Elicitation of Submerged Adventitious Root Cultures of Stevia rebaudiana with Cuscuta reflexa for Production of Biomass and Secondary Metabolites
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Biotic Elicitor on Growth Kinetics of Adventitious Root Culture of Stevia
2.2. The Effect of Cuscuta Reflexa Extract on Polyphenolics in Adventitious Roots Cultures
2.3. DPPH Radical Scavenging Activity (DRSA)
3. Materials and Methods
3.1. Seeds Collection and Germination
3.2. Explant Collection for Development of Stock Adventitious Root Cultures
3.3. Establishment of Adventitious Root Cultures
3.4. Adventitious Root Biomass Determination
3.5. Analytical Methods
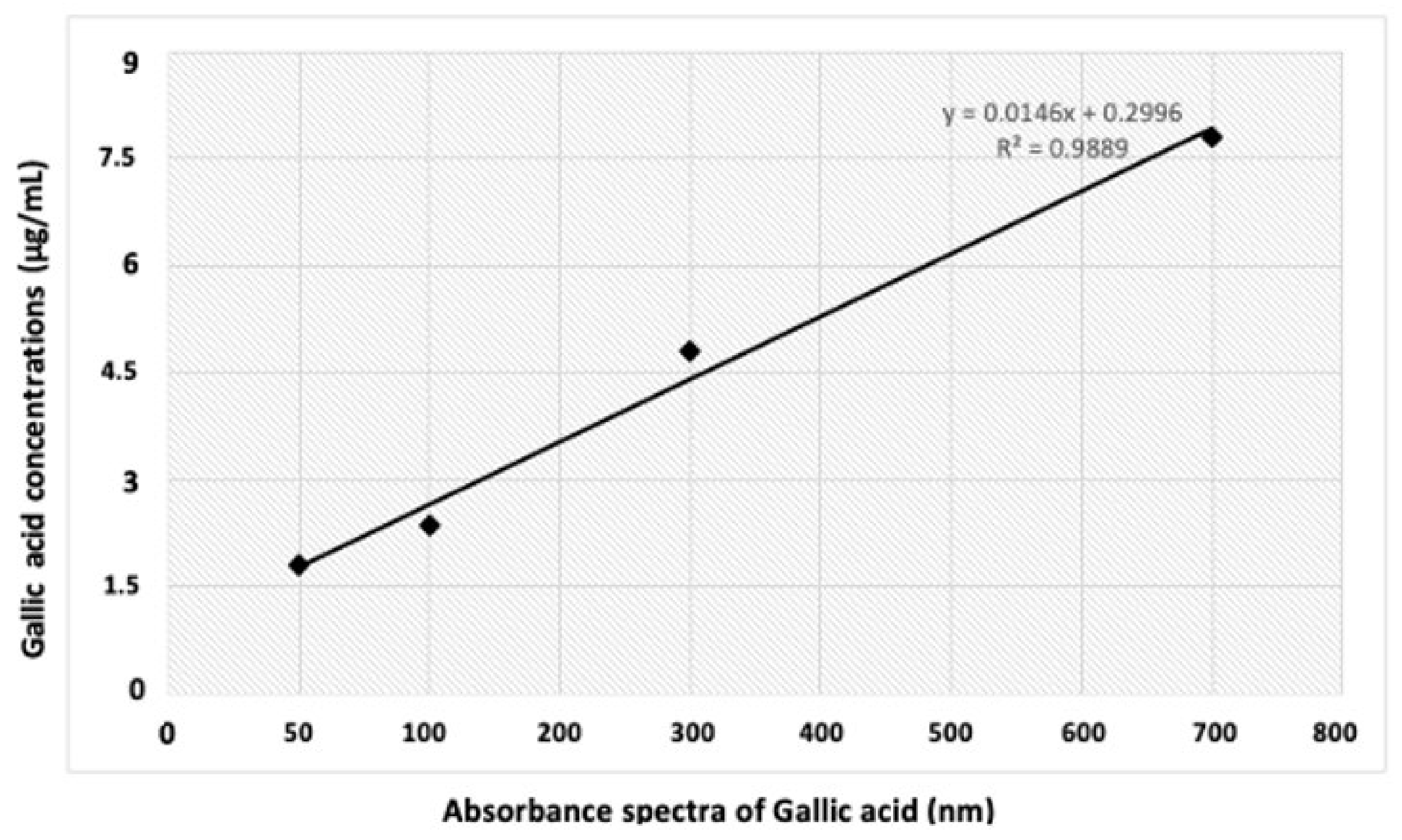
3.5.1. Extract Preparation
3.5.2. Total Phenolics Content (TPC)
3.5.3. Total Flavonoids Content (TFC)
3.5.4. DPPH Radical Scavenging Activity (DRSA)
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ahmad, N.; Rab, A.; Sajid, M.; Ahmad, N.; Fazal, H.; Ali, M.; Egertsdotter, U. Sucrose-dependent production of biomass and low-caloric Steviol glycosides in adventitious root cultures of Stevia rebaudiana (Bert.). Ind. Crops Prod. 2021, 164, 113382. [Google Scholar] [CrossRef]
- Ahmad, A.; Ali, H.; Khan, H.; Begam, A.; Khan, S.; Ali, S.S.; Ahmad, N.; Fazal, H.; Ali, M.; Hano, C.; et al. Effect of gibberellic acid on production of biomass, polyphenolics and Steviol glycosides in adventitious root cultures of Stevia rebaudiana (Bert.). Plants 2020, 9, 420. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Singh, S.; Dhyani, D.; Ahuja, P.S. A review on the improvement of stevia Stevia rebaudiana (Bertoni). Can. J. Plant Sci. 2011, 91, 1–27. [Google Scholar] [CrossRef]
- Carakostas, M.C.; Curry, L.L.; Boileau, A.C.; Brusick, D.J. Overview: The history, technical function and safety of rebaudioside A, a naturally occurring steviol glycoside, for use in food and beverages. Food Chem. Toxicol. 2008, 46, S1–S10. [Google Scholar] [CrossRef]
- Singh, S.D.; Rao, G.P. Stevia: The herbal sugar of 21st century. Sugar Technol. 2005, 7, 17–24. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Gregersen, S.; Roefsen, S.E. Anti-hyperglycaemic and blood pressure-reducing effects of stevioside in diabetic Gots-kakizaki rat. Metabolism 2003, 52, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Geuns, J.M.C. Molecules of interest, Stevioside. Phytochemistry 2003, 64, 913–921. [Google Scholar] [CrossRef]
- Hwang, S.J. Rapid in vitro propagation and enhanced stevioside accumulation in Stevia rebaudiana Bert. J. Plant Biol. 2006, 49, 267–270. [Google Scholar] [CrossRef]
- Khalil, S.A.; Zamir, R.; Ahmad, N. Selection of suitable propagation method for consistent plantlets production in Stevia rebaudiana (Bertoni). Saudi J. Biol. Sci. 2014, 21, 566–573. [Google Scholar] [CrossRef]
- Soejarto, D.D.; Compadre, C.M.; Medon, P.J.; Kamath, S.K.; Kinghorn, A.D. Potential sweetening agents of plant origin. II. Field search for sweet-tasting Stevia species. Econom. Bot. 1983, 37, 71–79. [Google Scholar] [CrossRef]
- Sairkar, P.; Shukla, N.P.; Mehrotra, N.N. Mass production of an economically important medicinal plant Stevia rebaudiana using in vitro propagation techniques. J. Med. Plants Res. 2009, 3, 266–270. [Google Scholar]
