SOMOphilic Alkynylation of Unreactive Alkenes Enabled by Iron-Catalyzed Hydrogen Atom Transfer
Abstract
:1. Introduction
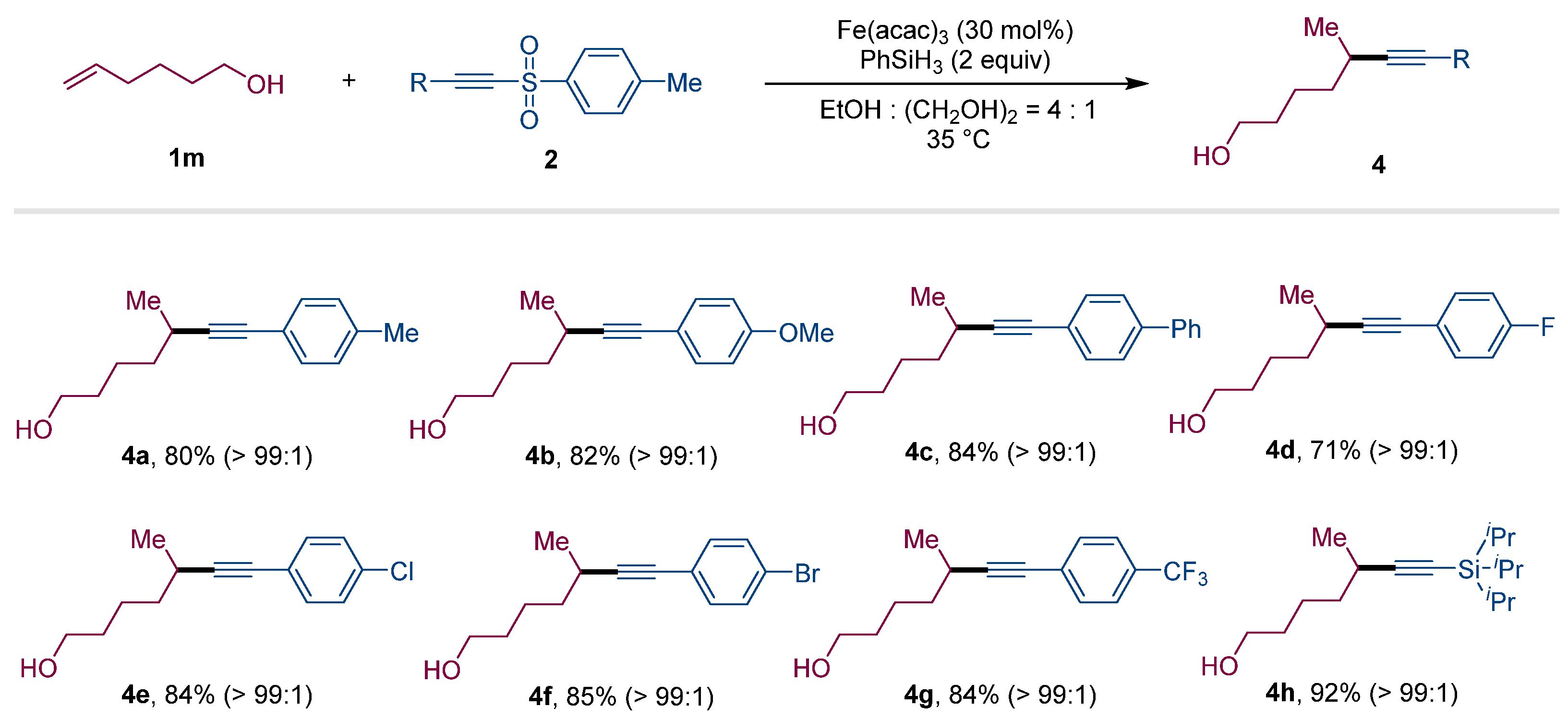
2. Results
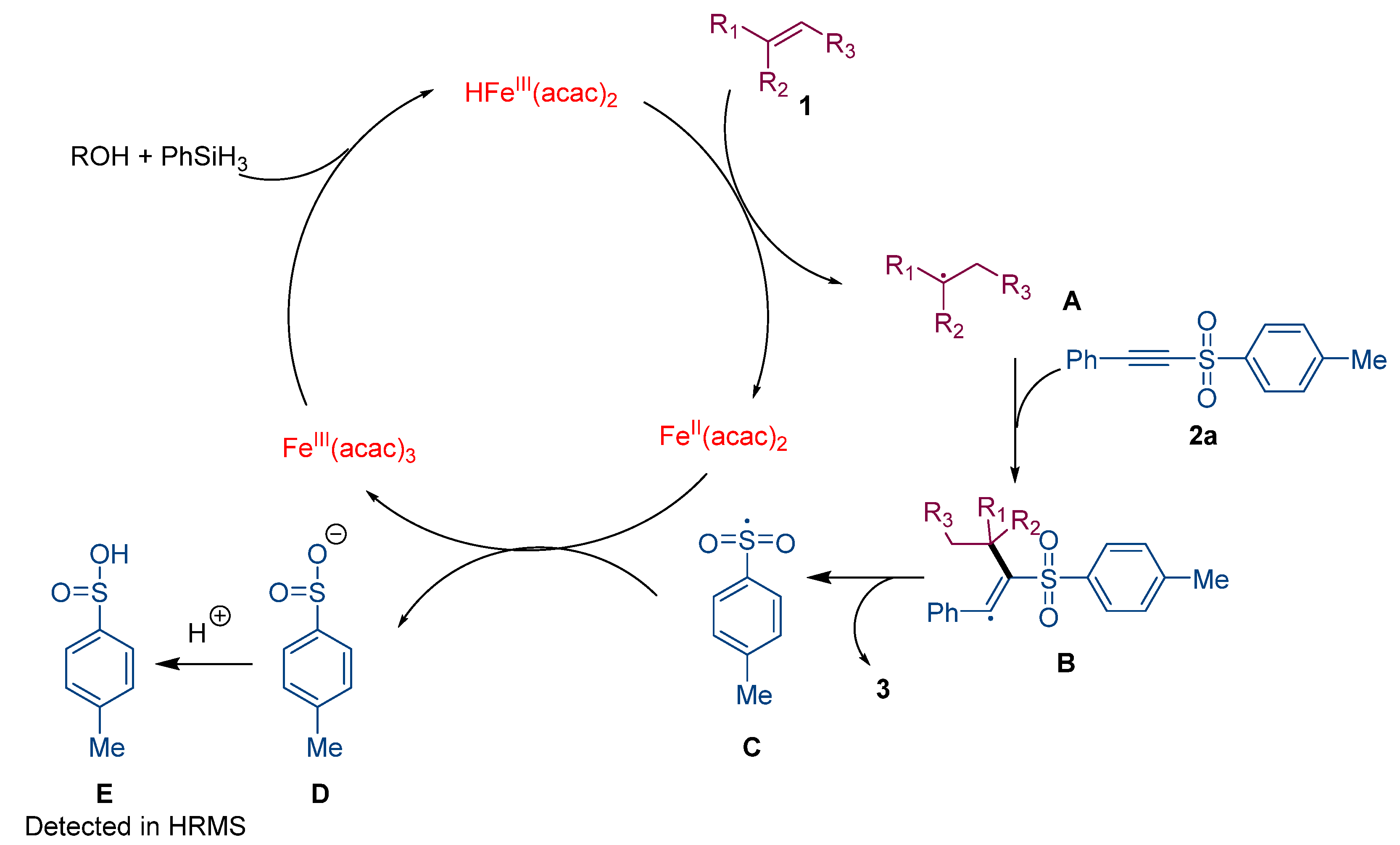
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. General Procedures of Iron-Catalyzed SOMOphilic Alkynylation
4.3. Characterization Data for Products
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Diederich, F.; Stang, P.J.; Tykwinski, R.R. Acetylene Chemistry; Wiley: New York, NY, USA, 2005. [Google Scholar]
- Trost, B.M.; Li, C.-J. Modern Alkyne Chemistry: Catalytic and Atom Economic Transformations; Wiley: New York, NY, USA, 2014. [Google Scholar]
- Talele, T.T. Acetylene group, friend or foe in medicinal chemistry. J. Med. Chem. 2020, 63, 5625. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, G.; Xu, Z.; Zhao, S.; Liu, W. Advances in alkynyl gold complexes for use as potential anticancer agents. Coord. Chem. Rev. 2020, 423, 213492. [Google Scholar] [CrossRef]
- Biyani, S.A.; Qi, Q.; Wu, J.; Moriuchi, Y.; Larocque, E.A.; Sintim, H.O.; Thompson, D.H. Use of High-Throughput Tools for Telescoped Continuous Flow Synthesis of an Alkynylnaphthyridine Anticancer Agent, HSN608. Org. Process Res. Dev. 2020, 24, 2240. [Google Scholar] [CrossRef]
- Trotuş, I.-T.; Zimmermann, T.; Schüth, F. Catalytic Reactions of Acetylene: A Feedstock for the Chemical Industry Revisited. Chem. Rev. 2014, 114, 1761–1782. [Google Scholar] [CrossRef]
- Boyarskiy, V.P.; Ryabukhin, D.S.; Bokach, N.A.; Vasilyev, A.V. Alkenylation of Arenes and Heteroarenes with Alkynes. Chem. Rev. 2016, 116, 5894–5986. [Google Scholar] [CrossRef] [PubMed]
- Haydl, A.M.; Breit, B.; Liang, T.; Krische, M.J. Alkynes as Electrophilic or Nucleophilic Allylmetal Precursors in Transition Metal Catalysis. Angew. Chem. Int. Ed. 2017, 56, 11312–11325. [Google Scholar] [CrossRef]
- Iha, R.K.; Wooley, K.L.; Nystrom, A.M.; Burke, D.J.; Kade, M.J.; Hawker, C.J. Applications of orthogonal “Click” chemistries in the synthesis of functional soft materials. Chem. Rev. 2009, 109, 5620–5686. [Google Scholar] [CrossRef] [Green Version]
