The Novel Function of Unsymmetrical Chiral CCN Pincer Nickel Complexes as Chemotherapeutic Agents Targeting Prostate Cancer Cells
Abstract
:1. Introduction
2. Results
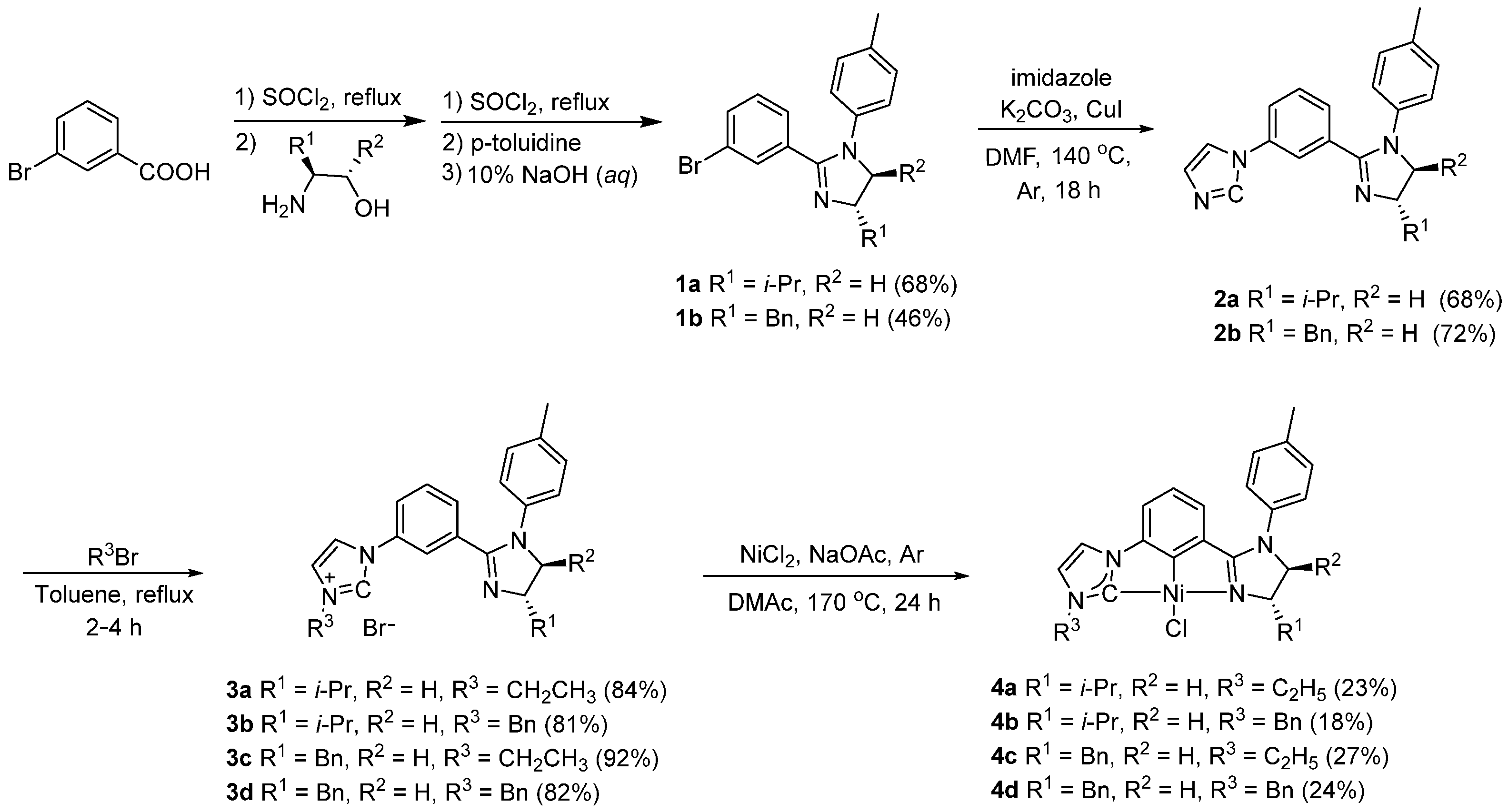
2.1. Chemical Synthesis
2.2. Cytotoxic Studies
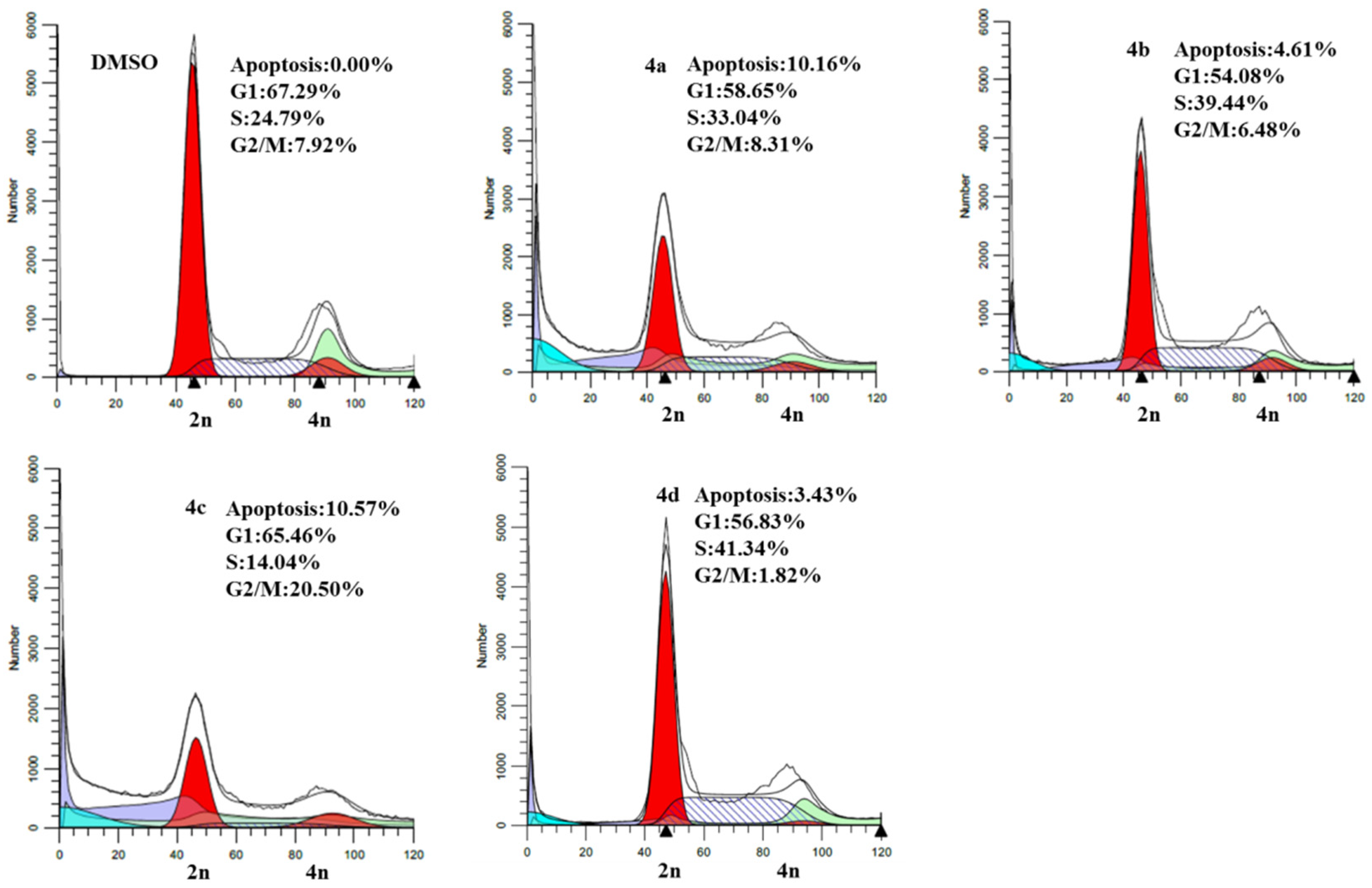
2.3. Cell Cycle Analysis
2.4. Cell Death Analysis
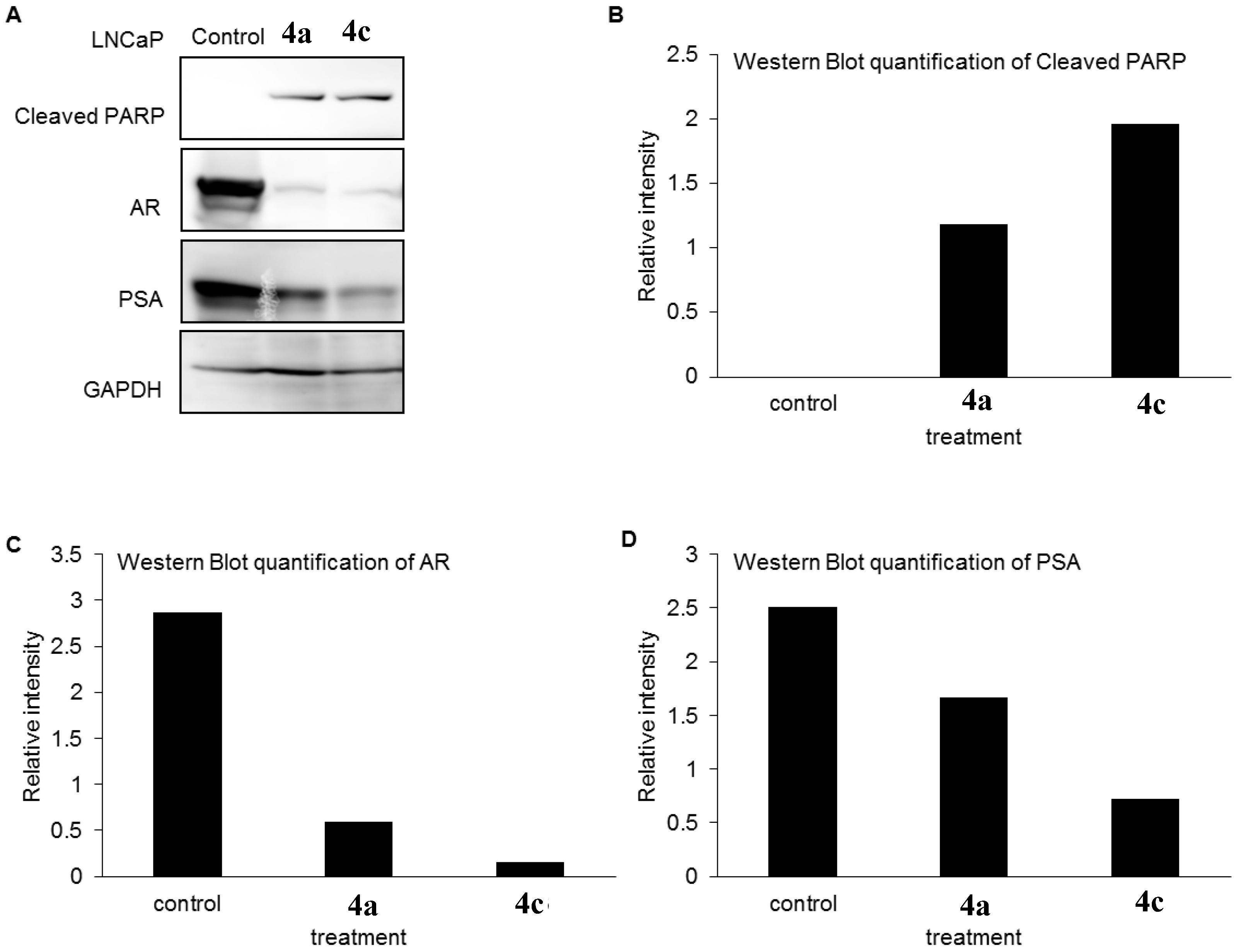
2.5. AR and PSA Expression Analysis
3. Discussion
4. Materials and Methods
4.1. Chemical Synthesis
4.2. Cell Culture and Reagents
4.3. Cell Proliferation Assay
4.4. Cell Cycle and Apoptosis Analysis
4.5. Antibodies and Western Blots
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G.; et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Agarwal, N.; Bjartell, A.; Chung, B.H.; Pereira de Santana Gomes, A.J.; Given, R.; Soto, A.J.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2019, 381, 13–24. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B.; et al. Abiraterone and Increased Survival in Metastatic Prostate Cancer REPLY. N. Engl. J. Med. 2011, 365, 767–768. [Google Scholar]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.