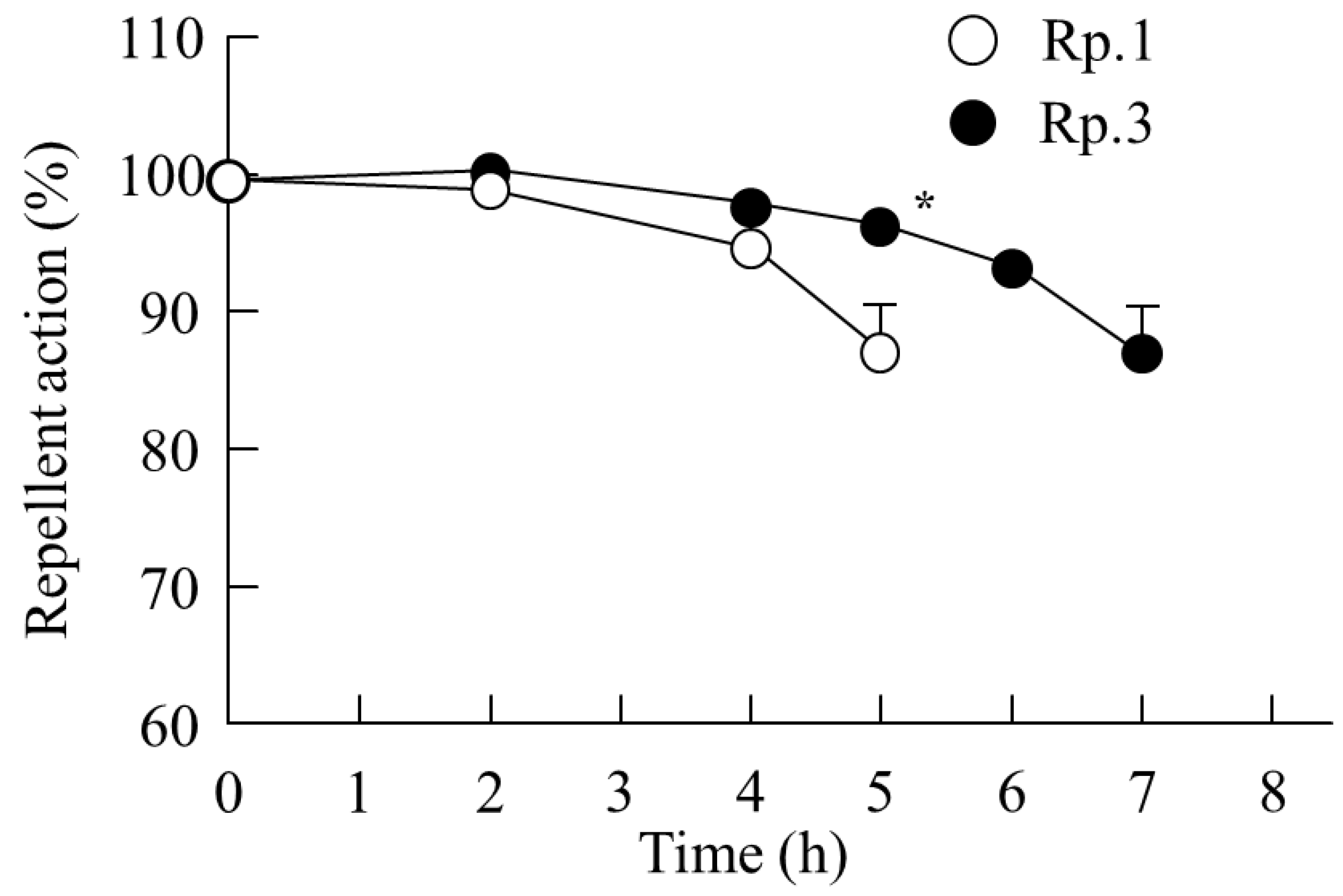
2.2. Changes in the Skin Penetration and Repellent Action of DEET/EtOH Formulations by the Addition of CDs
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Preparation of DEET/EtOH/CD Formulations
4.4. Characterization of DEET/EtOH/CD Formulations
4.5. Tensile Stress of DEET/EtOH/CD Formulations
4.6. Water Retention in DEET/EtOH/CD Formulations
4.7. Measurement of DEET on the Skin Surface, Stratum Corneum, Epithelium, and Dermis of Rats
4.8. Measurement of the Repellent Effect of DEET
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Paumgartten, F.J.R.; Delgado, I.F. Mosquito repellents, effectiveness in preventing diseases and safety during pregnancy. Vigil. Sanit. Debate 2016, 4, 97–104. [Google Scholar] [CrossRef]
- WHO. Guidelines for Efficacy Testing of Mosquito Repellents for Human Skin. pp. 1–6. Available online: Who/HTM/NTD/WHOPES/2009.4_eng.pdf (accessed on 3 February 2022).
