Mucuna pruriens Seed Aqueous Extract Improved Neuroprotective and Acetylcholinesterase Inhibitory Effects Compared with Synthetic L-Dopa
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Plant Sample Collection and Identification
2.3. Plant Extraction
2.4. HPLC Analysis of L-Dopa in M. pruriens Extract
2.5. Quantitative Analysis of Total Phenolic Content
2.6. Quantitative Analysis of Total Flavonoid Content
2.7. Cell Culture
2.8. Differentiation of P19 Cells into P19-Derived Neurons
2.9. Neuronal Cell Viability Assay
2.10. Neuroprotective Assay
2.10.1. Serum Deprivation Method
2.10.2. Co-Administration of H2O2 Assay
2.11. Acetylcholinesterase (AChE) Activity Assay
2.12. Statistical Analysis
3. Results
3.1. Yield of M. pruriens Aqueous Extract and L-Dopa Content in the Extract
3.2. Total Phenolic and Total Flavonoid Content in M. pruriens Aqueous Extract
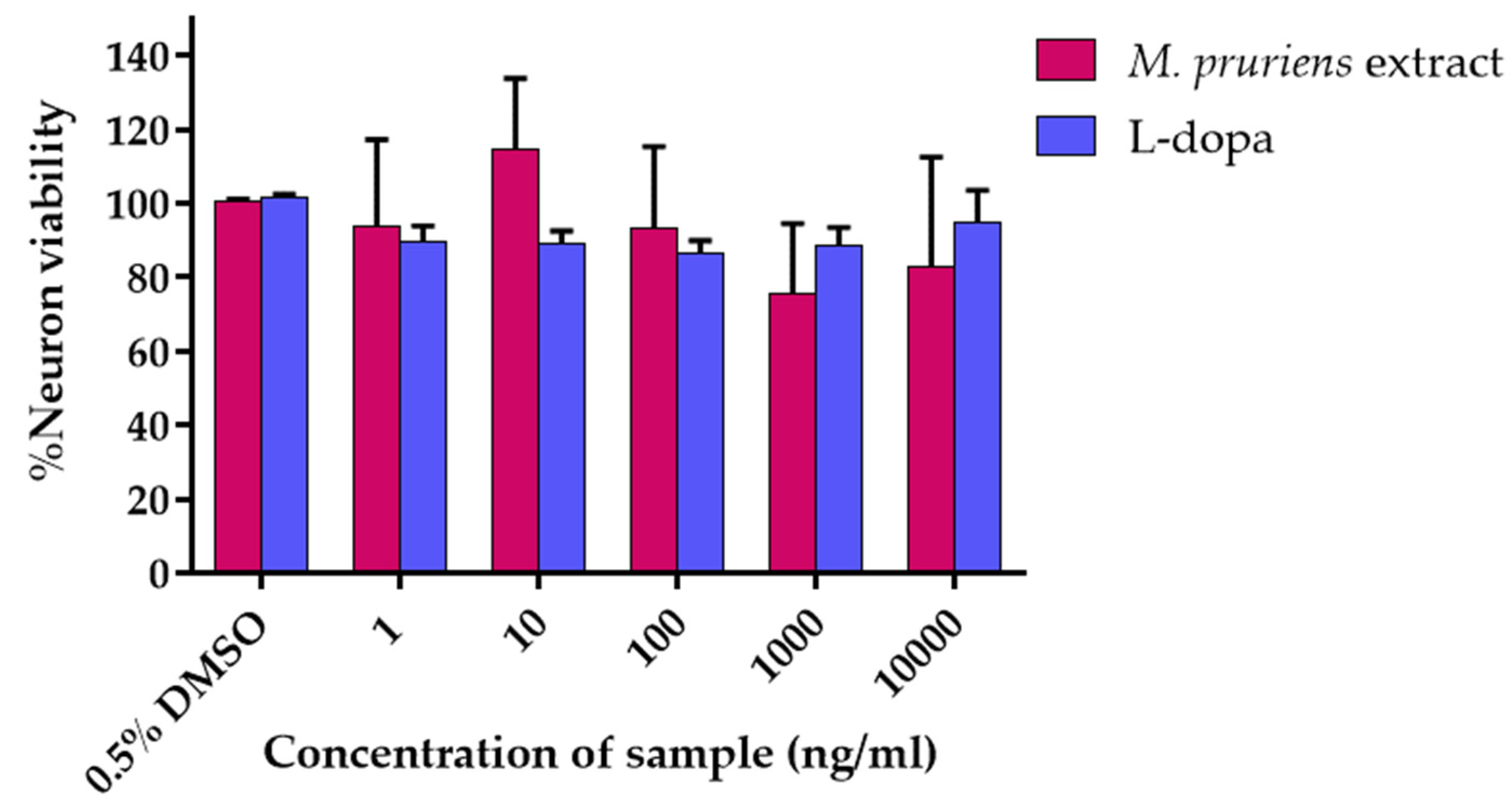
3.3. Cell Viability Assay
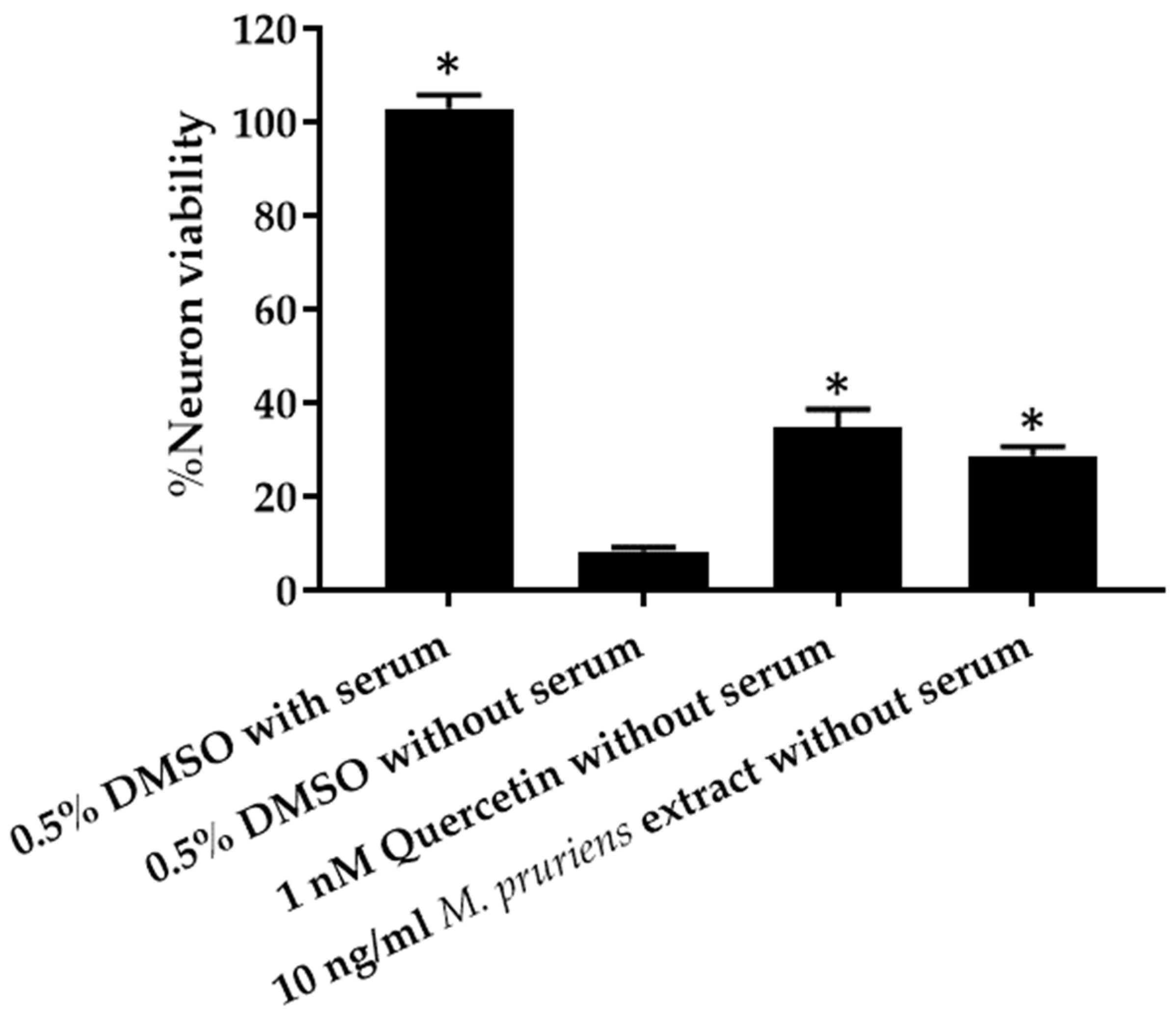
3.4. Serum Deprivation Method
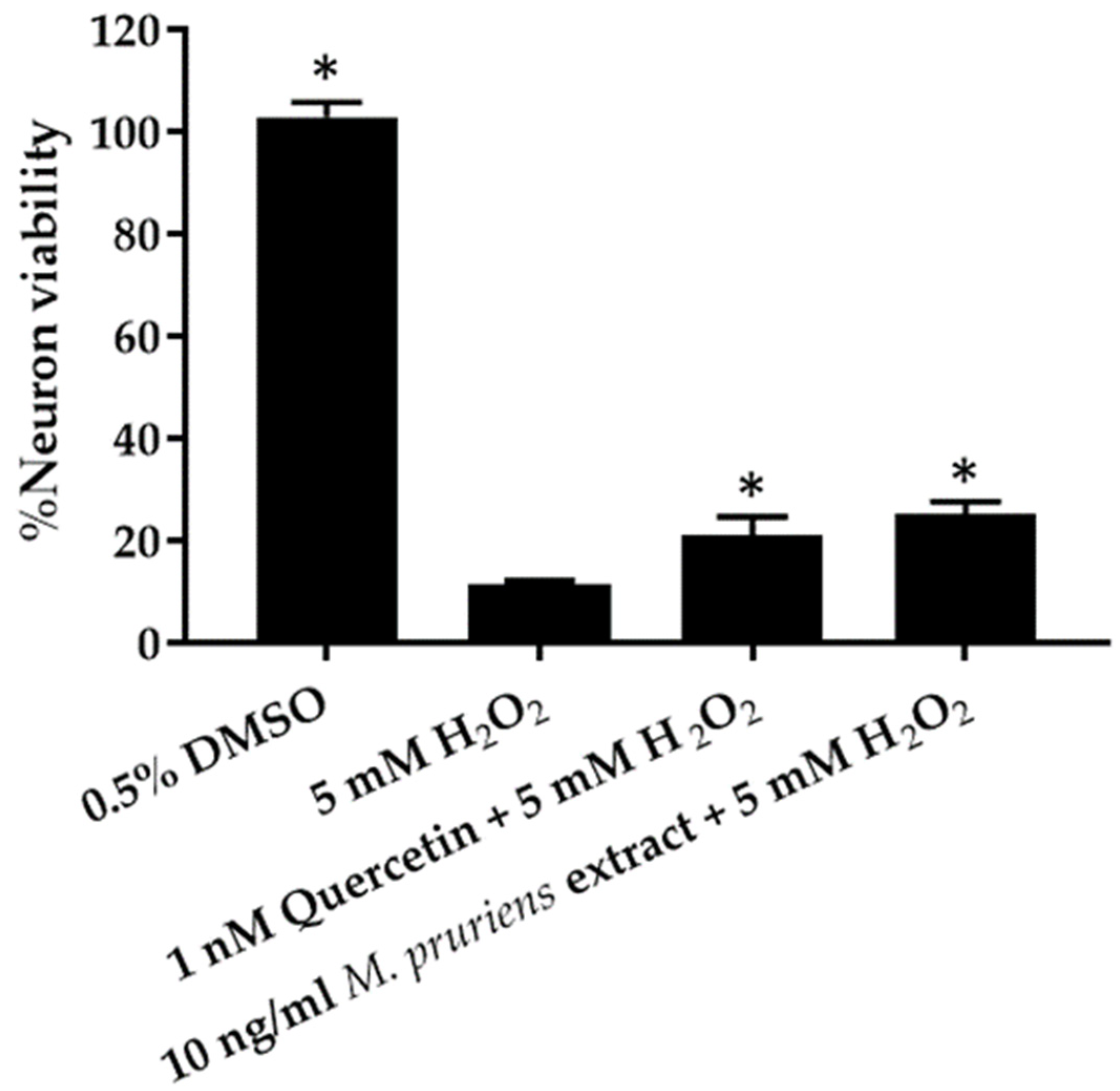
3.5. Co-Administration of H2O2 Assay
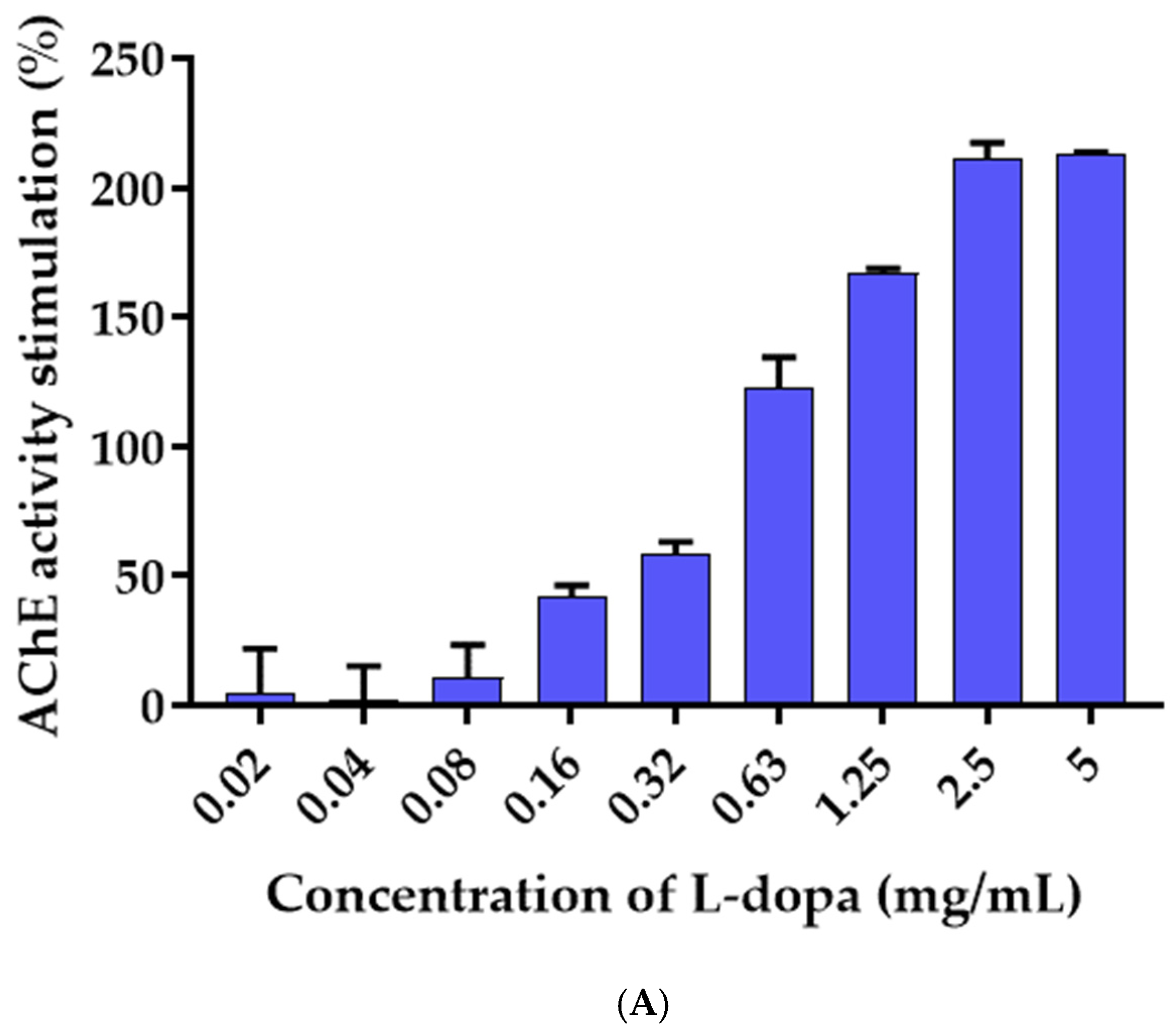
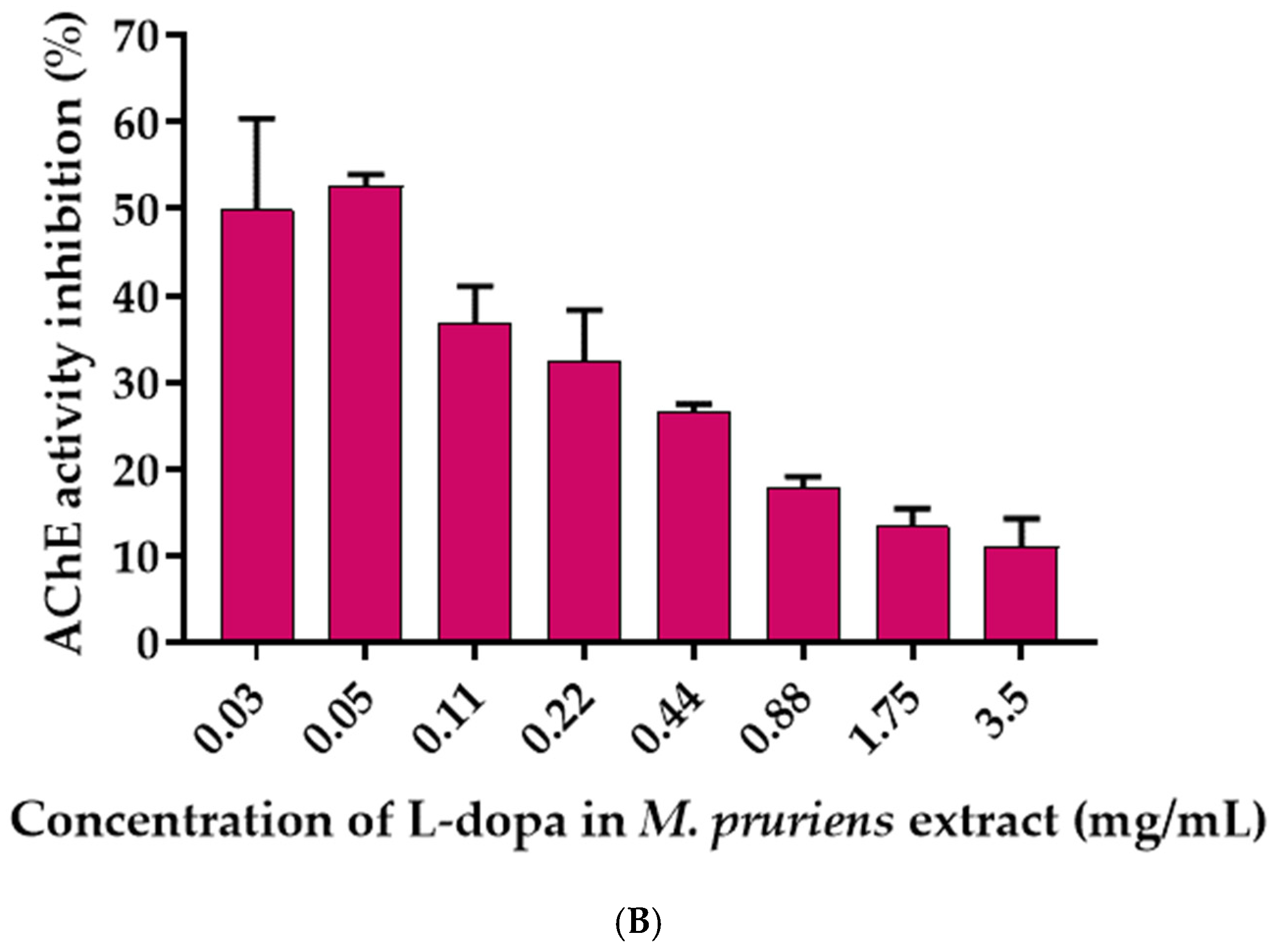
3.6. Effects of L-Dopa and M. pruriens Seed Aqueous Extract on the Acetylcholinesterase Enzyme Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Surguchov, A.; Bernal, L.; Surguchev, A.A. Phytochemicals as Regulators of Genes Involved in Synucleinopathies. Biomolecules 2021, 11, 624. [Google Scholar] [CrossRef] [PubMed]
