In Vitro Anti-Diabetic Activities and UHPLC-ESI-MS/MS Profile of Muntingia calabura Leaves Extract
Abstract
1. Introduction
2. Results and Discussion
2.1. Influence of Varied Drying Process and Ethanol Concentrations on Anti-Diabetic Activity
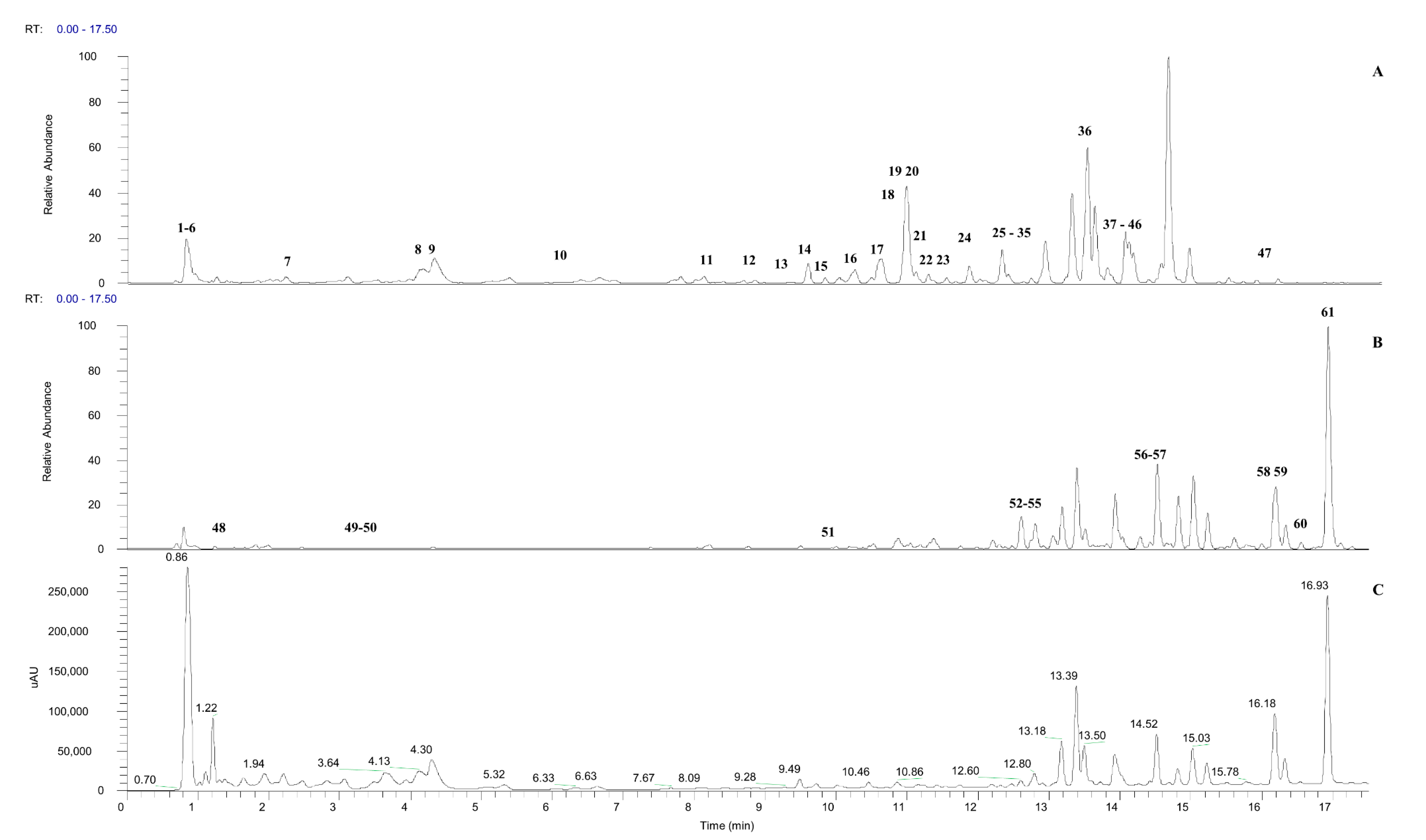
2.2. UHPLC-ESI-MS/MS Characterization of Phytoconstituents in the M. calabura Leaves Extract
2.2.1. Catechin Derivatives
2.2.2. Kaempferol Derivatives
2.2.3. Quercetin Derivatives
2.2.4. Apigenin Derivatives
2.2.5. Luteolin Derivatives
2.2.6. Daidzein Derivatives
2.2.7. Other Flavonoids
2.2.8. Anthocyanin
2.2.9. Chalcone
2.2.10. Quinone Derivatives
2.2.11. Lactone Derivatives
2.2.12. Terpene Glycoside
2.2.13. Alkaloid Derivatives
2.2.14. Sugar
2.2.15. Ellagitannin Derivative
| Peak No | tR | UV λ-Max | MF | Exact Mass | (M-H)− | (M+H)+ | Mass Error | MS/MS Fragment Ions | Tentative Identification | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.81 | 210, 274, 350, 370 | ND | ND | 683.2253 | − | ND | 341.1091, 179.0554, 161.0447, 143.0340 | Sucrose dimer | [40] |
| 2 | 0.94 | 210, 272, 350, 370 | C12H22O11 | 342.1162 | 341.1090 | − | 0.0072 | 179.0555, 161.0443, 143.0345 | Sucrose | [40] |
| 3 | 1.24 | 208, 266, 292, 350, 370 | C15H14O7 | 306.0740 | 305.0668 | − | 0.0072 | 289.0642, 245.0440, 219.0657, 167.0341 | Epigallocatechin | [40] |
| 4 | 1.36 | 210, 272 | C19H18O7 | 358.1053 | 357.1195 | − | −0.0142 | 270.8385, 224.8610, 179.0586 | 3′-Hydroxy-7,8,4′,5′-tetramethoxyflavone * | [46] |
| 5 | 1.39 | 208, 216, 276, 270 | C27H22O18 | 634.0806 | 633.0735 | − | 0.0071 | 481.1787, 313.0568, 169.0137 | Corilagin | [58] |
| 6 | 1.40 | 208, 274 | C15H14O7 | 306.0740 | 305.0668 | − | 0.0072 | 289.0233, 245.0284, 219.0655, 167.0338 | Gallocatechin | [40] |
| 7 | 2.42 | 208, 356 | C20H20O7 | 372.1209 | 371.0986 | − | 0.0223 | 296.8684, 240.8795, 231.0506 | 7,8,3′,4′,5′-Pentamethoxyflavone * | [46] |
| 8 | 4.05 | 206, 256, 350 | C21H21O12 | 465.1033 | 464.8049 | − | −0.7016 | 386.9921, 299.0193, 178.9979 | Myrtillin * | [40] |
| 9 | 4.21 | 206, 258, 350 | C21H21O12 | 465.1033 | 464.8049 | − | −0.7016 | 386.9907, 298.9871, 178.9980 | Myrtillin isomer | [40] |
| 10 | 6.34 | 210, 272, 350, 370 | C17H24O10 | 388.1369 | 387.1145 | − | 0.0224 | 284.0327, 255.0298, 224.8609 | Geniposide # | [40] |