- Eckhardt, M.; Fu, G.C. The First Applications of Carbene Ligands in Cross-Couplings of Alkyl Electrophiles: Sonogashira Reactions of Unactivated Alkyl Bromides and Iodides. J. Am. Chem. Soc. 2003, 125, 13642–13643. [Google Scholar] [CrossRef] [PubMed]
- Altenhoff, G.; Wurtz, S.; Glorius, F. The first palladium-catalyzed Sonogashira coupling of unactivated secondary alkyl bromides. Tetrahedron Lett. 2006, 47, 2925–2928. [Google Scholar] [CrossRef]
- Vechorkin, O.; Barmaz, D.; Proust, V.; Hu, X.L. Ni-Catalyzed Sonogashira Coupling of Nonactivated Alkyl Halides: Orthogonal Functionalization of Alkyl Iodides, Bromides, and Chlorides. J. Am. Chem. Soc. 2009, 131, 12078–12079. [Google Scholar] [CrossRef]
- Yi, J.; Lu, X.; Sun, Y.Y.; Xiao, B.; Liu, L. Nickel-Catalyzed Sonogashira Reactions of Non-activated Secondary Alkyl Bromides and Iodides. Angew. Chem. Int. Ed. 2013, 52, 12409–12413. [Google Scholar] [CrossRef]
- Hari, D.P.; Caramenti, P.; Waser, J. Cyclic hypervalent iodine reagents: Enabling tools for bond disconnection via reactivity umpolung. Acc. Chem. Res. 2018, 51, 3212–3225. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, G.; Gong, L.; Zhang, S.; Chen, Y. Visible-Light-Induced Chemoselective Deboronative Alkynylation under Biomolecule-Compatible Conditions. J. Am. Chem. Soc. 2014, 136, 2280–2283. [Google Scholar] [CrossRef]
- Le Vaillant, F.; Courant, T.; Waser, J. Room-Temperature Decarboxylative Alkynylation of Carboxylic Acids Using Photoredox Catalysis and EBX Reagents. Angew. Chem. Int. Ed. 2015, 54, 11200–11204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.-Q.; Guo, W.; Ding, W.; Wu, X.; Chen, X.; Lu, L.-Q.; Xiao, W.-J. Decarboxylative Alkynylation and Carbonylative Alkynylation of Carboxylic Acids Enabled by Visible-Light Photoredox Catalysis. Angew. Chem. Int. Ed. 2015, 54, 11196–11199. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Jiang, H.; Liu, B.; Chen, Z.; Zhou, P. Palladium-Catalyzed Bromoalkynylation of C-C Double Bonds: Ring-Structure-Dependent Synthesis of 7-Alkynyl Norbornanes and Cyclobutenyl Halides. Angew. Chem. Int. Ed. 2011, 50, 6341–6345. [Google Scholar] [CrossRef]