- Degennaro, M. The mysterious multi-modal repellency of DEET. Fly 2015, 9, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Debboun, M.; Frances, S.P.; Strickman, D. Insect Repellents Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015; p. 409. ISBN 978-1-4665-5355-2. [Google Scholar]
- Shrestha, B.; Lee, Y. Cellular and molecular mechanisms of DEET toxicity and disease-carrying insect vectors: A review. Genes Genom. 2020, 42, 1131–1144. [Google Scholar] [CrossRef]
- Tavares, M.; da Silva, M.R.M.; de Oliveira de Siqueira, L.B.; Rodrigues, R.A.S.; Bodjolle-d’Almeira, L.; Dós Santos, E.P.; Ricci-Júnior, E. Trends in insect repellent formulations: A review. Int. J. Pharm. 2018, 539, 190–209. [Google Scholar] [CrossRef]
- Diaz, J.H. Chemical and plant-based insect repellents: Efficacy, Safety, and Toxicity. Wilderness Environ. Med. 2016, 27, 153–163. [Google Scholar] [CrossRef]
- Barradas, T.N.; Lopes, L.M.A.; Ricci-Júnior, E.; de Holanda e Silva, K.G.; Mansur, C.R.E. Development and characterization of micellar systems for application as insect repellents. Int. J. Pharm. 2013, 454, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Antwi, F.B.; Shama, L.M.; Peterson, R.K.D. Risk assessments for the insect repellents DEET and picaridin. Regul. Toxicol. Pharmacol. 2008, 51, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.K.; Kim, K.C.; Cho, Y.; Gwon, Y.D.; Cho, H.S.; Heo, Y.; Park, K.; Lee, Y.W.; Kim, M.; Oh, Y.K.; et al. Comparison of Repellency Effect of Mosquito Repellents for DEET, Citronella, and Fennel Oil. J. Parasitol. Res. 2015, 2015, 361021. [Google Scholar] [CrossRef]
- Islam, J.; Zaman, K.; Duarah, S.; Raju, P.S.; Chattopadhyay, P. Mosquito repellents: An insight into the chronological perspectives and novel discoveries. Acta Trop. 2017, 167, 216–230. [Google Scholar] [CrossRef]
- Pinto, I.C.; Cerqueira-Coutinho, C.S.; Santos, E.P.; Carmo, F.A.; Ricci-Junior, E. Development and characterization of repellent formulations based on nanostructured hydrogels. Drug Dev. Ind. Pharm. 2017, 43, 67–73. [Google Scholar] [CrossRef]
