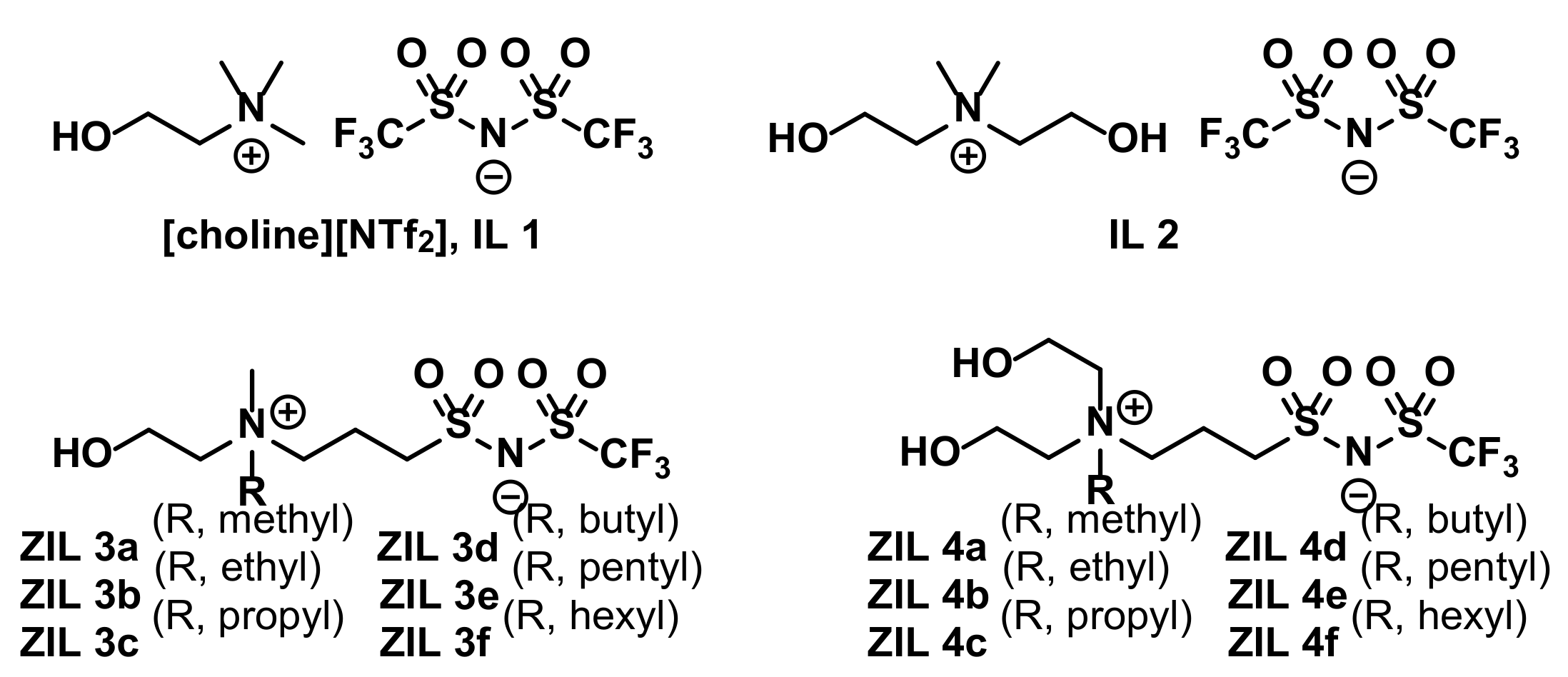
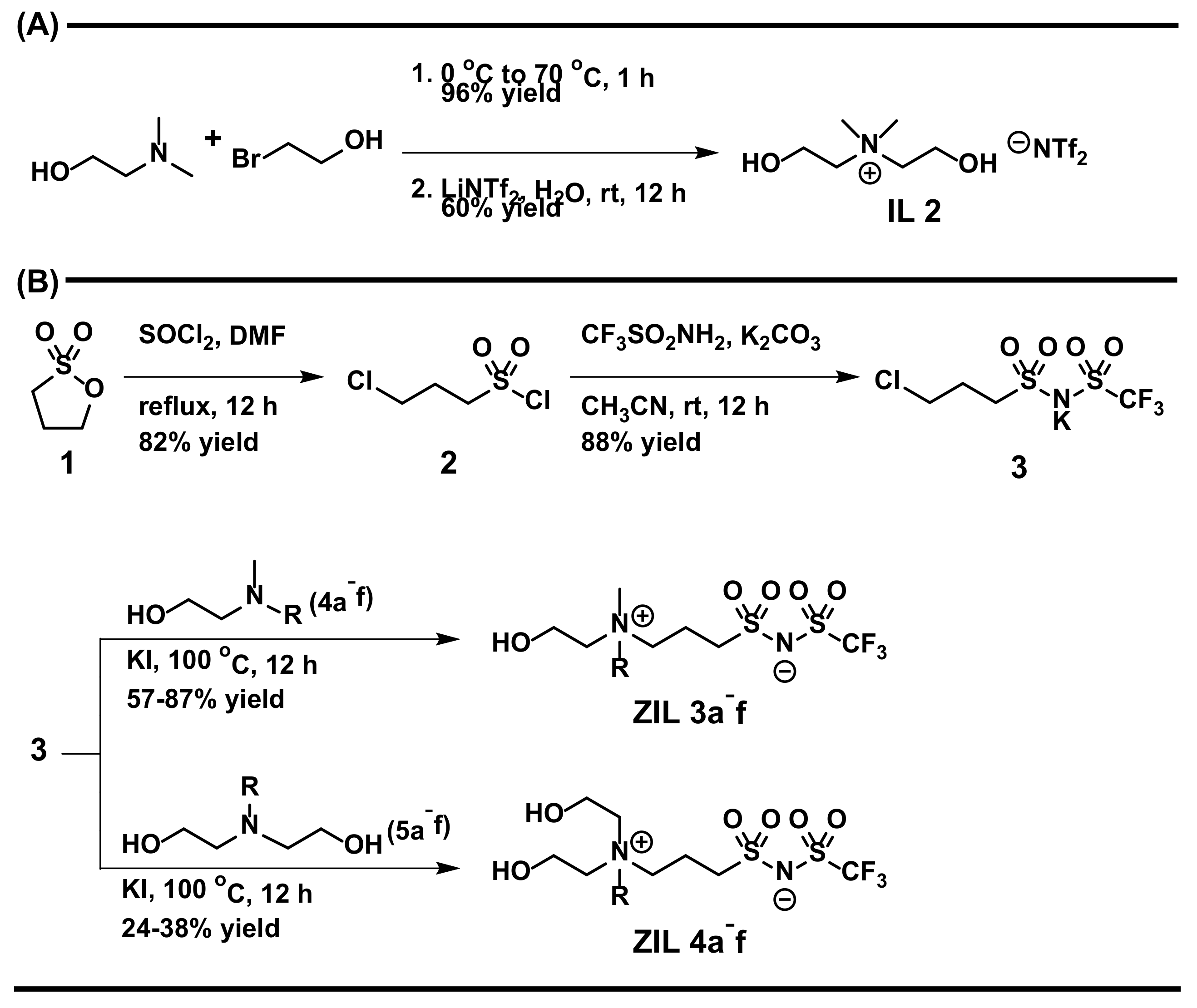
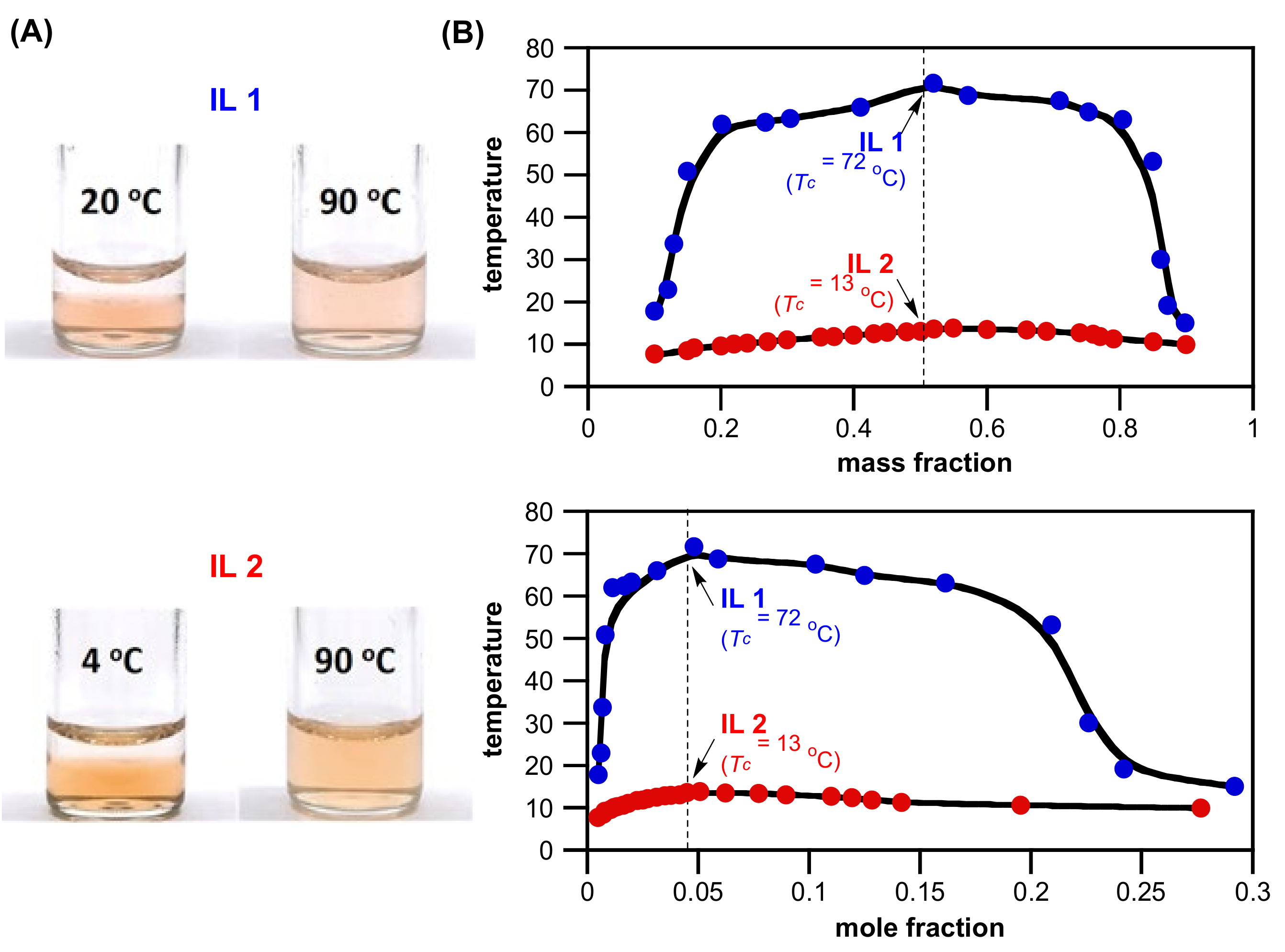
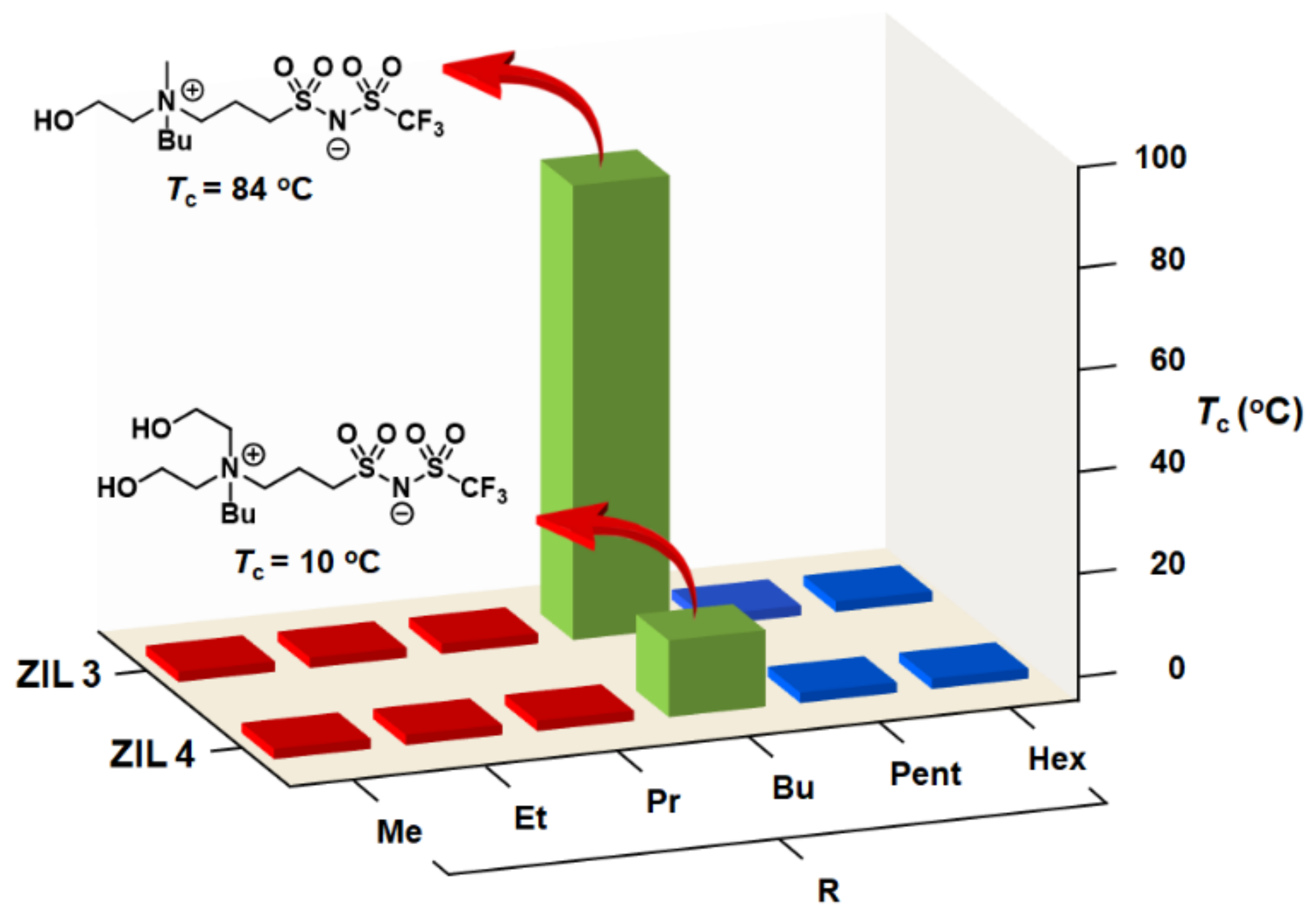