- Feng, Y.-S.; Xu, Z.-Q.; Mao, L.; Zhang, F.-F.; Xu, H.-J. Copper Catalyzed Decarboxylative Alkynylation of Quaternary α-Cyano Acetate Salts. Org. Lett. 2013, 15, 1472–1475. [Google Scholar] [CrossRef]
- Sun, F.; Gu, Z. Decarboxylative alkynyl termination of palladium-catalyzed catellani reaction: A facile synthesis of α–alkynyl anilines via ortho C–H amination and alkynylation. Org. Lett. 2015, 17, 2222–2225. [Google Scholar] [CrossRef]
- Jayaraman, A.; Lee, S. Selective monoand dialkynylation of 1–fluoro-2, 2- diiodovinylarenes using Pd-catalyzed decarboxylative coupling reactions. Org. Lett. 2019, 21, 7923–7927. [Google Scholar] [CrossRef]
- Back, T.G. The chemistry of acetylenic and allenic sulfones, Tetrahedron 2001, 57, 5263–5301. Tetrahedron 2001, 57, 5263–5301. [Google Scholar] [CrossRef]
- Vaillant, F.L.; Waser, J. Alkynylation of radicals: Spotlight on the “third way” to transfer triple bonds. Chem. Sci. 2019, 10, 8909–8923. [Google Scholar] [CrossRef]
- Guerrero-Robles, M.A.; Vilchis-Reyes, M.A.; Ramos-Rivera, E.M.; Alvarado, C. Synthesis of alkynyl sulfones. ChemistrySelect 2019, 4, 13698–13708. [Google Scholar] [CrossRef]
- Ge, D.; Wang, X.; Chu, X.-Q. SOMOphilic alkynylation using acetylenic sulfones as functional reagents. Org. Chem. Front. 2021, 8, 5145–5164. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, Y.; Zhang, H.; Wu, X.; Zhu, C. Radical-mediated sulfonyl alkynylation, allylation, and cyanation of propellane. Chem. Commun. 2021, 57, 6066–6069. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, J.; Qi, L.; Hu, C.; Chen, Y. Visible-light-induced chemoselective reductive decarboxylative alkynylation under biomolecule-compatible conditions. Chem. Commun. 2015, 51, 5275–5278. [Google Scholar] [CrossRef]
- Schwarz, J.; König, B. Decarboxylative Alkynylation of Biomass-Derived Compounds by Metal-Free Visible Light Photocatalysis. ChemPhotoChem 2017, 1, 237–242. [Google Scholar] [CrossRef]
- Ren, R.; Wu, Z.; Xu, Y.; Zhu, C. C–C Bond-Forming Strategy by Manganese-Catalyzed Oxidative Ring-Opening Cyanation and Ethynylation of Cyclobutanol Derivatives. Angew. Chem. Int. Ed. 2016, 55, 2866–2869. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.-L.; Wang, Z.; Zhang, R.; Wang, Y.; Wang, J. Visible-Light-Promoted Ring-Opening Alkynylation, Alkenylation, and Allylation of Cyclic Hemiacetals through β-Scission of Alkoxy Radicals. Chem. Eur. J. 2019, 25, 8992–8995. [Google Scholar] [CrossRef]