- Songkro, S.; Hayook, N.; Jaisawang, J.; Maneenuan, D.; Chuchome, T.; Kaewnopparat, N. Investigation of inclusion complexes of citronella oil, citronellal and citronellol with β-cyclodextrin for mosquito repellent. J. Incl. Phenom. Macrocycl. Chem. 2012, 72, 339–355. [Google Scholar] [CrossRef]
- Sakulku, U.; Nuchuchua, O.; Uawongyart, N.; Puttipipatkhachorn, S.; Soottitantawat, A.; Ruktanonchai, U. Characterization and mosquito repellent activity of citronella oil nanoemulsion. Int. J. Pharm. 2009, 372, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Maji, T.K.; Baruah, I.; Dube, S.; Hussain, M.R. Microencapsulation of zanthoxylum limonella oil (ZLO) in genipin crosslinked chitosan-gelatin complex for mosquito repellent application. Bioresour. Technol. 2007, 98, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Chittiteeranon, P.; Soontaros, S.; Pongsawasdi, P. Preparation and characterization of inclusion complexes containing fixolide, a synthetic musk fragrance and cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 69–73. [Google Scholar] [CrossRef]
- Hsieh, W.C.; Chang, C.P.; Gao, Y.L. Controlled release properties of Chitosan encapsulated volatile Citronella Oil microcapsules by thermal treatments. Colloids Surf. B Biointerfaces 2006, 53, 209–214. [Google Scholar] [CrossRef]
- Shrestha, B.; Nhuchhen Pradhan, R.; Nath, D.K.; Lee, Y. Cellular and molecular basis of IR3535 perception in Drosophila. Pest. Manag. Sci. 2022, 78, 793–802. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Conceição, J.; Adeoye, O.; Cabral-Marques, H.M.; Lobo, J.M.S. Cyclodextrins as Drug Carriers in Pharmaceutical Technology: The State of the Art. Curr. Pharm. Des. 2018, 24, 1405–1433. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, S.; Bao, F. Crystal structure of cyclomaltoheptaose (beta-cyclodextrin) complexes with p-aminobenzoic acid and o-aminobenzoic acid. Carbohydr. Res. 2008, 343, 2504–2508. [Google Scholar] [CrossRef]