| 11 | 8.70 | 222, 272 | C26H30O6 | 438.2042 | 437.0466 | − | 0.1576 | 296.8747, 288.7813, 242.8798 | Hiravanone * | [47] |
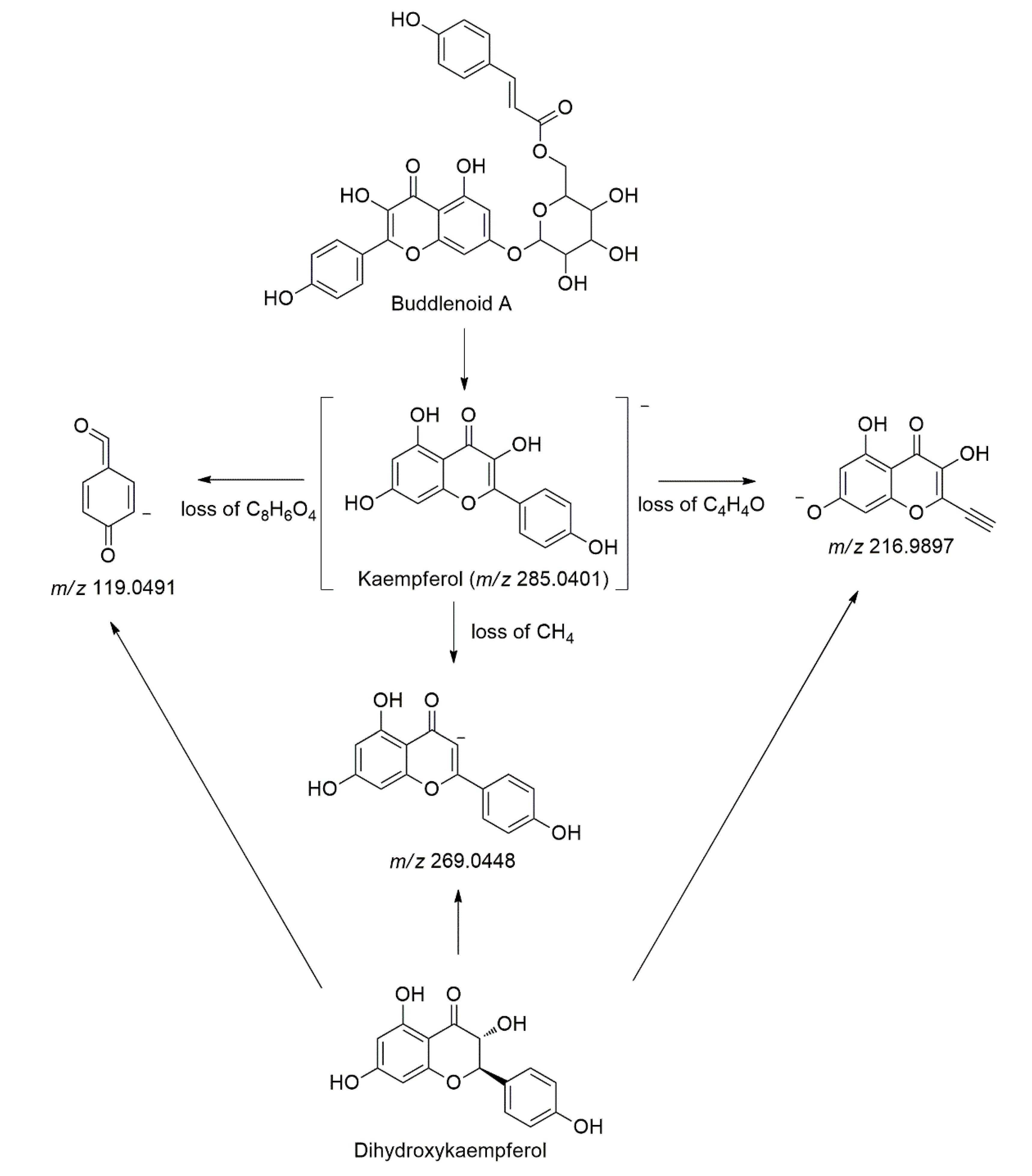
| 12 | 9.43 | 222, 274 | C30H26O13 | 594.1373 | 593.1304 | − | 0.0069 | 447.0935, 429.0823, 285.0404 | Buddlenoid A * | [41] |
| 13 | 9.49 | 222, 268, 314 | ND | ND | 1187.2678 | − | ND | 593.1306, 447.0932, 429.0825, 285.0408 | Buddlenoid A dimer | [41] |
| 14 | 9.75 | 222, 268, 296, 374 | C30H26O13 | 594.1373 | 593.1304 | − | 0.0069 | 447.0919, 429.0820, 285.0406 | Buddlenoid A isomer | [41] |
| 15 | 10.16 | 222, 280 | C15H12O4 | 256.0736 | 255.0662 | − | 0.0074 | 227.0477, 213.0526, 187.0655 | Isoliquiritigenin * | [51] |
| 16 | 10.47 | 222, 294 | C16H14O6 | 302.0790 | 301.0721 | − | 0.0069 | 286.0490, 269.0465, 211.0475 | 3,5,8-Trihydroxy-7-methoxyflavanone * | [16] |
| 17 | 10.63 | 224, 286 | C16H14O6 | 302.0790 | 301.0721 | − | 0.0069 | 286.0489, 269.0461, 211.0474 | Trihydroxymethoxyflavanone isomer | [16] |
| 18 | 10.78 | 222, 294 | C16H14O6 | 302.0790 | 301.0721 | − | 0.0069 | 286.0492, 269.0452, 211.0475 | Trihydroxymethoxyflavanone isomer | [16] |
| 19 | 10.80 | 222, 294 | C12H16O7 | 272.0896 | 271.0613 | − | 0.0283 | 253.0507, 197.0603, 161.0600 | Arbutin | [52] |
| 20 | 10.96 | 224, 276, 296, 376 | C12H16O7 | 272.0896 | 271.0613 | − | 0.0283 | 253.0509, 197.0603, 161.0600 | Arbutin isomer | [52] |
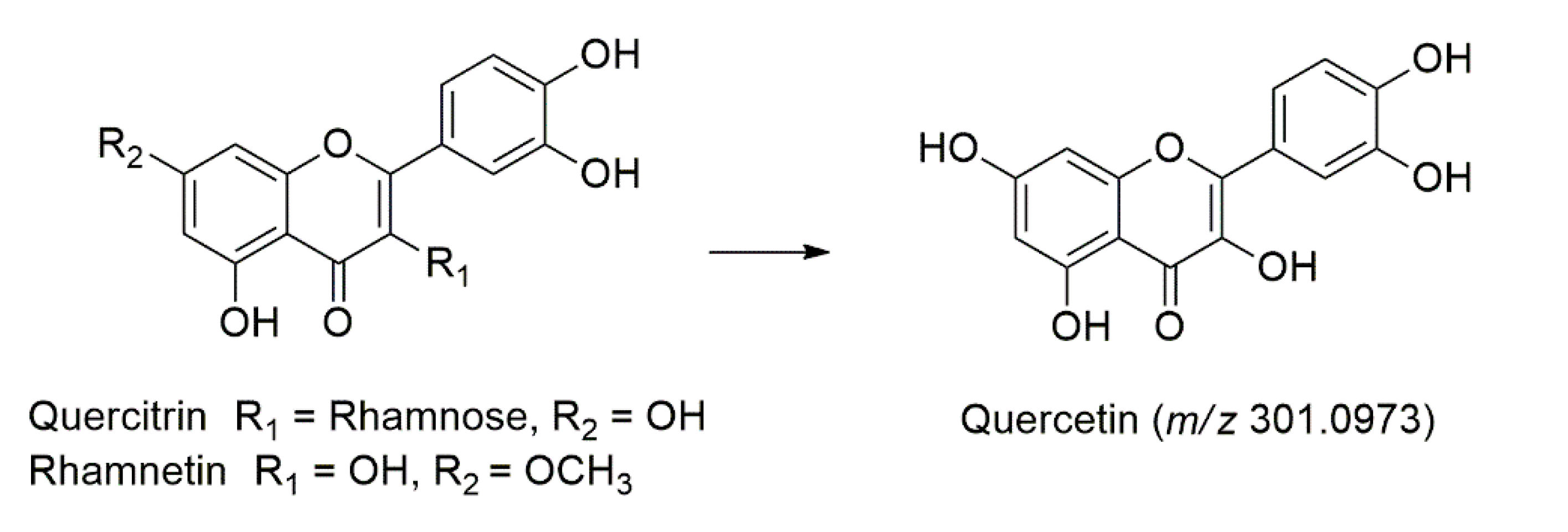
| 21 | 11.02 | 224, 282 | C21H20O11 | 448.1006 | 447.2237 | − | −0.1231 | 301.1495, 285.2063, 245.0717 | Quercitrin # | [40] |
| 22 | 11.13 | 224, 290, 298 | C15H10O3 | 238.0630 | 237.0555 | − | 0.0075 | 209.0591, 160.0157 | Hydroxyflavone | [40] |
| 23 | 11.17 | 224, 306 | C21H20O11 | 448.1006 | 447.2237 | − | −0.1231 | 301.0923, 285.0311, 245.0662 | Quercitrin isomer | [40] |
| 24 | 11.87 | 224, 282 | C15H10O6 | 286.0477 | 285.0401 | − | 0.0076 | 269.0448, 216.9897, 119.0491 | Kaempferol # | [40] |
| 25 | 12.30 | 224, 270, 292, 312 | C25H28O6 | 424.1886 | 423.0928 | − | 0.0958 | 353.2442, 287.6386, 251.0923 | 6,8-Diprenyleriodictyol * | [47] |
| 26 | 12.36 | 224, 290 | C27H26O13 | 558.4875 | 557.1458 | − | 0.3417 | 301.1331, 285.0776, 257.3555, 201.0187 | Piceatannol galloylglucoside | [57] |
| 27 | 12.50 | 224, 286 | C17H14O7 | 330.0740 | 329.0672 | − | 0.0068 | 314.0419, 299.0198, 285.0399 | Cirsiliol | [40] |