- Lampariello, L.R.; Cortelazzo, A.; Guerranti, R.; Sticozzi, C.; Valacchi, G. The Magic Velvet Bean of Mucuna pruriens. J. Tradit. Complement. Med. 2012, 2, 331–339. [Google Scholar] [CrossRef]
- Muthuraman, M.; Koirala, N.; Ciolac, D.; Pintea, B.; Glaser, M.; Groppa, S.; Tamás, G.; Groppa, S. Deep Brain Stimulation and L-DOPA Therapy: Concepts of Action and Clinical Applications in Parkinson’s Disease. Front. Neurol. 2018, 9, 711. [Google Scholar] [CrossRef] [PubMed]
- Misra, L.; Wagner, H. Extraction of bioactive principles from Mucuna pruriens seeds. Indian J. Biochem. Biophys. 2007, 44, 56–60. [Google Scholar] [PubMed]
- Kumar, D.S.; Muthu, A.; Smith, A.A.; Manavalan, R. In vitro antioxidant activity of various extracts of whole plant of Mucuna pruriens (Linn). Int. J. PharmTech Res. 2010, 2, 2063–2070. [Google Scholar]
- Emamzadeh, F.N.; Surguchov, A. Parkinson’s Disease: Biomarkers, Treatment, and Risk Factors. Front. Neurosci. 2018, 12, 1–14. [Google Scholar] [CrossRef]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The Role of Reactive Oxygen Species in the Pathogenesis of Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease: A Mini Review. Oxid. Med. Cell Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef]
- Ujowundu, C.O.; Kalu, F.N.; Emejulu, A.A.; Okafor, O.E.; Nkwonta, C.G.; Nwosunjoku, E.C. Evaluation of the chemical composition of Mucuna utilis leaves used in herbal medicine in Southeastern Nigeria. Afr. J. Pharm. Pharmacol. 2010, 4, 811–816. [Google Scholar]