- Stella, V.J.; Rao, V.M.; Zannou, E.A.; Vahid, Z. Mechanisms of drug release from cyclodextrin complexes. Adv. Drug Delivery Rev. 1999, 36, 3–16. [Google Scholar] [CrossRef]
- Campos-Candel, A.; Llobat-Estellés, M.; Mauri-Aucejo, A. Comparative evaluation of liquid chromatography versus gas chromatography using a beta-cyclodextrin stationary phase for the determination of BTEX in occupational environments. Talanta 2009, 78, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Romi, R.; Nostro, P.L.; Bocci, E.; Ridi, F.; Baglioni, P. Bioengineering of a Cellulosic Fabric for Insecticide Delivery via Grafted Cyclodextrin. Biotechnol. Prog. 2005, 21, 1724–1730. [Google Scholar] [CrossRef]
- Lis, M.J.; García Carmona, Ó.; García Carmona, C.; Maestá Bezerra, F. Inclusion Complexes of Citronella Oil with β-Cyclodextrin for Controlled Release in Biofunctional Textiles. Polymers 2018, 10, 1324. [Google Scholar] [CrossRef] [PubMed]
- Cal, K.; Centkowska, K. Use of cyclodextrins in topical formulations: Practical aspects. Eur. J. Pharm. Biopharm. 2008, 68, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Cecone, C.; Caldera, F.; Trotta, F.; Bracco, P.; Zanetti, M. Controlled Release of DEET Loaded on Fibrous Mats from Electrospun PMDA/Cyclodextrin Polymer. Molecules 2018, 23, 1694. [Google Scholar] [CrossRef]
- Proniuk, S.; Liederer, B.M.; Dixon, S.E.; Rein, J.A.; Kallen, M.A.; Blanchard, J. Topical formulation studies with DEET (N,N-diethyl-3-methylbenzamide) and cyclodextrins. J. Pharm. Sci. 2002, 91, 101–110. [Google Scholar] [CrossRef]
- Carneiro, S.B.; Costa Duarte, F.I.; Heimfarth, L.; de Souza Siqueira Quintans, J.; José Quintans-Júnior, L.; de Veiga Júnior, V.F.; de Lima, Á.A.N. Cyclodextrin–Drug Inclusion Complexes: In Vivo and In Vitro Approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef]