- Gao, C.; Li, J.; Yu, J.; Yang, H.; Fu, H. Visible-light photoredox synthesis of internal alkynes containing quaternary carbons. Chem. Commun. 2016, 52, 7292–7294. [Google Scholar] [CrossRef]
- Ociepa, M.; Turkowska, J.; Gryko, D. Redox-Activated Amines in C(sp3)−C(sp) and C(sp3)−C(sp2) Bond Formation Enabled by Metal-Free Photoredox Catalysis. ACS Catal. 2018, 8, 11362–11367. [Google Scholar] [CrossRef]
- Xia, Y.; Studer, A. Diversity-Oriented Desulfonylative Functionalization of Alkyl Allyl Sulfones. Angew. Chem. Int. Ed. 2019, 58, 9836–9840. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Sun, S.; Mao, Y.; Chen, L.; Lu, R.; Huang, J.; Liu, L. Iron(II)-Catalyzed Site-Selective Functionalization of Unactivated C(sp3)−H Bonds Guided by Alkoxyl Radicals. Angew. Chem. Int. Ed. 2018, 57, 11413–11417. [Google Scholar] [CrossRef]
- Paul, S.; Guin, J. Radical C(sp3)–H alkenylation, alkynylation and allylation of ethers and amides enabled by photocatalysis. Green Chem. 2017, 19, 2530–2534. [Google Scholar] [CrossRef]
- Guo, A.; Han, J.-B.; Zhu, L.; Wei, Y.; Tang, X.-Y. Site-Selective α-Alkoxyl Alkynation of Alkyl Esters Mediated by Boryl Radicals. Org. Lett. 2019, 21, 2927–2931. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhang, Y.; Zhang, S.; Wu, X.-F. Copper-Catalyzed Alkynylation of C(sp3)–H Bonds in N-Fluorosulfonamides. Adv. Synth. Catal. 2019, 361, 5478–5482. [Google Scholar] [CrossRef]
- Han, J.-B.; San, H.H.; Guo, A.; Wang, L.; Tang, X.-Y. Boryl Radical-Mediated C–H Activation of Inactivated Alkanes for the Synthesis of Internal Alkynes. Adv. Synth. Catal. 2021, 363, 2366–2370. [Google Scholar] [CrossRef]
- Capaldo, L.; Ravelli, D. Decatungstate as Direct Hydrogen Atom Transfer Photocatalyst for SOMOphilic Alkynylation. Org. Lett. 2021, 23, 2243–2247. [Google Scholar] [CrossRef] [PubMed]