| 28 | 12.66 | 224, 292, 376 | C17H14O7 | 330.0740 | 329.0670 | − | 0.0070 | 314.0435, 299.0198, 285.0421 | Cirsiliol isomer | [40] |
| 29 | 12.71 | 224, 292, 376 | C15H14O4 | 258.0892 | 257.0644 | − | 0.0248 | 239.0710, 213.0919, 197.0815 | Yangonin | [50] |
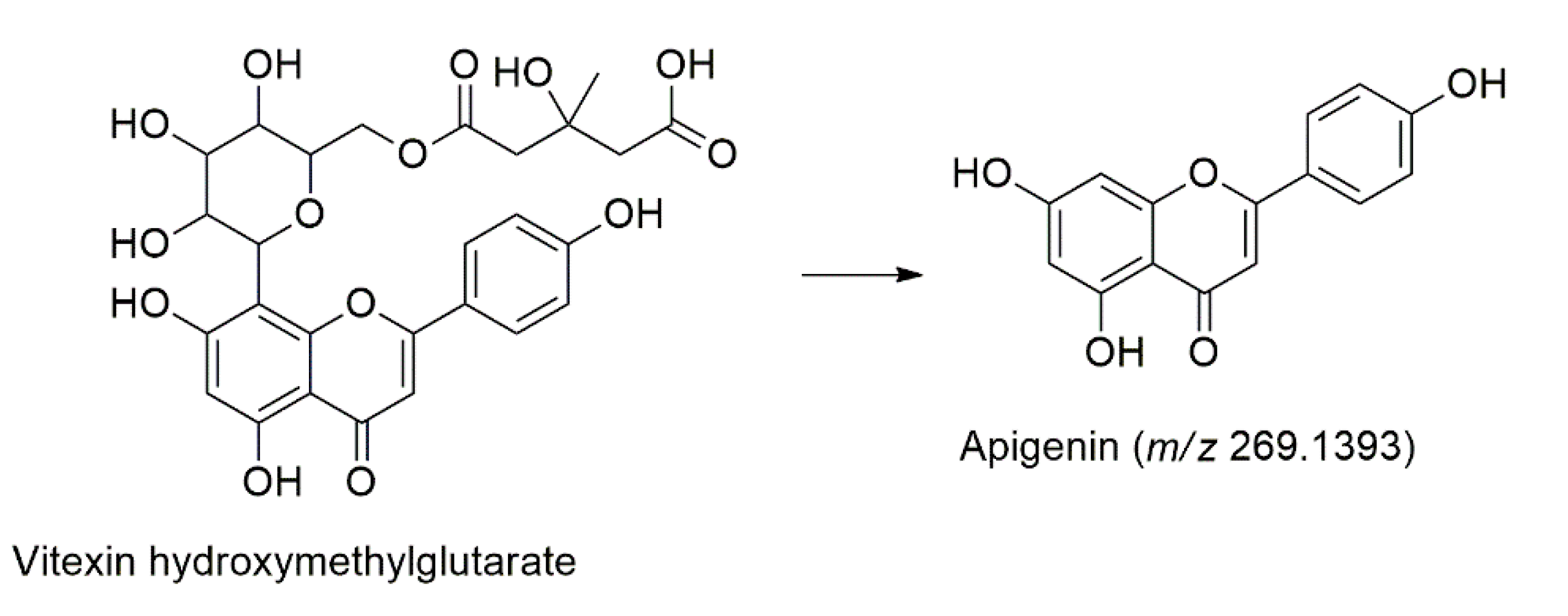
| 30 | 12.72 | 224, 292, 376 | C27H28O14 | 576.1479 | 575.1748 | − | −0.0269 | 513.0697, 341.1581, 269.1393, 231.1236 | Vitexin hydroxymethylglutarate | [43] |
| 31 | 12.73 | 224, 282, 332, 374 | C15H12O4 | 256.0736 | 255.0662 | − | 0.0074 | 227.0711, 213.0549, 187.0634 | Isoliquiritigenin isomer | [51] |
| 32 | 12.96 | 224, 332, 374 | C18H16O7 | 344.0896 | 343.0823 | − | 0.0073 | 327.0524, 313.0357, 256.9828 | 8,3′-Dihydroxy-7,4′,5′-trimethoxyflavone * | [46] |
| 33 | 13.31 | 224, 280, 378 | C17H16O5 | 300.0998 | 299.0196 | − | 0.0802 | 284.0326, 269.0457, 255.0300, 239.0348 | Dimethoxyhydroxyflavanone | [40] |
| 34 | 13.40 | 218, 274, 366 | C15H14O4 | 258.0892 | 257.0644 | − | 0.0248 | 239.0712, 213.0915, 197.0597 | Yangonin isomer | [50] |
| 35 | 13.45 | 224, 272, 360, 374 | C15H8O6 | 284.0321 | 282.9547 | − | 0.0774 | 267.0296, 239.0353, 211.0396 | Rhein | [53] |
| 36 | 13.47 | 222, 268, 312, 360 | C17H16O5 | 300.0998 | 299.0196 | − | 0.0802 | 284.0326, 269.0816, 255.0304, 239.0346 | Dimethoxyhydroxyflavanone isomer | [40] |
| 37 | 13.63 | 224, 278, 356, 376 | C15H12O6 | 288.0634 | 287.0539 | − | 0.0095 | 271.0607, 269.0723, 216.9894, 119.0077 | Dihydrokaempferol | [40] |
| 38 | 13.67 | 224, 286, 376 | C20H20O6 | 356.1260 | 355.1038 | − | 0.0222 | 285.0948, 255.0656, 241.1012 | Kievitone | [48] |
| 39 | 13.92 | 224, 274, 352 | C15H8O6 | 284.0321 | 282.9547 | − | 0.0774 | 267.0282, 239.0348, 211.0397 | Rhein isomer | [53] |
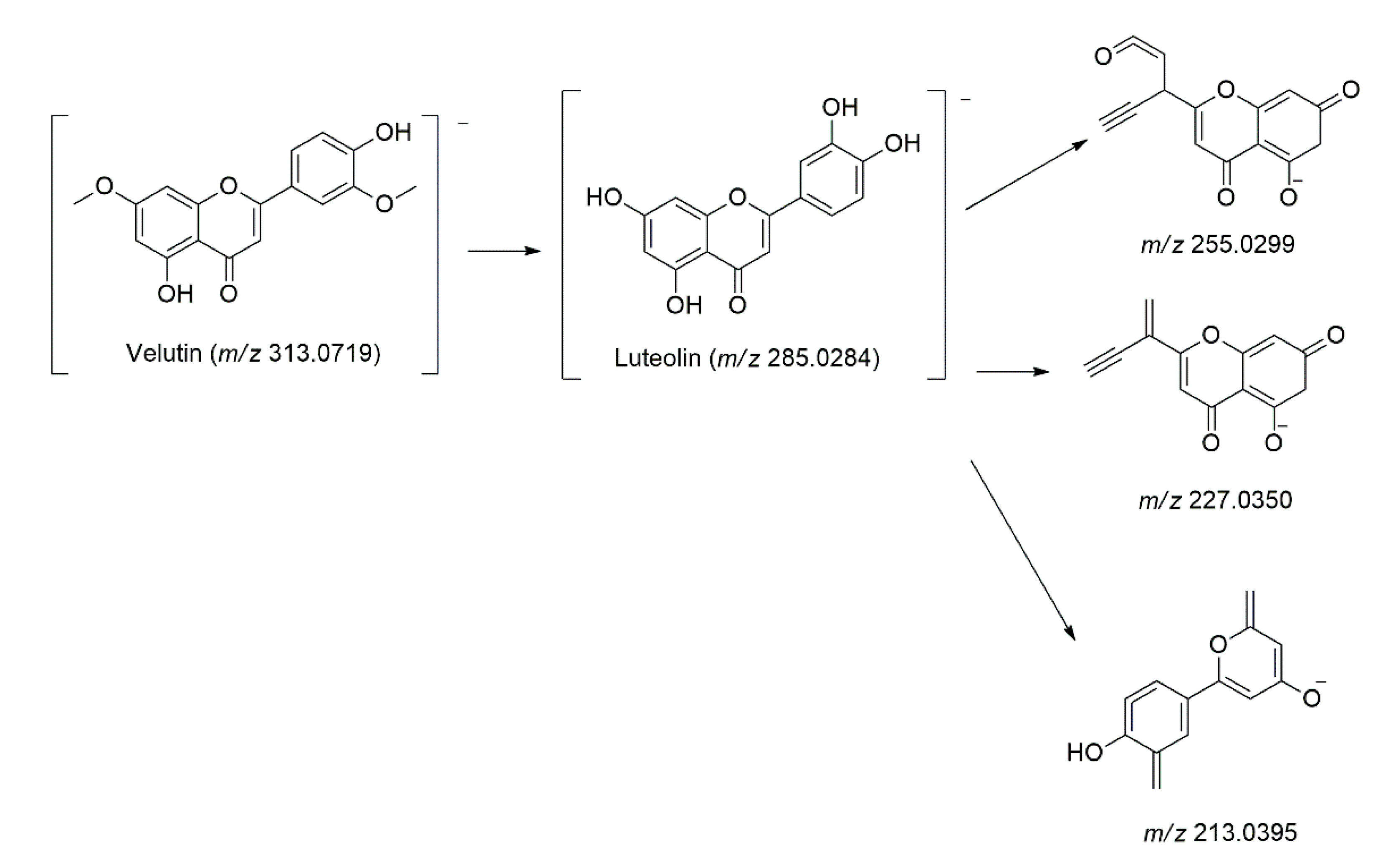
| 40 | 14.03 | 224, 268, 346 | C17H14O6 | 314.0790 | 313.0719 | − | 0.0071 | 299.0505, 285.0284, 255.0299, 227.0350, 213.0395 | Velutin | [40] |
| 41 | 14.23 | 224, 378 | C15H8O6 | 284.0321 | 282.9547 | − | 0.0774 | 267.0306, 239.0348, 211.0396 | Rhein isomer | [53] |
| 42 | 14.38 | 224, 286, 362, 376 | C15H14O3 | 242.0943 | 240.9106 | − | 0.1837 | 223.0756, 198.1011, 186.0566 | Lapachol | [54] |
| 43 | 14.61 | 224, 376 | C15H12O3 | 240.0786 | 239.0713 | − | 0.0073 | 211.0628, 197.0602, 136.0112 | 6-Hydroxyflavanone # | [40] |
