Characterization of the Volatile Compounds in Camellia oleifera Seed Oil from Different Geographic Origins
Abstract
:1. Introduction
2. Results and Discussion
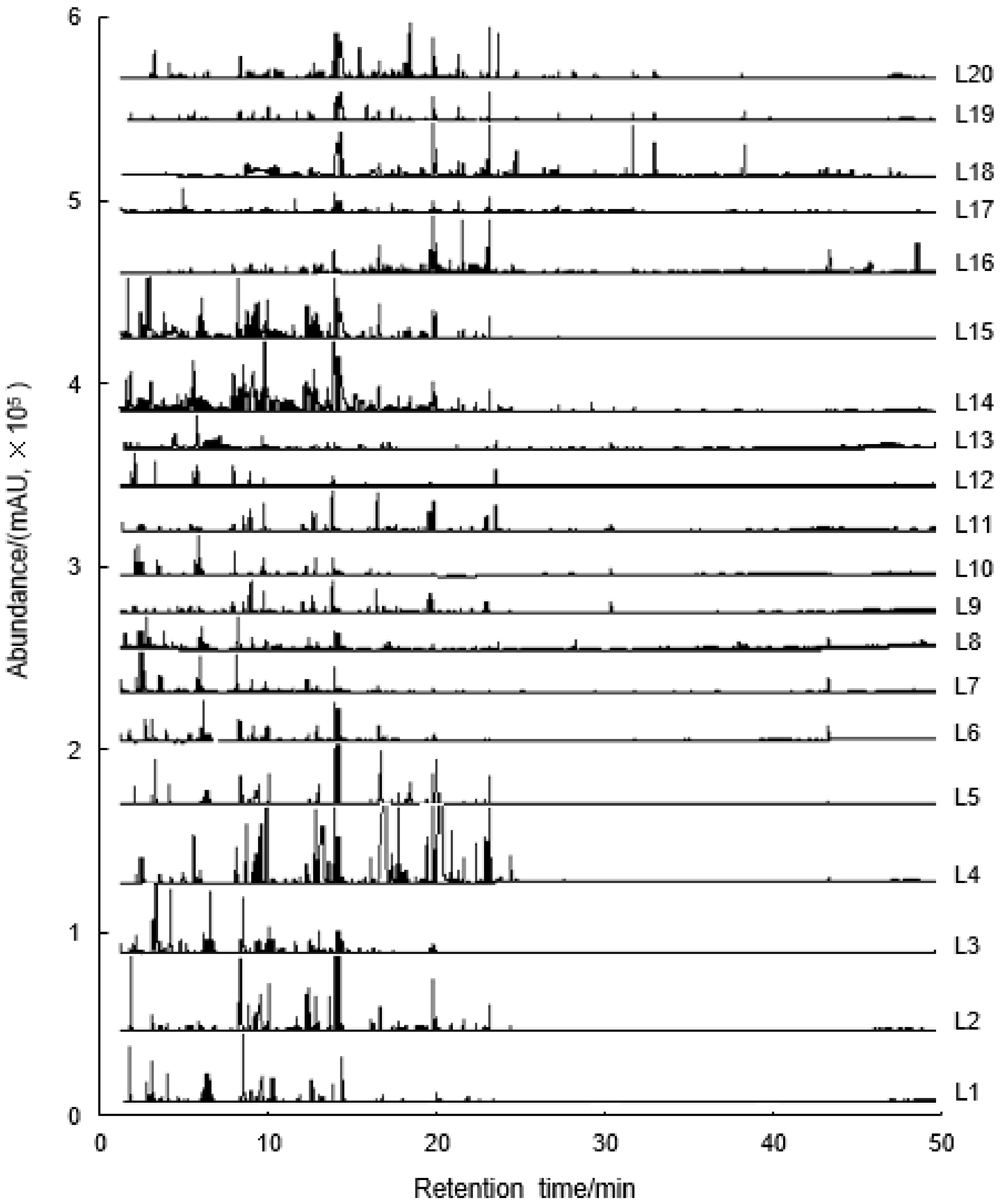
2.1. Comprehensive Analysis of Volatile Compounds
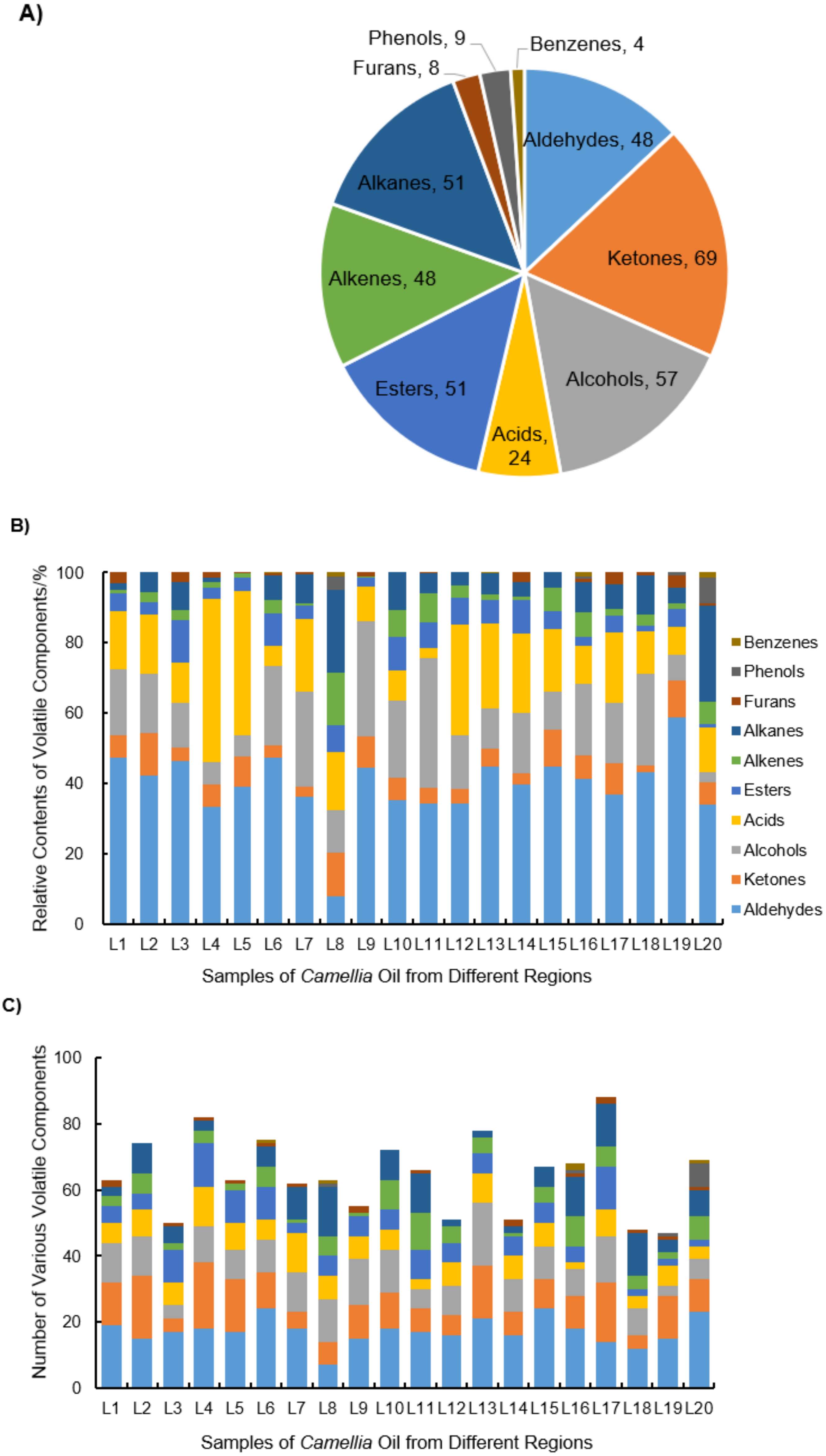
2.2. Composition Analysis of Volatile Components
2.2.1. Analysis of the Contents and Quantity of Volatile Compounds
2.2.2. Analysis of the Common and Unique Volatile Components
2.3. Multivariate Statistical Analysis
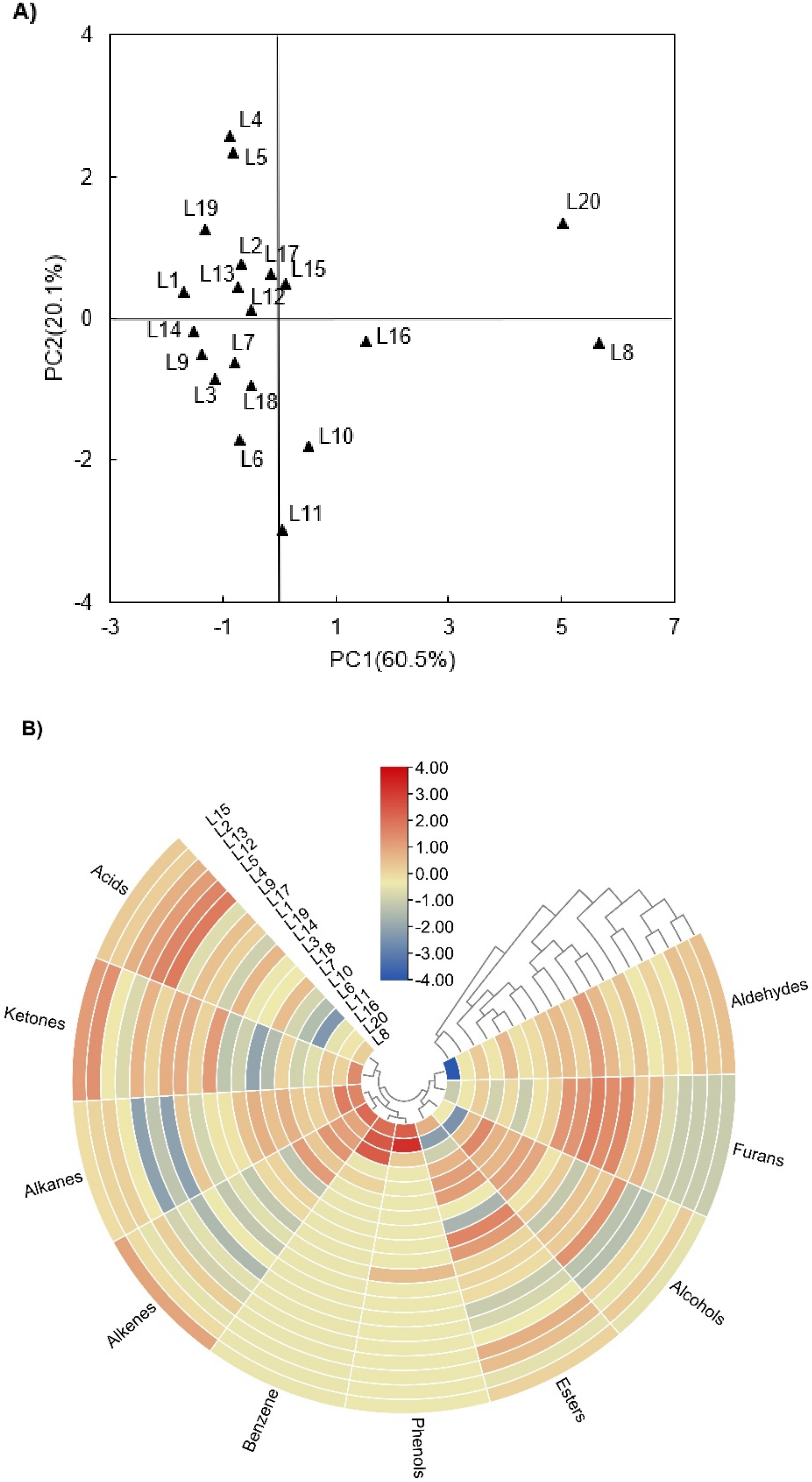
2.3.1. Principal Component Analysis
2.3.2. Heatmap Analysis
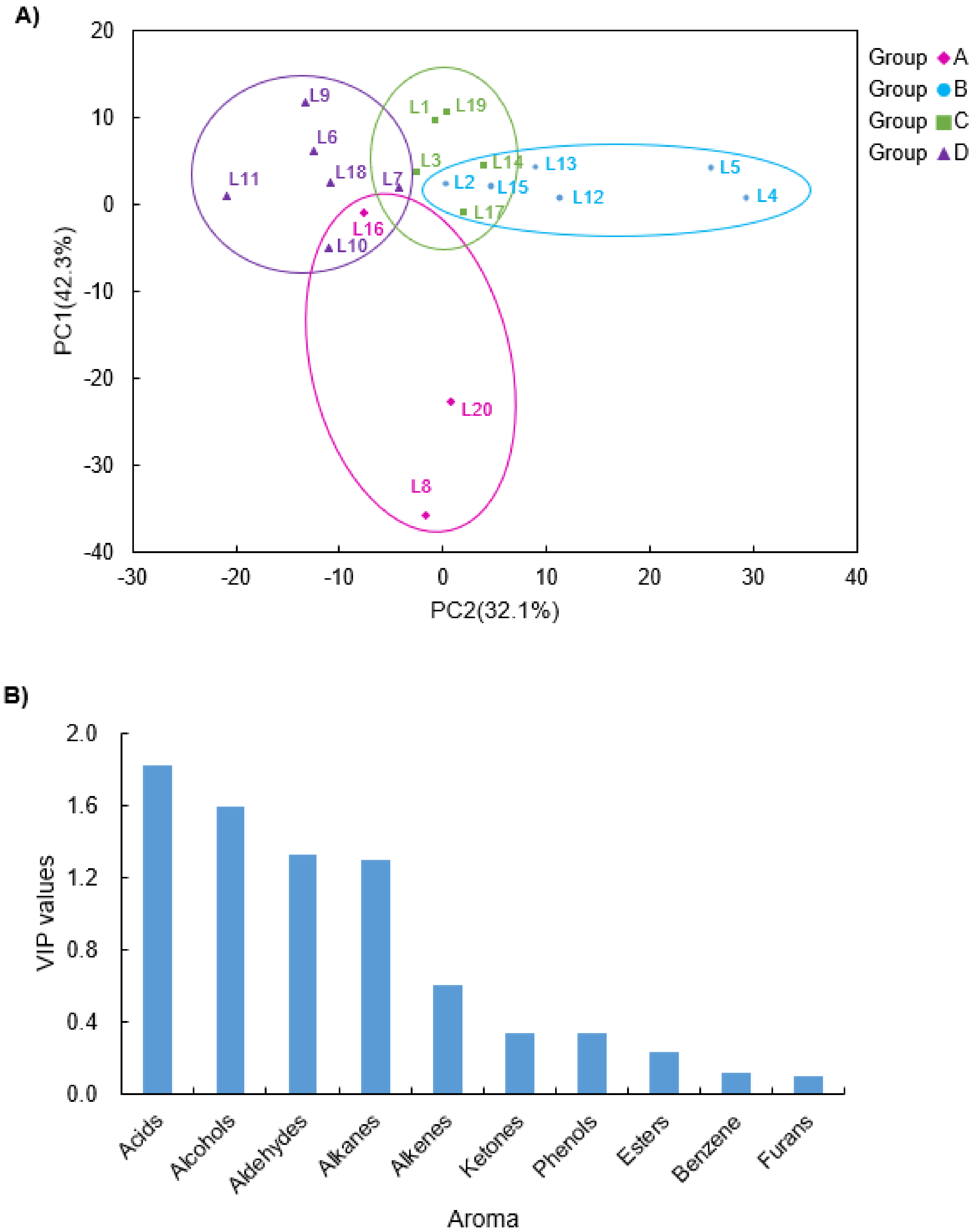
2.3.3. Partial Least Squares-Discrimination Analysis
3. Material and Methods
3.1. Materials
3.2. Oil Extraction
3.3. Volatile Compounds Analysis
3.3.1. HS-SPME
3.3.2. GC–MS Analysis
3.3.3. Qualitative Analysis
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wu, J.; Fan, X.; Huang, X.; Li, G.; Guan, J.; Tang, X.; Qiu, M.; Yang, S.; Lu, S. Effect of different drying treatments on the quality of camellia oleifera seed oil. S. Afr. J. Chem. Eng. 2021, 35, 8–13. [Google Scholar] [CrossRef]
- Zeng, W.; Endo, Y. Effect of cultivars and geography in China on the lipid characteristics of Camellia oleifera seeds. J. Oleo Sci. 2019, 68, 1051–1061. [Google Scholar] [CrossRef] [Green Version]
- Shen, M.; Zhao, S.; Zhang, F.; Huang, M.; Xie, J. Characterization and authentication of olive, camellia and other vegetable oils by combination of chromatographic and chemometric techniques: Role of fatty acids, tocopherols, sterols and squalene. Eur. Food Res. Technol. 2021, 247, 411–426. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, Q.; Verardo, V.; Del Mar Contreras, M. Fatty acid and sterol composition of tea seed oils: Their comparison by the “Fancy Tiles” approach. Food Chem. 2017, 233, 302–310. [Google Scholar] [CrossRef]
- Zeng, W.; Endo, Y. Lipid characteristics of Camellia seed oil. J. Oleo Sci. 2019, 68, 649–658. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zeng, Q.; Del Mar Contreras, M.; Wang, L. Profiling and quantification of phenolic compounds in Camellia seed oils: Natural tea polyphenols in vegetable oil. Food Res. Int. 2017, 102, 184–194. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, C.; Chen, H.; Zhou, H.; Ye, J. Prediction of fatty acid composition in Camellia oleifera oil by near infrared transmittance spectroscopy (NITS). Food Chem. 2013, 138, 1657–1662. [Google Scholar] [CrossRef]
- Wei, W.; Cheng, H.; Cao, X.; Zhang, X.; Feng, F. Triacylglycerols of camellia oil: Composition and positional distribution of fatty acids. Eur. J. Lipid Sci. Technol. 2015, 118, 1254–1255. [Google Scholar] [CrossRef]
- Chaikul, P.; Sripisut, T.; Chanpirom, S.; Sathirachawan, K.; Ditthawuthikul, N. Melanogenesis inhibitory and antioxidant effects of Camellia oleifera seed oil. Adv. Pharm. Bull. 2017, 7, 473–477. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-P.; Yen, G.-C. Antioxidant activity and bioactive compounds of tea seed (Camellia oleifera Abel.) oil. J. Agric. Food Chem. 2006, 54, 779–784. [Google Scholar] [CrossRef]
- Lee, W.-T.; Tung, Y.-Y.; Wu, C.C.; Tu, P.-S.; Yen, G.-C. Camellia oil (Camellia oleifera Abel.) modifies the composition of the gut microbiota and alleviates acetic acid-induced colitis in rats. J. Agric. Food Chem. 2018, 66, 7384–7392. [Google Scholar] [CrossRef]