- Bastide, M.F.; Meissner, W.G.; Picconi, B.; Fasano, S.; Fernagut, P.O.; Feyder, M.; Francardo, V.; Alcacer, C.; Ding, Y.; Brambilla, R.; et al. Pathophysiology of L-dopa-induced motor and non-motor complications in Parkinson’s disease. Prog. Neurobiol. 2015, 132, 96–168. [Google Scholar] [CrossRef]
- Ahlskog, J.E.; Muenter, M.D. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov. Disord. 2001, 16, 448–458. [Google Scholar] [CrossRef]
- Pan, H.; Zhang, J.; Wang, Y.; Cui, K.; Cao, Y.; Wang, L.; Wu, Y. Linarin improves the dyskinesia recovery in Alzheimer’s disease zebrafish by inhibiting the acetylcholinesterase activity. Life Sci. 2019, 222, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Fahn, S. How do you treat motor complications in Parkinson’s disease: Medicine, surgery, or both? Ann. Neurol. 2008, 64 (Suppl. S2), S56–S64. [Google Scholar] [CrossRef] [PubMed]
- Salat, D.; Tolosa, E. Levodopa in the treatment of Parkinson’s disease: Current status and new developments. J. Parkinsons Dis. 2013, 3, 255–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katzenschlager, R.; Evans, A.; Manson, A.; Patsalos, P.N.; Ratnaraj, N.; Watt, H.; Timmermann, L.; Van der Giessen, R.; Lees, A.J. Mucuna pruriens in Parkinson’s disease: A double blind clinical and pharmacological study. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1672–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, S.A.; Apine, O.A.; Surwase, S.N.; Jadhav, J.P. Biological sources of L-DOPA: An alternative approach. Adv. Parkinson’s Dis. 2013, 2, 81–87. [Google Scholar] [CrossRef]
- Hussian, G.; Manyam, B.V. Mucuna pruriens proves more effective than L-DOPA in Parkinson’s disease animal model. Phytother. Res. 1997, 11, 419–423. [Google Scholar] [CrossRef]