-E.; Sternberg, C.N.; Miller, K.; De Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased Survival with Enzalutamide in Prostate Cancer after Chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef] [Green Version]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef] [Green Version]
- Chiang, K.-C.; Tsui, K.-H.; Chung, L.-C.; Yeh, C.-N.; Feng, T.-H.; Chen, W.-T.; Chang, P.-L.; Chiang, H.-Y.; Juang, H.-H. Cisplatin modulates B-cell translocation gene 2 to attenuate cell proliferation of prostate carcinoma cells in both p53-dependent and p53-independent pathways. Sci. Rep. 2014, 4, 5511. [Google Scholar] [CrossRef] [Green Version]
- Yagoda, A.; Watson, R.C.; Natale, R.B.; Barzell, W.; Sogani, P.; Grabstald, H.; Whitmore, W.F. A critical analysis of response criteria in patients with prostatic cancer treated with cis-diamminedichloride platinum II. Cancer 1979, 44, 1553–1562. [Google Scholar] [CrossRef]
- Qazi, R.; Khandekar, J. Phase II study of cisplatin for metastatic prostatic carcinoma. An Eastern Cooperative Oncology Group study. Am. J. Clin. Oncol. 1983, 6, 203–205. [Google Scholar] [CrossRef]
- Merrin, C.E. Treatment of genitourinary tumours with cis-dichlorodiammineplatinum(II): Experience in 250 patients. Cancer Treat. Rep. 1979, 63, 1579–1584. [Google Scholar] [PubMed]
- Moore, M.R.; Troner, M.B.; de Simone, P.; Birch, R.; Irwin, L. Phase II evaluation of weekly cisplatin in metastatic hormone-resistant prostate cancer: A Southeastern Cancer Study Group Trial. Cancer Treat. Rep. 1986, 70, 541–542. [Google Scholar] [PubMed]
- de Sousa, I.H.; Campos, V.N.S.; Vale, A.A.M.; Maciel-Silva, V.L.; Leite, C.M.; Lopes, A.J.O.; Mourão, P.S.; Lima, F.D.C.A.; Batista, A.A.; Santos, A.P.S.D.A.D.; et al. Ruthenium (II) complexes with N, O-chelating proline and threonine ligands cause selective cytotoxicity by the induction of genomic instability, cell cycle arrest and apoptosis in breast and prostate tumor cells. Toxicol. Vitro 2020, 62, 104679. [Google Scholar] [CrossRef] [PubMed]