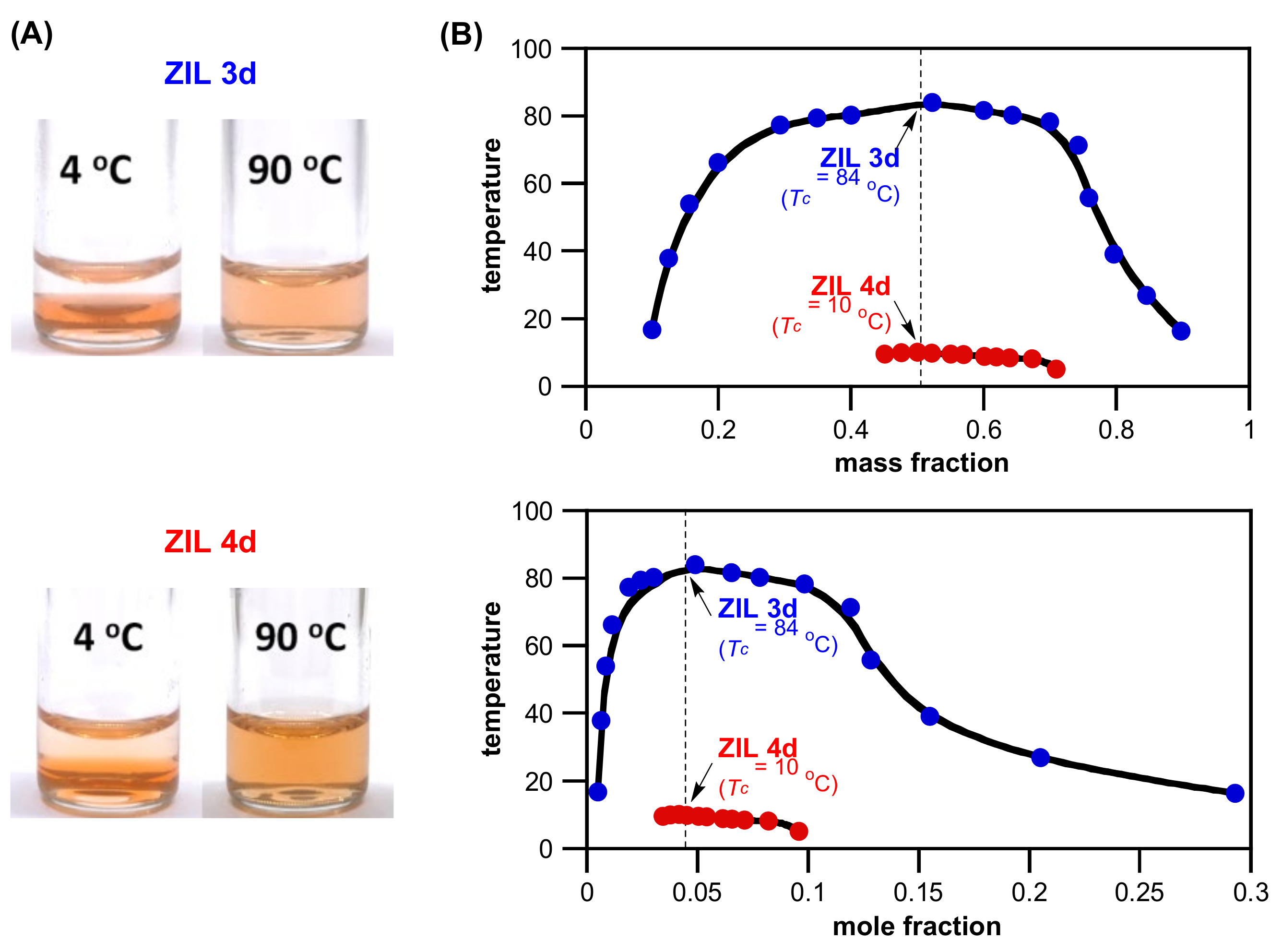
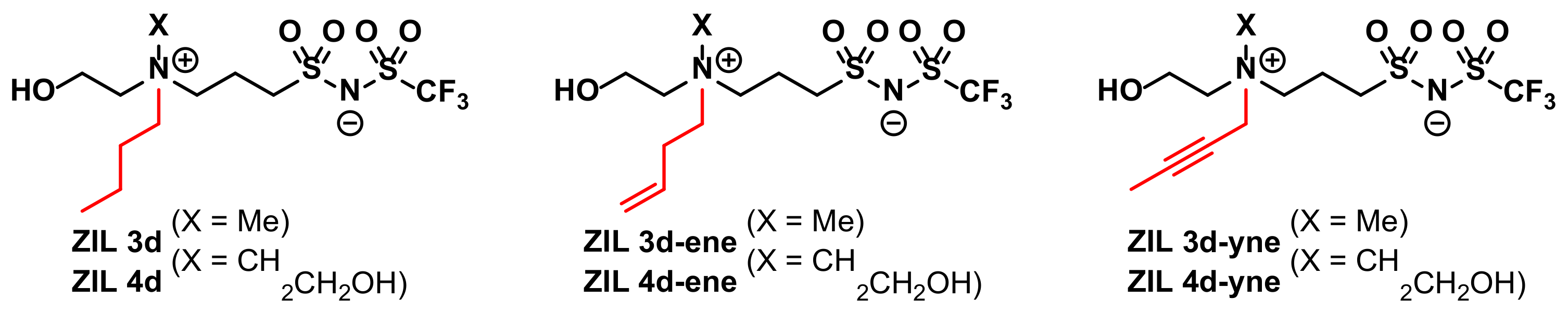
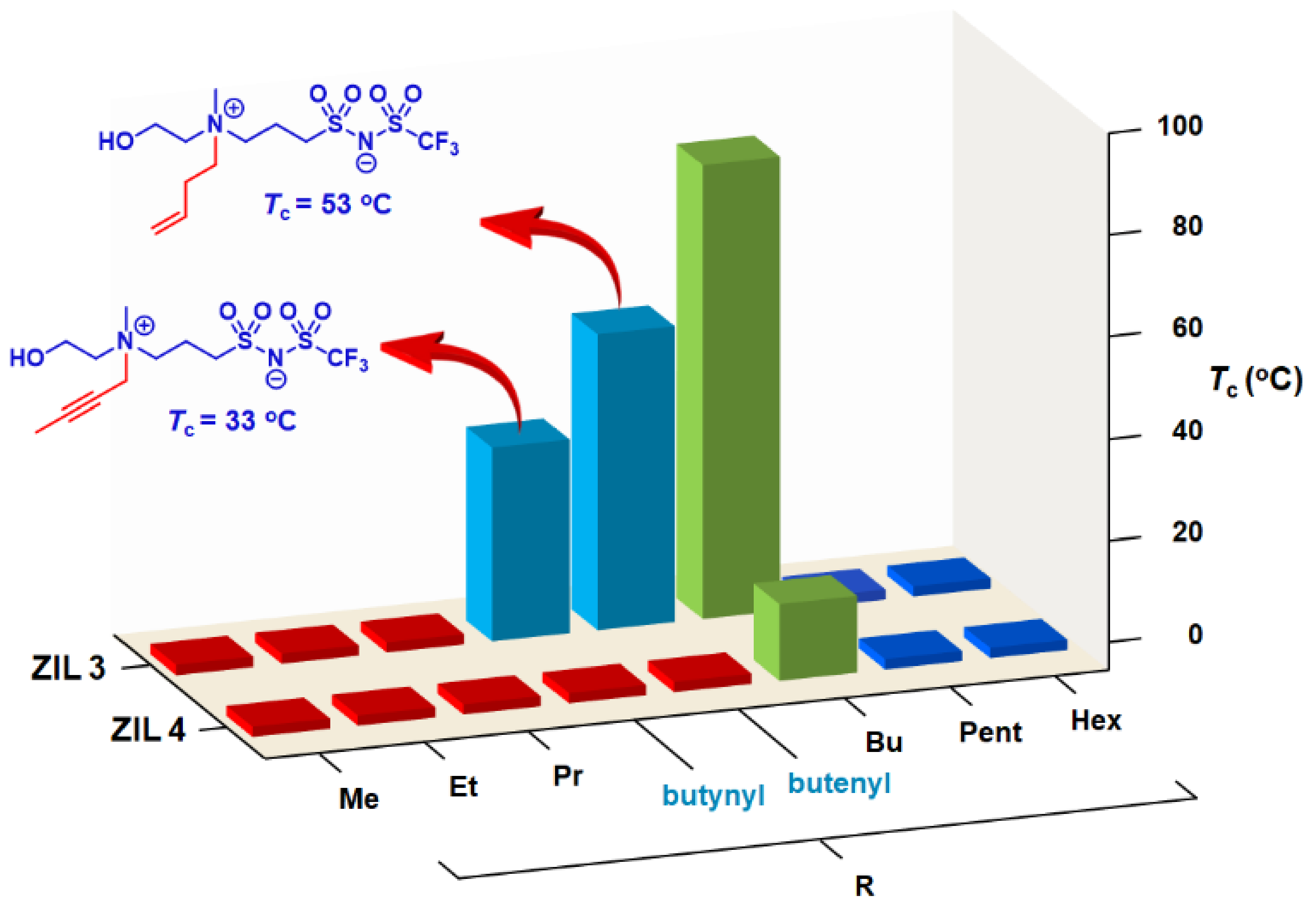
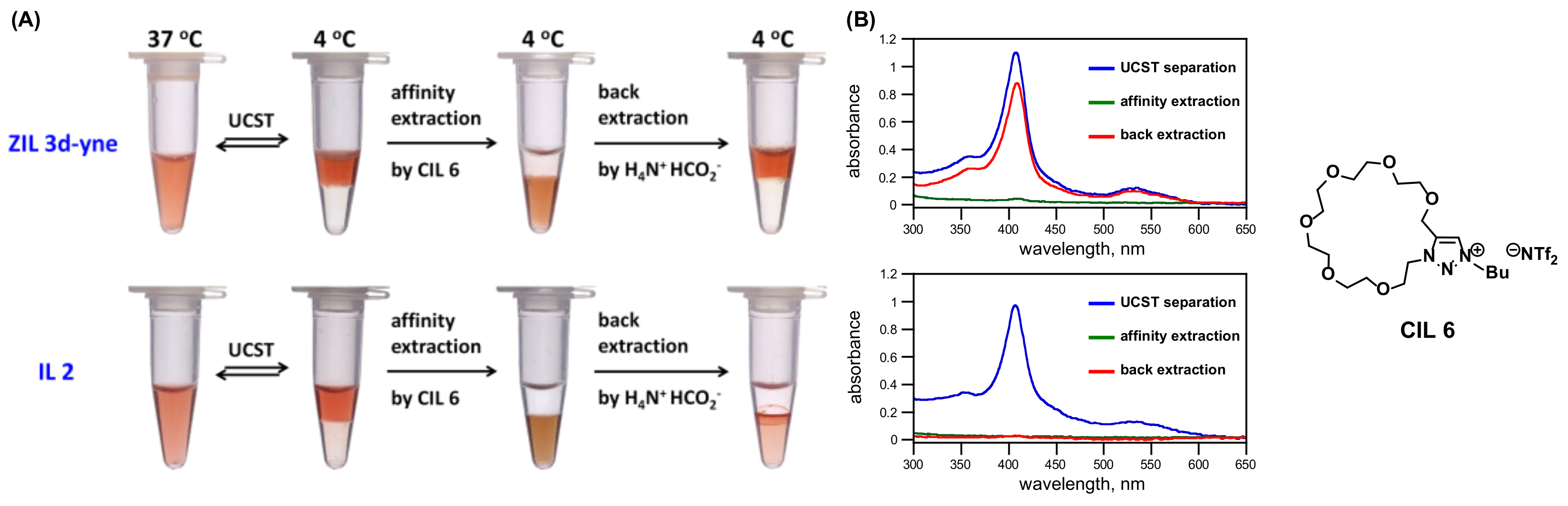
3.3. Synthesis of ZILs
3.3.1. Synthesis of 3-Chloropropane-1-Sulfonyl Chloride (2)
To a round-bottom flask containing 1,3-propanesultone 1 (3 g, 24.7 mmol) was added thionyl chloride (3.6 mL, 49.5 mmol) and DMF (0.2 mL). The mixture was refluxed for 12 h. The excess thionyl chloride was removed by vacuum to obtain sulfonyl chloride product as a pale yellow liquid 2 (3.58 g, 82% yield).