- Jones-Villeneuve, E.M.; McBurney, M.W.; Rogers, K.A.; Kalnins, V.I. Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells. J. Cell Biol. 1982, 94, 253–262. [Google Scholar] [CrossRef]
- MacPherson, P.A.; McBurney, M.W. P19 Embryonal Carcinoma Cells: A Source of Cultured Neurons Amenable to Genetic Manipulation. Methods 1995, 7, 238–252. [Google Scholar] [CrossRef]
- Jones-Villeneuve, E.M.; Rudnicki, M.A.; Harris, J.F.; McBurney, M.W. Retinoic acid-induced neural differentiation of embryonal carcinoma cells. Mol. Cell Biol. 1983, 3, 2271–2279. [Google Scholar]
- Tadtong, S.; Kanlayavattanakul, M.; Lourith, N. Neuritogenic and neuroprotective activities of fruit residues. Nat. Prod. Commun. 2013, 8, 1583–1586. [Google Scholar] [CrossRef] [Green Version]
- Supasuteekul, C.; Nonthitipong, W.; Tadtong, S.; Likhitwitayawuid, K.; Tengamnuay, P.; Sritularak, B. Antioxidant, DNA damage protective, neuroprotective, and α-glucosidase inhibitory activities of a flavonoid glycoside from leaves of Garcinia gracilis. Rev. Bras. Farmacogn. 2016, 26, 312–320. [Google Scholar] [CrossRef] [Green Version]
- Tadtong, S.; Chatsumpun, N.; Sritularak, B.; Jongbunprasert, V.; Ploypradith, P.; Likhitwitayawuid, K. Effects of oxyresveratrol and its derivatives on cultured P19-derived neurons. Trop. J. Pharm. Res. 2016, 15, 2619–2628. [Google Scholar] [CrossRef] [Green Version]