- Gupta, E.; Purwar, S.; Sundaram, S.; Rai, G.K. Nutritional and therapeutic values of Stevia rebaudiana: A review. J. Med. Plants Res. 2013, 7, 3343–3353. [Google Scholar]
- Ahmed, M.B.; Salahin, M.; Karim, R.; Razvy, M.A.; Hannan, M.M.; Sultana, R.; Hossain, M.; Islam, R. An efficient method for in vitro clonal propagation of a newly introduced sweetener plant (Stevia rebaudiana Bertoni.) in Bangladesh. Am.-Eurasian J. Sci. Res. 2007, 2, 121–125. [Google Scholar]
- Chan, P.; Tomlinson, B.; Chen, Y.J.; Liu, J.C.; Hsieh, M.H.; Cheng, J.T. A double-blind placebo-controlled study of the effectiveness and tolerability of oral stevioside in human hypertension. Br. J. Clin. Pharmacol. 2000, 50, 215–220. [Google Scholar] [CrossRef]
- Sreedhar, R.V.; Venkatachalam, L.; Thimmaraju, R.; Bhagyalakshmi, N.; Narayan, M.S.; Ravishankar, G.A. Direct organogenesis from leaf explants of Stevia rebaudiana and cultivation in bioreactor. Biol. Plant. 2008, 52, 355–360. [Google Scholar] [CrossRef]
- Kazmi, A.; Khan, M.A.; Mohammad, S.; Ali, A.; Kamil, A.; Arif, M.; Ali, H. Elicitation directed growth and production of Steviol glycosides in the adventitious roots of Stevia rebaudiana Bertoni. Ind. Crops Prod. 2019, 139, 111530. [Google Scholar] [CrossRef]
- Savita, S.M.; Sheela, K.; Sunanda, S.; Shankar, A.G.; Ramakrishna, P. Stevia rebaudiana—A functional component for food industry. J. Hum. Ecol. 2004, 15, 261–264. [Google Scholar] [CrossRef]
- Bondarev, N.; Reshetnyak, O.; Nosov, A. Effects of nutrient medium composition on development of Stevia rebaudiana shoots cultivated in the roller bioreactor and their production of Steviol glycosides. Plant Sci. 2003, 165, 845–850. [Google Scholar] [CrossRef]
- Dey, A.; Kundu, S.; Bandyopadhyay, A.; Bhattacharjee, A. Efficient micropropagation and chlorocholine chloride induced stevioside production of Stevia rebaudiana Bertoni. C. R. Biol. 2013, 336, 17–28. [Google Scholar] [CrossRef]
- Fazal, H.I.N.A.; Ahmad, N.; Ullah, I.; Inayat, H.; Khan, L.; Abbasi, B.H. Antibacterial potential in Parthenium hysterophorus, Stevia rebaudiana and Ginkgo biloba. Pak. J. Bot. 2011, 43, 1307–1313. [Google Scholar]
- Ahmad, N.; Fazal, H.; Abbasi, B.H.; Iqbal, M. In vitro larvicidal potential against Anopheles stephensi and antioxidative enzyme activities of Ginkgo biloba, Stevia rebaudiana and Parthenium hysterophorous. Asian Pac. J. Trop. Med. 2011, 4, 169–175. [Google Scholar] [CrossRef]
- Liu, J.C.; Kao, P.K.; Chan, P.; Hsu, Y.H.; Hou, C.C.; Lien, G.S.; Hsieh, M.H.; Chen, Y.J.; Cheng, J.T. Mechanism of the antihypertensive effect of stevioside in anesthetized dogs. Pharmacology 2003, 67, 14–20. [Google Scholar] [CrossRef]
- Tomita, T.; Sato, N.; Arai, T.; Shiraishi, H.; Sato, M.; Takeuchi, M.; Kamio, Y. Bactericidal activity of a fermented hot-water extract from Stevia rebaudiana Bertoni towards enterohemorrhagic Escherichia coli O157: H7 and other food-borne pathogenic bacteria. Microbiol. Immunol. 1997, 41, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-Y. The use of thidiazuron in tissue culture. In Vitro Cell. Dev. Biol. Plant. 1993, 29, 92–96. [Google Scholar] [CrossRef]
- Gregersen, S.; Jeppesen, P.B.; Holst, J.J.; Hermansen, K. Antihyperglycemic effects of stevioside in type 2 diabetic subjects. Metabolism 2004, 53, 73–76. [Google Scholar] [CrossRef]
