Ring-Opening Polymerization of ε-Caprolactone and Styrene Oxide–CO2 Coupling Reactions Catalyzed by Chelated Dehydroacetic Acid–Imine Aluminum Complexes
Abstract
:1. Introduction
2. Results and Discussion
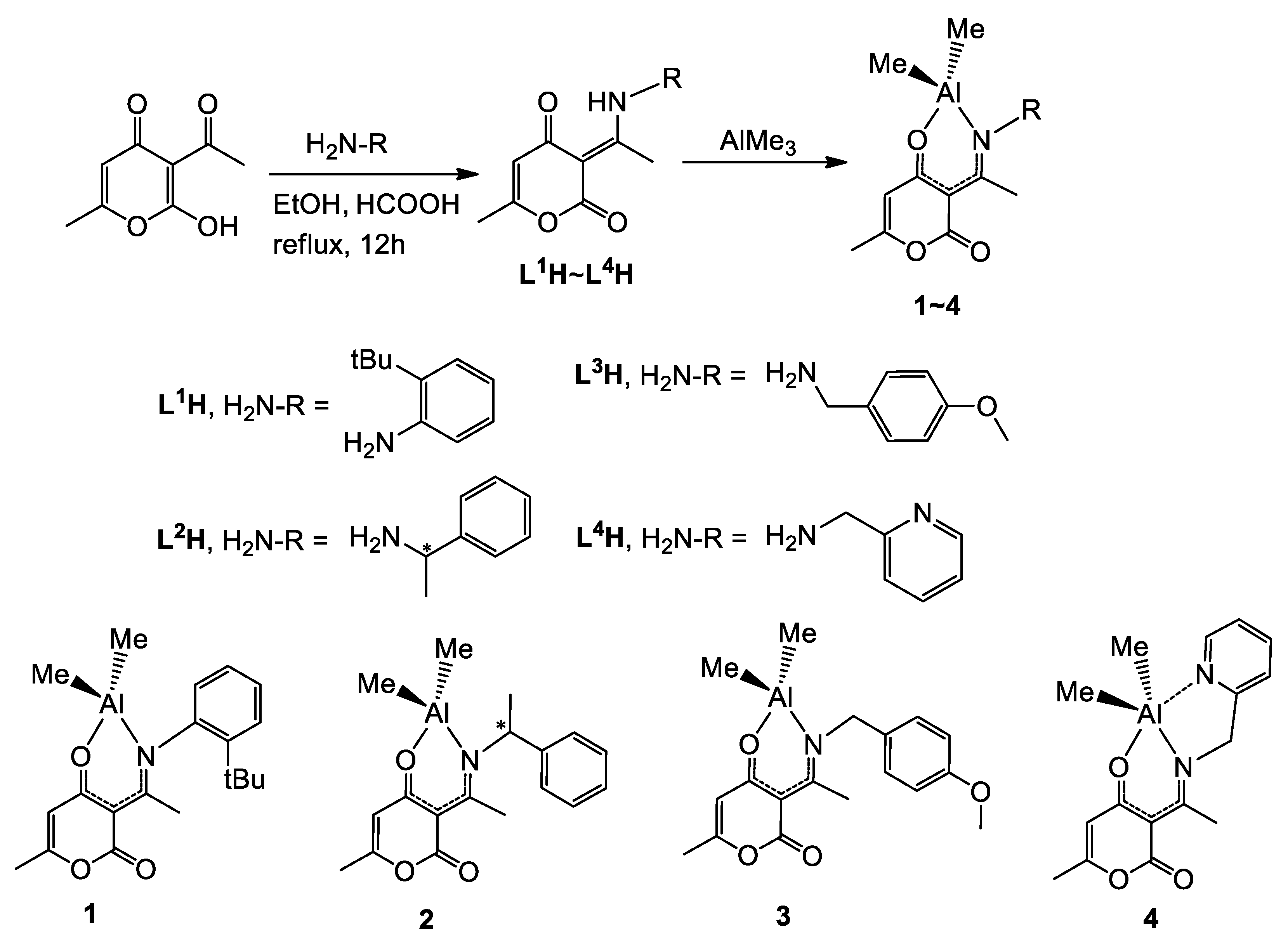
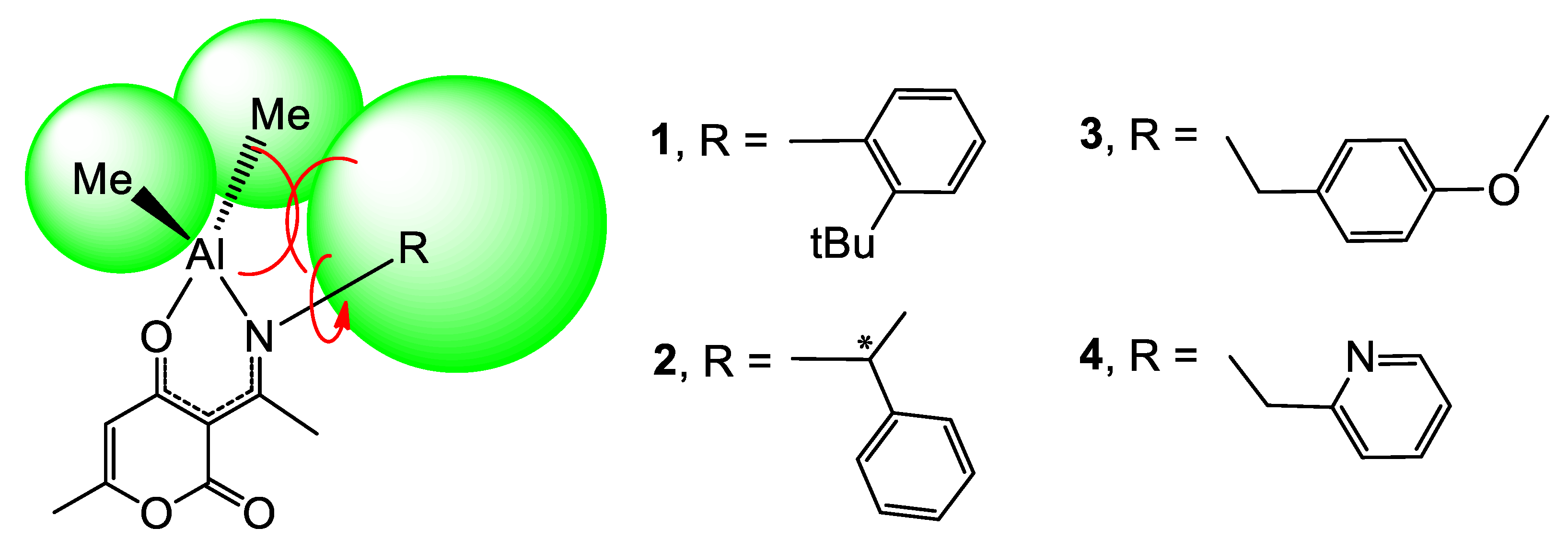