- Srivalli, K.M.R.; Mishra, B. Improved Aqueous Solubility and Antihypercholesterolemic Activity of Ezetimibe on Formulating with Hydroxypropyl-β-Cyclodextrin and Hydrophilic Auxiliary Substances. AAPS PharmSciTech 2016, 17, 272–283. [Google Scholar] [CrossRef]
- Folch-Cano, C.; Yazdani-Pedram, M.; Olea-Azar, C. Inclusion and functionalization of polymers with cyclodextrins: Current applications and future prospects. Molecules 2014, 19, 14066–14079. [Google Scholar] [CrossRef]
- Misiuk, W.; Zalewska, M. Investigation of inclusion complex of trazodone hydrochloride with hydroxypropyl-β-cyclodextrin. Carbohydr. Polym. 2009, 77, 482–488. [Google Scholar] [CrossRef]
- Gould, S.; Scott, R.C. 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD): A toxicology review. Food Chem. Toxicol. 2005, 43, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Legrand, F.X.; Sauthier, M.; Flahaut, C.; Hachani, J.; Elfakir, C.; Fourmentin, S.; Tilloy, S.; Monflier, E. Aqueous hydroformylation reraction mediated by randomly methylated β-cyclodextrin: How substitution degree influences catalytic activity and selectivity. J. Mol. Catal. A Chem. 2009, 303, 72–77. [Google Scholar] [CrossRef]
- Monfliera, E.; Tilloy, S.; Fremy, G.; Castanet, Y.; Morteux, A. A further breakthrough in biphasic, rhodium-catalyzed hydroformylation: The use of Per(2,6-di-O-methyl)-β-cyclodextrin as inverse phase transfer catalyst. Tetrahedron Lett. 1995, 36, 9481–9484. [Google Scholar] [CrossRef]
- Merkus, F.W.; Verhoef, J.C.; Marttin, E.; Romeijn, S.G.; van der Kuy, P.H.; Hermens, W.A.; Schipper, N.G. Cyclodextrins in nasal drug delivery. Adv. Drug Delivery Rev. 1999, 36, 41–57. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins. 1. drug solubilization and stabilization. J. Pharm. Sci. 1996, 85, 1017–1025. [Google Scholar] [CrossRef]
- Katz, T.M.; Miller, J.H.; Hebert, A.A. Insect repellents: Historical perspectives and new developments. J. Am. Acad. Dermatol. 2008, 58, 865–871. [Google Scholar] [CrossRef]