- de Grandis, R.A.; da Silva Dos Santos, P.W.; de Oliveira, K.M.; Machado, A.R.T.; Aissa, A.F.; Batista, A.A.; Antunes, L.M.G.; Pavan, F.R. Novel lawsone-containing ruthenium(II) complexes: Synthesis, characterization and anticancer activity on 2D and 3D spheroid models of prostate cancer cells. Bioorg. Chem. 2019, 85, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Mangadlao, J.D.; Wang, X.N.; McCleese, C.; Escamilla, M.; Ramamurthy, G.; Wang, Z.; Govande, M.; Basilion, J.P.; Burda, C. Prostate-Specific Membrane Antigen Targeted Gold Nanoparticles for Theranostics of Prostate Cancer. ACS Nano 2018, 12, 3714–3725. [Google Scholar] [CrossRef] [PubMed]
- Bordini, J.; Morisi, F.; Elia, A.R.; Santambrogio, P.; Pagani, A.; Cucchiara, V.; Ghia, P.; Bellone, M.; Briganti, A.; Camaschella, C.; et al. Iron Induces Cell Death and Strengthens the Efficacy of Antiandrogen Therapy in Prostate Cancer Models. Clin. Cancer Res. 2020, 26, 6387–6398. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Wang, Y.; Ding, W.; Xu, J.; Chen, R.; Xie, J.; Zhu, W.; Jia, L.; Ma, T. Anticancer activity and DNA-binding investigations of the Cu(II) and Ni(II) complexes with coumarin derivative. Chem. Biol. Drug Des. 2015, 85, 385–393. [Google Scholar] [CrossRef]
- Feld, C.J.; Johnson, A.; Xiao, Z.Y.; Suntharalingam, K. Breast Cancer Stem Cell Potency of Nickel(II)-Polypyridyl Complexes Containing Non-steroidal Anti-inflammatory Drugs. Chem. Eur. J. 2020, 26, 14011–14017. [Google Scholar] [CrossRef]
- Heng, M.P.; Sim, K.S.; Tan, K.W. Nickel and zinc complexes of testosterone N4-substituted thiosemicarbazone: Selective cytotoxicity towards human colorectal carcinoma cell line HCT 116 and their cell death mechanisms. J. Inorg. Biochem. 2020, 208, 111097. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Y.B.; Zhu, Z.H.; Li, Y.G.; Zhu, X.J.; Hao, X.Q.; Song, M.P. Synthesis, Characterization, and Catalytic Studies of Unsymmetrical Chiral NCC Pincer Pd(II) and Ni(II) Complexes Bearing (Imidazolinyl)aryl NHC Ligands. Organometallics 2018, 37, 2325–2334. [Google Scholar] [CrossRef]