- Dyrskog, S.E.U.; Jeppesen, P.B.; Colombo, M.; Abudula, R.; Hermansen, K. Preventive effects of a soy-based diet supplemented with stevioside on the development of the metabolic syndrome and type 2 diabetes in Zucker diabetic fatty rats. Metabolism 2004, 54, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Shivanna, N.; Naika, M.; Khanum, F.; Kaul, V.K. Antioxidant, anti-diabetic and renal protective properties of Stevia rebaudiana. J. Diab. Complic. 2013, 27, 103–113. [Google Scholar] [CrossRef]
- Saleh, O.M.; Awad, N.S.; Soliman, M.M.; Mansour, A.A.; Nassan, M.A. Insulin-mimetic activity of stevioside on diabetic rats: Biochemical, molecular and histopathological study. Afr. J. Trad. Compl. Alt. Med. 2016, 13, 156–163. [Google Scholar] [CrossRef][Green Version]
- Arpita, D.; Saikat, G.; Nirmal, M. Micropropagation of an elite medicinal plant: Stevia rebaudiana Bert. Inter. J. Agric. Res. 2011, 6, 40–48. [Google Scholar]
- Aman, H.; Hadi, F.; Khalil, S.A.; Zamir, R.; Ahmad, N. Efficient regeneration for enhanced steviol glycosides production in Stevia rebaudiana (Bertoni). C. R. Biol. 2013, 336, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, B.H.; Stiles, A.R.; Saxena, P.K.; Liu, C.-Z. Gibberellic acid increases secondary metabolite production in Echinacea purpurea hairy roots. Appl. Biochem. Biotechnol. 2012, 168, 2057–2066. [Google Scholar] [CrossRef]
- Ahmad, N.; Abbasi, B.H.; Fazal, H.; Rahman, U.R. Piper nigrum L.; micropropagation, antioxidative enzyme activities and chromatographic fingerprint analysis for quality control. Appl. Biochem. Biotechnol. 2013, 169, 2004–2015. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, B.H.; Saxena, P.K.; Murch, S.J.; Liu, C.Z. Echinacea biotechnology: Challenges and opportunities. In Vitro Cell. Dev. Biol. Plant. 2007, 43, 481–492. [Google Scholar] [CrossRef]
- Fazal, H.; Abbasi, B.H.; Ahmad, N. Optimization of adventitious root culture for production of biomass and secondary metabolites in Prunella vulgaris L. Appl. Biochem. Biotechnol. 2014, 174, 2086–2095. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.C. Shuler ML Large scale plant cell culture. Curr. Opin. Biotechnol. 1997, 8, 154–159. [Google Scholar] [CrossRef]
- Darvill, A.G.; Albersheim, P. Phytoalexins and their elicitors-a defense against microbial infection in plants. Ann. Rev. Plant Physiol. 1984, 35, 243–275. [Google Scholar] [CrossRef]
- Ahmad, N.; Abbasi, B.H.; Fazal, H.; Khan, M.A.; Afridi, M.S. Effect of reverse photoperiod on in vitro regeneration and piperine production in Piper nigrum L. C. R. Biol. 2014, 337, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Saini, P.; Mithal, R.; Menghani, E. A parasitic medicinal plant Cuscuta reflexa: An overview. Int. J. Sci. Eng. Res. 2015, 6, 951–959. [Google Scholar]
- Siwakoti, M.; Siwakoti, S. Ethnobotanical studies of Satars of Jhapa District, Nepal: A Case Study of Haldibari VDC; A report submitted to University Grants Commission, Kathmandu, Nepal; University Grants Commission: Kathmandu, Nepal, 1996. [Google Scholar]
- Pandit, S.; Chauhan, N.S.; Dixit, V.K. Effect of Cuscuta reflexa on androgen induced alopecia. J. Cosmet. Dermatol. 2008, 7, 199–204. [Google Scholar] [CrossRef]