- Cheng, Y.-T.; Lu, C.-C.; Yen, G.-C. Beneficial effects of Camellia oil (Camellia oleifera Abel.) on hepatoprotective and gastroprotective activities. J. Nutr. Sci. Vitaminol. 2015, 61, 100–102. [Google Scholar] [CrossRef] [Green Version]
- Bayrak, A.; Kiralan, M.; Kara, H.H. Determination of aroma profiles of olive oils from Turkish olive cultivars. J. Am. Oil Chem. Soc. 2013, 90, 1281–1300. [Google Scholar] [CrossRef]
- Nollet, L.M. Physical Characterization and Nutrient Analysis-Aroma Compounds. In Handbook of food Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004; Volume 1, p. 717. [Google Scholar]
- Kiritsakis, A.K. Flavor components of olive oil-a review. J. Am. Oil Chem. Soc. 1998, 75, 673–681. [Google Scholar] [CrossRef]
- Ridolfi, M.; Terenziani, S.; Patumi, M.; Fontanazza, G. Characterization of the lipoxygenases in some olive cultivars and determination of their role in volatile compounds formation. J. Agric. Food Chem. 2002, 50, 835–839. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavor development and quality: A critical review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Kesen, S.; Kelebek, H.; Selli, S. Characterization of the key aroma compounds in Turkish olive oils from different geographic origins by application of aroma extract dilution analysis (AEDA). Agric. Food Chem. 2014, 62, 391–401. [Google Scholar] [CrossRef]
- Ye, J.; Wang, W.; Ho, C.; Li, J.; Guo, X.; Zhao, M.; Jiang, Y.; Tu, P. Differentiation of two types of pu-erh teas by electronic nose and ultrasound-assisted extraction-dispersive liquid-liquid microextraction-gas chromatography-mass spectrometry. Anal. Methods 2016, 8, 593–604. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Yu, D.; Shu, C.; Chen, H.; Wang, H.; Xiao, Z. Comparison of aroma-active volatiles in oolong tea infusions using GC-olfactometry, GC-FPD, and GC-MS. J. Agric. Food Chem. 2015, 63, 7499–7510. [Google Scholar] [CrossRef]
- Zeng, L.; Zhou, Y.; Fu, X.; Mei, X.; Cheng, S.; Gui, J.; Dong, F.; Tang, J.; Ma, S.; Yang, Z. Does oolong tea (Camellia sinensis) made from a combination of leaf and stem smell more aromatic than leaf-only tea? Contribution of the stem to oolong tea aroma. Food Chem. 2017, 237, 488–498. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Q.; Zhong, C.; Guo, M. Analysis of volatile compounds responsible for kiwifruit aroma by desiccated headspace gas chromatography-mass spectrometry. J. Chromatogr. A 2016, 1440, 255–259. [Google Scholar] [CrossRef]
- Reale, S.; Biancolillo, A.; Gasparrini, C.; Di Martino, L.; Di Cecco, V.; Manzi, A.; Di Santo, M.; D’Archivio, A.A. Geographical discrimination of bell pepper (Capsicum annuum) spices by (HS)-SPME/GC-MS aroma profiling and chemometrics. Molecules 2021, 26, 6177. [Google Scholar] [CrossRef]
- Zarifikhosroshahi, M.; Murathan, Z.T.; Kafkas, E.; Okatan, V. Variation in volatile and fatty acid contents among Viburnum opulus L. fruits growing different locations. Sci. Hortic. 2020, 264, 109160. [Google Scholar] [CrossRef]
- Issaoui, M.; Flamini, G.; Brahmi, F.; Dabbou, S.; Hassine, K.B.; Taamali, A.; Chehab, H.; Ellouz, M.; Zarrouk, M.; Hammami, M. Effect of the growing area conditions on differentiation between Chemlali and Chétoui olive oils. Food Chem. 2010, 119, 220–225. [Google Scholar] [CrossRef]