- Crossley, S.W.M.; Obradors, C.; Martinez, R.M.; Shenvi, R.A. Mn-, Fe-, and Co-Catalyzed Radical Hydrofunctionalizations of Olefins. Chem. Rev. 2016, 116, 8912–9000. [Google Scholar] [CrossRef] [Green Version]
- Green, S.A.; Crossley, S.W.M.; Matos, J.L.M.; Vásquez-Céspedes, S.; Shevick, S.L.; Shenvi, R.A. The High Chemofidelity of Metal-Catalyzed Hydrogen Atom Transfer. Acc. Chem. Res. 2018, 51, 2628–2640. [Google Scholar] [CrossRef]
- Obradors, C.; Martinez, R.M.; Shenvi, R.A. Ph(i-PrO)SiH2: An Exceptional Reductant for Metal-Catalyzed Hydrogen Atom Transfers. J. Am. Chem. Soc. 2016, 138, 4962–4971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crossley, S.W.M.; Barabé, F.; Shenvi, R.A. Simple, Chemoselective, Catalytic Olefin Isomerization. J. Am. Chem. Soc. 2014, 136, 16788–16791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Herzon, S.B. Non-classical selectivities in the reduction of alkenes by cobalt-mediated hydrogen atom transfer. Chem. Sci. 2015, 6, 6250–6255. [Google Scholar] [CrossRef] [Green Version]
- Lo, J.C.; Gui, J.; Yabe, Y.; Pan, C.-M.; Baran, P.S. Functionalized olefin cross-coupling to construct carbon–carbon bonds. Nature 2014, 516, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.; Yabe, Y.; Baran, P.S. A Practical and Catalytic Reductive Olefin Coupling. J. Am. Chem. Soc. 2014, 136, 1304–1307. [Google Scholar] [CrossRef]
- Gaspar, B.; Carreira, E.M. Mild Cobalt-Catalyzed Hydrocyanation of Olefins with Tosyl Cyanide. Angew. Chem. Int. Ed. 2007, 46, 4519–4522. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.C.; Kim, D.; Pan, C.-M.; Edwards, J.T.; Yabe, Y.; Gui, J.; Qin, T.; Gutiérrez, S.; Giacoboni, J.; Smith, M.W.; et al. Fe-Catalyzed C−C Bond Construction from Olefins via Radicals. J. Am. Chem. Soc. 2017, 139, 2484–2503. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.L.M.; Green, S.A.; Chun, Y.; Dang, V.Q.; Dushin, R.G.; Richardson, P.; Chen, J.S.; Piotrowski, D.W.; Paegel, B.M.; Shenvi, R.A. Cycloisomerization of Olefins in Water. Angew. Chem. Int. Ed. 2020, 59, 12998–13003. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Bode, J.W. Olefin Amine (OLA) Reagents for the Synthesis of Bridged Bicyclic and Spirocyclic Saturated N-Heterocycles by Catalytic Hydrogen Atom Transfer (HAT) Reactions. J. Am. Chem. Soc. 2019, 141, 9739–9745. [Google Scholar] [CrossRef]