| 44 | 14.98 | 226, 274, 312, 378 | C25H30O4 | 394.5033 | 393.3017 | − | 0.2016 | 375.2910, 361.2715, 353.2982 | Trihydroxydiprenylisoflavan | [49] |
| 45 | 15.01 | 220, 274, 316 | C17H14O6 | 314.0790 | 313.0719 | − | 0.0071 | 299.0490, 285.0284, 255.0299, 227.0336, 213.0398 | Velutin isomer | [40] |
| 46 | 15.19 | 224, 270, 326, 374 | C17H14O6 | 314.0790 | 313.0719 | − | 0.0071 | 299.0466, 285.0287, 255.0299, 227.0347, 213.0396 | Velutin isomer | [40] |
| 47 | 16.18 | 216, 270 | C23H25NO9 | 459.4459 | 457.9947 | − | 0.4512 | 427.9972, 397.9993, 367.9799 | Narceinone | [56] |
| 48 | 1.68 | 208, 274 | C15H14O6 | 290.0790 | − | 291.0640 | 0.0150 | 244.9747, 207.0665, 139.0396 | Catechin | [40] |
| 49 | 3.66 | 208, 254, 356, 376 | C21H20O12 | 464.0955 | − | 465.1050 | −0.0095 | 447.3480, 303.0513, 285.0772 | Myricitrin | [40] |
| 50 | 3.82 | 208, 254, 356, 376 | C21H20O12 | 464.0955 | − | 465.1050 | −0.0095 | 447.3459, 303.0511, 285.0771 | Myricitrin isomer | [40] |
| 51 | 9.68 | 222, 296, 374, 386 | C22H18O11 | 458.0849 | − | 459.0942 | −0.0093 | 321.0591, 289.0771, 275.0818 | Epigallocatechin gallate | [40] |
| 52 | 12.58 | 222, 294, 344, 378 | C16H12O7 | 316.0583 | − | 317.0671 | −0.0088 | 301.0973, 285.0734, 245.1063 | Rhamnetin | [40] |
| 53 | 13.00 | 226, 292, 326 | C22H22O9 | 430.1260 | − | 431.1330 | −0.0070 | 269.1334, 254.0331, 227.0704, 213.0568, 201.0357 | Ononin | [40] |
| 54 | 13.13 | 224, 274, 376 | C15H10O4 | 254.0579 | − | 255.0661 | −0.0082 | 227.0694, 213.0553, 200.9240 | Daidzein # | [40] |
| 55 | 13.29 | 224, 280, 378 | C15H10O4 | 254.0579 | − | 255.0661 | −0.0082 | 227.0668, 213.0560, 200.9234 | Daidzein isomer | [40] |
| 56 | 14.38 | 224, 282, 356, 378 | C15H10O5 | 270.0528 | − | 271.0611 | −0.0083 | 254.0868, 227.0920, 213.0558, 201.0650 | 3′-Hydroxydaidzein * | [44] |
| 57 | 14.78 | 224, 338 | C15H10O5 | 270.0528 | − | 271.0611 | −0.0083 | 254.0666, 227.0918, 213.0557, 201.0810 | Hydroxydaidzein isomer | [44] |
| 58 | 16.13 | 226, 272, 378 | C16H12O4 | 268.0736 | − | 269.0819 | −0.0083 | 254.0582, 227.0666, 213.0548, 201.0915 | Formononetin # | [40] |
| 59 | 16.29 | 226, 266, 290, 354, 376 | C16H12O4 | 268.0736 | − | 269.0819 | −0.0083 | 254.0583, 227.0669, 213.0546, 201.0601 | Formononetin isomer | [40] |