- Jha, U.; Shelke, T. Hepatoprotective activity of hydroalcoholic extracts of Cuscuta reflexa roxb in paracetamol intoxicated albino rats. Int. J. Res. Ayurveda Pharm. 2011, 2, 1290–1293. [Google Scholar]
- Hajihashemi, S.; Ehsanpour, A.A. Antioxidant response of Stevia rebaudiana B. to polyethylene glycol and paclobutrazol treatments under in vitro culture. Appl. Biochem. Biotechnol. 2014, 172, 4038–4052. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Sharma, S.; Saxena, S. Biomass yield and Steviol glycoside production in callus and suspension culture of Stevia rebaudiana treated with proline and polyethylene glycol. Appl. Biochem. Biotechnol. 2015, 176, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Sharma, S.; Saxena, S. Effect of abiotic stress on growth parameters and steviol glycoside content in Stevia rebaudiana (Bertoni) raised in vitro. J. Appl. Res. Med. Arom. Plants 2016, 3, 160–167. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Geuns, J.M.C. Gene transcription and steviol glycoside accumulation in Stevia rebaudiana under polyethylene glycol-induced drought stress in greenhouse cultivation. FEBS Open Bio. 2016, 6, 937–944. [Google Scholar] [CrossRef]
- Ali, M.; Abbasi, B.H. Production of commercially important secondary metabolites and antioxidant activity in cell suspension cultures of Artemisia absinthium L. Ind. Crops Prod. 2013, 49, 400–406. [Google Scholar] [CrossRef]
- Kolewe, M.E.; Gaurav, V.; Roberts, S.C. Pharmaceutically active natural product synthesis and supply via plant cell culture technology. Mol. Pharm. 2008, 5, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Sivanandhan, G.M.; Arun, M.; Mayavan, S.; Rajesh, M.; Mariashibu, T.S.; Manickavasagam, M.; Selvaraj, N.; Ganapathi, A. Chitosan enhances withanolides production in adventitious root cultures of Withania somnifera (L.) Dunal. Ind. Crops Prod. 2012, 37, 124–129. [Google Scholar] [CrossRef]
- Vanisree, M.; Chen-Yue, L.; Shu-Fung, L.; Nalawade, S.M.; Lin, C.Y.; Hsin-Sheng, T. Studies on the production of some important secondary metabolites from medicinal plants by plant tissue cultures. Bot. Bull. Acad. Sin. 2004, 45, 1–22. [Google Scholar]
- Yue, W.; Qian-liang, M.; Bing, L.; Khalid, R.; Cheng-Jian, Z.; Ting, H.; Lu-ping, Q. Medicinal plant cell suspension cultures: Pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit. Rev. Biotechnol. 2016, 36, 215–232. [Google Scholar] [CrossRef]
- Fallah, F.; Nokhasi, F.; Ghaheri, M.; Kahrizi, D.; Beheshti, A.; Agha, A.; Ghorbani, T.; Kazemi, E.; Ansarypour, Z. Effect of salinity on gene expression, morphological and biochemical characteristics of Stevia rebaudiana Bertoni under in vitro conditions. Cell Mol. Biol. 2017, 63, 102–106. [Google Scholar] [CrossRef]
- Zeng, J.; Chen, A.; Li, D.; Yi, B.; Wu, W. Effects of salt stress on the growth, physiological responses, and glycoside contents of Stevia rebaudiana Bertoni. J. Agric. Food Chem. 2013, 61, 5720–5726. [Google Scholar] [CrossRef]
- Ahmad, N.; Rab, A.; Ahmad, N.; Fazal, H. Differential pH-induced biosynthesis of steviol glycosides and biochemical parameters in submerge root cultures of Stevia rebaudiana (Bert.). Sugar Tech. 2018, 20, 734–744. [Google Scholar] [CrossRef]