- Alkan, D.; Tokatli, F.; Ozen, B. Phenolic characterization and geographical classification of commercial extra virgin olive oils produced in Turkey. J. Am. Oil Soc. 2012, 78, 261–268. [Google Scholar] [CrossRef]
- Kiralan, M.; Ozkan, G.; Koyluoglu, F.; Ugurlu, H.A.; Bayrak, A.; Kiritsakis, A. Effect of cultivation area and climatic conditions on volatiles of virgin olive oil. Eur. J. Lipid Sci. Technol. 2012, 114, 552–557. [Google Scholar] [CrossRef]
- Tabaszewska, M.; Rutkowska, J.; Skoczylas, Ł.; Słupski, J.; Antoniewska, A.; Smoleń, S.; Łukasiewicz, M.; Baranowski, D.; Duda, I.; Pietsch, J. Red arils of Taxus Baccata L.—A new source of valuable fatty acids and nutrients. Molecules 2021, 26, 723. [Google Scholar] [CrossRef]
- Peng, L.; Men, S.; Liu, Z.; Tong, N.; Imran, M.; Shu, Q. Fatty acid composition, phytochemistry, antioxidant activity on seed coat and kernel of Paeonia ostii from main geographic production areas. Foods 2020, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Contreras, M.; Xu, D.; Jia, W.; Wang, L.; Yang, D. New insights into free and bound phenolic compounds as antioxidant cluster in tea seed oil: Distriction and contribution. LWT Food Sci. Technol. 2021, 136, 110315. [Google Scholar] [CrossRef]
- Angerosa, F.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G. Volatile compounds in virgin olive oil: Occurrence and their relationship with the quality. J. Chromatogr. A 2004, 1054, 17–31. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Dynamic headspace/GC-MS to control the aroma fingerprint of extra-virgin olive oil from the same and different olive varieties. Food Control 2012, 25, 684–695. [Google Scholar] [CrossRef]
- Kesen, S.; Amanpour, A.; Selli, S. Comparative evaluation of the fatty acids and aroma compounds in selected Iranian nut oils. Eur. J. Lipid Sci. Technol. 2018, 120, 1–27. [Google Scholar] [CrossRef]
- Cuicui, L.; Lixia, H. Review on volatile flavor components of roasted oilseeds and their products. Grain Oil Sci. Technol. 2018, 1, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Tomé-Rodríguez, S.; Ledesma-Escobar, C.A.; Penco-Valenzuela, J.M.; Priego-Capote, F. Influence of the fatty acid profile on the volatile components of virgin olive oil subjected to thermal stress. J. Sci. Food Agric. 2021, 101, 4829–4837. [Google Scholar] [CrossRef]
- Gómez-Rico, A.; Salvador, M.D.; La Greca, M.; Fregapane, G. Phenolic and volatile compounds of extra virgin olive oil (Olea europaea L. cv. Cornicabra) with regards to fruit ripening and irrigation management. J. Agric. Food Chem. 2006, 54, 7130–7136. [Google Scholar] [CrossRef]
- Dabbou, S.; Issaoui, M.; Brahmi, F.; Nakbi, A.; Chehab, H.; Mechri, B.; Hammami, M. Changes in volatile compounds during processing of Tunisian-style table olives. J. Am. Oil Chem. Soc. 2012, 89, 347–354. [Google Scholar] [CrossRef]
- He, J.; Wu, X.; Zhou, Y.; Chen, J. Effects of different preheat treatments on volatile compounds of camellia (Camellia oleifera Abel.) seed oil and formation mechanism of key aroma compounds. J. Food Biochem. 2021, 45, e13649. [Google Scholar] [CrossRef]