- He, S.-J.; Wang, J.-W.; Li, Y.; Xu, Z.-Y.; Wang, X.-X.; Lu, X.; Fu, Y. Nickel-Catalyzed Enantioconvergent Reductive Hydroalkylation of Olefins with α-Heteroatom Phosphorus or Sulfur Alkyl Electrophiles. J. Am. Chem. Soc. 2020, 142, 214–221. [Google Scholar] [CrossRef]
- Wang, J.-W.; Li, Y.; Nie, W.; Chang, Z.; Yu, Z.-A.; Zhao, Y.-F.; Lu, X.; Fu, Y. Catalytic asymmetric reductive hydroalkylation of enamides and enecarbamates to chiral aliphatic amines. Nat. Commun. 2021, 12, 1313. [Google Scholar] [CrossRef]
- Li, Y.; Nie, W.; Chang, Z.; Wang, J.-W.; Lu, X.; Fu, Y. Cobalt-catalysed enantioselective C(sp3)–C(sp3) coupling. Nat. Catal. 2021, 4, 901–911. [Google Scholar] [CrossRef]
- Shigehisa, H.; Aoki, T.; Yamaguchi, S.; Shimizu, N.; Hiroya, K. Hydroalkoxylation of Unactivated Olefins with Carbon Radicals and Carbocation Species as Key Intermediates. J. Am. Chem. Soc. 2013, 135, 10306–10309. [Google Scholar] [CrossRef]
- Liu, B.; Jin, F.; Wang, T.; Yuan, X.; Han, W. Wacker-Type Oxidation Using an Iron Catalyst and Ambient Air: Application to Late-Stage Oxidation of Complex Molecules. Angew. Chem. Int. Ed. 2017, 56, 12712–12717. [Google Scholar] [CrossRef] [PubMed]
- Touney, E.E.; Foy, N.J.; Pronin, S.V. Catalytic Radical−Polar Crossover Reactions of Allylic Alcohols. J. Am. Chem. Soc. 2018, 140, 16982–16987. [Google Scholar] [CrossRef] [PubMed]
- Ebisawa, K.; Izumi, K.; Ooka, Y.; Kato, H.; Kanazawa, S.; Komatsu, S.; Nishi, E.; Shigehisa, H. Catalyst- and Silane-Controlled Enantioselective Hydrofunctionalization of Alkenes by Cobalt-Catalyzed Hydrogen Atom Transfer and Radical-Polar Crossover. J. Am. Chem. Soc. 2020, 142, 13481–13490. [Google Scholar] [CrossRef]
- Puls, F.; Knölker, H.-J. Conversion of Olefins into Ketones by an Iron-Catalyzed Wacker-type Oxidation Using Oxygen as the Sole Oxidant. Angew. Chem. Int. Ed. 2018, 57, 1222–1226. [Google Scholar] [CrossRef]
- Girijavallabhan, V.; Alvarez, C.; Njoroge, F.G. Regioselective Cobalt-Catalyzed Addition of Sulfides to Unactivated Alkenes. J. Org. Chem. 2011, 76, 6442–6446. [Google Scholar] [CrossRef]