- Supasuteekul, C.; Tadtong, S.; Putalun, W.; Tanaka, H.; Likhitwitayawuid, K.; Tengamnuay, P.; Sritularak, B. Neuritogenic and neuroprotective constituents from Aquilaria crassna leaves. J. Food Biochem. 2017, 41, e12365. [Google Scholar] [CrossRef]
- Puksasook, T.; Kimura, S.; Tadtong, S.; Jiaranaikulwanitch, J.; Pratuangdejkul, J.; Kitphati, W.; Suwanborirux, K.; Saito, N.; Nukoolkarn, V. Semisynthesis and biological evaluation of prenylated resveratrol derivatives as multi-targeted agents for Alzheimer’s disease. J. Nat. Med. 2017, 71, 665–682. [Google Scholar] [CrossRef]
- Tangsaengvit, N.; Kitphati, W.; Tadtong, S.; Bunyapraphatsara, N.; Nukoolkarn, V. Neurite Outgrowth and Neuroprotective Effects of Quercetin from Caesalpinia mimosoides Lamk. on Cultured P19-Derived Neurons. Evid. Based Complement. Altern. Med. 2013, 2013, 838051. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.L.; Park, H.Y.; DaSilva, N.A.; Vattem, D.A.; Ma, H.; Seeram, N.P. Levodopa-Reduced Mucuna pruriens Seed Extract Shows Neuroprotective Effects against Parkinson’s Disease in Murine Microglia and Human Neuroblastoma Cells, Caenorhabditis elegans, and Drosophila melanogaster. Nutrients 2018, 10, 1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Dawson, V.L.; Dawson, T.M. What causes cell death in Parkinson’s disease? Ann. Neurol. 2008, 64 (Suppl. S2), S3–S15. [Google Scholar] [CrossRef]
- Shin, J.Y.; Park, H.J.; Ahn, Y.H.; Lee, P.H. Neuroprotective effect of L-dopa on dopaminergic neurons is comparable to pramipexol in MPTP-treated animal model of Parkinson’s disease: A direct comparison study. J. Neurochem. 