3.3.2. Synthesis of Potassium ((3-Chloropropyl)Sulfonyl)((Trifluoromethyl)Sulfonyl)Amide (3)
To a bottle containing trifluoromethanesulfonimide (1.0 g, 6.78 mmol) and K2CO3 (1.39 g, 10.18 mmol) was added acetonitrile (28 mL) and stirred at room temperature. Sulfonyl chloride product 2 (1.40 g, 7.5 mmol) was dissolved in acetonitrile (5 mL) and added dropwise to the reaction bottle. After 12 h, the solid salt was filtered off. The filtrate was concentrated under reduced pressure to obtain yellow solid crude product. Then, the crude product was washed with mixture solvent of EA and DCM (1/2) to afford white solid product 3 (1.95 g, 88% yield).
3.3.3. Synthesis of ZIL 3a
To a screw-cap septum vial containing potassium sulfonimide 3 (400 mg, 1.22 mmol) was added 2-(dimethylamino)ethanol 4a (5 equiv). The mixture was heated at 100 °C for 12 h. After completion of the reaction, acetonitrile was added to the vial for precipitation of potassium chloride produced. The solid salt was filtered off, and the filtrate was concentrated under reduced pressure to obtain yellow liquid crude product. Next the excess amine of crude product was washed out with sonication in ether. Then, the mixture solution of EA and MeOH (5/1, v/v) was added to the bottle containing crude product. White solid will precipitate in solvent. After collecting the white solid and washing it with EA several times, residual solvent was removed in vacuo to obtain ZIL 3a as white solid (295 mg, 70% yield).
White solid, m.p. 100 °C; 1H NMR (400 MHz, DMSO-d6) δ 2.05–2.16 (m, NCH2CH2, 2H), 3.05 (t, J = 7.2 Hz, CH2CH2S, 2H), 3.07 (s, 2 × N+CH3, 6H), 3.92 (t, J = 5.2 Hz, N+CH2CH2, 2H), 3.42–3.50 (m, HOCH2CH2, 2H), 3.78–3.86 (m, HOCH2CH2, 2H), 3.32 (bs, HOCH2CH2, H); 13C NMR (100 MHz, DMSO-d6) δ 17.69, 50.96, 51.22, 54.85, 62.31, 64.72; 19F NMR (376 MHz, DMSO-d6) δ −76.53 (CF3, 3F); ESI-HRMS m/z [M + H]+ calculated for C8H18F3N2O5S2 343.0604, found 343.0594 ([M + H]+), 365.0415 ([M + Na]+), 381.0155 ([M + K]+).
3.3.4. Synthesis of ZIL 3b–f, 3d-ene, and 3d-yne
To a screw cap septum vial containing potassium sulfonimide product 3 (200 mg, 0.613 mmol) and potassium iodide (20 mg, 0.2 equiv) was added tertiary amine 4b–f (for ZIL 3b–f), N-(but-3-enyl)-N-methylaminoethan-1-ol (for ZIL 3d-ene), or N-(but-2-ynyl)-N-methylaminoethan-1-ol (for ZIL 3d-yne) (3 equiv). The mixture was heated at 100 °C for 12 h. After completion of reaction, acetonitrile was added to the vial for precipitation of potassium chloride. The solid salt was filtered off, and filtrate was concentrated under reduced pressure to obtain yellow liquid crude product. Next the excess amine of crude product was washed out with sonication in ether. The crude product was dissolved in a mixture solution of EA and MeOH (5/1, 30 mL), then was poured into a bottle containing ether (150 mL). The mixture solution will become white turbid solution. After centrifugation of the white turbid solution, pale yellow ZIL will precipitate in the solvent. After collecting the precipitate and washing it with ether several times, residual was purified by silica gel column chromatography (ethyl acetate/methanol = 5/1) to afford pale-yellow liquid.
ZIL 3b pale yellow liquid (87% yield); 1H NMR (400 MHz, DMSO-d6) δ 1.24 (t, J = 7.0 Hz, N+CH2CH3, 3H), 2.02–2.16 (m, N+CH2CH2CH2S, 2H), 3.02 (s, N+CH3, 3H), 3.07 (t, J = 7.2 Hz, CH2CH2S, 2H), 3.32–3.50 (m, N+CH2CH2 + N+CH2CH3 + HOCH2CH2, 6H), 3.76–3.87 (m, HOCH2CH2, 2H), 5.28 (t, J = 4.8 Hz, HOCH2CH2, 1H); 13C NMR (100 MHz, DMSO-d6) δ 7.53, 17.35, 47.87, 51.17, 54.68, 57.29, 59.02, 62.06, 120.06 (q, JCF = 322 Hz); 19F NMR (376 MHz, DMSO-d6) δ −76.56 (CF3, 3F); ESI-HRMS m/z [M + H]+ calculated for C9H20F3N2O5S2 357.0760, found 357.0757 ([M + H]+), 379.0581 ([M + Na]+).