- Snow, O.; Lallous, N.; Singh, K.; Lack, N.; Rennie, P.; Cherkasov, A. Androgen receptor plasticity and its implications for prostate cancer therapy. Cancer Treat. Rep. 2019, 81, 101871. [Google Scholar] [CrossRef] [PubMed]
- Komura, K.; Sweeney, C.J.; Inamoto, T.; Ibuki, N.; Azuma, H.; Kantoff, P.W. Current treatment strategies for advanced prostate cancer. Int. J. Urol. 2018, 25, 220–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
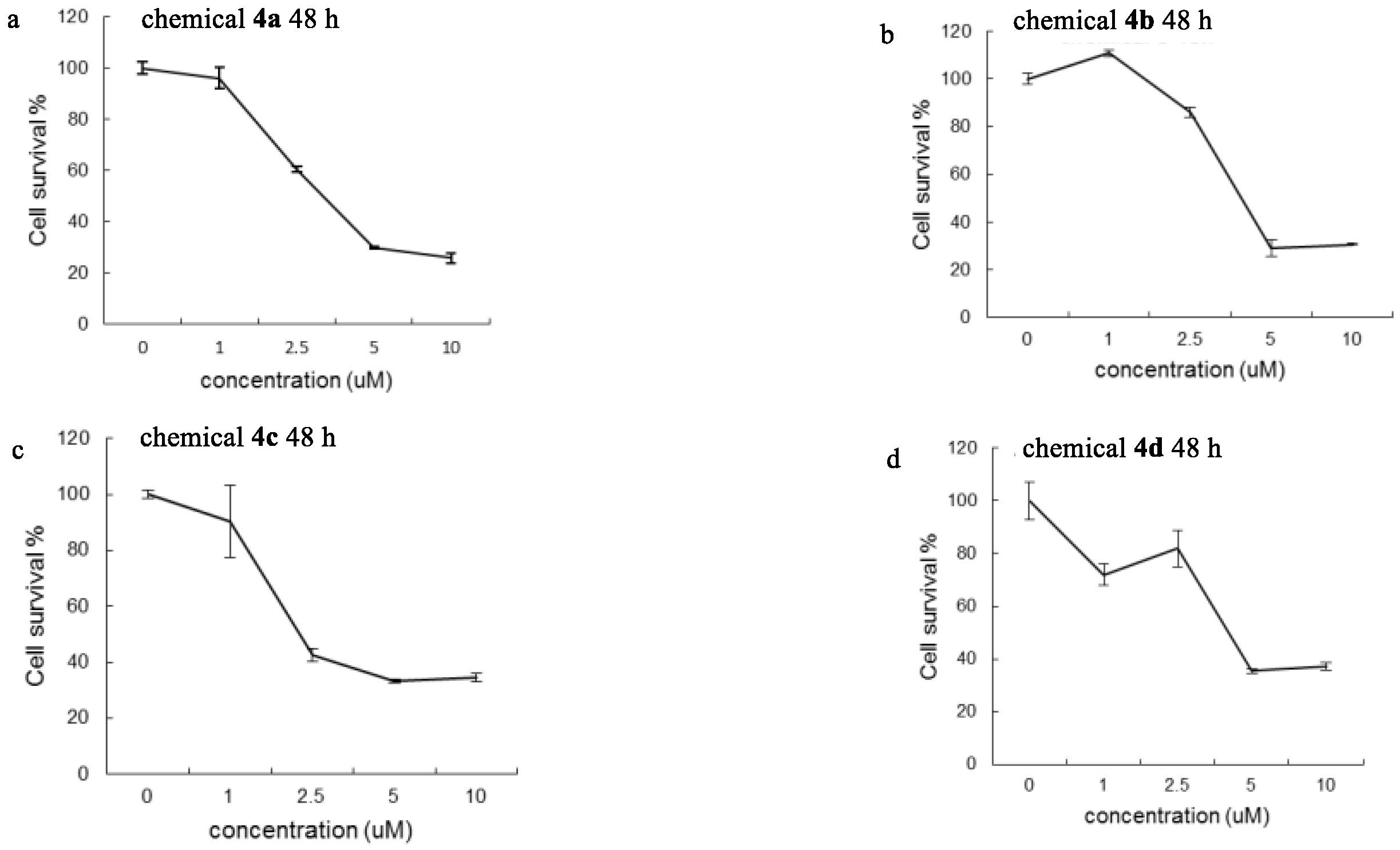
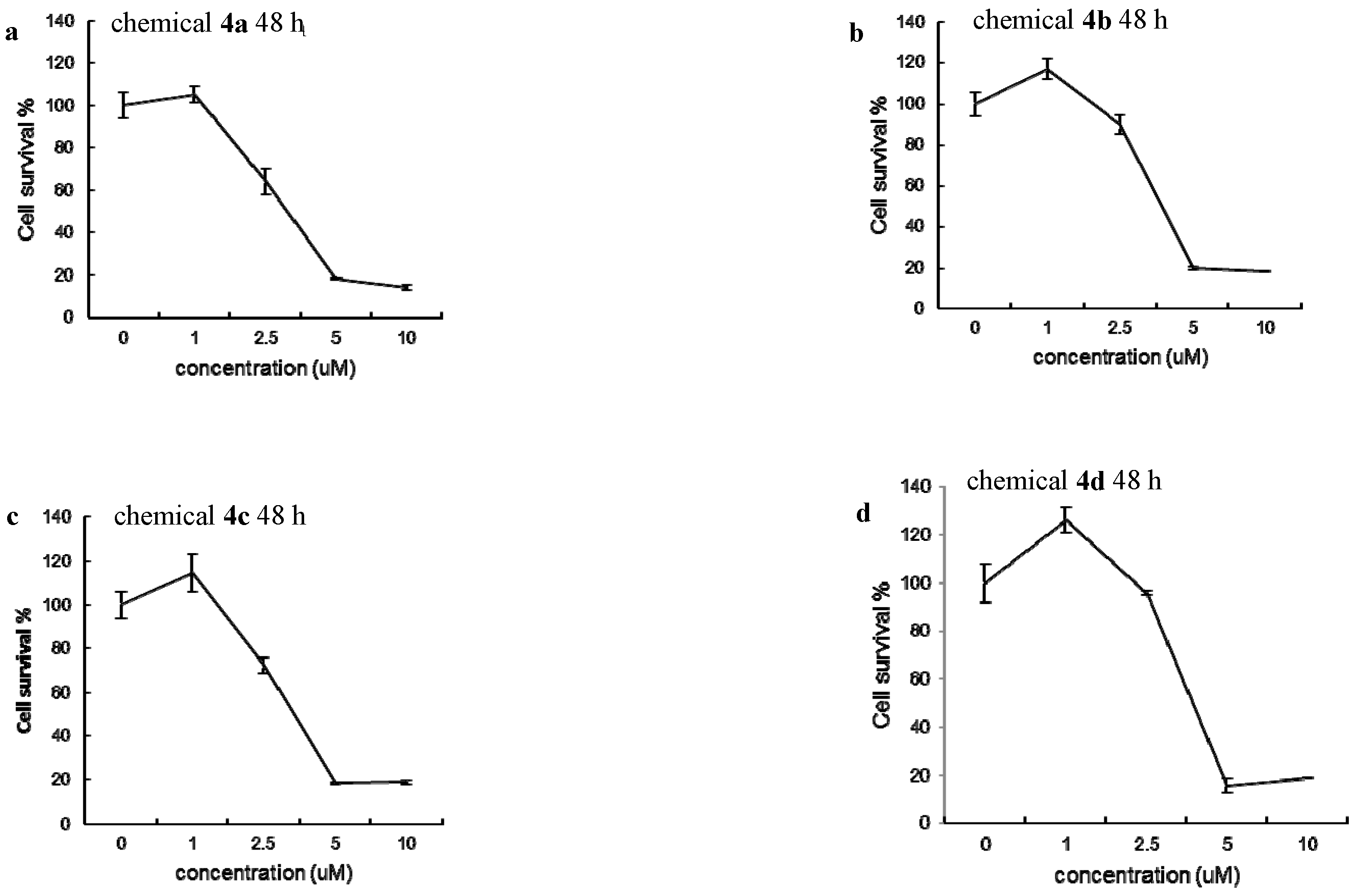

| Drugs | IC50 (μM) |
|---|---|
| Chemical 4a | 5.23 μM |
| Chemical 4b | 4.68 μM |
| Chemical 4c | 5.33 μM |
| Chemical 4d | 4.33 μM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, J.-J.; Shi, L.-L.; Wang, Y.-B.; Yan, J.; Shao, T.; Hao, X.-Q.; Wang, J.-X.; Zhang, H.-Y.; Gong, J.-F.; Song, B. The Novel Function of Unsymmetrical Chiral CCN Pincer Nickel Complexes as Chemotherapeutic Agents Targeting Prostate Cancer Cells. Molecules 2022, 27, 3106. https://doi.org/10.3390/molecules27103106