- Ghazal, B.; Saif, S.; Farid, K.; Khan, A.; Rehman, S.; Reshma, A.; Fazal, H.; Ali, M.; Ahmad, A.; Rahman, L.; et al. Stimulation of secondary metabolites by copper and gold nanoparticles in submerge adventitious root cultures of Stevia rebaudiana (Bert.). IET Nanobiotechnol. 2018, 12, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Jehnsen, J. Effects on Artemisia annua growth with the addition of gibberellic acid, salicylic acid, and methyl jasmonate in hydroponic systems. UC. Berkeley Spring 2010, 1–23. [Google Scholar] [CrossRef]
- Pazuki, A.; Fatemeh, A.; Buhara, Y.; Songül, G. Effects of cytokinins, gibberellic acid 3, and gibberellic acid 4/7 on in vitro growth, morphological traits, and content of Steviol glycosides in Stevia rebaudiana. Plant Physiol. Biochem. 2019, 137, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Hiroshi, K.; Hiroshi, S.; Hiroshi, H. Effects of gibberellic acid on hairy root growth in Datura innoxia. J. Plant Physiol. 1989, 134, 633–636. [Google Scholar] [CrossRef]
- Subroto, M.A.; Pauline, M.D. Production of steroidal alkaloids by hairy roots of Solanum aviculare and the effect of gibberellic acid. Plant Cell Tiss. Org. Cult. 1994, 38, 93–102. [Google Scholar] [CrossRef]
- Ahmad, N.; Rab, A.; Ahmad, N. Light-induced biochemical variations in secondary metabolite production and antioxidant activity in callus cultures of Stevia rebaudiana (Bert). J. Photochem. Photobiol. B Biol. 2016, 154, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Idrees, M.; Sania, B.; Hafsa, B.; Kumari, S.; Khan, H.; Fazal, H.; Ahmad, I.; Akbar, F.; Ahmad, N.; Ali, S.; et al. Spectral lights trigger biomass accumulation and production of antioxidant secondary metabolites in adventitious root cultures of Stevia rebaudiana (Bert.). C. R. Biol. 2018, 341, 334–342. [Google Scholar] [CrossRef]
- Bertoldi, D.; Annalisa, T.; Lucia, M.; Nello, B. Polyamines and somatic embryogenesis in two Vitis vinifera cultivars. Physiol. Plant. 2004, 120, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Kevers, C.; Thomas, G.; Jacques, D. The beneficial role of different auxins and polyamines at successive stages of somatic embryo formation and development of Panax ginseng in vitro. Plant Cell Tiss. Org. Cult. 2002, 70, 181–188. [Google Scholar] [CrossRef]
- Bais, H.P.; Ravishankar, G.A. Synergistic effect of auxins and polyamines in hairy roots of Cichorium intybus L. during growth, coumarin production and morphogenesis. Acta Physiol. Plant. 2003, 25, 193–208. [Google Scholar] [CrossRef]
- Suresh, B.; Rudrappa, T.; Neelwarne, B.; Ravishankar, G.A. Polyamine and methyl jasmonate-influenced enhancement of betalaine production in hairy root cultures of Beta vulgaris grown in a bubble column reactor and studies on efflux of pigments. Process Biochem. 2004, 39, 2091–2096. [Google Scholar] [CrossRef]