ZIL 3c pale yellow liquid (68% yield); 1H NMR (400 MHz, DMSO-d6) δ 0.88 (t, J = 7.2 Hz, N+CH2 CH2CH3, 3H), 1.62–1.73 (m, N+CH2CH2CH3, 2H), 2.03–2.16 (m, N+CH2CH2CH2S, 2H), 3.03 (s, N+CH3, 3H), 3.06 (t, J = 7.2 Hz, CH2CH2S, 2H), 3.23–3.32 (m, N+CH2CH2CH3, 2H), 3.35–3.41 (m, N+CH2CH2CH2S, 2H), 3.40–3.48 (m, HOCH2CH2, 2H), 3.77–3.86 (m, HOCH2CH2, 2H), 5.35 (bs, HOCH2CH2, 1H); 13C NMR (100 MHz, DMSO-d6) δ 10.40, 15.04, 17.39, 48.36, 51.12, 54.62, 59.62, 62.62, 62.99, 120.03 (q, JCF = 322 Hz); 19F NMR (376 MHz, DMSO-d6) δ −76.57 (CF3, 3F); ESI-HRMS m/z [M + H]+ calculated for C10H22F3N2O5S2 371.0917, found 371.0918 ([M + H]+), 393.0739 ([M + Na]+), 409.0479 ([M + K]+).
ZIL 3d pale yellow liquid (64% yield); 1H NMR (400 MHz, DMSO-d6) δ 0.92 (t, J = 7.2 Hz, N+CH2CH2CH2CH3, 3H), 1.21–1.37 (m, N+CH2CH2CH2CH3, 2H), 1.57–1.72 (m, N+CH2CH2CH2CH3, 2H), 2.02–2.16 (m, N+CH2CH2CH2S, 2H), 3.03 (s, N+CH3, 3H), 3.06 (t, J = 7.2 Hz, CH2CH2S, 2H), 3.26–3.34 (m, N+CH2CH2CH2CH3, 2H), 3.34–3.40 (m, N+CH2CH2CH2S, 2H), 3.40–3.48 (m, HOCH2CH2, 2H), 3.77–3.85 (m, HOCH2CH2, 2H), 5.26 (t, J = 5.2 Hz, HOCH2CH2, 1H); 13C NMR (100 MHz, DMSO-d6) δ 13.43, 17.42, 19.16, 23.34, 48.40, 51.13, 54.68, 59.55, 61.48, 62.62, 120.04 (q, JCF = 323 Hz); 19F NMR (376 MHz, DMSO-d6) δ −76.59 (CF3, 3F); ESI-HRMS m/z [M + H]+ calculated for C11H24F3N2O5S2 385.1073, found 385.1075 ([M + H]+), 407.0895 ([M + Na]+).
ZIL 3d-ene pale yellow liquid (73% yield); 1H NMR (400 MHz, DMSO-d6) δ 2.03–2.19 (m, N+CH2CH2CH2S, 2H), 2.42–2.59 (m, N+CH2CH2CH = CH2, 2H), 3.02–3.12 (m, CH2CH2S, 2H), 3.06 (s, N+CH3, 3H), 3.26–3.34 (m, N+CH2CH2CH = CH2 + N+CH2CH2CH2S, 2H + HOCH2CH2, 6H), 3.77–3.88 (m, HOCH2CH2, 2H), 5.10–5.27 (m, N+CH2CH2CH = CH2, 2H), 5.30 (t, J = 4.4 Hz, HOCH2CH2, 1H), 5.66–5.81 (m, N+CH2CH2CH = CH2, 1H); 13C NMR (100 MHz, DMSO-d6) δ 17.41, 26.08, 48.42, 51.08, 54.68, 59.60, 60.42, 62.72, 118.40, 120.02 (q, JCF = 323 Hz); 19F NMR (376 MHz, DMSO-d6) δ −76.54 (CF3, 3F); ESI-HRMS m/z [M + H]+ calculated for C11H22F3N2O5S2 383.0917, found 383.0911 ([M + H]+), 405.0729 ([M + Na]+).
ZIL 3d-yne pale yellow liquid (80% yield); 1H NMR (400 MHz, DMSO-d6) δ 1.93 (s, N+CH2C ≡ CCH3, 3H), 2.06–2.19 (m, N+CH2CH2CH2S, 2H), 3.03–3.15 (m, CH2CH2S, 2H), 3.08 (s, N+CH3, 3H), 3.41–3.48 (m, N+CH2CH2CH2S, 2H), 3.48–3.60 (m, HOCH2CH2, 2H), 3.78–3.89 (m, HOCH2CH2, 2H), 4.30–4.39 (m, N+CH2C ≡ CCH3, 2H), 5.33 (t, J = 4.8 Hz, HOCH2CH2, 1H); 13C NMR (100 MHz, DMSO-d6) δ 3.34, 17.55, 48.23, 51.19, 53.11, 54.69, 59.75, 62.29, 67.46, 88.77, 120.04 (q, JCF = 322 Hz); 19F NMR (376 MHz, DMSO-d6) δ −76.52 (CF3, 3F); ESI-HRMS m/z [M + H]+ calculated for C11H20F3N2O5S2 381.0760, found 381.0765 ([M + H]+), 403.0581 ([M + Na]+).
ZIL 3e pale yellow liquid (57% yield); 1H NMR (400 MHz, DMSO-d6) δ 0.89 (t, J = 7.0 Hz, N+CH2CH2(CH2)2CH3, 3H), 1.18–1.40 (m, N+CH2CH2(CH2)2CH3, 4H), 1.59–1.74 (m, N+CH2CH2(CH2)2CH3, 2H), 2.02–2.16 (m, N+CH2CH2CH2S, 2H), 3.03 (s, N+CH3, 3H), 3.06 (t, J = 6.8 Hz, CH2CH2S, 2H), 3.25–3.33 (m, N+CH2CH2(CH2)2CH3, 2H), 3.33–3.50 (m, N+CH2CH2CH2S + HOCH2CH2, 4H), 3.76–3.86 (m, HOCH2CH2, 2H), 5.27 (t, J = 4.8 Hz, HOCH2CH2, 1H); 13C NMR (100 MHz, DMSO-d6) δ 13.71, 17.42, 21.07, 21.61, 27.88, 48.37, 51.15, 54.69, 59.57, 61.70, 62.61, 120.05 (q, JCF = 322 Hz); 19F NMR (376 MHz, DMSO-d6) δ −76.57 (CF3, 3F); ESI-HRMS m/z [M + Na]+ calculated for C12H25F3N2NaO5S2 421.1049, found 421.1044.