- Date, S.; Hamasaki, K.; Sunagawa, K.; Koyama, H.; Sebe, C.; Hiroya, K.; Shigehisa, H. Catalytic Direct Cyclization of Alkenyl Thioester. ACS Catal. 2020, 10, 2039–2045. [Google Scholar] [CrossRef]
- Gui, J.; Pan, C.-M.; Jin, Y.; Qin, T.; Lo, J.C.; Lee, B.J.; Spergel, S.H.; Mertzman, M.E.; Pitts, W.J.; La Cruz, T.E.; et al. Practical olefin hydroamination with nitroarenes. Science 2015, 348, 886–891. [Google Scholar] [CrossRef] [Green Version]
- Shigehisa, H.; Koseki, N.; Shimizu, N.; Fujisawa, M.; Niitsu, M.; Hiroya, K. Catalytic Hydroamination of Unactivated Olefins Using a Co Catalyst for Complex Molecule Synthesis. J. Am. Chem. Soc. 2014, 136, 13534–13537. [Google Scholar] [CrossRef]
- Yin, Y.-N.; Ding, R.-Q.; Ouyang, D.-C.; Zhang, Q.; Zhu, R. Highly chemoselective synthesis of hindered amides via cobalt-catalyzed intermolecular oxidative hydroamidation. Nat. Commun. 2021, 12, 2552. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Mimata, N.; Terada, Y.; Sebe, C.; Shigehisa, H. Catalytic Dealkylative Synthesis of Cyclic Carbamates and Ureas via Hydrogen Atom Transfer and Radical-Polar Crossover. Org. Lett. 2020, 22, 5522–5527. [Google Scholar] [CrossRef]
- Leggans, E.K.; Barker, T.J.; Duncan, K.K.; Boger, D.L. Iron(III)/NaBH4-Mediated Additions to Unactivated Alkenes: Synthesis of Novel 200-Vinblastine Analogues. Org. Lett. 2012, 14, 1428–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, T.J.; Boger, D.L. Fe(III)/NaBH4-Mediated Free Radical Hydrofluorination of Unactivated Alkenes. J. Am. Chem. Soc. 2012, 134, 13588. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Sun, P.-W.; Li, Y.; Wang, S.; Ye, M.; Li, Z. Ligand-Promoted Iron(III)-Catalyzed Hydrofluorination of Alkenes. Angew. Chem. Int. Ed. 2019, 58, 7097–7101. [Google Scholar] [CrossRef]
- Schaffner, A.-P.; Darmency, V.; Renaud, P. Radical-Mediated Alkenylation, Alkynylation, Methanimination, and Cyanation of B-Alkylcatecholboranes. Angew. Chem. Int. Ed. 2006, 45, 5847–5849. [Google Scholar] [CrossRef] [PubMed]
- Saladrigas, M.; Bosch, C.; Saborit, G.V.; Bonjoch, J.; Bradshaw, B. Radical Cyclization of Alkene-Tethered Ketones Initiated by Hydrogen-Atom Transfer. Angew. Chem. Int. Ed. 2018, 57, 182–186. [Google Scholar] [CrossRef]