- Wei, C.Q.; Liu, W.Y.; Xi, W.P.; Cao, D.; Zhang, H.J.; Ding, M.; Chen, L.; Xu, Y.Y.; Huang, K.X. Comparison of volatile compounds of hot-pressed, cold-pressed and solvent-extracted flaxseed oils analyzed by SPME-GC/MS combined with electronic nose: Major volatiles can be used as markers to distinguish differently processed oils. Eur. J. Lipid Sci. Technol. 2015, 117, 320–330. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, Y.; Hong, B.; Zhao, X.; Gu, Z. Characteristic volatile fingerprints of four chrysanthemum teas determined by HS-GC-IMS. Molecules 2021, 26, 7113. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; CRC Press Inc.: Boca Raton, FL, USA, 2016. [Google Scholar]
- Chen, L.; Li, D.; Zhu, C.; Ma, X.; Rong, Y. Fatty acids and flavor components in the oil extracted from golden melon seeds. Eur. J. Lipid Sci. Technol. 2021, 123, 2000233. [Google Scholar] [CrossRef]
- Bonvehí, J.S. Investigation of aromatic compounds in roasted cocoa powder. Eur. Food Res. Technol. 2005, 221, 19–29. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, M.; Yin, H.; Deng, Y.; Jiang, Y.; Yuan, H.; Dong, C.; Li, J.; Hua, J.; Wang, J. Rapid profiling of volatile compounds in green teas using Micro-Chamber/Thermal Extractor combined with thermal desorption couple to gas chromatography-mass spectrometry followed by multivariate statistical analysis. LWT-Food Sci. Technol. 2018, 96, 42–50. [Google Scholar] [CrossRef]
- Gao, J.; Wu, B.; Gao, L.; Liu, H.; Zhang, B.; Sun, C.; Chen, K. Glycosidically bound volatiles as affected by ripening stages of Satsuma mandarin fruit. Food Chem. 2018, 240, 1097–1105. [Google Scholar] [CrossRef]
- Giannetti, V.; Mariani, M.B.; Mannino, P.; Marini, F. Volatile fraction analysis by HS-SPME/GC-MS and chemometric modeling for traceability of apples cultivated in the Northeast Italy. Food Control 2017, 78, 215–221. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, H.; Xie, Y.; Liao, Q.; Lin, Y.; Liu, Y.; Liu, Y.; Xiao, H.; Gao, Z.; Hu, S. Postharvest processing and storage methods for Camellia oleifera seeds. Food Rev. Int. 2019, 36, 319–339. [Google Scholar] [CrossRef]
- Huang, D.; Tao, Y.; Li, W.; Sherif, S.A.; Tang, X. Heat transfer characteristics and kinetics of Camellia oleifera seeds during hot-air drying. J. Thermal. Sci. Eng. Appl. 2020, 12, 031017. [Google Scholar] [CrossRef]
- Wang, J.; Tang, X.; Zhang, Y.; Wang, M.; Xu, B.; Zhang, M. Optimization of headspace solid phase micro-extraction of volatile components from Camellia oleifera seeds oil by response surface methodology. Food Res. Dev. 2020, 41, 93–100. [Google Scholar] [CrossRef]
| NO. | Volatile Compound | CAS | Formula | Retention Index | Sensory Descriptions a | Unrecognized Samples |
|---|---|---|---|---|---|---|
| Aldehydes | ||||||
| 1 | Decanal | 112-89-0 | C10H20O | 1204 | Sweet, waxy | —— |
| 2 | 2,4-Decadienal | 2363-88-4 | C10H16O | 1220 | Deep-fried | —— |
| 3 | (E)-2-Decenal | 3913-81-3 | C10H18O | 1212 | Fatty, green | —— |
| 4 | 2-Undecenal | 2463-77-6 | C11H20O | 1311 | Strong fresh aldehyde | —— |
| 5 | Hexanal | 66-25-1 | C6H12O | 806 | Cut grassy, apple | L8 |
| 6 | (E)-2-Nonenal | 18829-56-6 | C9H16O | 1112 | Green, fatty | L8 |
| 7 | Octanal | 124-13-0 | C8H16O | 1005 | Vanilla, orange | L19 |
| 8 | Heptanal | 111-71-7 | C7H14O | 905 | Green plant, oily | L8, L18 |