- Murthy, H.N.; Eun-Jung, L.; Kee-Yoeup, P. Production of secondary metabolites from cell and organ cultures: Strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tiss. Org. Cult. 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Xu, S.; Wenwu, Z.; Sarah, P.; Ian, T.B. Herbivore associated elicitor-induced defences are highly specific among closely related Nicotiana species. BMC Plant Biol. 2015, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Muhammad, A.U.; Laurine, G.; Hano, C.; Abbasi, B.H. Synergistic effects of melatonin and distinct spectral lights for enhanced production of anti-cancerous compounds in callus cultures of Fagonia indica. J. Photochem. Photobiol. B Biol. 2019, 190, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Abbasi, B.H. Light-induced fluctuations in biomass accumulation, secondary metabolites production and antioxidant activity in cell suspension cultures of Artemisia absinthium L. J. Photochem. Photobiol. B Biol. 2014, 140, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Hasbullah, N.A.; Rosna, M.; Taha, A.S.; Noraini, M. Irradiation effect on in vitro organogenesis, callus growth and plantlet development of Gerbera jamesonii. Horticult. Bras. 2012, 30, 252–257. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [PubMed]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Younas, M.; Drouet, S.; Nadeem, M.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Differential accumulation of silymarin induced by exposure of Silybum marianum L. callus cultures to several spectres of monochromatic lights. J. Photochem. Photobiol. B Biol. 2018, 184, 61–70. [Google Scholar] [CrossRef]
- Roy, A.; Bharadvaja, N. Establishment of root suspension culture of Plumbago zeylanica and enhanced production of plumbagin. Ind. Crops Prod. 2019, 137, 419–427. [Google Scholar] [CrossRef]
- Fan, M.Z.; An, X.L.; Cui, X.H.; Jiang, X.L.; Piao, X.C.; Jin, M.Y.; Lian, M.L. Production of eurycomanone and polysaccharides through adventitious root culture of Eurycoma longifolia in a bioreactor. Biochem. Eng. J. 2021, 171, 108013. [Google Scholar] [CrossRef]
- Krishnan, M.L.; Roy, A.; Bharadvaja, N. Elicitation effect on the production of asiaticoside and asiatic acid in shoot, callus, and cell suspension culture of Centella asiatica. J. Appl. Pharm. Sci. 2019, 9, 67–74. [Google Scholar]
- Kundu, K.; Roy, A.; Saxena, G.; Kumar, L.; Bharadvaja, N. Effect of different carbon sources and elicitors on shoot multiplication in accessions of Centella asiatica. Med. Aromat. Plants 2016, 5, 2167-0412. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Ahmad, N.; Abbasi, B.H.; Fazal, H. Evaluation of antioxidant activity and its association with plant development in Silybum marianum L. Ind. Crop. Prod. 2013, 49, 164–168. [Google Scholar] [CrossRef]
- Smith, T.C.; Weathers, P.J.; Cheetham, R.D. Effects of Gibberellic acid on hairy root cultures of Artemisia annua: Growth and artemisinin production. In Vitro Cell. Dev. Biol. Plant. 1997, 33, 75–79. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, L.; Cui, G.; Mao, Y.; He, X. Effect of gibberellins and its synthetic inhibitor on metabolism of tanshinones. Chin. J. Exp. Trad. Med. Formulae 2008, 6, 2. [Google Scholar]
- Banyai, W.; Mii, M.; Supaibulwatana, K. Enhancement of artemisinin content and biomass in Artemisia annua by exogenous GA3 treatment. Plant Growth Regul. 2011, 63, 45–54. [Google Scholar] [CrossRef]