2009, 111, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, Y.M.; Zhang, B.; Guo, C.Y. Protective Effect of Total Phenolic Compounds from Inula helenium on Hydrogen Peroxide-induced Oxidative Stress in SH-SY5Y Cells. Indian J. Pharm. Sci. 2015, 77, 163–169. [Google Scholar]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Ovais, M.; Ullah, I.; Shahid, M. Flavonoids as Prospective Neuroprotectants and Their Therapeutic Propensity in Aging Associated Neurological Disorders. Front. Aging Neurosci. 2019, 11, 155. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.K.; Rai, S.N.; Singh, S.P. Mucuna pruriens reduces inducible nitric oxide synthase expression in Parkinsonian mice model. J. Chem. Neuroanat. 2017, 80, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Manyam, B.V.; Dhanasekaran, M.; Hare, T.A. Neuroprotective effects of the antiparkinson drug Mucuna pruriens. Phytother. Res. 2004, 18, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Quik, M.; Cox, H.; Parameswaran, N.; O’Leary, K.; Langston, J.W.; Di Monte, D. Nicotine reduces levodopa-induced dyskinesias in lesioned monkeys. Ann. Neurol. 2007, 62, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Bordia, T.; Campos, C.; Huang, L.; Quik, M. Continuous and Intermittent Nicotine Treatment Reduces L-3,4-Dihydroxyphenylalanine (L-DOPA)-Induced Dyskinesias in a Rat Model of Parkinson’s Disease. J. Pharmacol. Exp. Ther. 2008, 327, 239–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.Z.; Campos, C.; Ly, J.; Ivy Carroll, F.; Quik, M. Nicotinic receptor agonists decrease L-dopa-induced dyskinesias most effectively in partially