| 60 | 16.86 | 226, 270 | C17H14O5 | 298.0841 | − | 299.0925 | −0.0084 | 283.0610, 267.0578, 237.0548 | 3-Hydroxy-3′,4′-dimethoxyflavone * | [40] |
| 61 | 17.02 | 226 | C17H14O5 | 298.0841 | − | 299.0925 | −0.0084 | 283.0611, 267.0571, 237.0637 | Hydroxydimethoxyflavone isomer | [40] |
2.3. UHPLC Absolute Quantification
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Materials
3.3. In Vitro Anti-Diabetic Activity
3.3.1. α-Glucosidase Inhibition Assay
3.3.2. α-Amylase Inhibition Assay
3.4. UHPLC-ESI-MS/MS Analysis
3.5. Absolute Quantification from FD Leaves Extracted with 50% Ethanol by UHPLC
- Specificity: the retention time of the standards and extract are complementary with no contaminants or impurities detected in the eluent, with the equal volume (2.0 µL) of sample, standards, and solvent injected into the chromatography system.
- Repeatability precision: all the relative standard deviation (RSD) were <2%, indicating high precision. The repeatability precision test was acquired by three-times injections at three concentration levels (5, 20, and 40 µg/mL) for each standard (daidzein, quercitrin, kaempferol, formononetin, 6-hydroxyflavanone, and geniposide).
- Linearity and range: the calibration curve was obtained by three data points at 5, 20, and 40 µg/mL. Each calibration curve was determined by the averaging triplicate value of each concentration. Table 4 shows the concentration range, regression equation, and correlation coefficient (R2).
- Limit of detection (LOD) and limit of quantification (LOQ) were determined by calculating the signal:noise ratio, established at 3.3:1 (LOD) and 10:1 (LOQ), based on the calibration curve with the following formula:LOD = (SD × 3.3)/Mwhere SD is the standard deviation of the response, and M is the slope of calibration curve. Table 4 demonstrates the LOD and LOQ value for the six tested standards.LOQ = (SD × 10)/M
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Moini, J. Pathophysiology of diabetes. In Epidemiology of Diabetes; Elsevier: Oxford, UK, 2019; pp. 25–43. [Google Scholar]
- Rajendiran, D.; Packirisamy, S.; Gunasekaran, K. A review on role of antioxidants in diabetes. Asian J. Pharm. Clin. Res. 2018, 11, 48–53. [Google Scholar] [CrossRef]
- Chandran, A.; Abdullah, M.N.; Abdul, F. National Diabetes Registry Report 2013–2019; Ministry of Health Malaysia: Putrajaya, Malaysia, 2019; pp. 1–34.