2.1. Synthesis and Characterization of L1H~L4H and Compounds 1–4
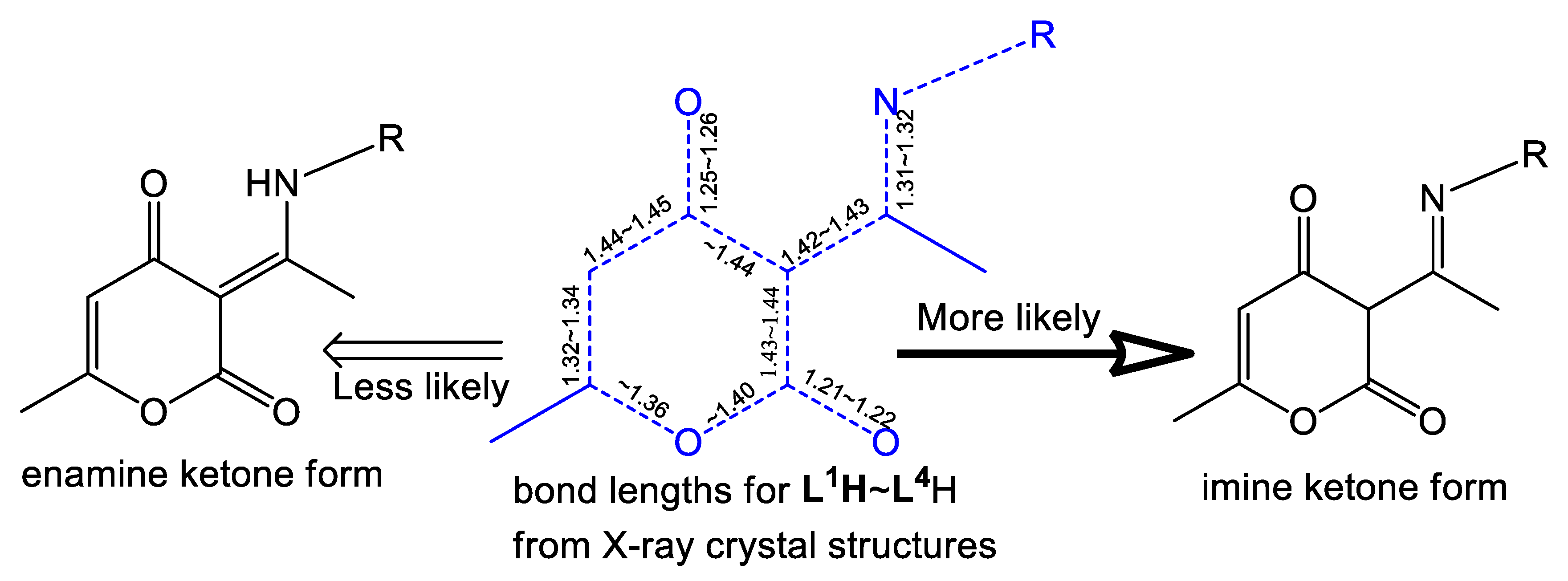
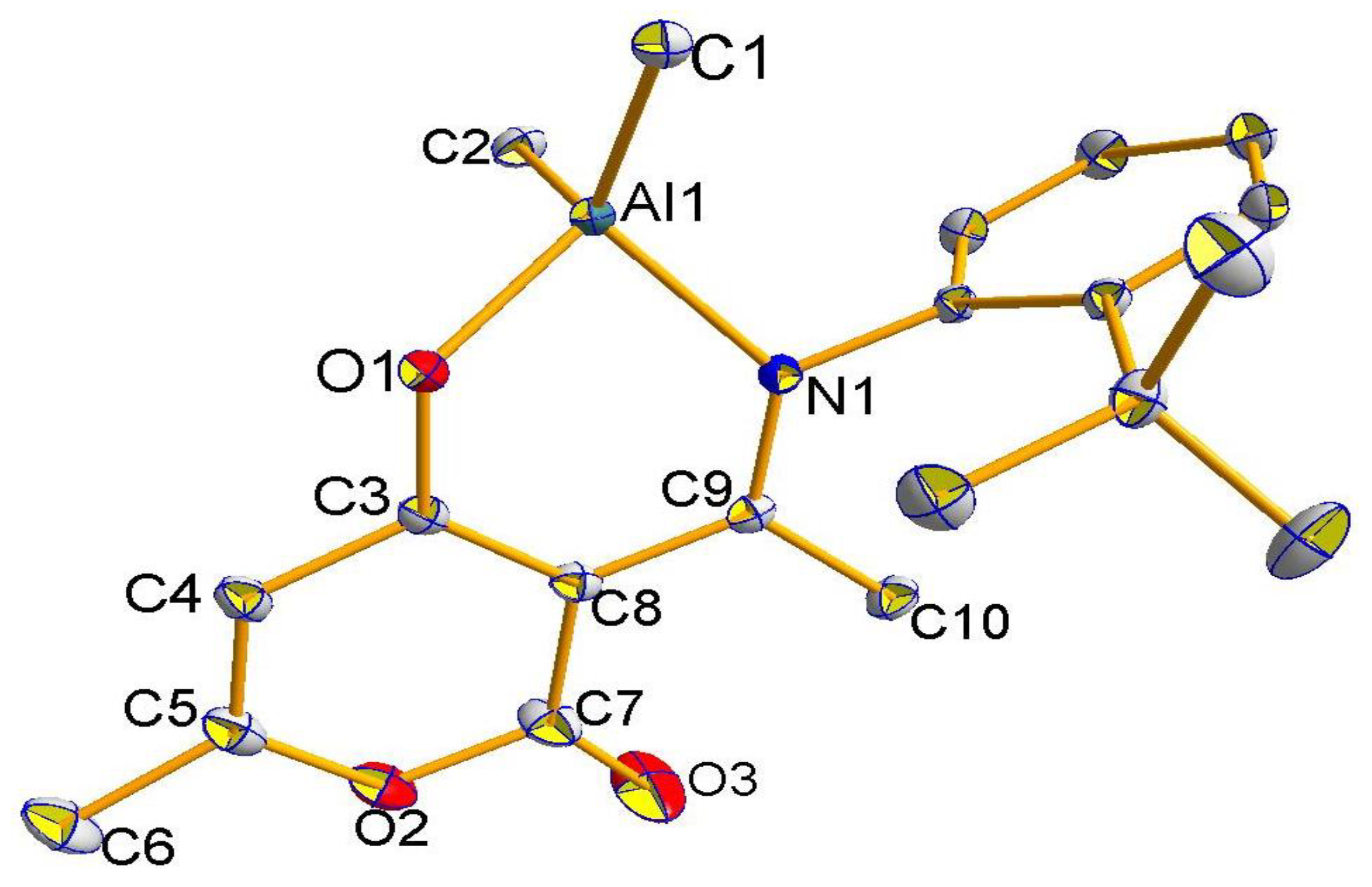
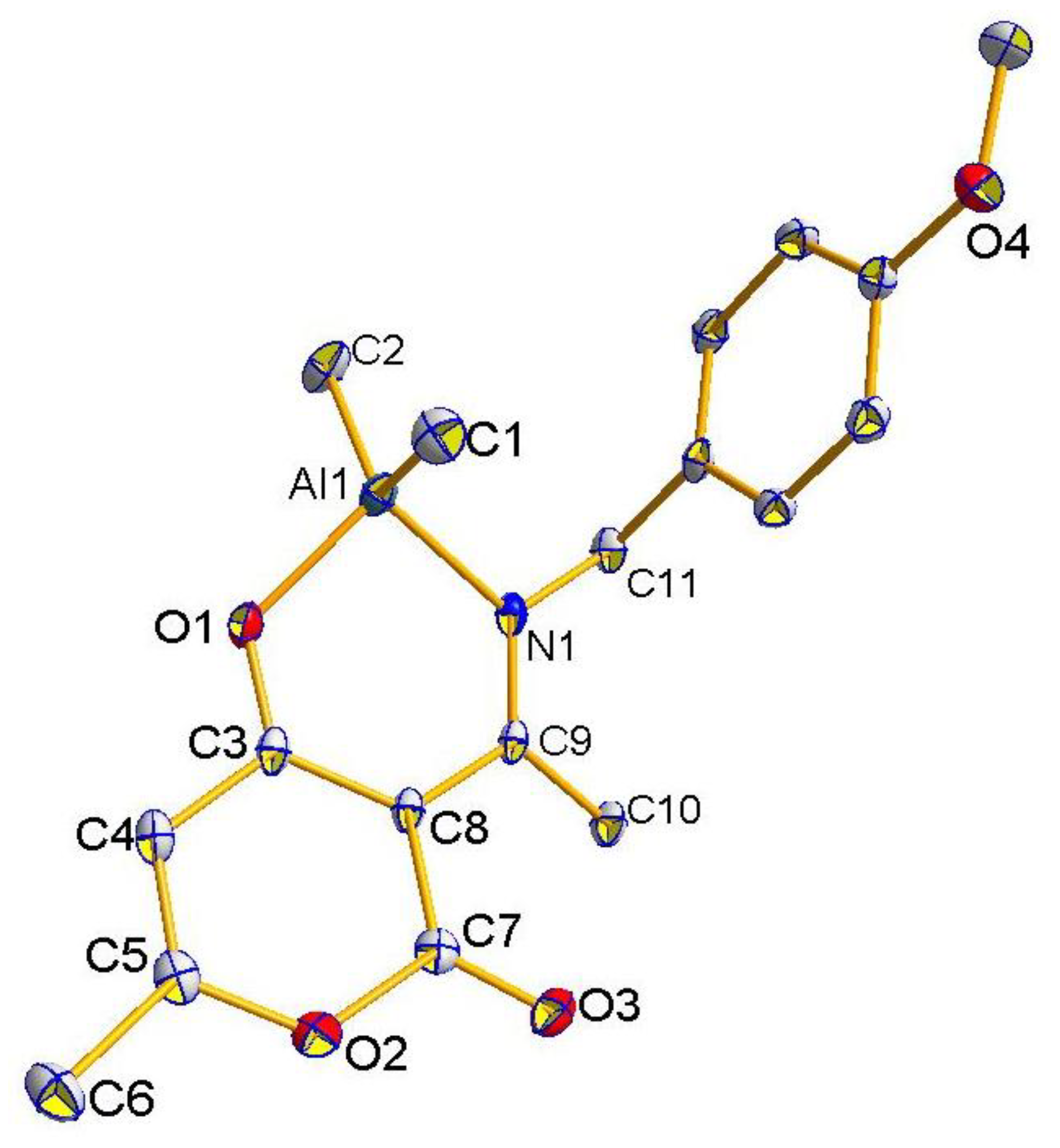
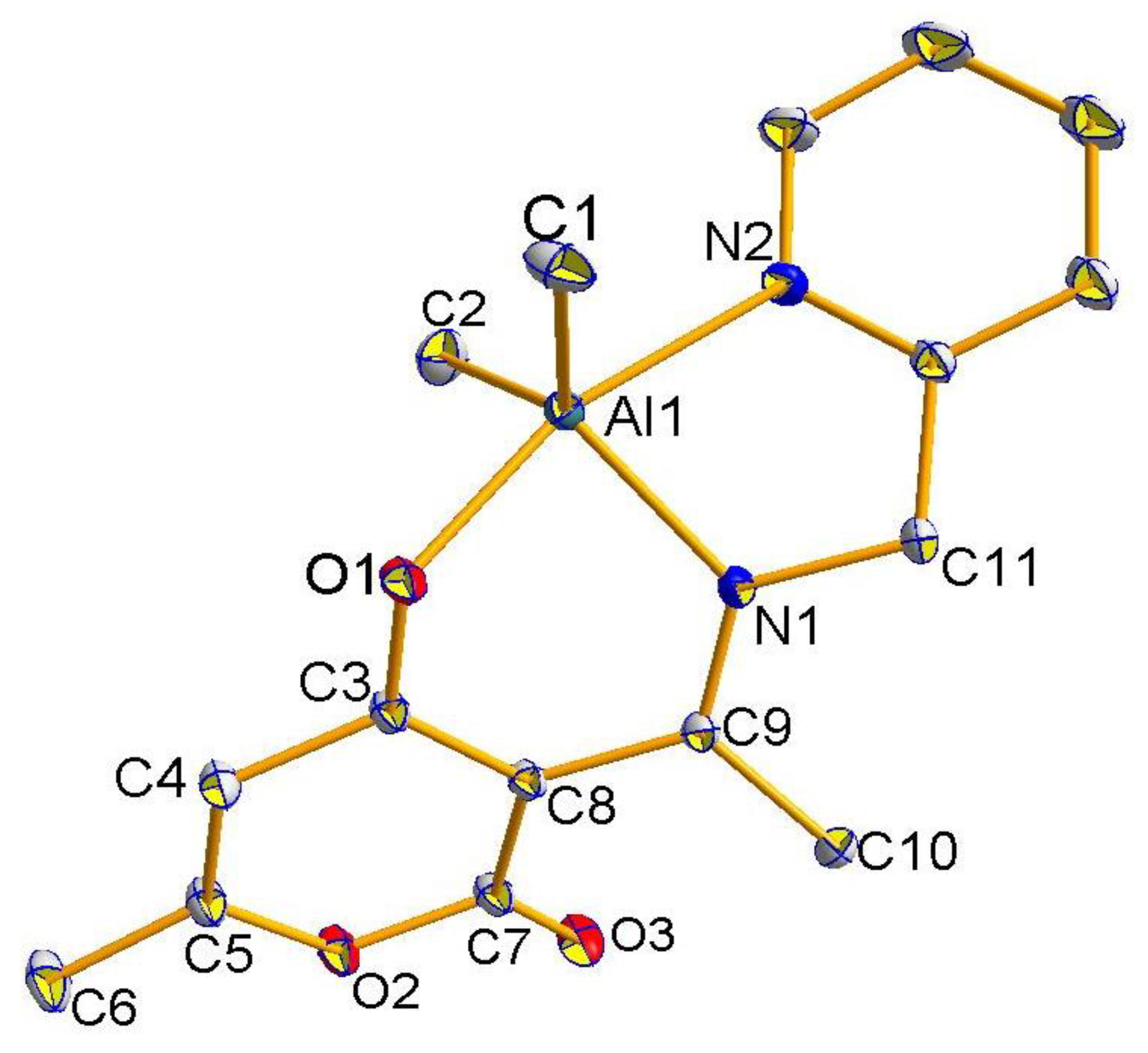
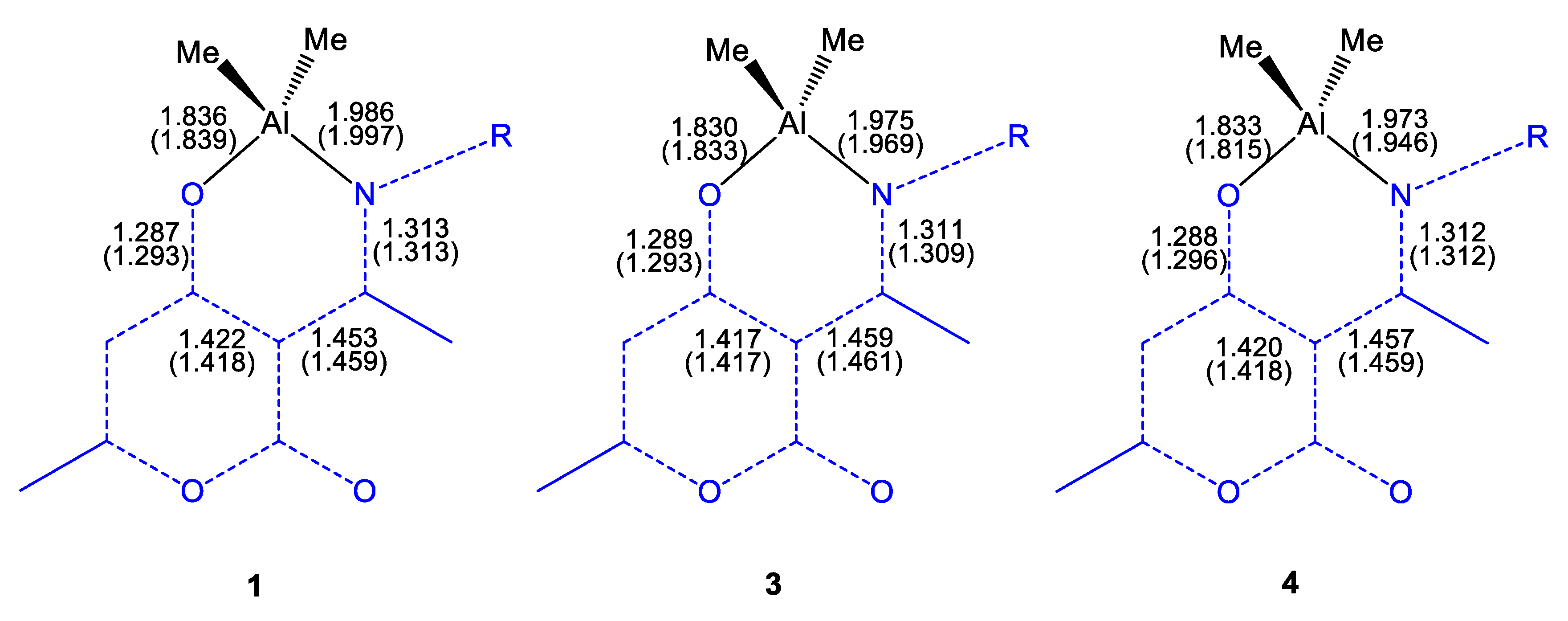