| 9 | Nonanal | 124-19-6 | C9H18O | 1104 | Grassy, Almond | L11, L14 |
| 10 | (Z)-2-Heptenal | 57266-86-1 | C7H12O | 913 | Oxidised, pungent | L14, L15, L19 |
| 11 | Furfural | 98-01-1 | C5H4O2 | 831 | Almond | L8, L15, L16, L20 |
| 12 | (E)-2-Octenal | 2548-87-0 | C8H14O | 1013 | Green, floral | L3, L8, L16, L19 |
| Ketones | ||||||
| 13 | γ-Octanoic lactone | 104-50-7 | C8H14O2 | 1184 | Peach, coconut, oatmeal bread | L3, L6, L18 |
| Alcohols | ||||||
| 14 | 1-Heptanol | 111-70-6 | C7H16O | 960 | Fresh, woody | L15, L16, L19 |
| 15 | 2-Furan methanol | 98-00-0 | C5H6O2 | 885 | Bitterness | L8, L15, L16, L20 |
| 16 | Benzyl alcohol | 100-51-6 | C7H8O | 1036 | Aromatic | L5, L8, L12, L18, L19 |
| Acids | ||||||
| 17 | Octanoic acid | 124-07-2 | C8H16O2 | 1173 | Oily, fatty | L8 |
| 18 | Hexanoic acid | 142-62-1 | C6H12O2 | 974 | Sweet, pungent | L8, L20 |
| 19 | Nonanoic acid | 112-05-0 | C9H18O2 | 1272 | Cheese, sweet | L8, L9, L18 |
| 20 | 4-Hydroxybutanoic acid | 591-81-1 | C4H8O3 | 1018 | Buttery, rancid | L8, L15, L16, L20 |
| Ester | ||||||
| 21 | Methyl cinnamate | 103-26-4 | C10H10O2 | 1267 | Cherry, balsamic flavor | L4, L9, L14, L20 |
| Alkenes | ||||||
| 22 | 8-Methyl-1-undecene | 74630-40-3 | C12H24 | 1140 | NF | L8, L10, L18, L20 |
| Phenols | ||||||
| 23 | Maltol | 118-71-8 | C6H6O3 | 1063 | Caramel | L2, L4, L8, L17, L20 |
| NO. | Volatile Compound | CAS | Formula | Retention Index | Similarity (%) | Recognized Sample |
|---|---|---|---|---|---|---|
| Aldehydes | ||||||
| 1 | 2,3-Dihydro-4-carboxaldehyde | 37414-43-0 | C10H10O | 1348 | 81 | L6 |
| 2 | 3-Hydroxy-4-methoxy-benzaldehyde | 621-59-0 | C8H8O3 | 1392 | 90 | L20 |
| 3 | (Z)-13-Octadecenal | 58594-45-9 | C18H34O | 2007 | 86 | L6 |
| 4 | 13-Tetradecenal | 85896-31-7 | C14H26O | 1591 | 80 | L10 |
| 5 | (Z)-4-Undecenal | 68820-32-6 | C11H20O | 1311 | 88 | L15 |
| Ketones | ||||||
| 6 | γ-Butyrolactone | 96-48-0 | C4H6O2 | 825 | 85 | L19 |
| 7 | Cyclopentadecanone | 502-72-7 | C15H28O | 1970 | 85 | L6 |
| 8 | 1,4-Cyclooctanedione | 55794-45-1 | C8H12O2 | 1302 | 84 | L4 |
| 9 | 2,3-Dihydro-3,5-dihydroxy-6-methyl-4(4H)-pyranone | 29446-10-4 | C6H8O4 | 1269 | 92 | L19 |
| 10 | Dihydro-5-methyl-3(2H)-furanone | 34003-72-0 | C5H8O2 | 821 | 83 | L11 |
| 11 | 3-Nonanone | 925-78-0 | C9H18O | 1053 | 93 | L13 |
| 12 | 4-Dodecanone | 6137-26-4 | C12H24O | 1350 | 83 | L15 |
| 13 | 2,5-Dimethyl-4-hydroxy-3(2H)-furanone | 3658-77-3 | C6H8O3 | 1022 | 93 | L19 |
| 14 | 5-Hexyldihydro-2(3H)-furanone | 706-14-9 | C10H18O2 | 1383 | 85 | L10 |
| 15 | 1-Hydroxy-2-butanone | 5077-67-8 | C4H8O2 | 798 | 85 | L9 |
| 16 | 9-Hydroxy-2-nonanone | 25368-56-3 | C9H18O2 | 1295 | 83 | L2 |
| 17 | 1-Indanone | 83-33-0 | C9H8O | 1218 | 81 | L20 |
| 18 | 5-Isopropylfuran-2(3H)-one | 1315481-67-4 | C7H10O2 | 956 | 80 | L2 |
| 19 | 4-Methyl-cyclopentadecanone | 34894-60-5 | C16H30O | 2031 | 85 | L6 |
| 20 | 4-Methyl-2-hexanone | 105-42-0 | C7H14O | 789 | 90 | L20 |
| 21 | 4-Methyl-2-oxepanone | 2549-60-2 | C7H12O2 | 1126 | 89 | L7 |
| 22 | 4-Methyl-4-penten-2-one | 3744-02-3 | C6H10O | 721 | 86 | L19 |
| 23 | (E)-3-Octen-2-one | 18402-82-9 | C8H14O | 960 | 95 | L17 |
| 24 | 3-Pentylcyclopentanone | 85163-13-9 | C10H18O | 1145 | 88 | L13 |
| 25 | Solavetivone | 54878-25-0 | C15H22O | 1645 | 85 | L11 |