- Janani, C.; Kumari, B.D.R. PPAR gamma gene—A review. Diabetes Metab. Syndr. Clin. Res. Rev. 2014, 9, 46–50. [Google Scholar] [CrossRef]
- Sola, D.; Rossi, L.; Piero, G.; Schianca, C.; Maffioli, P.; Bigliocca, M.; Mella, R.; Corlianò, F.; Fra, G.P.; Bartoli, E.; et al. Sulfonylureas and their use in clinical practice. Arch. Med. Sci. 2015, 4, 840–848. [Google Scholar] [CrossRef]
- Thrasher, J. Pharmacologic management of type 2 diabetes mellitus: Available therapies. Am. J. Med. 2017, 130, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Herman, G.A.; Stevens, C.; Van Dyck, K.; Bergman, A.; Yi, B.; De Smet, M.; Snyder, K.; Hilliard, D.; Tanen, M.; Tanaka, W.; et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: Results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin. Pharmacol. Ther. 2005, 78, 675–688. [Google Scholar] [CrossRef]
- Murai, A.; Iwamura, K.; Takada, M.; Ogawa, K.; Usui, T.; Okumura, J.I. Control of postprandial hyperglycaemia by galactosyl maltobionolactone and its novel anti-amylase effect in mice. Life Sci. 2002, 71, 1405–1415. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Pohjola, M.; Lindström, J.; Aro, A. Acarbose and nutrient intake in non-insulin dependent diabetes mellitus. Diabetes Res. Clin. Pract. 1994, 26, 215–222. [Google Scholar] [CrossRef]
- Mohamad-Yusof, M.I.; Salleh, M.Z.; Kek, T.L.; Ahmat, N.; Nik-Azmin, N.F.; Zakaria, Z.A. Activity-guided isolation of bioactive constituents with antinociceptive activity from Muntingia calabura L. leaves using the formalin test. Evid. Based Complement. Altern. Med. 2013, 2013, 715074. [Google Scholar] [CrossRef] [PubMed]
- Balan, T.; Sani, M.H.M.; Mumtaz-Ahmad, S.H.; Suppaiah, V.; Mohtarrudin, N.; Zakaria, Z.A. Antioxidant and anti-inflammatory activities contribute to the prophylactic effect of semi-purified fractions obtained from the crude methanol extract of Muntingia calabura leaves against gastric ulceration in rats. J. Ethnopharmacol. 2015, 164, 1–15. [Google Scholar] [CrossRef]
- Sufian, A.S.; Ramasamy, K.; Ahmat, N.; Zakaria, Z.A.; Yusof, M.I.M. Isolation and identification of antibacterial and cytotoxic compounds from the leaves of Muntingia calabura L. J. Ethnopharmacol. 2013, 146, 198–204. [Google Scholar] [CrossRef]
- Zakaria, Z.A.; Mohamed, A.M.; Mohd-Jamil, N.S.; Rofiee, M.S.; Hussain, M.K.; Sulaiman, M.R.; Teh, L.K.; Salleh, M.Z. In vitro antiproliferative and antioxidant activities of the extracts of Muntingia calabura leaves. Am. J. Chin. Med. 2011, 39, 183–200. [Google Scholar] [CrossRef]
- Aligita, W.; Susilawati, E.; Sukmawati, I.K.; Holidayanti, L.; Riswanti, J. Antidiabetic activities of Muntingia calabura L. leaves water extract in type 2 diabetes mellitus animal models. Indones. Biomed. J. 2018, 10, 165–170. [Google Scholar] [CrossRef]
- Chen, J.; Lin, R.W.; Du, C.Y.; Huang, H.Y.; Chen, I.S. Flavones and cytotoxic constituents from the stem bark of Muntingia calabura. J. Chin. Chem. Soc. 2004, 51, 665–670. [Google Scholar] [CrossRef]
- Su, B.; Jung, E.; Schunke, J.; Graham, J.G.; Cabieses, F.; Fong, H.H.S.; Pezzuto, J.M.; Kinghorn, A.D. Activity-guided isolation of the chemical constituents of Muntingia calabura using a quinone reductase induction assay. Phytochemistry 2003, 63, 335–341. [Google Scholar] [CrossRef]
- Zakaria, Z.A.; Nor Hazalin, N.A.M.; Zaid, S.N.H.M.; Ghani, M.A.; Hassan, M.H.; Gopalan, H.K.; Sulaiman, M.R. Antinociceptive, anti-inflammatory and antipyretic effects of Muntingia calabura aqueous extract in animal models. J. Nat. Med. 2007, 61, 443–448. [Google Scholar] [CrossRef]
- Wu, S.Y.; Wang, G.F.; Liu, Z.Q.; Rao, J.J.; Lü, L.; Xu, W.; Wu, S.G.; Zhang, J.J. Effect of geniposide, a hypoglycemic glucoside, on hepatic regulating enzymes in diabetic mice induced by a high-fat diet and streptozotoci. Acta Pharmacol. Sin. 2009, 30, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Jung, U.J.; Yeo, J.; Kim, M.J.; Lee, M.K. Genistein and daidzein prevent diabetes onset by elevating insulin level and altering hepatic gluconeogenic and lipogenic enzyme activities in non-obese diabetic (NOD) mice. Diabetes Metab. Res. Rev. 2008, 24, 74–81. [Google Scholar] [CrossRef]
- Babujanarthanam, R.; Kavitha, P.; Pandian, M.R. Quercitrin, a bioflavonoid improves glucose homeostasis in streptozotocin-induced diabetic tissues by altering glycolytic and gluconeogenic enzymes. Fundam. Clin. Pharmacol. 2010, 24, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Mikell, J.R.; Herath, W.; Khan, I.A. Eleven microbial metabolites of 6-hydroxyflavanone. Chem. Pharm. Bull. 2015, 63, 579–583. [Google Scholar] [CrossRef][Green Version]
- Chandramohan, G.; Al-numair, K.S.; Alsaif, M.A.; Veeramani, C. Antidiabetic effect of kaempferol a flavonoid compound, on streptozotocin-induced diabetic rats with special reference to glycoprotein components. Prog. Nutr. 2015, 17, 50–57. [Google Scholar]
- Vishnuvathan, V.J.; Karunanidhi, S.L.; Srividya, A.R. Medicinal uses of formononetin-A review. J. Ethnobiol. Tradit. Med. 2016, 126, 1197–1209. [Google Scholar]
- Abu-Bakar-Sajak, A.; Abas, F.; Ismail, A.; Khatib, A. Effect of different drying treatments and solvent ratios on phytochemical constituents of Ipomoea aquatica and correlation with α-glucosidase inhibitory activity. Int. J. Food Prop. 2016, 19, 2817–2831. [Google Scholar] [CrossRef]
