Green Bioanalytical Applications of Graphene Oxide for the Extraction of Small Organic Molecules
Abstract
1. Introduction
2. Preparation of GO and GO Derived Materials
3. Bioanalytical Applications of Graphene Oxide for the Extraction of Small Organic Molecules
3.1. Solid-Phase Microextraction
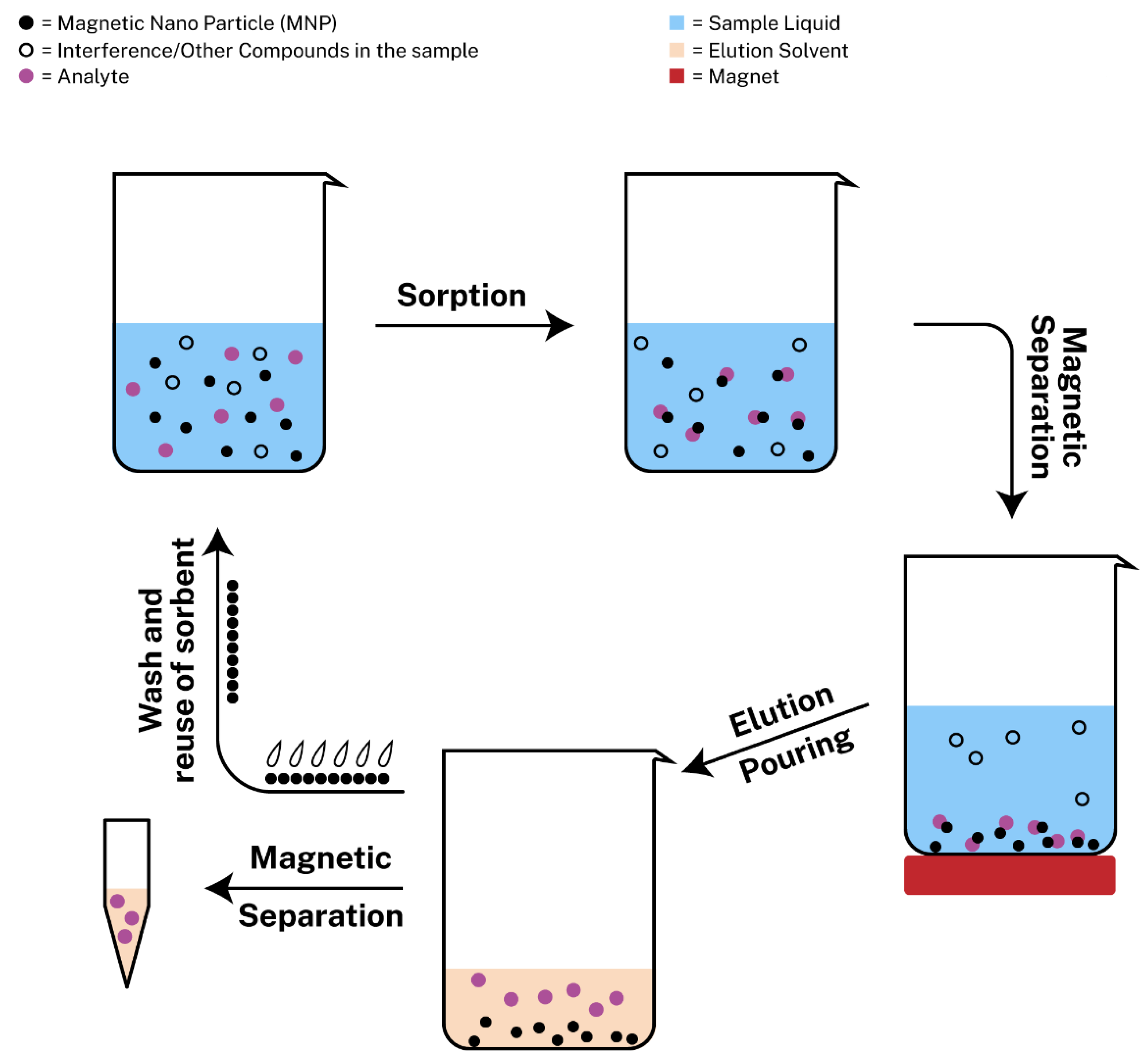
3.2. Magnetic Solid-Phase Extraction
3.3. Stir Bar Sorptive Extraction
3.4. Pipette Tip Solid-Phase Extraction
3.5. Other Extraction Techniques
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Li, P.; Bartlett, M.G. A review of sample preparation methods for quantitation of small-molecule analytes in brain tissue by liquid chromatography tandem mass spectrometry (LC-MS/MS). Anal. Methods 2014, 6, 6183–6207. [Google Scholar] [CrossRef]
- Cajka, T.; Fiehn, O. Toward Merging Untargeted and Targeted Methods in Mass Spectrometry-Based Metabolomics and Lipidomics. Anal. Chem. 2016, 88, 524–545. [Google Scholar] [CrossRef]
- James, C.A.; Breda, M.; Baratt, S.; Casati, M.; Grassi, S.; Pellegatta, B.; Sarati, S.; Frigerio, E. Analysis of Drugs and Metabolites in Tissues and Other Solid Matrices. Chromatographia 2004, 59, S149–S156. [Google Scholar] [CrossRef]
- Samanidou, V.F.; Metaxa, A.S.; Papadoyannis, I.N. Direct simultaneous determination of uremic toxins: Creatine, creatinine, uric acid, and xanthine in human biofluids by HPLC. J. Liq. Chromatogr. Relat. Technol. 2002, 25, 43–57. [Google Scholar] [CrossRef]
- Samanidou, V.F.; Nikolaidou, K.I.; Papadoyannis, I.N. Development and validation of an HPLC confirmatory method for the determination of tetracycline antibiotics residues in bovine muscle according to the European Union regulation 2002/657/EC. J. Sep. Sci. 2005, 28, 2247–2258. [Google Scholar] [CrossRef]
- Manousi, N.; Samanidou, V. Green sample preparation of alternative biosamples in forensic toxicology. Sustain. Chem. Pharm. 2021, 20, 100388. [Google Scholar] [CrossRef]
- Kataoka, H. New trends in sample preparation for clinical and pharmaceutical analysis. TrAC Trends Anal. Chem. 2003, 22, 232–244. [Google Scholar] [CrossRef]
- Manousi, N.; Raber, G.; Papadoyannis, I. Recent Advances in Microextraction Techniques of Antipsychotics in Biological Fluids Prior to Liquid Chromatography Analysis. Separations 2017, 4, 18. [Google Scholar] [CrossRef]
- Patteet, L.; Cappelle, D.; Maudens, K.E.; Crunelle, C.L.; Sabbe, B.; Neels, H. Advances in detection of antipsychotics in biological matrices. Clin. Chim. Acta 2015, 441, 11–22. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Samanidou, V.F. Recent Trends in the Development of Green Microextraction Techniques for the Determination of Hazardous Organic Compounds in Wine. Curr. Anal. Chem. 2019, 15, 788–800. [Google Scholar] [CrossRef]
- Armenta, S.; Garrigues, S.; de la Guardia, M. The role of green extraction techniques in Green Analytical Chemistry. TrAC Trends Anal. Chem. 2015, 71, 2–8. [Google Scholar] [CrossRef]
- Pasandideh, Y.; Razmi, H. Introduction of a biowaste/graphene oxide nanocomposite as a coating for a metal alloy based SPME fiber: Application to screening of polycyclic aromatic hydrocarbons. Arab. J. Chem. 2020, 13, 8499–8512. [Google Scholar] [CrossRef]
- Liu, H.; Dasgupta, P.K. Analytical chemistry in a drop. solvent extraction in a microdrop. Anal. Chem. 1996, 68, 1817–1821. [Google Scholar] [CrossRef]
- Ścigalski, P.; Kosobucki, P. Recent Materials Developed for Dispersive Solid Phase Extraction. Molecules 2020, 25, 4869. [Google Scholar] [CrossRef]