- Liang, Z.; Ma, Y.; Xu, T.; Cui, B.; Liu, Y.; Guo, Z.; Yang, D. Effects of abscisic acid, gibberellin, ethylene and their interactions on production of phenolic acids in Salvia miltiorrhiza bunge hairy roots. PLoS ONE 2013, 8, e72806. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, M.J.C.; Parr, A.J.; Giulietti, A.; Aird, E.L.H. Influence of exogenous hormones on the growth and secondary metabolite formation in transformed root cultures. In Primary and Secondary Metabolism of Plants and Cell Cultures III; Springer: Dordrecht, The Netherlands, 1994; pp. 143–151. [Google Scholar]
- Gavidia, I.; Segura, J.; Perez-Bermudez, P. Effects of gibberellic acid on morphogenesis and cardenolide accumulation in juvenile and adult Digitalis obscura cultures. J. Plant Physiol. 1993, 142, 373–376. [Google Scholar] [CrossRef]
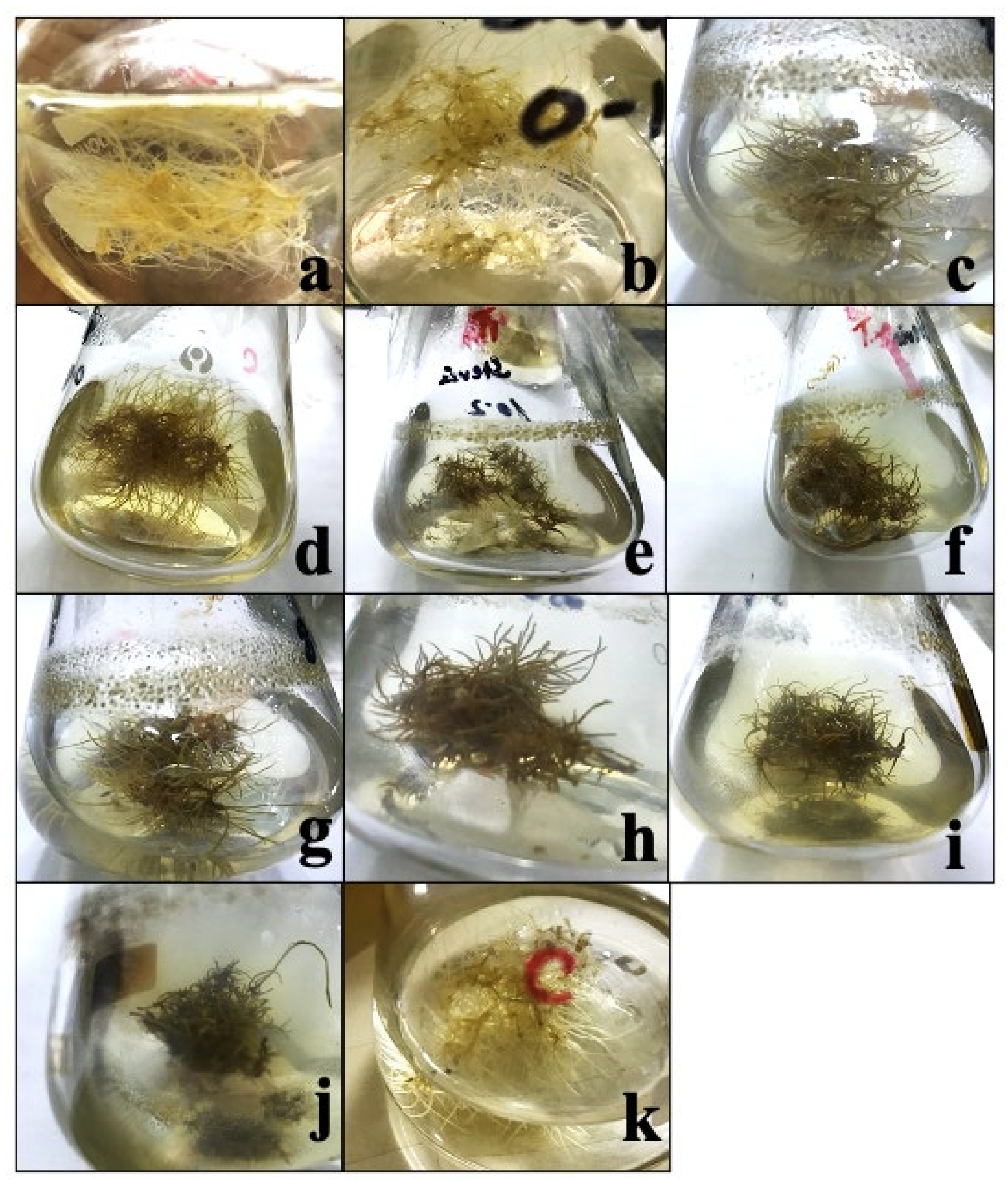
| Culture Period | 7 Days | 14 Days | 21 Days | 28 Days | 35 Days | 42 Days | 49 Days |
|---|---|---|---|---|---|---|---|
| ADR Biomass (g/3 Flasks) | ADR Biomass (g/3 Flasks) | ADR Biomass (g/3 Flasks) | ADR Biomass (g/3 Flasks) | ADR Biomass (g/3 Flasks) | ADR Biomass (g/3 Flasks) | ADR Biomass (g/3 Flasks) | |
| Cr (10 mg/3 flasks) | 2.97 ± 0.01 c | 4.05 ± 0.05 b | 5.13 ± 0.03 ab | 5.67 ± 0.01 ab | 6.2 ± 0.1 a | 7.29 ± 0.09 a | 7.83 ± 0.08 a |
| Cr (20 mg/3 flasks) | 2.43 ± 0.01 b | 3.5 ± 0.03 b | 4.05 ± 0.02 ab | 5.13 ± 0.03 ab | 5.49 ± 0.08 ab | 6.12 ± 0.05 a | 7.29 ± 0.08 a |
| Cr (30 mg/3 flasks) | 1.35 ± 0.02 c | 2.16 ± 0.04 b | 3.24 ± 0.03 b | 4.32 ± 0.02 ab | 4.86 ± 0.03 ab | 5.13 ± 0.02 a | 5.94 ± 0.02 a |
| Cr (40 mg/3 flasks) | 1.08 ± 0.01 c | 1.89 ± 0.01 c | 2.41 ± 0.01 b | 3.78 ± 0.03 b | 4.59 ± 0.02 ab | 4.86 ± 0.05 a | 5.67 ± 0.04 a |
| Cr (50 mg/3 flasks) | 0.99 ± 0.01 c | 1.52 ± 0.01 b | 2.32 ± 0.01 b | 2.7 ± 0.02 b | 4.56 ± 0.02 a | 4.6 ± 0.04 a | 5.2 ± 0.03 a |
| Cr (60 mg/3 flasks) | 0.81 ± 0.01 c | 1.35 ± 0.01 c | 2.16 ± 0.01 bc | 2.43 ± 0.02 bc | 3.78 ± 0.03 b | 4.59 ± 0.06 a | 5.86 ± 0.04 a |
| Cr (70 mg/3 flasks) | 0.54 ± 0.01 c | 1.08 ± 0.01 b | 2.05 ± 0.01 b | 2.32 ± 0.01 b | 3.27 ± 0.0 ab | 4.6 ± 0.03 a | 4.8 ± 0.02 a |
| Cr (80 mg/3 flasks) | 0.27 ± 0 c | 1.08 ± 0.01 bc | 1.86 ± 0 bc | 2.2 ± 0.01 b | 2.9 ± 0.02 b | 3.97 ± 0.03 b | 4.5 ± 0.03 a |
| Cr (90 mg/3 flasks) | 0.27 ± 0 c | 0.54 ± 0 c | 1.73 ± 0 bc | 2.1 ± 0.01 b | 2.5 ± 0.02 b | 3.5 ± 0.01 ab | 4.1 ± 0.02 a |
| Cr (100 mg/3 flasks) | 0.27 ± 0 c | 0.54 ± 0 bc | 1.4 ± 0 b | 1.86 ± 0.01 b | 2.3 ± 0.01 ab | 3.3 ± 0.03 a | 3.74 ± 0.03 a |
| Control | 0.34 ± 0 e | 1.17 ± 0 d | 3.27 ± 0 cd | 5.33 ± 0.01 c | 6.94 ± 0.01 b | 7.77 ± 0.02 ab | 8.16 ± 0.01 a |
| Plant Extracts | Fresh Weight (g/3 Flask) | Dry Weight (g/3 Flask) |
|---|---|---|
| C. reflexa (10 mg/3 flasks) | 9.14 ± 1.2 a | 0.84 ± 0.1 a |
| C. reflexa (20 mg/3 flasks) | 8.9 ± 0.7 ab | 0.74 ± 0.02 ab |
| C. reflexa (30 mg/3 flasks) | 8.84 ± 0.3 ab | 0.82 ± 0.02 a |
| C. reflexa (40 mg/3 flasks) | 8.66 ± 0.1 ab | 0.64 ± 0.03 ab |
| C. reflexa (50 mg/3 flasks) | 8.14 ± 0.3 ab | 0.72 ± 0.01 ab |
| C. reflexa (60 mg/3 flasks) | 8.04 ± 0.7 ab | 0.62 ± 0.04 ab |
| C. reflexa (70 mg/3 flasks) | 7.4 ± 0.2 b | 0.6 ± 0.03 ab |
| C. reflexa (80 mg/3 flasks) | 7.54 ± 0.2 b | 0.68 ± 0.02 ab |
| C. reflexa (90 mg/3 flasks) | 7.26 ± 0.3 b | 0.66 ± 0.01 ab |
| C. reflexa (100 mg/3 flasks) | 5.42 ± 0.2 c | 0.42 ± 0.01 c |
| Control | 10.23 ± 0.3 a | 0.877 ± 0.02 a |
| Plant Extract | TPC mg/g-GAE-DW | TPP g/3 Flask | TFC mg/g-QE-DW | TFP g/3 Flask | TPPC g/3 Flask | TPPP g/3 Flask |