- Caldera, F.; Tannous, M.; Cavalli, R.; Zanetti, M.; Trotta, F. Evolution of Cyclodextrin Nanosponges. Int. J. Pharm. 2017, 531, 470–479. [Google Scholar] [CrossRef]
- Ross, J.S.; Shah, J.C. Reduction in skin permeation of N,N-diethyl-m-toluamide (DEET) by altering the skin/vehicle partition coefficient. J. Control Release 2000, 67, 211–221. [Google Scholar] [CrossRef]
- Bos, J.D.; Meinardi, M.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef]
- Yano, T.; Nakagawa, A.; Tsuji, M.; Noda, K. Skin permeability of various non-steroidal anti-inflammatory drugs in man. Life Sci. 1986, 39, 1043–1050. [Google Scholar] [CrossRef]
- Scheuplein, R.J.; Blank, I.H.; Brauner, G.J.; MacFarlane, D.J. Percutaneous absorption of steroids. J. Investig. Dermatol. 1969, 52, 63–70. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Flynn, G.L.; Yalkowsky, S.H. Correlation and prediction of mass transport across membranes. I. Influence of alkyl chain length on flux-determining properties of barrier and diffusant. J. Pharm. Sci. 1972, 61, 838–852. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ogata, F.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Design of a transdermal formulation containing raloxifene nanoparticles for osteoporosis treatment. Int. J. Nanomed. 2018, 13, 5215–5229. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ogata, F.; Yamaguchi, M.; Fukuoka, Y.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Combination with l-Menthol Enhances Transdermal Penetration of Indomethacin Solid Nanoparticles. Int. J. Mol. Sci. 2019, 20, 3644. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ogata, F.; Ishii, M.; Fukuoka, Y.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Involvement of Endocytosis in the Transdermal Penetration Mechanism of Ketoprofen Nanoparticles. Int. J. Mol. Sci. 2018, 19, 2138. [Google Scholar] [CrossRef]

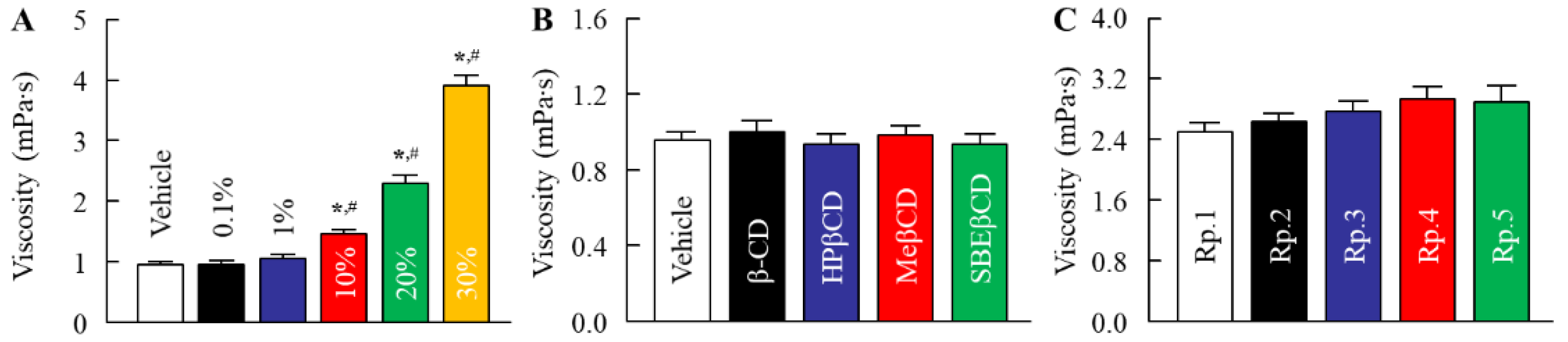
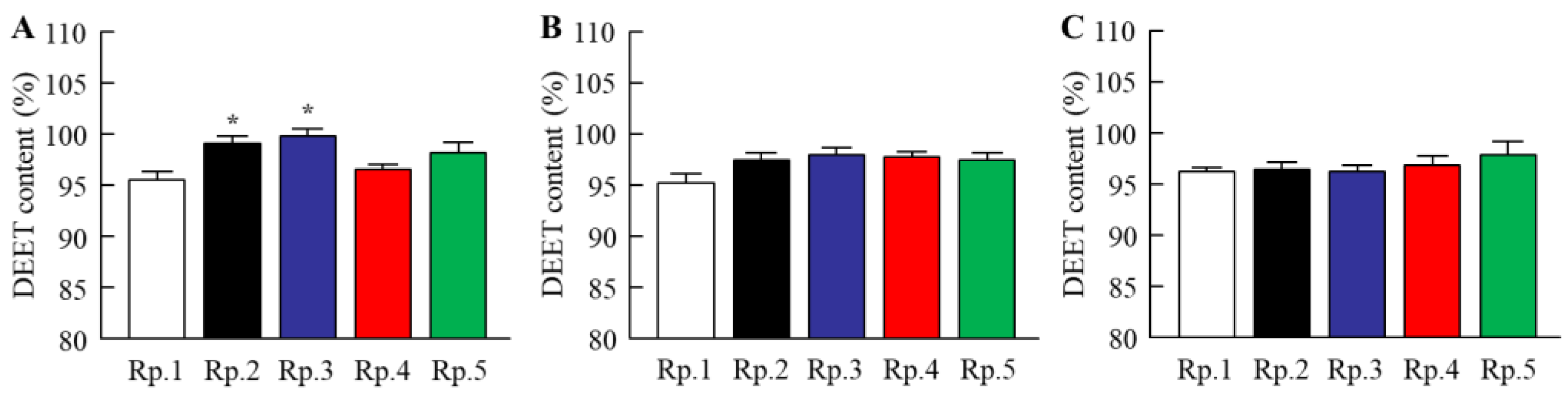
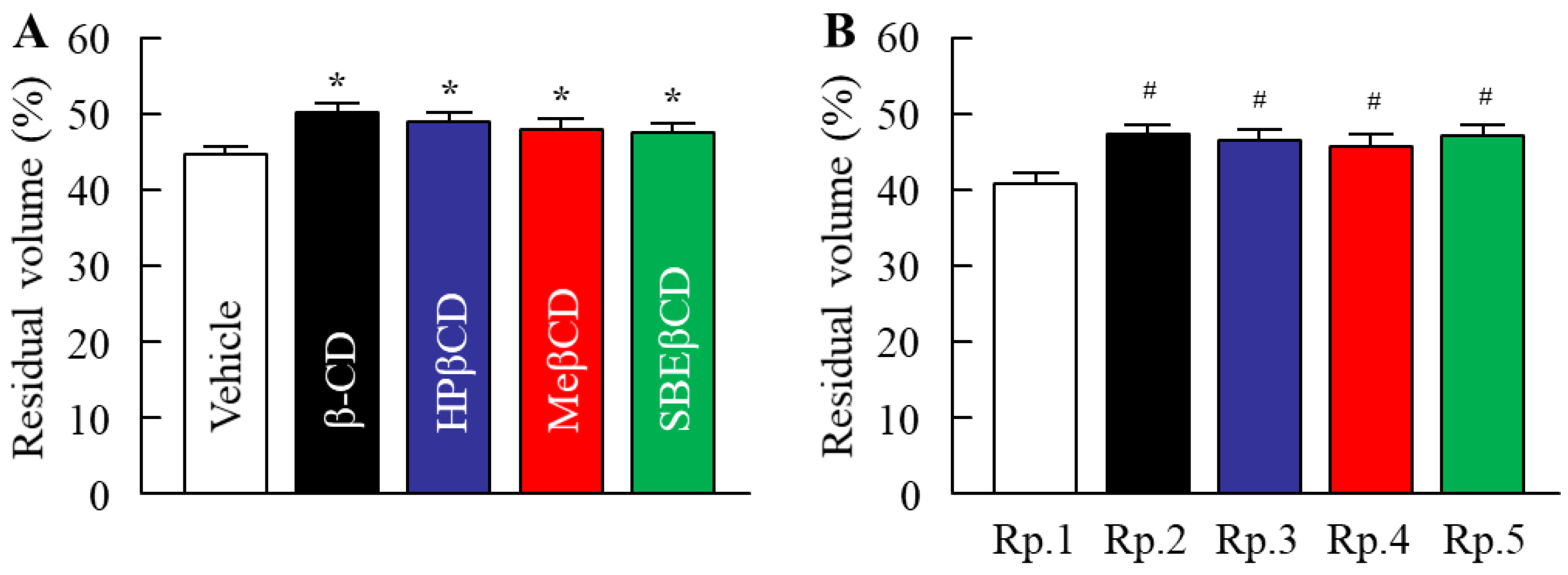