- Karageorgou, E.; Armeni, M.; Moschou, I.; Samanidou, V.F. Ultrasound-assisted dispersive extraction for the high pressure liquid chromatographic determination of tetracyclines residues in milk with diode array detection. Food Chem. 2014, 150, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Karageorgou, E.G.; Samanidou, V.F.; Papadoyannis, I.N. Ultrasound-assisted matrix solid phase dispersive extraction for the simultaneous analysis of β-lactams (four penicillins and eight cephalosporins) in milk by high performance liquid chromatography with photodiode array detection. J. Sep. Sci. 2012, 35, 2599–2607. [Google Scholar] [CrossRef]
- Filippou, O.; Deliyanni, E.A.; Samanidou, V.F. Fabrication and evaluation of magnetic activated carbon as adsorbent for ultrasonic assisted magnetic solid phase dispersive extraction of bisphenol A from milk prior to high performance liquid chromatographic analysis with ultraviolet detection. J. Chromatogr. A 2017, 1479, 20–31. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, R.; Chen, Z. Stir bar sorptive extraction with a graphene oxide framework-functionalized stainless-steel wire for the determination of Sudan dyes in water samples. Anal. Methods 2019, 11, 2050–2056. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.-G.; Chen, W.-S.; Cheng, H.-L.; Zeng, X.-Q.; Zhu, Y. Three-dimensional ionic liquid-ferrite functionalized graphene oxide nanocomposite for pipette-tip solid phase extraction of 16 polycyclic aromatic hydrocarbons in human blood sample. J. Chromatogr. A 2018, 1552, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zilfidou, E.; Kabir, A.; Furton, K.G.; Samanidou, V. An improved fabric phase sorptive extraction method for the determination of five selected antidepressant drug residues in human blood serum prior to high performance liquid chromatography with diode array detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1125, 121720. [Google Scholar] [CrossRef]
- Samanidou, V.; Kehagia, M.; Kabir, A.; Furton, K.G. Matrix molecularly imprinted mesoporous sol–gel sorbent for efficient solid-phase extraction of chloramphenicol from milk. Anal. Chim. Acta 2016, 914, 62–74. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Jiang, G. Application of graphene in analytical sample preparation. TrAC Trends Anal. Chem. 2012, 37, 1–11. [Google Scholar] [CrossRef]
- Zhang, B.-T.; Zheng, X.; Li, H.-F.; Lin, J.-M. Application of carbon-based nanomaterials in sample preparation: A review. Anal. Chim. Acta 2013, 784, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Scida, K.; Stege, P.W.; Haby, G.; Messina, G.A.; García, C.D. Recent applications of carbon-based nanomaterials in analytical chemistry: Critical review. Anal. Chim. Acta 2011, 691, 6–17. [Google Scholar] [CrossRef]
- Chen, X.; Hai, X.; Wang, J. Graphene/graphene oxide and their derivatives in the separation/isolation and preconcentration of protein species: A review. Anal. Chim. Acta 2016, 922, 1–10. [Google Scholar] [CrossRef]
- Manousi, N.; Rosenberg, E.; Deliyanni, E.A.; Zachariadis, G.A. Sample Preparation Using Graphene-Oxide-Derived Nanomaterials for the Extraction of Metals. Molecules 2020, 25, 2411. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Jiang, H.-L.; Wang, X.; Wang, X.; Xu, G.; Zhang, B.; Wang, L.; Zhao, R.-S.; Lin, J.-M. Recent advances in graphene-based magnetic composites for magnetic solid-phase extraction. TrAC Trends Anal. Chem. 2018, 102, 60–74. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, G.; Liu, S.; Zhang, T.; Wu, J.; Wang, F.; Xu, J.; Liu, Y.; Zhu, F.; Ouyang, G. Bioinspired Polyelectrolyte-Assembled Graphene-Oxide-Coated C18 Composite Solid-Phase Microextraction Fibers for In Vivo Monitoring of Acidic Pharmaceuticals in Fish. Anal. Chem. 2016, 88, 5841–5848. [Google Scholar] [CrossRef]