lesioned parkinsonian rats. Neuropharmacology 2011, 60, 861–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajput, A.H. Factors predictive of the development of levodopa-induced dyskinesia and Wearing-Off in Parkinson’s disease. Mov. Disord. 2014, 29, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinson’s Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.Y.; Liu, C.M.; Tsai, H.H.; Jong, Y.J.; Chen, I.J.; Lo, Y.C. KMUP-1 attenuates serum deprivation-induced neurotoxicity in SH-SY5Y cells: Roles of PKG, PI3K/Akt and Bcl-2/Bax pathways. Toxicology 2010, 268, 46–54. [Google Scholar] [CrossRef]
- Longhi, J.G.; Pérez, E.S.; Lima, J.J.D.; Cândido, L.M.B. In vitro evaluation of Mucuna pruriens (L.) DC. antioxidant activity. Braz. J. Pharm. Sci. 2011, 47, 535–544. [Google Scholar] [CrossRef]
- Giarola, A.; Auber, A.; Chiamulera, C. Acetylcholinesterase inhibitors partially generalize to nicotine discriminative stimulus effect in rats. Behav. Pharmacol. 2011, 22, 1–6. [Google Scholar] [CrossRef]
- Sousa, B.L.D.; Leite, J.P.; Mendes, T.A.; Varejão, E.V.; Chaves, A.; Silva, J.G.D.; Agrizzi, A.T.; Ferreira, P.G.; Pilau, E.J.; Silva, E.; et al. Inhibition of acetylcholinesterase by coumarin-linked amino acids synthetized via triazole associated with molecule partition coefficient. J. Braz. Chem. Soc. 2021, 32, 652–664. [Google Scholar] [CrossRef]