2.2. Molecular Structures of Compounds L1H~L4H, 1, 3, and 4
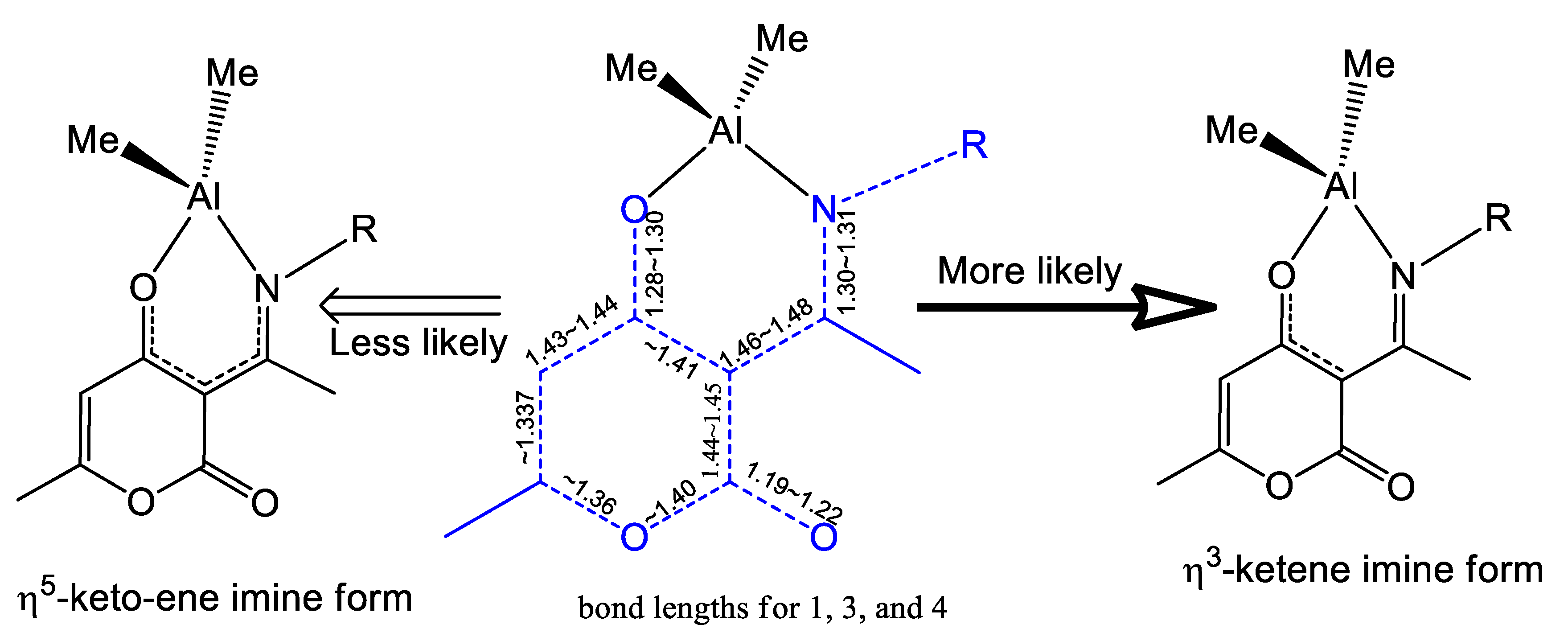
2.3. The Structure Resonance Forms of Ligands and Complexes in Solution
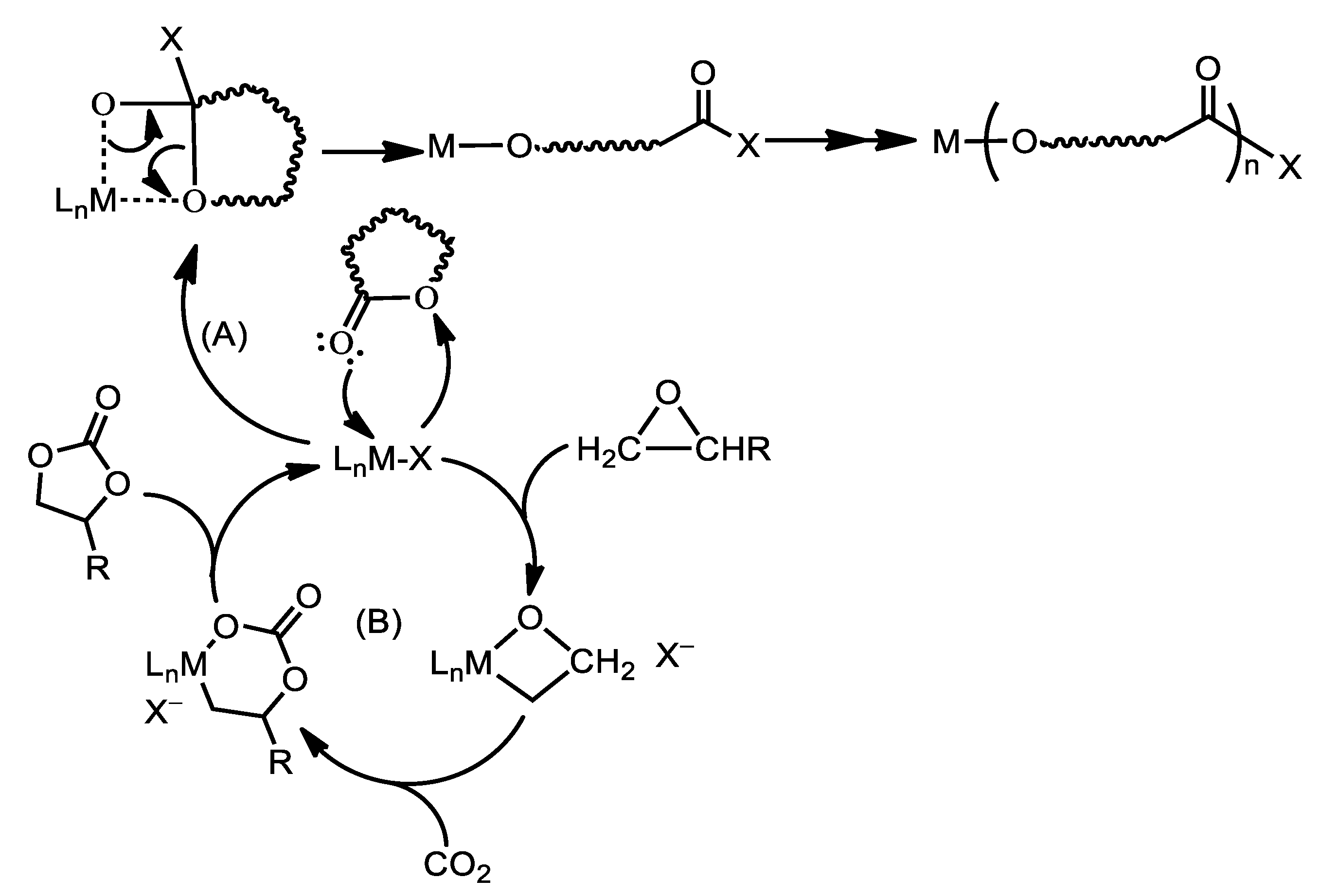
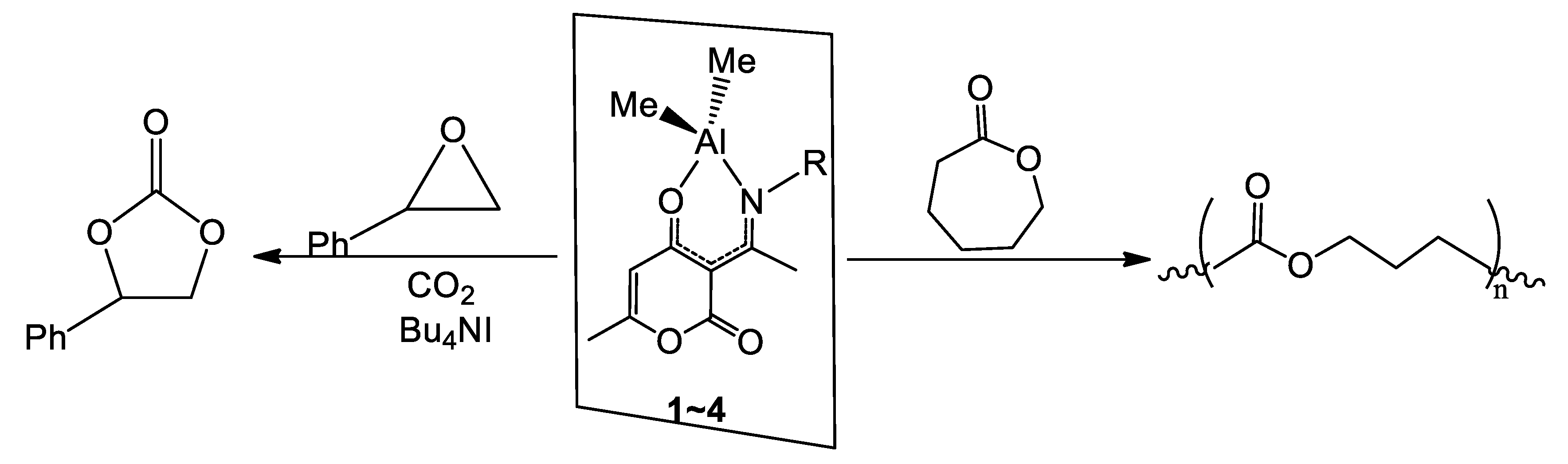
2.4. Catalytic Ring-Opening Polymerization of ε-Caprolactone and Coupling Reactions of Styrene Oxide with CO2
3. Conclusions
4. Experimental Section
4.1. Physical Experiments and Reagents
4.2. Synthesis of Ligands L1H~L4H and Complexes 1~4
4.3. Ring-Opening Polymerization of ε-Caprolactone
4.4. CO2 Coupling Reactions with Styrene Oxide
4.5. X-ray Crystallography
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Beil, L. Pollution Killed 9 Million People in 2015. (15 November 2017). Available online: Sciencenews.org (accessed on 1 December 2017).