- Thangamuthu, M.; Hsieh, K.Y.; Kumar, P.V.; Chen, G.-Y. Graphene- and Graphene Oxide-Based Nanocomposite Platforms for Electrochemical Biosensing Applications. Int. J. Mol. Sci. 2019, 20, 2975. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.; Kim, S.; Min, D.-H. Biosensors based on graphene oxide and its biomedical application. Adv. Drug Deliv. Rev. 2016, 105, 275–287. [Google Scholar] [CrossRef]
- Manousi, N.; Rosenberg, E.; Deliyanni, E.; Zachariadis, G.A.; Samanidou, V. Magnetic Solid-Phase Extraction of Organic Compounds Based on Graphene Oxide Nanocomposites. Molecules 2020, 25, 1148. [Google Scholar] [CrossRef]
- Ibrahim, W.A.W.; Nodeh, H.R.; Sanagi, M.M. Graphene-Based Materials as Solid Phase Extraction Sorbent for Trace Metal Ions, Organic Compounds, and Biological Sample Preparation. Crit. Rev. Anal. Chem. 2016, 46, 267–283. [Google Scholar] [CrossRef]
- Ye, N.; Shi, P. Applications of Graphene-Based Materials in Solid-Phase Extraction and Solid-Phase Microextraction. Sep. Purif. Rev. 2014, 44, 183–198. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Alkhouzaam, A.; Qiblawey, H.; Khraisheh, M.; Atieh, M.; Al-Ghouti, M. Synthesis of graphene oxides particle of high oxidation degree using a modified Hummers method. Ceram. Int. 2020, 46, 23997–24007. [Google Scholar] [CrossRef]
- Ahammad, A.S.; Islam, T.; Hasan, M. Biomedical Applications of Graphene and 2D Nanomaterials; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 249–282. [Google Scholar]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Fan, X.; Peng, W.; Li, Y.; Li, X.; Wang, S.; Zhang, G.; Zhang, F. Deoxygenation of Exfoliated Graphite Oxide under Alkaline Conditions: A Green Route to Graphene Preparation. Adv. Mater. 2008, 20, 4490–4493. [Google Scholar] [CrossRef]
- Toh, S.Y.; Loh, K.S.; Kamarudin, S.K.; Daud, W.R.W. Graphene production via electrochemical reduction of graphene oxide: Synthesis and characterisation. Chem. Eng. J. 2014, 251, 422–434. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Koduru, J.R.; Karri, R.R. A comprehensive review of applications of magnetic graphene oxide based nanocomposites for sustainable water purification. J. Environ. Manag. 2019, 231, 622–634. [Google Scholar] [CrossRef]
- Haw, C.Y.; Mohamed, F.; Chia, C.H.; Radiman, S.; Zakaria, S.; Huang, N.M.; Lim, H.N. Hydrothermal synthesis of magnetite nanoparticles as MRI contrast agents. Ceram. Int. 2010, 36, 1417–1422. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Travlou, N.A.; Kalogirou, O.; Deliyanni, E.A. Magnetic Graphene Oxide: Effect of Preparation Route on Reactive Black 5 Adsorption. Materials 2013, 6, 1360–1376. [Google Scholar] [CrossRef]
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Jalili, V.; Barkhordari, A.; Ghiasvand, A. A comprehensive look at solid-phase microextraction technique: A review of reviews. Microchem. J. 2020, 152, 104319. [Google Scholar] [CrossRef]
- Hajebi, N.; Seidi, S.; Ramezani, M.; Manouchehri, M. Electrospun polyamide/graphene oxide/polypyrrole composite nanofibers: An efficient sorbent for headspace solid phase microextraction of methamphetamine in urine samples followed by GC-MS analysis. New J. Chem. 2020, 44, 14429–14437. [Google Scholar] [CrossRef]
- Junting, L.; Peng, C.; Suzuki, O. Solid-phase microextraction (SPME) of drugs and poisons from biological samples. Forensic Sci. Int. 1998, 97, 93–100. [Google Scholar] [CrossRef]
- Kataoka, H. In-tube solid-phase microextraction: Current trends and future perspectives. J. Chromatogr. A 2021, 1636, 461787. [Google Scholar] [CrossRef] [PubMed]