| 26 | Tetrahydro-6-pentenyl-pyran-2-one | 25524-95-2 | C10H16O2 | 1205 | 82 | L5 |
| 27 | 2-Tridecanone | 593-08-8 | C13H26O | 1449 | 94 | L15 |
| 28 | 3,3,6-Trimethyl-1,5-heptadien-4-one | 546-49-6 | C10H16O | 1042 | 84 | L4 |
| Alcohols | ||||||
| 29 | [S-(R*,R*)]-2,3-Butanediol | 5341-95-7 | C4H10O2 | 743 | 96 | L14 |
| 30 | Diglycerol | 59113-36-9 | C6H14O5 | 1504 | 93 | L16 |
| 31 | Glycerine | 56-81-5 | C3H8O3 | 967 | 96 | L16 |
| 32 | 1,5-Heptadiene-3,4-diol | 51945-98-3 | C7H12O2 | 1040 | 91 | L13 |
| 33 | (Z)-9-Hexadecen-1-ol | 10378-01-5 | C16H32O | 1862 | 93 | L10 |
| 34 | 6-Methyl-5-hepten-2-ol | 1569-60-4 | C8H16O | 924 | 88 | L5 |
| 35 | 6-Methyl-2-hepten-4-ol | 153665-39-5 | C8H16O | 923 | 88 | L5 |
| 36 | 2-Methyl-2-nonen-1-ol | 43161-19-9 | C10H20O | 1243 | 89 | L4 |
| 37 | 2-Octanol | 123-96-6 | C8H18O | 1060 | 97 | L9 |
| 38 | E-2-Tetradecen-1-ol | 75039-86-0 | C14H28O | 1664 | 91 | L8 |
| 39 | 2,4-Undecadien-1-ol | 59376-58-8 | C11H20O | 1373 | 92 | L15 |
| Acids | ||||||
| 40 | 2-Decenoic acid | 3913-85-7 | C10H18O2 | 1380 | 98 | L4 |
| 41 | Dodecanoic acid | 143-07-7 | C12H24O2 | 1570 | 97 | L8 |
| 42 | Heptanoic acid | 111-14-8 | C7H14O2 | 1074 | 97 | L13 |
| 43 | 2-Heptenoic acid | 18999-28-5 | C7H12O2 | 1081 | 96 | L4 |
| 44 | (E)-3-Hexenoic acid | 1577-18-0 | C6H10O2 | 982 | 95 | L19 |
| 45 | (E)-2-Methyl-2-butenoic acid | 80-59-1 | C5H8O2 | 860 | 92 | L8 |
| 46 | 2-Methyl-propanoic acid | 79-31-2 | C4H8O2 | 711 | 89 | L8 |
| 47 | (E)-2-Octenoic acid | 1871-67-6 | C8H14O2 | 1181 | 93 | L4 |
| 48 | Tetradecanoic acid | 544-63-8 | C14H28O2 | 1769 | 94 | L8 |
| Esters | ||||||
| 49 | 2-Butenoic acid, 3-methyl-, pentyl ester | 56922-72-6 | C10H18O2 | 1168 | 84 | L15 |
| 50 | Butyric acid, 1-propylpentyl ester | 20286-46-8 | C12H24O2 | 1317 | 85 | L13 |
| 51 | Cyclobutanecarboxylic acid, 2-methylpropanyl ester | 87661-19-6 | C9H16O2 | 1141 | 82 | L15 |
| 52 | Cyclobutanecarboxylic acid, 2-pentyl ester | 925444-74-2 | C10H18O2 | 1141 | 84 | L3 |
| 53 | Dibutyl phthalate | 84-74-2 | C16H22O4 | 2037 | 83 | L3 |
| 54 | 1,2-Ethanediol, dipropanoate | 123-80-8 | C8H14O4 | 1151 | 85 | L9 |
| 55 | Formic acid, heptyl ester | 112-23-2 | C8H16O2 | 1081 | 89 | L4 |
| 56 | (Z)-9-Hexadecen-1-ol acetate | 34010-20-3 | C18H34O2 | 1822 | 81 | L4 |
| 57 | Octanoic acid, ethyl ester | 106-32-1 | C10H20O2 | 1183 | 89 | L17 |
| 58 | Octanoic acid, pentyl ester | 638-25-5 | C13H26O2 | 1481 | 88 | L4 |
| 59 | Oxalic acid, butyl propyl ester | 26404-30-8 | C9H16O4 | 1250 | 87 | L9 |
| 60 | 2-Phenylacetic acid,2-ethylhexyl ester | 5421-30-7 | C16H24O2 | 1758 | 88 | L4 |
| 61 | 2-Propenoic acid, tridecyl ester | 2495-25-2 | C17H32O2 | 1814 | 90 | L14 |
| Alkenes | ||||||
| 62 | trans-α-Bergamotene | 13474-59-4 | C15H24 | 1430 | 81 | L11 |
| 63 | 3,7-Decadiene | 72015-36-2 | C10H18 | 1032 | 90 | L4 |
| 64 | Decahydro-1,1,4,7-tetramethyl-1H-cycloprop[e]azulene | 6790-78-9 | C15H26 | 1380 | 83 | L8 |
| 65 | 3,4-Dimethylpent-1-ene | 7385-78-6 | C7H14 | 1030 | 84 | L4 |
| 66 | (E)-7,11-Dimethyl-3-methylene-1,6,10-dodecatriene | 18794-84-8 | C15H24 | 1440 | 82 | L11 |
| 67 | 3,3-Dimethyl-1-octene | 74511-51-6 | C10H20 | 921 | 88 | L1 |
| 68 | 1,5-Dodecadiene | 84348-04-9 | C12H22 | 1212 | 89 | L3 |
| 69 | 1-Ethoxy-4,4-dimethyl-2-pentene | 55702-60-8 | C9H18O | 915 | 84 | L11 |