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Gentile, G.; Sorrentino, L.L.; Ambrosio, L. Polycaprolactone: Synthesis, properties, and applications. Encycl. Poly. Sci. Technol. 2017, 1–36. [Google Scholar]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Lyubov, D.M.; Tolpygin, A.O.; Trifonov, A.A. Rare-earth metal complexes as catalysts for ring-opening polymerization of cyclic esters. Coord. Chem. Rev. 2019, 392, 83–145. [Google Scholar] [CrossRef]
- Fuoco, T.; Pappalardo, D. Aluminum alkyl complexes bearing salicylaldiminato ligands: Versatile initiators in the ring-opening polymerization of cyclic esters. Catalysts 2017, 7, 64. [Google Scholar] [CrossRef]
- Guillaume, S.M. Recent advances in ring-opening polymerization strategies toward α,ω-Hydroxy telechelic polyesters and resulting copolymers. Eur. Polym. J. 2013, 49, 768–779. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the valorization of exhaust carbon: From CO2 to chemicals, materials, and fuels. Technological use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [CrossRef]
- Mandal, M. Group 4 complexes as catalysts for the transformation of CO2 into polycarbonates and cyclic carbonates. J. Organomet. Chem. 2020, 907, 121067. [Google Scholar] [CrossRef]
- Castro-Osma, J.A.; North, M. Synthesis of cyclic carbonates from polyols and carbon dioxide, Uuea or carbon monoxide. Curr. Green Chem. 2014, 1, 257–272. [Google Scholar] [CrossRef]
- Castro-Osma, A.J.; North, M.; Wu, X. Synthesis of cyclic carbonates catalysed by chromium and aluminium salphen complexes. Chem. Eur. J. 2016, 22, 2100–2107. [Google Scholar] [CrossRef]
- Indran, V.P.; Saud, A.S.H.; Maniam, G.P.; Yuso, M.M.; Taufiq-Yap, Y.H.; Rahim, M.H.A. Versatile boiler ash containing potassium silicate for the synthesis of organic carbonates. RSC Adv. 2016, 6, 34877–34884. [Google Scholar] [CrossRef] [Green Version]
- North, M.; Pasquale, R.; Young, C. Synthesis of cyclic carbonates from epoxides and CO2. Green Chem. 2010, 12, 1514–1539. [Google Scholar] [CrossRef]
- Martín, C.; Fiorani, G.; Kleij, A.W. Recent advances in the catalytic preparation of cyclic organic carbonates. ACS Catal. 2015, 5, 1353–1370. [Google Scholar] [CrossRef]
- Lopes, E.J.C.; Ribeir, A.P.C.; Martins, L.M.D.R.S. New trends in the conversion of CO2 to cyclic carbonates. Catalysts 2020, 10, 479. [Google Scholar] [CrossRef]
- Gaona, M.A.; de la Cruz-Martínez, F.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Alonso-Moreno, C.; Rodríguez, A.M.; Rodríguez-Diéguez, A.; Castro-Osma, J.A.; Otero, A.; Lara-Sánchez, A. Synthesis of helical aluminium catalysts for cyclic carbonate formation. Dalton Trans. 2019, 48, 4218–4227. [Google Scholar] [CrossRef]
- Castro-Osma, J.A.; Lara-Sánchez, A.; North, M.; Otero, A.; Villuendasa, P. Synthesis of cyclic carbonates using monometallic, and helical bimetallic, aluminium complexes. Catal. Sci. Technol. 2012, 2, 1021–1026. [Google Scholar] [CrossRef]
- Castro-Osma, J.A.; Alonso-Moreno, C.; Lara-Sánchez, A.; Martínez, J.; North, M.; Otero, A. Synthesis of cyclic carbonates catalysed by aluminium heteroscorpionate complexes. Catal. Sci. Technol. 2014, 4, 1674–1684. [Google Scholar] [CrossRef]
- Gibson, C.; Marshall, E.L. Metal complexes as catalysts for polymerization reactions. In Comprehensive Coordination Chemistry II; Ward, M.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 9, pp. 1–74. [Google Scholar]
- Arbaoui, A.; Redshaw, C. Metal catalysts for ε-caprolactone polymerization. Polym. Chem. 2010, 1, 801–826. [Google Scholar] [CrossRef]
- Stevels, W.M.; Ankoné, M.J.K.; Dijkstra, P.J.; Feijen, J. Kinetics and mechanism of ε-caprolactone polymerization using yttrium alkoxides as initiators. Macromolecules 1996, 29, 8296–8303. [Google Scholar] [CrossRef]
- Chamberlain, B.M.; Jazdzewsky, B.A.; Pink, M.; Hillmyer, M.A.; Tolman, W.B. Controlled polymerization of DL-lactide and ε-caprolactone by structurally well-defined alkoxo-bridged di- and triyttrium(III) complexes. Macromolecules 2000, 33, 3970–3977. [Google Scholar] [CrossRef]
- Babu, H.V.; Muralidharan, K. Zn(II), Cd(II) and Cu(II) complexes of 2,5-bis{N-(2,6-diisopropylphenyl)iminomethyl}pyrrole: Synthesis, structures and their high catalytic activity for efficient cyclic carbonate synthesis. Dalton Trans. 2013, 42, 1238–1248. [Google Scholar] [CrossRef]
- Tian, D.; Liu, B.; Gan, Q.; Li, H.; Darensbourg, D.J. Formation of cyclic carbonates from carbon dioxide and epoxides coupling reactions efficiently catalyzed by robust, recyclable one-component aluminum-salen complexes. ACS Catal. 2012, 2, 2029–2035. [Google Scholar] [CrossRef]
- Wu, M.-C.; Hu, T.-C.; Lo, Y.-C.; Lee, T.-Y.; Lin, C.-H.; Lu, W.-Y.; Lin, C.-C.; Datta, A.; Huang, J.-H. New types of bi- and tri-dentate pyrrole-piperazine ligands and related zinc compounds: Synthesis, characterization, reaction study, and ring-opening polymerization of ε-caprolactone. J. Organomet. Chem. 2015, 791, 141–147. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Chuang, S.-J.; Chuang, N.-T.; Hsiao, C.-S.; Datta, A.; Chen, S.-J.; Hu, C.-H.; Huang, J.-H.; Lee, T.-Y.; Lin, C.-H. Aluminium complexes containing bidentate and symmetrical tridentate pincer type pyrrolyl ligands: Synthesis, reactions and ring opening polymerization. Dalton Trans. 2011, 40, 7423–7433. [Google Scholar] [CrossRef]