- Manousi, N.; Tzanavaras, P.D.; Zacharis, C.K. Bioanalytical HPLC Applications of In-Tube Solid. Molecules 2020, 25, 2096. [Google Scholar] [CrossRef]
- Shamsayei, M.; Yamini, Y.; Asiabi, H. Polythiophene/graphene oxide nanostructured electrodeposited coating for on-line electrochemically controlled in-tube solid-phase microextraction. J. Chromatogr. A 2016, 1475, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, A.M.; Yamini, Y. Electrodeposition of poly-ethylenedioxythiophene-graphene oxide nanocomposite in a stainless steel tube for solid phase microextraction of letrozole in plasma samples. J. Sep. Sci. 2020, 43, 4338–4346. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xu, H. Simultaneous HPLC-MS determination of 8-hydroxy-2′-deoxyguanosine, 3-hydroxyphenanthrene and 1-hydroxypyrene after online in-tube solid phase microextraction using a graphene oxide/poly(3,4-ethylenedioxythiophene)/polypyrrole composite. Microchim. Acta 2019, 186, 300. [Google Scholar] [CrossRef]
- Darvishnejad, F.; Raoof, J.B.; Ghani, M. In-situ synthesis of nanocubic cobalt oxide @ graphene oxide nanocomposite reinforced hollow fiber-solid phase microextraction for enrichment of non-steroidal anti-inflammatory drugs from human urine prior to their quantification via high-performance liquid chromatography-ultraviolet detection. J. Chromatogr. A 2021, 1641, 461984. [Google Scholar] [CrossRef] [PubMed]
- Rezaeifar, Z.; Es’Haghi, Z.; Rounaghi, G.H.; Chamsaz, M. Hyperbranched polyglycerol/graphene oxide nanocomposite reinforced hollow fiber solid/liquid phase microextraction for measurement of ibuprofen and naproxen in hair and waste water samples. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1029–1030, 81–87. [Google Scholar] [CrossRef]
- Šafaříková, M.; Šafařík, I. Magnetic solid-phase extraction. J. Magn. Magn. Mater. 1999, 194, 108–112. [Google Scholar] [CrossRef]
- Plastiras, O.-E.; Deliyanni, E.; Samanidou, V. Applications of Graphene-Based Nanomaterials in Environmental Analysis. Appl. Sci. 2021, 11, 3028. [Google Scholar] [CrossRef]
- Li, N.; Chen, J.; Shi, Y.-P. Magnetic nitrogen-doped reduced graphene oxide as a novel magnetic solid-phase extraction adsorbent for the separation of bisphenol endocrine disruptors in carbonated beverages. Talanta 2019, 201, 194–203. [Google Scholar] [CrossRef]
- Lotfi, Z.; Mousavi, H.Z.; Sajjadi, S.M. Amino-terminated hyper-branched polyamidoamine polymer grafted magnetic graphene oxide nanosheets as an efficient sorbent for the extraction of selective serotonin reuptake inhibitors from plasma samples. Anal. Methods 2017, 9, 4504–4513. [Google Scholar] [CrossRef]
- Asgharinezhad, A.A.; Ebrahimzadeh, H. Poly(2-aminobenzothiazole)-coated graphene oxide/magnetite nanoparticles composite as an efficient sorbent for determination of non-steroidal anti-inflammatory drugs in urine sample. J. Chromatogr. A 2016, 1435, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhao, H.; Xiao, D.; Chuong, P.-H.; He, J.; He, H. Mixed hemimicelles solid-phase extraction of cephalosporins in biological samples with ionic liquid-coated magnetic graphene oxide nanoparticles coupled with high-performance liquid chromatographic analysis. J. Chromatogr. A 2016, 1454, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lamei, N.; Ezoddin, M.; Ardestani, M.S.; Abdi, K. Dispersion of magnetic graphene oxide nanoparticles coated with a deep eutectic solvent using ultrasound assistance for preconcentration of methadone in biological and water samples followed by GC–FID and GC–MS. Anal. Bioanal. Chem. 2017, 409, 6113–6121. [Google Scholar] [CrossRef]