- Singh, R.P.; Heldman, D.R. Packaging concepts. In Introduction to Food Engineering; Taylor, S.L., Ed.; Elsevier: Berkeley, CA, USA, 2013; Volume 84, pp. 745–769. [Google Scholar]
- Mediani, A.; Abas, F.; Maulidiani, M.; Shaari, K.; Choi, Y.H.; Lajis, N.H. 1H-NMR-based metabolomics approach to understanding the drying effects on the phytochemicals in Cosmos caudatus. Food Res. Int. 2012, 49, 763–770. [Google Scholar] [CrossRef]
- Azizan, A.; Ahamad-Bustamam, M.S.; Maulidiani, M.; Shaari, K.; Ismail, I.S.; Nagao, N.; Abas, F. Metabolite profiling of the microalgal diatom Chaetoceros alcitrans and correlation with antioxidant and nitric oxide inhibitory activities via 1H NMR-based metabolomics. Mar. Drugs 2018, 16, 154. [Google Scholar] [CrossRef]
- De-Sales, P.M.; De-Souza, P.M.; Simeoni, L.A.; Magalhães, P.D.O.; Silveira, D. α-Amylase inhibitors: A review of raw material and isolated compounds from plant source. J. Pharm. Pharm. Sci. 2012, 15, 141–183. [Google Scholar] [CrossRef]
- Matsui, T.; Yoshimoto, C.; Osajima, K.; Oki, T.; Osajima, Y. In vitro survey of α–glucosidase inhibitory food components. Biosci. Biotechnol. Biochem. 1996, 60, 2019–2022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Lv, J.; Li, G. A colorimetric method for α-glucosidase activity assay and its inhibitor screening based on aggregation of gold nanoparticles induced by specific recognition between phenylenediboronic acid and 4-aminophenyl-α-d-glucopyranoside. Nano Res. 2015, 8, 920–930. [Google Scholar] [CrossRef]
- Keharom, S.; Mahachai, R.; Chanthai, S. The optimization study of α-amylase activity based on central composite design-response surface methodology by dinitrosalicylic acid method. Int. Food Res. J. 2016, 23, 10–17. [Google Scholar]
- Telagari, M.; Hullatti, K. In-vitro α-amylase and α-glucosidase inhibitory activity of Adiantum caudatum Linn. and Celosia argentea Linn. extracts and fractions. Indian J. Pharmacol. 2015, 47, 425–429. [Google Scholar]
- Spigno, G.; Tramelli, L.; Faveri, D.M. De Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Tapani, E.; Taavitsainen, M.; Lindros, K.; Vehmas, T.; Lehtonen, E. Toxicity of ethanol in low concentrations: Experimental evaluation in cell culture. Acta Radiol. 1996, 37, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.C.; Lim, Y.Y.; Wong, S.K.; Lim, K.K.; Tan, S.P.; Lianto, F.S.; Yong, M.Y. Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chem. 2009, 113, 166–172. [Google Scholar] [CrossRef]
- Khoo, L.W.; Mediani, A.; Zolkeflee, N.K.Z.; Leong, S.W.; Ismail, I.S.; Khatib, A.; Shaari, K.; Abas, F. Phytochemical diversity of Clinacanthus nutans extracts and their bioactivity correlations elucidated by NMR based metabolomics. Phytochem. Lett. 2015, 14, 123–133. [Google Scholar] [CrossRef]
- Lee, S.Y.; Abas, F.; Khatib, A.; Ismail, I.S.; Shaari, K.; Zawawi, N. Metabolite profiling of Neptunia oleracea and correlation with antioxidant and α-glucosidase inhibitory activities using 1H NMR-based metabolomics. Phytochem. Lett. 2016, 16, 23–33. [Google Scholar] [CrossRef]
- Nakbanpote, W.; Ruttanakorn, M.; Sukadeetad, K.; Sakkayawong, N.; Damrianant, S. Effects of drying and extraction methods on phenolic compounds and in vitro assays of Eclipta prostrata Linn. leaf extracts. Sci. Asia 2019, 45, 127–137. [Google Scholar] [CrossRef]
- Slatnar, A.; Klancar, U.; Stampar, F.; Veberic, R. Effect of drying of figs (Ficus carica L.) on the contents of sugars, organic acids, and phenolic compounds. J. Agric. Food Chem. 2011, 59, 11696–11702. [Google Scholar] [CrossRef] [PubMed]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef]
- Kubo, I.; Yokokawa, Y. Two tyrosinase inhibiting flavonol glycosides from Buddleia coriacea. Phytochemistry 1992, 31, 1075–1077. [Google Scholar] [CrossRef]
- Nshimo, C.M.; Pezzuto, J.M.; Kinghorn, A.D.; Farnsworth, N.R. Cytotoxic constituents of Muntingia calabura leaves and stems collected in Thailand. Int. J. Pharmacogn. 1993, 31, 77–81. [Google Scholar] [CrossRef]
- Kim, C.S.; Koh, H.S.; Fukami, H.; Irie, R. Antifeedants of finger millet, Eleusine coracana GAERTN, against brown planthopper, Nilaparvata lugens (STÅL). Biosci. Biotechnol. Biochem. 1994, 58, 380–383. [Google Scholar] [CrossRef][Green Version]
- Yang, R.; Lan, Y.; Huang, Z.; Shao, C.; Liang, H. Isoflavonoids from Sophora tonkinensis. Chem. Nat. Compd. 2012, 48, 674–676. [Google Scholar] [CrossRef]
- Matsuda, H.; Morikawa, T.; Xu, F.; Ninomiya, K.; Yoshikawa, M. New isoflavones and pterocarpane with hepatoprotective activity from the stems of Erycibe expansa. Planta Med. 2004, 70, 1201–1209. [Google Scholar] [CrossRef]
- Kaneda, N.; Prezzuto, J.M.; Soejarto, D.D.; Kinghorn, A.D.; Farnsworth, N.R. Plant anticancer agents, XLVIII. New cytotoxic flavonoids from Muntingia calabura roots. J. Nat. Prod. 1991, 54, 196–206. [Google Scholar] [CrossRef]
- Seo, E.K.; Silva, G.L.; Chai, H.B.; Chagwedra, T.E.; Farnsworth, N.R.; Cordell, G.A.; Pezzuto, J.M.; Kinghorn, A.D. Cytotoxic prenylated flavanones from Monotes engleri. Phytochemistry 1997, 45, 509–515. [Google Scholar] [CrossRef]
- Ingham, J.L. Systematic aspects of phytoalexin formation within tribe phaseoleae of the Leguminosae (subfamily Papilionoideae). Biochem. Syst. Ecol. 1990, 18, 329–343. [Google Scholar] [CrossRef]