- Hsueh, L.-F.; Chuang, N.-T.; Lee, C.-Y.; Datta, A.; Huang, J.-H.; Lee, T.-Y. Magnesium complexes containing η1- and η3-pyrrolyl ligands or ketiminate ligands: Synthesis, structural investigation and ε-caprolactone ring opening polymerization. Eur. J. Inorg. Chem. 2011, 5530–5537. [Google Scholar] [CrossRef]
- Chen, K.-H.; Lin, T.-H.; Hsu, T.-E.; Li, Y.-J.; Chen, G.-H.; Leu, W.-J.; Guh, J.-H.; Lin, C.-H.; Huang, J.-H. Ruthenium (II) complexes containing dehydroacetic acid and its imine derivative ligands. Synthesis, characterization and cancer cell growth anti-proliferation activity (GI50) study. J. Organomet. Chem. 2018, 871, 150–158. [Google Scholar] [CrossRef]
- Bergman, J.J.; Chandler, W.D.A. Study of the barriers to rotation in some highly substituted diphenyl ethers. Can. J. Chem. 1972, 50, 353–363. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.; Kim, J.; Won, D.; Chang, S.-K.; Cha, W.; Jeong, K.; Ahn, S.; Kwak, K. Electronic effect on the molecular motion of aromatic amides: Combined studies dsing VT-NMR and quantum calculations. Molecules 2018, 23, 2294. [Google Scholar] [CrossRef] [Green Version]
- Baaziz, S.; Bellec, N.; Le Gal, Y.; Kaoua, R.; Camerel, F.; Bakhta, S.; Nedjar-Kolli, B.; Roisnel, T.; Dorcet, V.; Jeannin, O.; et al. Difluoroboron Complexes of functionalized dehydroacetic acid: Electrochemical and luminescent properties. Tetrahedron 2016, 72, 464–471. [Google Scholar] [CrossRef]
- Amar, A.; Meghezzi, H.; Boucekkine, A.; Kaoua, R.; Kolli, B. How to drive imine–enamine tautomerism of pyronic derivatives of biological interest–A theoretical and experimental study of substituent and solvent effects. Comptes Rendus Chim. 2010, 13, 553–560. [Google Scholar] [CrossRef]
- Seijas, J.A.; Crecente-Campo, J.; Feás, X.; Vázquez-Tato, M.P. Microwave assisted synthesis, crystal structure and modelling of cytotoxic dehydroacetic acid enamine: A natural alkaloid from Fusarium Incarnatum (HKI0504). RSC Adv. 2014, 4, 17054–17059. [Google Scholar] [CrossRef]
- Kwocz, A.; Kochel, A.; Chudoba, D.; Filarowski, A. Tautomeric design of ortho-hydroxyheterocyclic Schiff bases. J. Mol. Struct. 2015, 1080, 52–56. [Google Scholar] [CrossRef]
- Đilović, I.; Užarević, K. Conformational adaptations of acyclic receptor templated by weakly coordinating anions. CrystEngComm 2015, 17, 3153–3161. [Google Scholar] [CrossRef] [Green Version]
- Užarević, K.; Halasz, I.; Đilović, I.; Bregović, N.; Rubčić, M.; Matković-Čalogović, D.; Tomišic, V. Dynamic molecular recognition in solid state for separating mixtures of isomeric dicarboxylic acids. Angew. Chem. Int. Ed. 2013, 52, 5504–5508. [Google Scholar] [CrossRef] [PubMed]
- Gilli, P.; Bertolasi, V.; Ferretti, V.; Gilli, G. Evidence for intramolecular N−H···O resonance-assisted hydrogen bonding in β-enaminones and related heterodienes. A combined crystal-structural, IR and NMR spectroscopic, and quantum-mechanical investigation. J. Am. Chem. Soc. 2000, 122, 10405–10417. [Google Scholar] [CrossRef]
- Teo, S.-B.; Teoh, S.-G.; Okechukwu, R.C.; Wei, C. The preparation and crystal structure of dimethyl [[N-(pentanone-4-(2-mercaptophenyl)iminato](2-)-N,O,S]tin(IV). J. Coord. Chem. 1993, 28, 81–87. [Google Scholar] [CrossRef]
- Podlahová, J.; Haber, V.; Knížek, K.; Loub, J.; Malý, K. Structure of {4-[2-(2-amino ethylamino)ethylimino]pentan-2-onato-N,N′,N″,O}nickel(II) iodide monohydrate. Acta Crystallogr. Sec. C 1989, 45, 1216–1218. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, X.-F.; Huo, L.-H.; Zhao, H. [Benzoylacetone (4-methoxybenzoyl)hydrazonato-κ3O,N2,O′]dichloro iron(III). Acta Crystallogr. Sec. E 2004, 60, m1447–m1449. [Google Scholar] [CrossRef]
- Kratochvíl, B.; Ondráček, J.; Maixner, J.; Macíček, J.; Haber, V. Structure of a palladium(II) complex with a non-symmetrical tetradentate Schiff base, [Pd(C14H20N3O)]NCS. Collect Czechoslov. Chem. Commun. 1991, 56, 1900–1907. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Wang, S.; Zhou, S. Aluminum alkyl complexes: Synthesis, structure, and application in ROP of cyclic esters. Dalton Trans. 2016, 45, 4471–4485. [Google Scholar] [CrossRef]
- Chen, C.-T.; Hung, C.-C.; Chang, Y.-J.; Peng, K.-F.; Chen, M.-T. Magnesium and zinc complexes containing pendant pyrazolylephenolate ligands as catalysts for ring opening polymerisation of cyclic esters. J. Organomet. Chem. 2013, 738, 1–9. [Google Scholar] [CrossRef]
- Chien, K.-M.; Hu, T.-C.; Lin, C.-H.; Lo, Y.-C.; Lee, T.-Y.; Huang, J.-H. Synthesis and characterization of tri-dentate pyrrole-morpholine ligands and their corresponding aluminum, magnesium, and zinc compounds. J. Organomet. Chem. 2015, 779, 39–44. [Google Scholar] [CrossRef]
- Hsiao, H.-C.; Datta, A.; Chen, Y.-F.; Chang, W.; Lee, T.-Y.; Lin, C.-H.; Huang, J.-H. Structural aspects and ring opening polymerization of ε-caprolactone using mono- and di-aluminum compounds incorporating bidentate pyrrole-morpholine ligands. J. Organomet. Chem. 2016, 804, 35–41. [Google Scholar] [CrossRef]
- Lulio, C.D.; Jones, M.D.; Mahon, M.F.; Apperley, D.C. Zinc(II) silsesquioxane complexes and their application for the ring-opening polymerization of rac-Lactide. Inorg. Chem. 2010, 49, 10232–10234. [Google Scholar]