Qu J-J, Shi L-L, Wang Y-B, Yan J, Shao T, Hao X-Q, Wang J-X, Zhang H-Y, Gong J-F, Song B. The Novel Function of Unsymmetrical Chiral CCN Pincer Nickel Complexes as Chemotherapeutic Agents Targeting Prostate Cancer Cells. Molecules. 2022; 27(10):3106. https://doi.org/10.3390/molecules27103106
Chicago/Turabian StyleQu, Jing-Jing, Lin-Lin Shi, Yan-Bing Wang, Jing Yan, Tian Shao, Xin-Qi Hao, Jia-Xiang Wang, Hong-Yu Zhang, Jun-Fang Gong, and Bing Song. 2022. "The Novel Function of Unsymmetrical Chiral CCN Pincer Nickel Complexes as Chemotherapeutic Agents Targeting Prostate Cancer Cells" Molecules 27, no. 10: 3106. https://doi.org/10.3390/molecules27103106
APA StyleQu, J.-J., Shi, L.-L., Wang, Y.-B., Yan, J., Shao, T., Hao, X.-Q., Wang, J.-X., Zhang, H.-Y., Gong, J.-F., & Song, B. (2022). The Novel Function of Unsymmetrical Chiral CCN Pincer Nickel Complexes as Chemotherapeutic Agents Targeting Prostate Cancer Cells. Molecules, 27(10), 3106. https://doi.org/10.3390/molecules27103106