- Taghvimi, A.; Hamishehkar, H.; Ebrahimi, M. Magnetic nano graphene oxide as solid phase extraction adsorbent coupled with liquid chromatography to determine pseudoephedrine in urine samples. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1009, 66–72. [Google Scholar] [CrossRef]
- Taghvimi, A.; Hamishehkar, H.; Ebrahimi, M. The application of magnetic nano graphene oxide in determination of methamphetamine by high performance liquid chromatography of urine samples. J. Iran. Chem. Soc. 2016, 13, 1471–1480. [Google Scholar] [CrossRef]
- Ghorbani, M.; Chamsaz, M.; Rounaghi, G.H.; Aghamohammadhasani, M.; Seyedin, O.; Lahoori, N.A. Development of a novel ultrasonic-assisted magnetic dispersive solid-phase microextraction method coupled with high performance liquid chromatography for determination of mirtazapine and its metabolites in human urine and water samples employing experimen. Anal. Bioanal. Chem. 2016, 408, 7719–7729. [Google Scholar] [CrossRef]
- Sereshti, H.; Bakhtiari, S. Three-dimensional graphene/Fe3O4-based magnetic solid phase extraction coupled with high performance liquid chromatography for determination of carvedilol in human blood plasma. RSC Adv. 2016, 6, 75757–75765. [Google Scholar] [CrossRef]
- Ferrone, V.; Carlucci, M.; Ettorre, V.; Cotellese, R.; Palumbo, P.; Fontana, A.; Siani, G.; Carlucci, G. Dispersive magnetic solid phase extraction exploiting magnetic graphene nanocomposite coupled with UHPLC-PDA for simultaneous determination of NSAIDs in human plasma and urine. J. Pharm. Biomed. Anal. 2018, 161, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Barati, A.; Kazemi, E.; Dadfarnia, S.; Shabani, A.M.H. Synthesis/characterization of molecular imprinted polymer based on magnetic chitosan/graphene oxide for selective separation/preconcentration of fluoxetine from environmental and biological samples. J. Ind. Eng. Chem. 2017, 46, 212–221. [Google Scholar] [CrossRef]
- Pashaei, Y.; Ghorbani-Bidkorbeh, F.; Shekarchi, M. Superparamagnetic graphene oxide-based dispersive-solid phase extraction for preconcentration and determination of tamsulosin hydrochloride in human plasma by high performance liquid chromatography-ultraviolet detection. J. Chromatogr. A 2017, 1499, 21–29. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Meng, X.; Shi, Y.; Wang, R.; Xiao, D.; He, H. Solid phase extraction based on porous magnetic graphene oxide/β-cyclodextrine composite coupled with high performance liquid chromatography for determination of antiepileptic drugs in plasma samples. J. Chromatogr. A 2017, 1524, 49–56. [Google Scholar] [CrossRef]
- Daryakenary, M.A.; Zeeb, M. Trace determination of chlorpheniramine in human plasma using magnetic dispersive solid-phase extraction based on a graphene oxide/Fe3O4@polythionine nanocomposite combined with high-performance liquid chromatography. RSC Adv. 2017, 7, 53210–53218. [Google Scholar] [CrossRef]
- Zeeb, M.; Farahani, H. Graphene oxide/Fe3O4@polythionine nanocomposite as an efficient sorbent for magnetic solid-phase extraction followed by high-performance liquid chromatography for the determination of duloxetine in human plasma. Chem. Pap. 2017, 72, 15–27. [Google Scholar] [CrossRef]
- Hua, X.; Gao, G.; Pan, S. High-affinity graphene oxide-encapsulated magnetic Zr-MOF for pretreatment and rapid determination of the photosensitizers hematoporphyrin and hematoporphyrin monomethyl ether in human urine prior to UPLC-HRMS. Anal. Bioanal. Chem. 2018, 410, 7749–7764. [Google Scholar] [CrossRef]
- Pourbahman, F.; Zeeb, M.; Monzavi, A.; Homami, S.S. Simultaneous trace monitoring of prokinetic drugs in human plasma using magnetic dispersive micro-solid phase extraction based on a new graphene oxide/metal–organic framework-74/Fe3O4/polytyramine nanoporous composite in combination with HPLC. Chem. Pap. 2019, 73, 3135–3150. [Google Scholar] [CrossRef]