ZIL 3f pale yellow liquid (58% yield); 1H NMR (400 MHz, DMSO-d6) δ 0.87 (t, J = 6.8 Hz, N+CH2CH2(CH2)3CH3, 3H), 1.17–1.37 (m, N+CH2CH2(CH2)3CH3, 4H), 1.55–1.73 (m, N+CH2CH2(CH2)3CH3, 2H), 1.96–2.17 (m, N+CH2CH2CH2S, 2H), 3.03 (s, N+CH3, 3H), 3.06 (t, J = 6.8 Hz, CH2CH2S, 2H), 3.25–3.33 (m, N+CH2CH2(CH2)3CH3, 2H), 3.33–3.49 (m, N+CH2CH2CH2S + HOCH2CH2, 4H), 3.75–3.87 (m, HOCH2CH2, 2H), 5.26 (t, J = 4.8 Hz, HOCH2CH2, 1H); 13C NMR (100 MHz, DMSO-d6) δ 13.84, 17.46, 21.36, 21.92, 25.46, 30.66, 48.41, 51.18, 54.73, 59.59, 61.74, 62.64, 120.09 (q, JCF = 322 Hz); 19F NMR (376 MHz, DMSO-d6) δ −76.58 (CF3, 3F); ESI-HRMS m/z [M + H]+ calculated for C13H28F3N2O5S2 413.1386, found 413.1386 ([M + H]+), 435.1208 ([M + Na]+), 451.0948 ([M + K]+).
3.3.5. Synthesis of ZIL 4a
To a screw cap septum vial containing potassium sulfonimide product 3 (200 mg, 0.613 mmol) and potassium iodide (20 mg, 0.2 equiv) was added tertiary amine 5a (3 equiv). The mixture was heated at 100 °C for 12 h. After completion of the reaction, acetonitrile was added to the vial for precipitation of potassium chloride. The solid salt was filtered off, and filtrate was concentrated under reduced pressure to obtain yellow liquid crude product. Next the excess amine of crude product was washed out with sonication in ether. Then, the mixture solution of EA and MeOH (5/1) was added to bottle containing crude product. The mixture solution will become white turbid solution. After centrifugation of the white turbid solution, pale-yellow ZIL will precipitate in the solvent. After collecting the precipitate and washing it with ether several times, residual solvent was removed in vacuo to obtain ZIL 4a as pale-yellow liquid (80 mg, 35% yield).
Pale-yellow liquid; 1H NMR (400 MHz, DMSO-d6) δ 2.05–2.18 (m, NCH2CH2, 2H), 3.05 (t, J = 7.2 Hz, CH2CH2S, 2H), 3.10 (s, N+CH3, 3H), 3.41–3.55 (m, NCH2CH2 + 2 × HOCH2CH2, 6H), 3.77–3.87 (m, 2 × HOCH2CH2, 4H), 3.32 (t, J = 4.8 Hz, 2 × HOCH2CH2, 2H); 13C NMR (100 MHz, DMSO-d6) δ 17.48, 49.17, 51.23, 54.71, 60.49, 63.41, 120.05 (q, JCF = 322 Hz); 19F NMR (376 MHz, DMSO-d6) δ −76.52 (CF3, 3F); ESI-HRMS m/z [M + H]+ calculated for C9H20F3N2O6S2 373.0709, found 373.0707 ([M + H]+), 395.0532 ([M + Na]+).
3.3.6. Synthesis of ZIL 4b–f, 4d-ene, and 4d-yne
To a screw cap septum vial containing potassium sulfonimide product 3 (200 mg, 0.613 mmol) and potassium iodide (20 mg, 0.2 equiv) was added tertiary amine 5b–f (for ZIL 4b–f), N-(but-3-enyl)-N-methylaminoethan-1-ol (for ZIL 4d-ene), or N-(but-2-ynyl)-N-methylaminoethan-1-ol (for ZIL 4d-yne) (3 equiv). The mixture was heated at 100 °C for 12 h. After completion of reaction, acetonitrile was added to the vial for precipitation of potassium chloride. The solid salt was filtered off, and filtrate was concentrated under reduced pressure to obtain yellow liquid crude product. Next the excess amine of crude product was washed out with sonication in ether. The crude product was dissolved in a mixture solution of EA and MeOH (5/1, 30 mL), then was poured into a bottle containing ether (150 mL). The mixture solution will become white turbid solution. After centrifugation of the white turbid solution, pale-yellow ZIL will precipitate in the solvent. After collecting the precipitate and washing it with ether several times, the crude ZIL product was purified by silica gel column chromatography (ethyl acetate/methanol = 5/1) to afford pale yellow liquid.
ZIL 4b pale yellow liquid (32% yield); 1H NMR (400 MHz, DMSO-d6) δ 1.22 (t, J = 6.4 Hz, N+CH2CH3, 3H), 2.00–2.15 (m, N+CH2CH2CH2S, 2H), 3.06 (t, J = 6.8 Hz, CH2CH2S, 2H), 3.38-3.51 (m, N+CH2CH3 + N+CH2CH2CH2S + 2 × HOCH2CH2, 8H), 3.75–3.87 (m, 2 × HOCH2CH2, 4H), 5.26 (t, J = 4.8 Hz, HOCH2CH2, 1H); 13C NMR (100 MHz, DMSO-d6) δ 7.40, 17.09, 51.14, 54.54, 55.02, 56.90, 59.66, 61.70, 62.61, 120.08 (q, JCF = 322 Hz); 19F NMR (376 MHz, DMSO-d6) δ −76.54 (CF3, 3F); ESI-HRMS m/z [M + H]+ calculated for C10H22F3N2O6S2 387.0866, found 387.0860 ([M + H]+), 409.0678 ([M + Na]+).
ZIL 4c pale yellow liquid (24% yield); 1H NMR (400 MHz, DMSO-d6) δ 0.87 (t, J = 7.2 Hz, N+CH2CH2CH3, 3H), 1.60–1.73 (m, N+CH2CH2CH3, 2H), 2.02–2.14 (m, N+CH2CH2CH2S, 2H), 3.06 (t, J = 7.2 Hz, CH2CH2S, 2H), 3.27–3.38 (m, N+CH2CH2CH3, 2H), 3.38–3.55 (m, N+CH2CH2CH2S + 2 × HOCH2CH2, 6H), 3.74–3.86 (m, 2 × HOCH2CH2, 4H), 5.25 (t, J = 4.8 Hz, HOCH2CH2, 1H); 13C NMR (100 MHz, DMSO-d6) δ 10.79, 15.28, 17.59, 51.57, 54.98, 57.86, 60.68, 61.26, 120.51 (q, JCF = 322 Hz); 19F NMR (376 MHz, DMSO-d6) δ −76.57 (CF3, 3F); ESI-HRMS m/z [M + H]+ calculated for C11H24F3N2O6S2 401.0122, found 401.1017 ([M + H]+), 423.0835 ([M + Na]+).