| 70 | 8-Heptadecene | 16369-12-3 | C17H34 | 1719 | 89 | L10 |
| 71 | 1-Heptadecyne | 26186-00-5 | C17H32 | 1709 | 92 | L10 |
| 72 | 10-Heneicosene | 95008-11-0 | C21H42 | 2117 | 93 | L16 |
| 73 | 1,15-Hexadecadiene | 21964-51-2 | C16H30 | 1592 | 91 | L6 |
| 74 | 1,2,3,5,6,7,8,8a-Octahydro-1,4-dimethyl-7-(1-methylethenyl)-azulene | 489-81-6 | C15H24 | 1490 | 81 | L11 |
| 75 | 7-Oxabicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic anhydride | 6118-51-0 | C8H6O4 | 1248 | 81 | L6 |
| 76 | (Z)-5-Tetradecene | 41446-62-2 | C14H28 | 1421 | 90 | L13 |
| 77 | 3,7,7-Trimethyl-11-methylenespiro[5.5]undec-2-ene | 15401-86-2 | C15H24 | 1507 | 83 | L11 |
| Alkanes | ||||||
| 78 | 1-Butyl-2-ethylcyclopentane | 72993-32-9 | C11H22 | 999 | 84 | L15 |
| 79 | 1-Cyclopropylpentane | 2511-91-3 | C8H16 | 819 | 82 | L13 |
| 80 | 1,1-Dimethyl-3-methylidene-2-prop-2-enylidenecyclohexane | 99647-15-1 | C12H18 | 788 | 83 | L2 |
| 81 | 3,7-Dimethyl-nonane | 17302-32-8 | C11H24 | 986 | 94 | L7 |
| 82 | 1,2-Epoxydodecane | 2855-19-8 | C12H24O | 1304 | 91 | L4 |
| 83 | 5-Ethylundecane | 17453-94-0 | C13H28 | 1249 | 94 | L11 |
| 84 | 3-Methyl-5-propylnonane | 31081-18-2 | C13H28 | 1185 | 92 | L16 |
| 85 | (S)-{[4-(Phenylmethoxy)phenoxy]methyl}-oxirane | 122797-04-0 | C16H16O3 | 410 | 88 | L17 |
| 86 | n-Nonylcyclohexane | 2883-02-5 | C15H30 | 1576 | 89 | L10 |
| 87 | cis-2-Phenyl-1-(2-methyl-1-propenyl)cyclopropane | 89486-56-6 | C13H16 | 1078 | 83 | L3 |
| 88 | Propyl-cyclopropane | 2415-72-7 | C6H12 | 620 | 88 | L2 |
| 89 | 2,2,3,3-Tetramethylhexane | 13475-81-5 | C10H22 | 846 | 97 | L19 |
| 90 | (S)-2-Tridecyloxirane | 96938-07-7 | C15H30O | 1603 | 84 | L19 |
| Furans | ||||||
| 91 | Dibenzofuran | 132-64-9 | C12H8O | 1483 | 90 | L20 |
| 92 | Furan | 110-00-9 | C4H4O | 553 | 96 | L6 |
| 93 | 2-Hexyl-2-methyl-5-(propan-2-ylidene)tetrahydrofuran | 124099-79-2 | C14H26O | 1147 | 91 | L4 |
| 94 | Octahydro-2,3’-bifuran | 73373-15-6 | C8H14O2 | 1079 | 87 | L1 |
| Phenols | ||||||
| 95 | 4-Ethyl-2-methoxy-phenol | 2785-89-9 | C9H12O2 | 1303 | 85 | L20 |
| 96 | 2-Ethylphenol | 90-00-6 | C8H10O | 1114 | 89 | L20 |
| 97 | 2-Methoxy-4-methyl-phenol | 93-51-6 | C8H10O2 | 1203 | 89 | L20 |
| 98 | 2,4-bis(1,1-dimethylethyl)-Phenol | 96-76-4 | C14H22O | 1555 | 86 | L8 |
| Samples | Collected Location | Cultivars | Latitude | Longitude | Altitude (m) | Annual Average Temperature (°C) | Annual Rainfall (mm) | Annual Sunshine Duration (h) | Climate |
|---|---|---|---|---|---|---|---|---|---|
| L1 | Sihui, Zhaoqing, Guangdong | Camellia semiserrata Chi | 23°35′ N | 112°33′ E | ≤1000 | 20–22 | 1750 | 1600 | Subtropical climate |
| L2 | Lianzhou, Qingyuan, Guangdong | Camellia meiocarpa Hu | 25°05′ N | 112°37′ E | ≤1000 | 19–21 | 1625 | 1510 | Central Asia monsoon climate |
| L3 | Qingxin, Qingyuan, Guangdong | Camellia oleifera Abel | 23°44′ N | 113°0′ E | ≤1000 | 19–21 | 1625 | 1510 | Central Asia monsoon climate |
| L4 | Yangchun, Yangjiang, Guangdong | Camellia oleifera Abel | 22°19′ N | 111°51′ E | ≤200 | 21–28 | 2380 | 2000 | Subtropical rainforest climate |
| L5 | Qujiang, Shaoguan, Guangdong | Camellia oleifera Abel | 24°42′ N | 113°49′ E | ≤200 | 18–26 | 1700 | 1660 | Subtropical monsoon climate |