- Peng, J.; Tian, H.; Du, Q.; Hui, X.; He, H. A regenerable sorbent composed of a zeolite imidazolate framework (ZIF-8), Fe3O4 and graphene oxide for enrichment of atorvastatin and simvastatin prior to their determination by HPLC. Microchim. Acta 2018, 185, 2–10. [Google Scholar] [CrossRef]
- Abdolmohammad-Zadeh, H.; Zamani, A.; Shamsi, Z. Preconcentration of morphine and codeine using a magnetite/reduced graphene oxide/silver nano-composite and their determination by high-performance liquid chromatography. J. Chromatogr. A 2019, 1590, 2–9. [Google Scholar] [CrossRef]
- Abdolmohammad-Zadeh, H.; Zamani, A.; Shamsi, Z. Extraction of four endocrine-disrupting chemicals using a Fe3O4/graphene oxide/di-(2-ethylhexyl) phosphoric acid nano-composite, and their quantification by HPLC-UV. Microchem. J. 2020, 157, 104964. [Google Scholar] [CrossRef]
- Lu, Q.; Guo, H.; Zhang, Y.; Tang, X.; Lei, W.; Qi, R.; Chu, J.; Li, D.; Zhao, Q. Graphene oxide-Fe3O4 nanocomposite magnetic solid phase extraction followed by UHPLC-MS/MS for highly sensitive determination of eight psychoactive drugs in urine samples. Talanta 2020, 206, 120212. [Google Scholar] [CrossRef]
- Yuvali, D.; Narin, I.; Soylak, M.; Yilmaz, E. Green synthesis of magnetic carbon nanodot/graphene oxide hybrid material (Fe3O4@C-nanodot@GO) for magnetic solid phase extraction of ibuprofen in human blood samples prior to HPLC-DAD determination. J. Pharm. Biomed. Anal. 2020, 179, 113001. [Google Scholar] [CrossRef] [PubMed]
- Baltussen, E.; Sandra, P.; David, F.; Cramers, C. Stir bar sorptive extraction (SBSE), a novel extraction technique for aqueous samples: Theory and principles. J. Microcolumn Sep. 1999, 11, 737–747. [Google Scholar] [CrossRef]
- Nazyropoulou, C.; Samanidou, V. Stir bar sorptive extraction applied to the analysis of biological fluids. Bioanalysis 2015, 7, 2241–2250. [Google Scholar] [CrossRef] [PubMed]
- Hasan, C.K.; Ghiasvand, A.; Lewis, T.W.; Nesterenko, P.N.; Paull, B. Recent advances in stir-bar sorptive extraction: Coatings, technical improvements, and applications. Anal. Chim. Acta 2020, 1139, 222–240. [Google Scholar] [CrossRef]
- Gilart, N.; Marcé, R.M.; Borrull, F.; Fontanals, N. New coatings for stir-bar sorptive extraction of polar emerging organic contaminants. TrAC Trends Anal. Chem. 2014, 54, 11–23. [Google Scholar] [CrossRef]
- Taghvimi, A.; Hamishehkar, H. Developed nano carbon-based coating for simultaneous extraction of potent central nervous system stimulants from urine media by stir bar sorptive extraction method coupled to high performance liquid chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1125, 121701. [Google Scholar] [CrossRef]
- Fan, W.; He, M.; You, L.; Zhu, X.; Chen, B.; Hu, B. Water-compatible graphene oxide/molecularly imprinted polymer coated stir bar sorptive extraction of propranolol from urine samples followed by high performance liquid chromatography-ultraviolet detection. J. Chromatogr. A 2016, 1443, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, E.; Shabani, A.M.H.; Dadfarnia, S. Application of graphene oxide-silica composite reinforced hollow fibers as a novel device for pseudo-stir bar solid phase microextraction of sulfadiazine in different matrices prior to its spectrophotometric determination. Food Chem. 2017, 221, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, P.; Masrournia, M.; Es’Haghi, Z.; Pordel, M. Hollow fiber coated Fe3O4@Maleamic acid-functionalized graphene oxide as a sorbent for stir bar sorptive extraction of ibuprofen, aspirin, and venlafaxine in human urine samples before determining by gas chromatography–mass spectrometry. J. Iran. Chem. Soc. 2021, 1–11. [Google Scholar] [CrossRef]