- Sun, J.; Fujita, S.-I.; Zhao, F.; Arai, M. Synthesis of styrene carbonate from styrene oxide and carbon dioxide in the presence of zinc bromide and ionic liquid under mild conditions. Green Chem. 2004, 6, 613–616. [Google Scholar] [CrossRef]
- Fujita, S.; Nishiura, M.; Arai, M. Synthesis of styrene carbonate from carbon dioxide and styrene oxide with various zinc halide-based ionic liquids. Cataal. Lett. 2010, 135, 263–268. [Google Scholar] [CrossRef] [Green Version]
- Martic, C.; Kleij, A.W. Comparing kinetic profiles between bifunctional and binary type of Zn(salen)-based catalysts for organic carbonate formation. J. Org. Chem. 2014, 10, 1817–1825. [Google Scholar]
- Whiteoak, C.J.; Kielland, N.; Laserna, V.; Escudero-Adan, E.C.; Martin, E.; Kleij, A.W. A powerful aluminum catalyst for the synthesis of highly functional organic carbonates. J. Am. Chem. Soc. 2013, 135, 1228–1235. [Google Scholar] [CrossRef]
- Janeta, M.; Lis, T.; Szafert, S. Zinc imine polyhedral oligomeric silsesquioxane as a quattro-site catalyst for the synthesis of cyclic carbonates from epoxides and low-pressure CO2. Chem. Eur. J. 2020, 26, 13686–13697. [Google Scholar] [CrossRef] [PubMed]
- Castro-Osma, J.A.; Lamb, K.J.; North, M. Cr(salophen) complex catalyzed cyclic carbonate synthesis at ambient temperature and pressure. ACS Catal. 2016, 6, 5012–5025. [Google Scholar] [CrossRef]
- SADABS. Area-Detector Absorption Correction; Bruker AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Sheldrick, G.M. SHELX97-Programs for Crystal Structure Analysis: Structure Determination (SHELXS); University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELX97-Programs for Crystal Structure Analysis: Refinement (SHELXL); University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]

| Entry | Solvent | Time (h) | Temp (C) | BnOH b | Conv. c (%) |
|---|---|---|---|---|---|
| 1 | THF | 24 | 30 | - | 7.4 |
| 2 | DCM | 24 | 30 | - | 3.8 |
| 3 | toluene | 24 | 30 | - | 12.6 |
| 4 | toluene | 1 | 30 | - | NR |
| 5 | toluene | 1 | 80 | - | 15 |
| 6 | toluene | 3 | 80 | - | 47 |
| 7 | toluene | 6 | 80 | - | 56 |
| 8 | toluene | 1 | 30 | 1 | NR |
| 9 | toluene | 1 | 60 | 1 | 66 |
| 10 | toluene | 1 | 80 | 1 | 99 |
| Entry | Initiator | [M]/[I] | Mn, exp. b (g/mol) | Mn, theo. (g/mol) | Mw | PDI | Conv. c (%) |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 50/1 | 5900 | 5700 | 12,000 | 1.14 | 99 |
| 2 | 1 | 100/1 | 14,800 | 11,300 | 45,600 | 1.51 | 99 |
| 3 | 1 | 150/1 | 16,900 | 16,900 | 44,300 | 1.67 | 99 |
| 4 | 1 | 200/1 | 27,900 | 22,600 | 78,700 | 1.56 | 99 |
| 5 | 1 | 250/1 | 28,200 | 25,700 | 66,800 | 1.34 | 90 |
| 6 | 2 | 50/1 | 6200 | 5700 | 13,100 | 1.19 | 99 |
| 7 | 2 | 100/1 | 9700 | 10,800 | 22,200 | 1.28 | 95 |
| 8 | 2 | 150/1 | 6600 | 14,700 | 21,300 | 1.79 | 86 |
| 9 | 2 | 200/1 | 27,200 | 22,600 | 75,000 | 1.54 | 99 |
| 10 | 2 | 250/1 | 26,100 | 28,000 | 98,000 | 2.10 | 99 |
| 11 | 3 | 50/1 | 7600 | 5700 | 16,400 | 1.20 | 99 |
| 12 | 3 | 100/1 | 10,800 | 11,300 | 31,800 | 1.65 | 99 |
| 13 | 3 | 150/1 | 15,200 | 16,900 | 48,900 | 1.80 | 99 |
| 14 | 3 | 200/1 | 17,200 | 21,700 | 44,700 | 1.39 | 95 |
| 15 | 3 | 250/1 | 17,900 | 28,300 | 47,300 | 1.54 | 99 |
| 16 | 4 | 50/1 | 7500 | 5700 | 20,000 | 1.48 | 98 |
| 17 | 4 | 100/1 | 8000 | 11,000 | 19,900 | 1.38 | 97 |
| 18 | 4 | 150/1 | 13,000 | 11,300 | 33,900 | 1.46 | 99 |
| 19 | 4 | 200/1 | 14,800 | 16,900 | 39,500 | 1.48 | 95 |
| 20 | 4 | 250/1 | 21,500 | 28,300 | 69,200 | 1.75 | 99 |
| Entry | Catalyst | Temp. (°C) | Time (h) | Conv.% b |
|---|---|---|---|---|
| 1 | 1 | 90 | 3 | 2.9 |
| 2 | 1 | 90 | 6 | 42 |
| 3 | 1 | 90 | 12 | 85 |
| 4 | 2 | 90 | 3 | 4.7 |
| 5 | 2 | 90 | 6 | 11 |
| 6 | 2 | 90 | 12 | 91 |
| 7 | 3 | 90 | 3 | 2.8 |
| 8 | 3 | 90 | 6 | 23 |
| 9 | 3 | 90 | 12 | 95 |
| 10 | 4 | 90 | 3 | 5.9 |
| 11 | 4 | 90 | 6 | 31 |
| 12 | 4 | 90 | 12 | 93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.-Y.; Su, Y.-C.; Ko, B.-T.; Hsu, Y.; Zeng, Y.-F.; Hu, C.-H.; Datta, A.; Huang, J.-H. Ring-Opening Polymerization of ε-Caprolactone and Styrene Oxide–CO2 Coupling Reactions Catalyzed by Chelated Dehydroacetic Acid–Imine Aluminum Complexes. Molecules 2022, 27, 164. https://doi.org/10.3390/molecules27010164
Wang T-Y, Su Y-C, Ko B-T, Hsu Y, Zeng Y-F, Hu C-H, Datta A, Huang J-H. Ring-Opening Polymerization of ε-Caprolactone and Styrene Oxide–CO2 Coupling Reactions Catalyzed by Chelated Dehydroacetic Acid–Imine Aluminum Complexes. Molecules. 2022; 27(1):164. https://doi.org/10.3390/molecules27010164
Chicago/Turabian StyleWang, Ting-Yen, Yu-Chia Su, Bao-Tsan Ko, Yu Hsu, Yu-Fang Zeng, Ching-Han Hu, Amitabha Datta, and Jui-Hsien Huang. 2022. "Ring-Opening Polymerization of ε-Caprolactone and Styrene Oxide–CO2 Coupling Reactions Catalyzed by Chelated Dehydroacetic Acid–Imine Aluminum Complexes" Molecules 27, no. 1: 164. https://doi.org/10.3390/molecules27010164
APA StyleWang, T.-Y., Su, Y.-C., Ko, B.-T., Hsu, Y., Zeng, Y.-F., Hu, C.-H., Datta, A., & Huang, J.-H. (2022). Ring-Opening Polymerization of ε-Caprolactone and Styrene Oxide–CO2 Coupling Reactions Catalyzed by Chelated Dehydroacetic Acid–Imine Aluminum Complexes. Molecules, 27(1), 164. https://doi.org/10.3390/molecules27010164