- Fukai, T.; Shen, C.B.; Horikoshi, T.; Nomura, T. Isoprenylated flavonoids from underground parts of Glycyrrhia glabra. Phytochemistry 1996, 43, 1119–1124. [Google Scholar] [CrossRef]
- Puri, B.; Hall, A. Phytochemical Dictionary: A Handbook of Bioactive Compounds from Plants; Baxter, H., Harborne, J.B., Moss, G.P., Eds.; CRC Press: Boca Raton, FL, USA, 1998; 976p. [Google Scholar]
- Hsu, Y.L.; Kuo, P.L.; Lin, L.T.; Lin, C.C. Isoliquiritigenin inhibits cell proliferation and induces apoptosis in human hepatoma cells. Planta Med. 2005, 71, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, H.; Kasai, R.; Otsuka, H.; Hirata, E.; Shinzato, T.; Aramoto, M.; Takeda, Y. Terpenic and phenolic glycosides from leaves of Breynia officinalis Hemsl. Chem. Pharm. Bull. 2004, 52, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.M.; Wang, Z.B.; Xin, P.; Wang, Q.H.; Bu, H.; Kuang, H.X. Phytochemistry and pharmacology of genus Ephedra. Chin. J. Nat. Med. 2018, 16, 811–828. [Google Scholar] [CrossRef]
- Kanchanapoom, T.; Kasai, R.; Yamasaki, K. Phenolic glycosides from Markhamia stipulata. Phytochemistry 2002, 59, 557–563. [Google Scholar] [CrossRef]
- Liang, Z.; Yang, M.; Xu, X.; Xie, Z.; Huang, J.; Li, X.; Yang, D. Isolation and purification of geniposide, crocin-1, and geniposidic acid from the fruit of Gardenia jasminoides Ellis by high-speed counter-current chromatography. Sep. Sci. Technol. 2014, 49, 1427–1433. [Google Scholar] [CrossRef]
- Chaudhuri, P.K.; Thakur, R.S. Narceinone, an alkaloid from Papaver somniferum. Phytochemistry 1989, 28, 2002–2003. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Della Tan, S.L.; Shiekh, K.A.; Benjakul, S.; Nirmal, N.P. Ethanolic guava leaf extracts with different chlorophyll removal processes: Anti-melanosis, antibacterial properties and the impact on qualities of Pacific white shrimp during refrigerated storage. Food Chem. 2021, 341, 128251. [Google Scholar] [CrossRef] [PubMed]
- Fogliani, B.; Raharivelomanana, P.; Bianchini, J.P.; Bouraïma-Madjèbi, S.; Hnawia, E. Bioactive ellagitannins from Cunonia macrophylla, an endemic Cunoniaceae from New Caledonia. Phytochemistry 2005, 66, 241–247. [Google Scholar] [CrossRef]
- Piszcz, P.; Wozniak, M.; Asztemborska, M.; Glod, B.K. Comparative analysis of antioxidative activity of flavonoids using HPLC–ED and photometric assays. Food Anal. Methods 2014, 7, 1474–1480. [Google Scholar] [CrossRef]
- Abd-Ghafar, S.Z.; Mediani, A.; Ramli, N.S.; Abas, F. Antioxidant, α-glucosidase, and nitric oxide inhibitory activities of Phyllanthus acidus and LC–MS/MS profile of the active extract. Food Biosci. 2018, 25, 134–140. [Google Scholar] [CrossRef]
- Shabir, G.A. Step-by-step analytical methods validation and protocol in the quality system compliance industry. Rev. Saude Publica 2006, 40, 951–961. [Google Scholar]


| Drying Method | Ethanol:Water Ratio | α-Glucosidase Inhibitory Assay IC50 (µg /mL) | α-Amylase Inhibitory Assay IC50 (µg /mL) |
|---|---|---|---|
| OD | 100 | 1.13 ± 0.13 Ba | 59.39 ± 2.47 Ba |
| 50 | 0.81 ± 0.09 Ca | 45.77 ± 2.46 Ca | |
| 0 | 2.76 ± 0.09 Aa | 105.95 ± 1.57 Ac | |
| AD | 100 | 1.07 ± 0.06 Ba | 53.34 ± 1.64 Bb |
| 50 | 0.59 ± 0.14 Bb | 35.32 ± 2.35 Cb | |
| 0 | 2.41 ± 1.00 Aa | 114.43 ± 2.22 Ab | |
| FD | 100 | 0.65 ± 0.04 Bb | 23.84 ± 1.85 Bc |
| 50 | 0.46 ± 0.05 Bb | 26.39 ± 3.93 Bc | |
| 0 | 2.01 ± 0.86 Aa | 185.17 ± 2.11 Aa | |
| Standard | Quercetin | 2.15 ± 0.26 | − |
| Acarbose | − | 0.68 ± 0.14 |
| Metabolites | Concentration (µg/mg of Extract) |
|---|---|
| Geniposide | 650.01 ± 0.12 |
| Daidzein | 231.65 ± 0.31 |
| Quercitrin | 223.24 ± 0.59 |
| Kaempferol | 75.22 ± 0.72 |
| Formononetin | 56.58 ± 0.28 |
| 6-Hydroxyflavanone | 196.43 ± 0.28 |
| Standards | Concentration Range (µg/mL) | Regression Equation | Correlation Coefficient (R2) | LOD (µg/mL) | LOQ (µg/mL) |
|---|---|---|---|---|---|
| Daidzein | 5.1592–40.2551 | Y = 2.67 × 104X − 2.33 × 104 | 0.999 | 0.10 | 0.30 |
| Quercitrin | 4.7603–39.9357 | Y = 1.83 × 104X − 2.45 × 104 | 0.999 | 0.04 | 0.11 |
| Kaempferol | 5.4330–40.6827 | Y = 1.82 × 104X − 3.33 × 104 | 0.999 | 0.17 | 0.52 |
| Formononetin | 5.1813–40.1937 | Y = 2.05 × 104X − 1.45 × 104 | 0.999 | 0.19 | 0.56 |
| 6-Hydroxyflavanone | 5.1507–40.1779 | Y = 1.58 × 104X− 4.56 × 104 | 0.999 | 0.03 | 0.10 |
| Geniposide | 5.7511~40.7790 | Y = 4.16 × 103X − 6.01 × 103 | 0.999 | 0.32 | 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zolkeflee, N.K.Z.; Ramli, N.S.; Azlan, A.; Abas, F. In Vitro Anti-Diabetic Activities and UHPLC-ESI-MS/MS Profile of Muntingia calabura Leaves Extract. Molecules 2022, 27, 287. https://doi.org/10.3390/molecules27010287
Zolkeflee NKZ, Ramli NS, Azlan A, Abas F. In Vitro Anti-Diabetic Activities and UHPLC-ESI-MS/MS Profile of Muntingia calabura Leaves Extract. Molecules. 2022; 27(1):287. https://doi.org/10.3390/molecules27010287
Chicago/Turabian StyleZolkeflee, Nur Khaleeda Zulaikha, Nurul Shazini Ramli, Azrina Azlan, and Faridah Abas. 2022. "In Vitro Anti-Diabetic Activities and UHPLC-ESI-MS/MS Profile of Muntingia calabura Leaves Extract" Molecules 27, no. 1: 287. https://doi.org/10.3390/molecules27010287
APA StyleZolkeflee, N. K. Z., Ramli, N. S., Azlan, A., & Abas, F. (2022). In Vitro Anti-Diabetic Activities and UHPLC-ESI-MS/MS Profile of Muntingia calabura Leaves Extract. Molecules, 27(1), 287. https://doi.org/10.3390/molecules27010287