- Bordin, D.C.M.; Alves, M.N.R.; de Campos, E.G.; de Martinis, B.S. Disposable pipette tips extraction: Fundamentals, applications and state of the art. J. Sep. Sci. 2016, 39, 1168–1172. [Google Scholar] [CrossRef]
- Mirzaee, M.T.; Seidi, S.; Alizadeh, R. Pipette-tip SPE based on Graphene/ZnCr LDH for Pb(II) analysis in hair samples followed by GFAAS. Anal. Biochem. 2021, 612, 113949. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Sun, N.; Yan, H.; Han, D.; Row, K.H. Determination of indometacin and acemetacin in human urine via reduced graphene oxide—Based pipette tip solid-phase extraction coupled to HPLC. Microchim. Acta 2015, 183, 799–804. [Google Scholar] [CrossRef]
- Yuan, Y.; Han, Y.; Yang, C.; Han, D.; Yan, H. Deep eutectic solvent functionalized graphene oxide composite adsorbent for miniaturized pipette-tip solid-phase extraction of toluene and xylene exposure biomarkers in urine prior to their determination with HPLC-UV. Microchim. Acta 2020, 187, 1–9. [Google Scholar] [CrossRef]
- Manouchehri, M.; Seidi, S.; Rouhollahi, A.; Shanehsaz, M. Porphyrin-functionalized graphene oxide sheets: An efficient nanomaterial for micro solid phase extraction of non-steroidal anti-inflammatory drugs from urine samples. J. Chromatogr. A 2019, 1607, 460387. [Google Scholar] [CrossRef]
- Sereshti, H.; Bakhtiari, S.; Najarzadekan, H.; Samadi, S. Electrospun polyethylene terephthalate/graphene oxide nanofibrous membrane followed by HPLC for the separation and determination of tamoxifen in human blood plasma. J. Sep. Sci. 2017, 40, 3383–3391. [Google Scholar] [CrossRef]
- Ahmadi, M.; Moein, M.M.; Madrakian, T.; Afkhami, A.; Bahar, S.; Abdel-Rehim, M. Reduced graphene oxide as an efficient sorbent in microextraction by packed sorbent: Determination of local anesthetics in human plasma and saliva samples utilizing liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1095, 177–182. [Google Scholar] [CrossRef]
- Abdel-Rehim, M. New trend in sample preparation: On-line microextraction in packed syringe for liquid and gas chromatography applications. I. Determination of local anaesthetics in human plasma samples using gas chromatography-mass spectrometry. J. Chromatogr. B 2004, 801, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Lioupi, A.; Kabir, A.; Furton, K.G.; Samanidou, V. Fabric phase sorptive extraction for the isolation of five common antidepressants from human urine prior to HPLC-DAD analysis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1118, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Samanidou, V.; Galanopoulos, L.-D.; Kabir, A.; Furton, K.G. Fast extraction of amphenicols residues from raw milk using novel fabric phase sorptive extraction followed by high-performance liquid chromatography-diode array detection. Anal. Chim. Acta 2015, 855, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Kazantzi, V.; Anthemidis, A. Fabric Solgel Phase Sorptive Extraction Technique: A Review. Separation 2017, 4, 20. [Google Scholar] [CrossRef]
- Bruheim, I.; Liu, X.; Pawliszyn, J. Thin-Film Microextraction. Anal. Chem. 2003, 75, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Ghani, M.; Haghdoostnejad, K. Woven cotton yarn-graphene oxide-layered double hydroxide composite as a sorbent for thin film microextraction of nonsteroidal anti-inflammatory drugs followed by quantitation through high performance liquid chromatography. Anal. Chim. Acta 2020, 1097, 94–102. [Google Scholar] [CrossRef]