- van Laar, T.; De Deyn, P.P.; Aarsland, D.; Barone, P.; Galvin, J.E. Effects of cholinesterase inhibitors in Parkinson’s disease dementia: A review of clinical data. CNS Neurosci. Ther. 2011, 17, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.A.; Lobb, B.M.; Nutt, J.G.; Horak, F.B. Effects of a central cholinesterase inhibitor on reducing falls in Parkinson disease. Neurology 2010, 75, 1263–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamkaen, N.; Chittasupho, C.; Vorarat, S.; Tadtong, S.; Phrompittayarat, W.; Okonogi, S.; Kwankhao, P. Mucuna pruriens Seed Aqueous Extract Improved Neuroprotective and Acetylcholinesterase Inhibitory Effects Compared with Synthetic L-Dopa. Molecules 2022, 27, 3131. https://doi.org/10.3390/molecules27103131
Kamkaen N, Chittasupho C, Vorarat S, Tadtong S, Phrompittayarat W, Okonogi S, Kwankhao P. Mucuna pruriens Seed Aqueous Extract Improved Neuroprotective and Acetylcholinesterase Inhibitory Effects Compared with Synthetic L-Dopa. Molecules. 2022; 27(10):3131. https://doi.org/10.3390/molecules27103131
Chicago/Turabian StyleKamkaen, Narisa, Chuda Chittasupho, Suwanna Vorarat, Sarin Tadtong, Watoo Phrompittayarat, Siriporn Okonogi, and Pakakrong Kwankhao. 2022. "Mucuna pruriens Seed Aqueous Extract Improved Neuroprotective and Acetylcholinesterase Inhibitory Effects Compared with Synthetic L-Dopa" Molecules 27, no. 10: 3131. https://doi.org/10.3390/molecules27103131
APA StyleKamkaen, N., Chittasupho, C., Vorarat, S., Tadtong, S., Phrompittayarat, W., Okonogi, S., & Kwankhao, P. (2022). Mucuna pruriens Seed Aqueous Extract Improved Neuroprotective and Acetylcholinesterase Inhibitory Effects Compared with Synthetic L-Dopa. Molecules, 27(10), 3131. https://doi.org/10.3390/molecules27103131