|---|---|---|---|---|---|---|
| Cr (10 mg/L) | 0.06 ± 0.01 d | 0.05 ± 0 c | 0.01 ± 0 c | 0.08 ± 0.01 c | 0.07 ± 0.01 c | 0.06 ± 0.01 c |
| Cr (20 mg/L) | 0.1 ± 0.01 c | 0.07 ± 0.01 c | 0.04 ± 0 c | 0.03 ± 0 c | 0.14 ± 0.01 c | 0.11 ± 0 b |
| Cr (30 mg/L) | 0.17 ± 0.02 b | 0.14 ± 0.01 ab | 0.07 ± 0.01 c | 0.06 ± 0.01 c | 0.24 ± 0.02 b | 0.19 ± 0.01 b |
| Cr (40 mg/L) | 0.19 ± 0.02 b | 0.12 ± 0.01 b | 0.092 ± 0.02 a | 0.058 ± 0.02 a | 1.11 ± 0.09 a | 0.07 ± 0.01 c |
| Cr (50 mg/L) | 0.19 ± 0.01 b | 0.14 ± 0.01 ab | 0.12 ± 0.01 b | 0.08 ± 0.01 c | 0.32 ± 0.02 b | 0.23 ± 0.01 ab |
| Cr (60 mg/L) | 0.21 ± 0.02 b | 0.13 ± 0.01 b | 0.16 ± 0.01 b | 0.09 ± 0.01 c | 0.37 ± 0.03 b | 0.23 ± 0.02 ab |
| Cr (70 mg/L) | 0.25 ± 0.02 ab | 0.15 ± 0.01 ab | 0.19 ± 0.02 b | 0.12 ± 0 b | 0.44 ± 0.02 b | 0.26 ± 0.01 ab |
| Cr (80 mg/L) | 0.28 ± 0.02 a | 0.19 ± 0.02 a | 0.21 ± 0.01 b | 0.14 ± 0 b | 0.48 ± 0.01 b | 0.33 ± 0.02 a |
| Cr (90 mg/L) | 0.29 ± 0.01 a | 0.19 ± 0.02 a | 0.21 ± 0.02 b | 0.14 ± 0.01 b | 0.49 ± 0.03 b | 0.33 ± 0.01 a |
| Cr (100 mg/L) | 0.31 ± 0.03 a | 0.15 ± 0.03 ab | 0.22 ± 0.02 b | 0.11 ± 0 b | 0.53 ± 0.02 b | 0.22 ± 0.01 ab |
| Control | 0.18 ± 0.01 b | 0.12 ± 0.02 b | 0.11 ± 0.01 b | 0.11 ± 0 b | 0.27 ± 0.01 b | 0.17 ± 0 b |
| Plant Extract | Antioxidant Activity (%) |
|---|---|
| C. reflexa (10 mg/3 flasks) | 54.2 ± 0.2 g |
| C. reflexa (20 mg/3 flasks) | 57.7 ± 0.7 f |
| C. reflexa (30 mg/3 flasks) | 61.6 ± 0.4 e |
| C. reflexa (40 mg/3 flasks) | 67.9 ± 1.0 d |
| C. reflexa (50 mg/3 flasks) | 72.1 ± 0.4 c |
| C. reflexa (60 mg/3 flasks) | 77.1 ± 1.3 b |
| C. reflexa (70 mg/3 flasks) | 79.8 ± 0.9 ab |
| C. reflexa (80 mg/3 flasks) | 81.3 ± 2.3 ab |
| C. reflexa (90 mg/3 flasks) | 84.7 ± 3.1 a |
| C. reflexa (100 mg/3 flasks) | 85.5 ± 3.3 a |
| Control w/o extract | 74.4 ± 2.1 bc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, N.; Khan, P.; Khan, A.; Usman, M.; Ali, M.; Fazal, H.; Durrishahwar; Uddin, M.N.; Hano, C.; Abbasi, B.H. Elicitation of Submerged Adventitious Root Cultures of Stevia rebaudiana with Cuscuta reflexa for Production of Biomass and Secondary Metabolites. Molecules 2022, 27, 14. https://doi.org/10.3390/molecules27010014
Ahmad N, Khan P, Khan A, Usman M, Ali M, Fazal H, Durrishahwar, Uddin MN, Hano C, Abbasi BH. Elicitation of Submerged Adventitious Root Cultures of Stevia rebaudiana with Cuscuta reflexa for Production of Biomass and Secondary Metabolites. Molecules. 2022; 27(1):14. https://doi.org/10.3390/molecules27010014
Chicago/Turabian StyleAhmad, Nisar, Palwasha Khan, Abdullah Khan, Maliha Usman, Mohammad Ali, Hina Fazal, Durrishahwar, Muhammad Nazir Uddin, Christophe Hano, and Bilal Haider Abbasi. 2022. "Elicitation of Submerged Adventitious Root Cultures of Stevia rebaudiana with Cuscuta reflexa for Production of Biomass and Secondary Metabolites" Molecules 27, no. 1: 14. https://doi.org/10.3390/molecules27010014
APA StyleAhmad, N., Khan, P., Khan, A., Usman, M., Ali, M., Fazal, H., Durrishahwar, Uddin, M. N., Hano, C., & Abbasi, B. H. (2022). Elicitation of Submerged Adventitious Root Cultures of Stevia rebaudiana with Cuscuta reflexa for Production of Biomass and Secondary Metabolites. Molecules, 27(1), 14. https://doi.org/10.3390/molecules27010014