| L6 | Guangning, Zhaoqing, Guangdong | Camellia oleifera Abel | 23°39′ N | 112°21′ E | ≤300 | 20–22 | 1720 | 1613 | Transitional climate between South Asia and central subtropics |
| L7 | Yunan, Yunfu, Guangdong | Camellia oleifera Abel | 22°56′ N | 111°53′ E | ≤1000 | 20–25 | 1580 | 1480 | Subtropical monsoon climate |
| L8 | Longchuan, Heyuan, Guangdong | Camellia oleifera Abel | 24°19′ N | 115°15′ E | ≤500 | 18–27 | 1500 | 1700 | Subtropical monsoon climate |
| L9 | Xingning, Meizhou, Guangdong | Camellia meiocarpa Hu | 24°25′ N | 115°37′ E | ≤400 | 19–26 | 1520 | 1900 | Transitional climate between South Asia and central subtropics |
| L10 | Tianhe, Guangzhou, Guangdong | Camellia gauchowensis Change | 23°11′ N | 113°22′ E | ≤100 | 20–28 | 2000 | 1620 | Subtropical marine monsoon climate |
| L11 | Xuwen, Zhanjiang, Guangdong | Camellia gauchowensis Change | 20°19′ N | 110°19′ E | ≤100 | 20–25 | 2000 | 2100 | Tropical monsoon climate |
| L12 | Gaozhou, Maoming, Guangdong | Camellia gauchowensis Change | 21°42′ N | 110°36′ E | ≤1600 | 20–25 | 1900 | 1950 | Subtropical monsoon climate |
| L13 | You, Zhuzhou, Hunan | Camellia oleifera Abel | 26°46′ N | 113°09′ E | ≤1400 | 16–18 | 1400 | NF | Mid-subtropical humid monsoon climate |
| L14 | Yuanzhou, Yichun, Jiangxi | Camellia oleifera Abel | 27°33′ N | 113°54′ E | ≤1800 | 15–20 | 1680 | 1740 | Mid-subtropical monsoon climate |
| L15 | Zhanggong, Ganzhou, Jiangxi | Camellia oleifera Abel | 24°29′ N | 113°54′ E | 300–500 | 18–22 | 1320 | NF | Subtropical monsoon climate |
| L16 | Qingshanhu, Nanchang, Jiangxi | Camellia oleifera Abel | 28°10′ N | 115°27′ E | ≤1000 | 17–18 | 1650 | 1800 | Subtropical monsoon climate |
| L17 | Xixiangtang, Nanning, Guangxi | Camellia gauchowensis Change | 22°48′ N | 108°22′ E | 300–600 | 20–23 | 1300 | NF | Subtropical monsoon climate |
| L18 | Xiuying, Haikou, Hainan | Camellia vietnamensis Huang ex Hu | 19°31′ N | 110°24′ E | ≤100 | 27–29 | 2040 | 2160 | Tropical monsoon climate |
| L19 | Beireng, Qionghai, Hainan | Camellia vietnamensis Huang ex Hu | 18°58′ N | 110°7′ E | ≤100 | 27–28 | 2040 | 2155 | Tropical monsoon climate |
| L20 | Panlong, Kunming, Yunnan | Camellia oleifera Abel | 25°02′ N | 102°42′ E | 1500–2800 | 13–18 | 1035 | 2200 | Subtropical highland monsoon climate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Tang, X.; Chu, Q.; Zhang, M.; Zhang, Y.; Xu, B. Characterization of the Volatile Compounds in Camellia oleifera Seed Oil from Different Geographic Origins. Molecules 2022, 27, 308. https://doi.org/10.3390/molecules27010308
Wang J, Tang X, Chu Q, Zhang M, Zhang Y, Xu B. Characterization of the Volatile Compounds in Camellia oleifera Seed Oil from Different Geographic Origins. Molecules. 2022; 27(1):308. https://doi.org/10.3390/molecules27010308
Chicago/Turabian StyleWang, Jing, Xuxiao Tang, Qiulu Chu, Mengyu Zhang, Yingzhong Zhang, and Baohua Xu. 2022. "Characterization of the Volatile Compounds in Camellia oleifera Seed Oil from Different Geographic Origins" Molecules 27, no. 1: 308. https://doi.org/10.3390/molecules27010308
APA StyleWang, J., Tang, X., Chu, Q., Zhang, M., Zhang, Y., & Xu, B. (2022). Characterization of the Volatile Compounds in Camellia oleifera Seed Oil from Different Geographic Origins. Molecules, 27(1), 308. https://doi.org/10.3390/molecules27010308