ZIL 4d pale yellow liquid (34% yield); 1H NMR (400 MHz, DMSO-d6) δ 0.92 (t, J = 7.2 Hz, N+CH2CH2CH2CH3, 3H), 1.21–1.37 (m, N+CH2CH2CH2CH3, 2H), 1.58–1.72 (m, N+CH2CH2CH2CH3, 2H), 2.02–2.16 (m, N+CH2CH2CH2S, 2H), 3.06 (t, J = 6.4 Hz, CH2CH2S, 2H), 3.30–3.40 (m, N+CH2CH2CH2CH3, 2H), 3.40–3.53 (m, N+CH2CH2CH2S + 2 × HOCH2CH2, 6H), 3.75–3.87 (m, 2 × HOCH2CH2, 4H), 5.24 (t, J = 4.8 Hz, HOCH2CH2, 1H); 13C NMR (100 MHz, DMSO-d6) δ 13.45, 17.16, 19.13, 23.09, 51.11, 54.56, 57.39, 59.29, 60.19, 120.06 (q, JCF = 322 Hz); 19F NMR (376 MHz, DMSO-d6) δ −76.56 (CF3, 3F); ESI-HRMS m/z [M + H]+ calculated for C12H26F3N2O6S2 415.1179, found 415.1183 ([M + H]+), 437.0991 ([M + Na]+).
ZIL 4d-ene pale yellow liquid (25% yield); 1H NMR (400 MHz, DMSO-d6) δ 2.02–2.17 (m, N+CH2CH2CH2S, 2H), 2.43–2.55 (m, N+CH2CH2CH = CH2), 3.06 (t, J = 6.8 Hz, CH2CH2S, 2H), 3.39–3.60 (m, N+CH2CH2CH = CH2 + N+CH2CH2CH2S + 2 × HOCH2CH2, 8H), 3.74–3.93 (m, 2 × HOCH2CH2, 4H), 5.08–5.27 (m, N+CH2CH2CH = CH2, 2H), 5.32 (t, J = 4.8 Hz, HOCH2CH2, 1H), 5.65-5.78 (m, N+CH2CH2CH=CH2, 1H); 13C NMR (100 MHz, DMSO-d6) δ 17.18, 25.93, 51.07, 54.59, 60.29, 62.61, 118.52, 120.06 (q, JCF = 322 Hz), 132.73; 19F NMR (376 MHz, DMSO-d6) δ −76.53 (CF3, 3F); ESI-HRMS m/z [M + H]+ calculated for C12H24F3N2O6S2 413.1022, found 413.1025 ([M + H]+), 435.0845 ([M + Na]+).
ZIL 4d-yne pale yellow liquid (22% yield); 1H NMR (400 MHz, DMSO-d6) δ 1.93 (s, N+CH2C ≡ CCH3, 3H), 2.02–2.14 (m, N+CH2CH2CH2S, 2H), 3.07 (t, J = 6.8 Hz, CH2CH2S, 2H), 3.44–3.61 (m, N+CH2CH2CH2S + 2 × HOCH2CH2, 6H), 3.77–3.88 (m, 2 × HOCH2CH2, 4H), 4.37–4.44 (m, N+CH2C ≡ CCH3, 2H), 5.33 (t, J = 4.8 Hz, HOCH2CH2, 1H); 13C NMR (100 MHz, DMSO-d6) δ 3.40, 17.40, 51.22, 51.29, 54.61, 58.10, 60.56, 67.40, 88.79, 120.06 (q, JCF = 322 Hz); 19F NMR (376 MHz, DMSO-d6) δ −76.52 (CF3, 3F); ESI-HRMS m/z [M + H]+ calculated for C12H22F3N2O6S2 411.0866, found 411.0871 ([M + H]+), 433.0690 ([M + Na]+).
ZIL 4e pale yellow liquid (36% yield); 1H NMR (400 MHz, DMSO-d6) δ 0.88 (t, J = 7.2 Hz, N+CH2CH2(CH2)2CH3, 3H), 1.16–1.41 (m, N+CH2CH2(CH2)2CH3, 2H), 1.59–1.75 (m, N+CH2CH2(CH2)2CH3, 4H), 2.01–2.16 (m, N+CH2CH2CH2S, 2H), 3.06 (t, J = 6.8 Hz, CH2CH2S, 2H), 3.29–3.38 (m, N+CH2CH2(CH2)2CH3, 2H), 3.39–3.55 (m, N+CH2CH2CH2S + 2 × HOCH2CH2, 6H), 3.72–3.90 (m, 2 × HOCH2CH2, 4H), 5.25 (t, J = 4.8 Hz, HOCH2CH2, 1H); 13C NMR (100 MHz, DMSO-d6) δ 13.75, 17.13, 20.80, 21.61, 27.85, 51.08, 54.52, 57.33, 59.45, 60.13, 120.04 (q, JCF = 322 Hz); 19F NMR (376 MHz, DMSO-d6) δ −76.57 (CF3, 3F); ESI-HRMS m/z [M + H]+ calculated for C13H28F3N2O6S2 429.1335, found 429.1337 ([M + H]+), 451.1157 ([M + Na]+).
ZIL 4f pale yellow liquid (38% yield); 1H NMR (400 MHz, DMSO-d6) δ 0.87 (t, J = 6.8 Hz, N+CH2CH2(CH2)3CH3, 3H), 1.19–1.36 (m, N+CH2CH2(CH2)3CH3, 6H), 1.60–1.72 (m, N+CH2CH2(CH2)3CH3, 2H), 2.02–2.14 (m, N+CH2CH2CH2S, 2H), 3.06 (t, J = 7.2 Hz, CH2CH2S, 2H), 3.28–3.40 (m, N+CH2CH2(CH2)3CH3, 2H), 3.40–3.55 (m, N+CH2CH2CH2S + 2 × HOCH2CH2, 6H), 3.73–3.86 (m, 2 × HOCH2CH2, 4H), 5.24 (t, J = 4.8 Hz, HOCH2CH2, 1H); 13C NMR (100 MHz, DMSO-d6) δ 13.78, 17.12, 21.03, 21.86, 25.34, 30.58, 51.07, 54.51, 57.34, 59.47, 60.13, 120.03 (q, JCF = 322 Hz); 19F NMR (376 MHz, DMSO-d6) δ −76.57 (CF3, 3F); ESI-HRMS m/z [M + H]+ calculated for C14H30F3N2O6S2 443.1492, found 443.1497 ([M + H]+), 465.1309 ([M + Na]+).