- Dao, H.T.; Li, C.; Michaudel, Q.; Maxwell, B.D.; Baran, P.S. Hydromethylation of Unactivated Olefins. J. Am. Chem. Soc. 2015, 137, 8046–8049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meesin, J.; Katrun, P.; Pareseecharoen, C.; Pohmakotr, M.; Reutrakul, V.; Soorukram, D.; Kuhakarn, C. Iodine-catalyzed Sulfonylation of Arylacetylenic Acids and Arylacetylenes with Sodium Sulfinates: Synthesis of Arylacetylenic Sulfones. J. Org. Chem. 2016, 81, 2744–2752. [Google Scholar] [CrossRef]


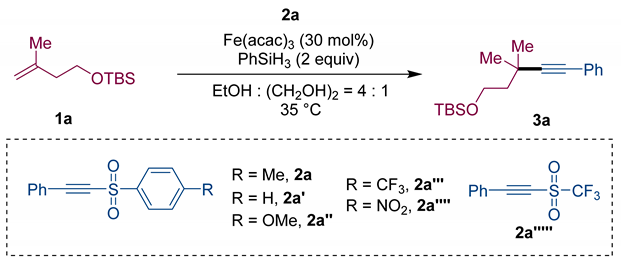
| Entry | Variation from the “Standard Conditions” | Yield (%) b |
|---|---|---|
| Entry 1 | none | 85 (81) c |
| Entry 2 | 2a′ instead of 2a | 80 (72) c |
| Entry 3 | 2a″ instead of 2a | 71 |
| Entry 4 | 2a‴ instead of 2a | 49 |
| Entry 5 | 2a⁗ instead of 2a | ND |
| Entry 6 | 2a′′′′′ instead of 2a | ND |
| Entry 7 | Only EtOH instead of EtOH and (CH2OH)2 | 62 |
| Entry 8 | Fe(acac)3 (20 mol%) instead of Fe(acac)3 (30 mol%) | 60 |
| Entry 9 | In(acac)3 instead of Fe(acac)3 | ND |
| Entry 10 | Co(acac)3 instead of Fe(acac)3 | ND |
| Entry 11 | FeCl3 instead of Fe(acac)3 | 45 |
| Entry 12 | 1a (0.2 mmol), 2a (0.3 mmol) | 65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, B.; Zhu, T.; Ma, M.; Shi, Z. SOMOphilic Alkynylation of Unreactive Alkenes Enabled by Iron-Catalyzed Hydrogen Atom Transfer. Molecules 2022, 27, 33. https://doi.org/10.3390/molecules27010033
Zhao B, Zhu T, Ma M, Shi Z. SOMOphilic Alkynylation of Unreactive Alkenes Enabled by Iron-Catalyzed Hydrogen Atom Transfer. Molecules. 2022; 27(1):33. https://doi.org/10.3390/molecules27010033
Chicago/Turabian StyleZhao, Binlin, Tianxiang Zhu, Mengtao Ma, and Zhuangzhi Shi. 2022. "SOMOphilic Alkynylation of Unreactive Alkenes Enabled by Iron-Catalyzed Hydrogen Atom Transfer" Molecules 27, no. 1: 33. https://doi.org/10.3390/molecules27010033
APA StyleZhao, B., Zhu, T., Ma, M., & Shi, Z. (2022). SOMOphilic Alkynylation of Unreactive Alkenes Enabled by Iron-Catalyzed Hydrogen Atom Transfer. Molecules, 27(1), 33. https://doi.org/10.3390/molecules27010033