- Ghani, M.; Ghoreishi, S.M.; Azamati, M. Magnesium-aluminum-layered double hydroxide-graphene oxide composite mixed-matrix membrane for the thin-film microextraction of diclofenac in biological fluids. J. Chromatogr. A 2018, 1575, 11–17. [Google Scholar] [CrossRef]
- Zohdi, Z.; Hashemi, M.; Uheida, A.; Moein, M.M.; Abdel-Rehim, M. Graphene Oxide Tablets for Sample Preparation of Drugs in Biological Fluids: Determination of Omeprazole in Human Saliva for Liquid Chromatography Tandem Mass Spectrometry. Molecules 2019, 24, 1191. [Google Scholar] [CrossRef]
- Bagheri, H.; Zavareh, A.F.; Koruni, M.H. Graphene oxide assisted electromembrane extraction with gas chromatography for the determination of methamphetamine as a model analyte in hair and urine samples. J. Sep. Sci. 2016, 39, 1182–1188. [Google Scholar] [CrossRef]
| Adsorbent | Analyte(s) | Applications | LODs (ng·mL−1) | EF 1 | Reference |
|---|---|---|---|---|---|
| Poly(2-aminobenzothiazole)-mGO | NSAIDs | Urine | 0.07–0.3 | 35.7–37.7 | [58] |
| mGO-IL | Cephalosporins | Spiked urine | 0.6–1.9 | 4 | [59] |
| mGO-DES | Methadone | Urine, plasma | 2.5–14.3 × 10−3 | 500 | [60] |
| Nano mGO | Pseudoephedrine | Urine | 25 | NA 2 | [61] |
| Nano mGO | Methamphetamine | Urine | 30 | 168 | [62] |
| mGO-polyaniline | Mirtazapine, 8-hydroxy mirtazapine, N-desmethyl mirtazapine | Urine | 0.4–1.1 | 158, 124, 109 | [63] |
| 3D-m Graphene | Carvedilol | Plasma | 0.5 | NA | [64] |
| 3D-m Graphene | NSAIDs | Urine, plasma | 0.61–1.2 | 10 | [65] |
| GO-magnetic chitosan | Fluoxetine | Urine | 0.03 | 500 | [66] |
| Superpara-mGO | Tamsulosin hydrochloride | Plasma | 0.17 | 10 | [67] |
| mGO-β-cyclodextrine | Antiepileptic drugs | Plasma | 11.89–47.1 | NA | [68] |
| mGO-polythione | Chloropheniramine | Plasma | 0.4 | 16.7–18.3 | [69] |
| mGO-polythione | Duloxetine | Plasma | 0.5 | NA | [70] |
| mGO-dendrimer | Serotonin reuptake inhibitors | Plasma | 0.3–0.9 | 30 | [57] |
| mGO-Zr-MOF | Hematoporphyrin, hematoporphyrin monomethyl ether | Urine | 3.6, 4.2 | NA | [71] |
| mGO-MOF-74 | Prokinetic drugs | Plasma | 0.4, 1.1 | 18, 17.6 | [72] |
| mGO-ZIF-8 | Atorvastatin, simvastatin | Urine | 116 × 10−3, 387 × 10−3 | 169.4–191.4 | [73] |
| m-rGO-Ag | Codeine, morphine | Blood, urine | 1.8 × 10−3, 2.1 × 10−3 | 1000 | [74] |
| mGO-di-(2-ethylhexyl) phosphoric acid | Methyl & propyl paraben, phenol, bisphenol A | Breast milk, urine | 2.5–14.3 × 10−3 | 500 | [75] |
| mGO | Psychoactive drugs | Urine | 0.02–0.2 | NA | [76] |
| mGO-carbon nanodot | Ibuprofen | Plasma | 8 | 7.5 | [77] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manousi, N.; Plastiras, O.-E.; Deliyanni, E.A.; Zachariadis, G.A. Green Bioanalytical Applications of Graphene Oxide for the Extraction of Small Organic Molecules. Molecules 2021, 26, 2790. https://doi.org/10.3390/molecules26092790
Manousi N, Plastiras O-E, Deliyanni EA, Zachariadis GA. Green Bioanalytical Applications of Graphene Oxide for the Extraction of Small Organic Molecules. Molecules. 2021; 26(9):2790. https://doi.org/10.3390/molecules26092790
Chicago/Turabian StyleManousi, Natalia, Orfeas-Evangelos Plastiras, Eleni A. Deliyanni, and George A. Zachariadis. 2021. "Green Bioanalytical Applications of Graphene Oxide for the Extraction of Small Organic Molecules" Molecules 26, no. 9: 2790. https://doi.org/10.3390/molecules26092790
APA StyleManousi, N., Plastiras, O.-E., Deliyanni, E. A., & Zachariadis, G. A. (2021). Green Bioanalytical Applications of Graphene Oxide for the Extraction of Small Organic Molecules. Molecules, 26(9), 2790. https://doi.org/10.3390/molecules26092790