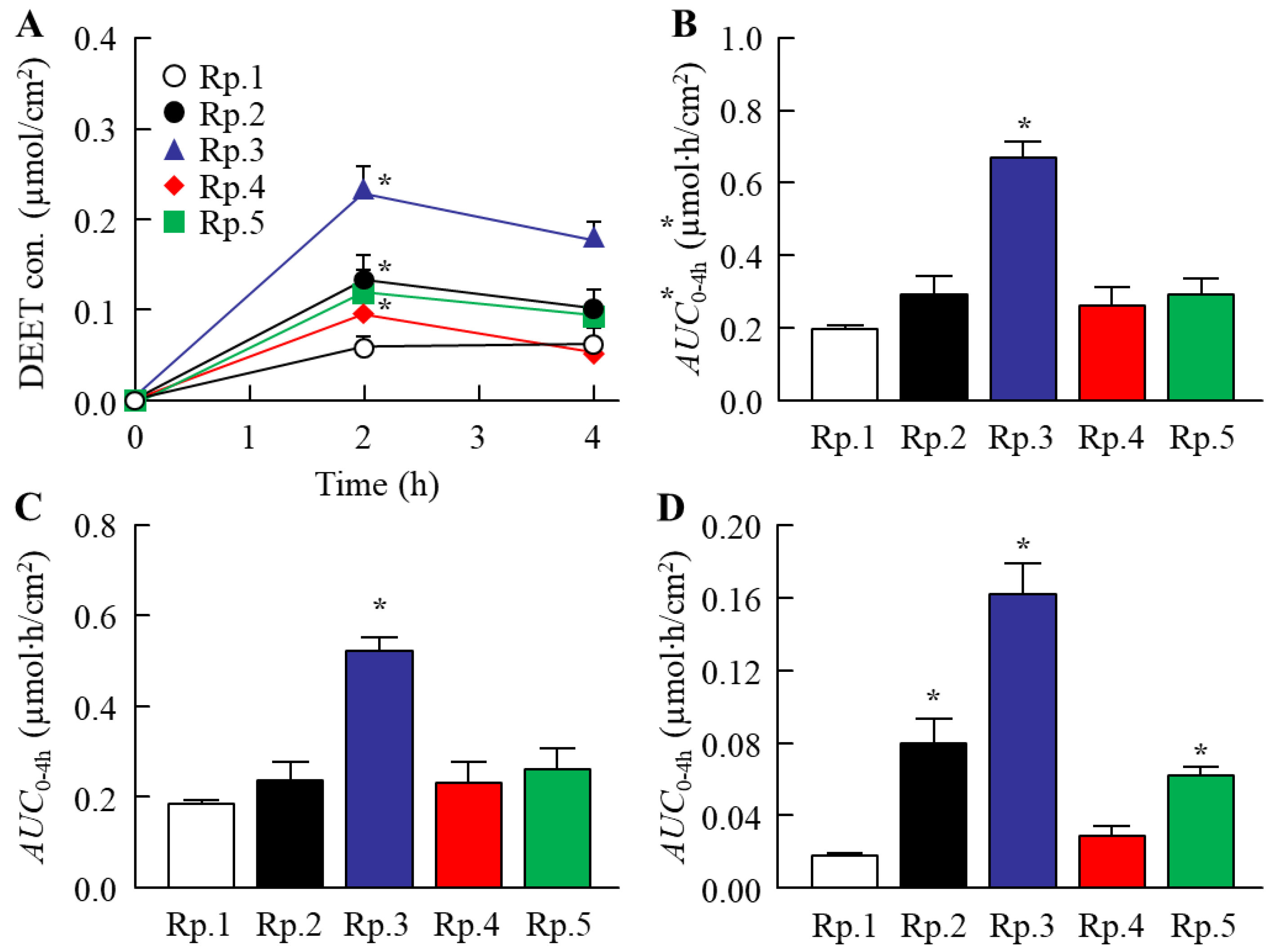

| Formulation | Content (w/v%) | ||||||
|---|---|---|---|---|---|---|---|
| DEET | EtOH | β-CD | HPβCD | MeβCD | SBEβCD | Distilled Water ad. | |
| Rp. 1 DEET/EtOH | 10 | 40 | 100 | ||||
| Rp. 2 DEET/EtOH/βCD | 10 | 40 | 0.1 | 100 | |||
| Rp. 3 DEET/EtOH/HPβCD | 10 | 40 | 0.1 | 100 | |||
| Rp. 4 DEET/EtOH/MeβCD | 10 | 40 | 0.1 | 100 | |||
| Rp. 5 DEET/EtOH/SBEβCD | 10 | 40 | 0.1 | 100 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagai, N.; Kawaguchi, M.; Minami, M.; Matsumoto, K.; Sasabe, T.; Nobuhara, K.; Matsubara, A. N,N-Diethyl-3-toluamide Formulation Based on Ethanol Containing 0.1% 2-Hydroxypropyl-β-cyclodextrin Attenuates the Drug’s Skin Penetration and Prolongs the Repellent Effect without Stickiness. Molecules 2022, 27, 3174. https://doi.org/10.3390/molecules27103174
Nagai N, Kawaguchi M, Minami M, Matsumoto K, Sasabe T, Nobuhara K, Matsubara A. N,N-Diethyl-3-toluamide Formulation Based on Ethanol Containing 0.1% 2-Hydroxypropyl-β-cyclodextrin Attenuates the Drug’s Skin Penetration and Prolongs the Repellent Effect without Stickiness. Molecules. 2022; 27(10):3174. https://doi.org/10.3390/molecules27103174
Chicago/Turabian StyleNagai, Noriaki, Mayu Kawaguchi, Misa Minami, Kana Matsumoto, Tatsuji Sasabe, Kenji Nobuhara, and Akira Matsubara. 2022. "N,N-Diethyl-3-toluamide Formulation Based on Ethanol Containing 0.1% 2-Hydroxypropyl-β-cyclodextrin Attenuates the Drug’s Skin Penetration and Prolongs the Repellent Effect without Stickiness" Molecules 27, no. 10: 3174. https://doi.org/10.3390/molecules27103174
APA StyleNagai, N., Kawaguchi, M., Minami, M., Matsumoto, K., Sasabe, T., Nobuhara, K., & Matsubara, A. (2022). N,N-Diethyl-3-toluamide Formulation Based on Ethanol Containing 0.1% 2-Hydroxypropyl-β-cyclodextrin Attenuates the Drug’s Skin Penetration and Prolongs the Repellent Effect without Stickiness. Molecules, 27(10), 3174. https://doi.org/10.3390/molecules27103174





