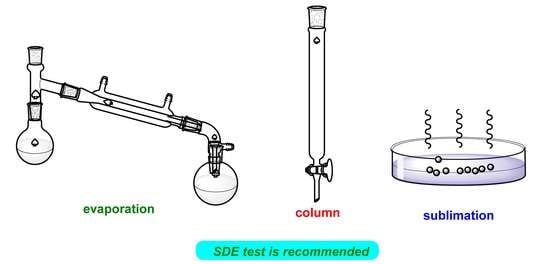
Recommended Tests for the Self-Disproportionation of Enantiomers (SDE) to Ensure Accurate Reporting of the Stereochemical Outcome of Enantioselective Reactions
Abstract
1. Introduction
2. Preview of the SDE
2.1. Origin and Purpose
2.2. Areas of SDE Manifestation
2.2.1. Physicochemical Phase Transitions
- There is very likely to be a euamotic point, since the vast majority of compounds are likely to be racemic compounds by sublimation in concert with their crystallization behavior,
- Below the euamotic point, the more volatile portion enriches the sublimate and conversely above it, and
- The portion with the lower melting point (mp) is generally the more volatile (i.e., there is a correlation between mp and volatility) and which is typically the enantiomer.
2.2.2. Force Field Applications
2.2.3. Chromatography
2.3. SDE-Phoric Groups
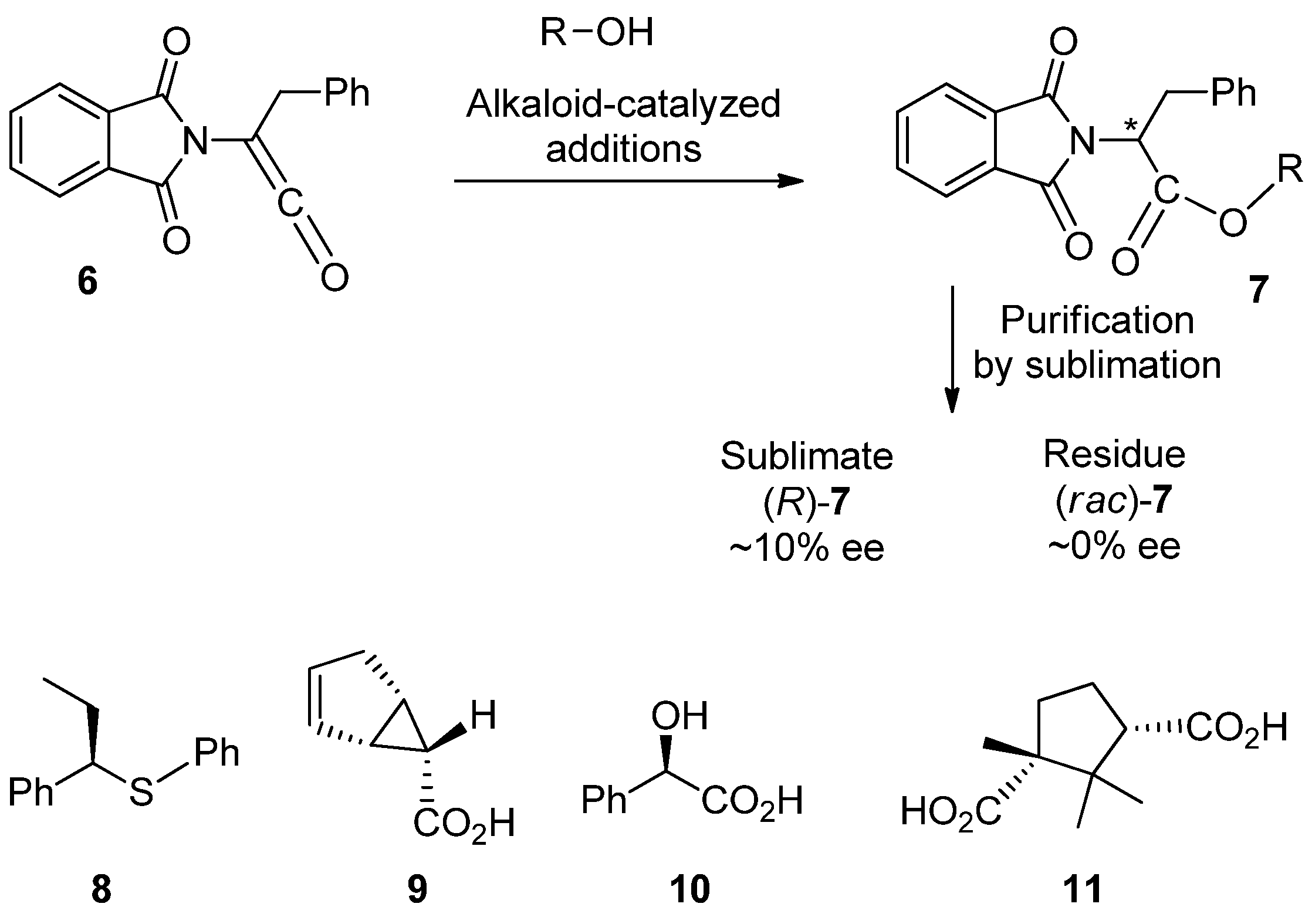
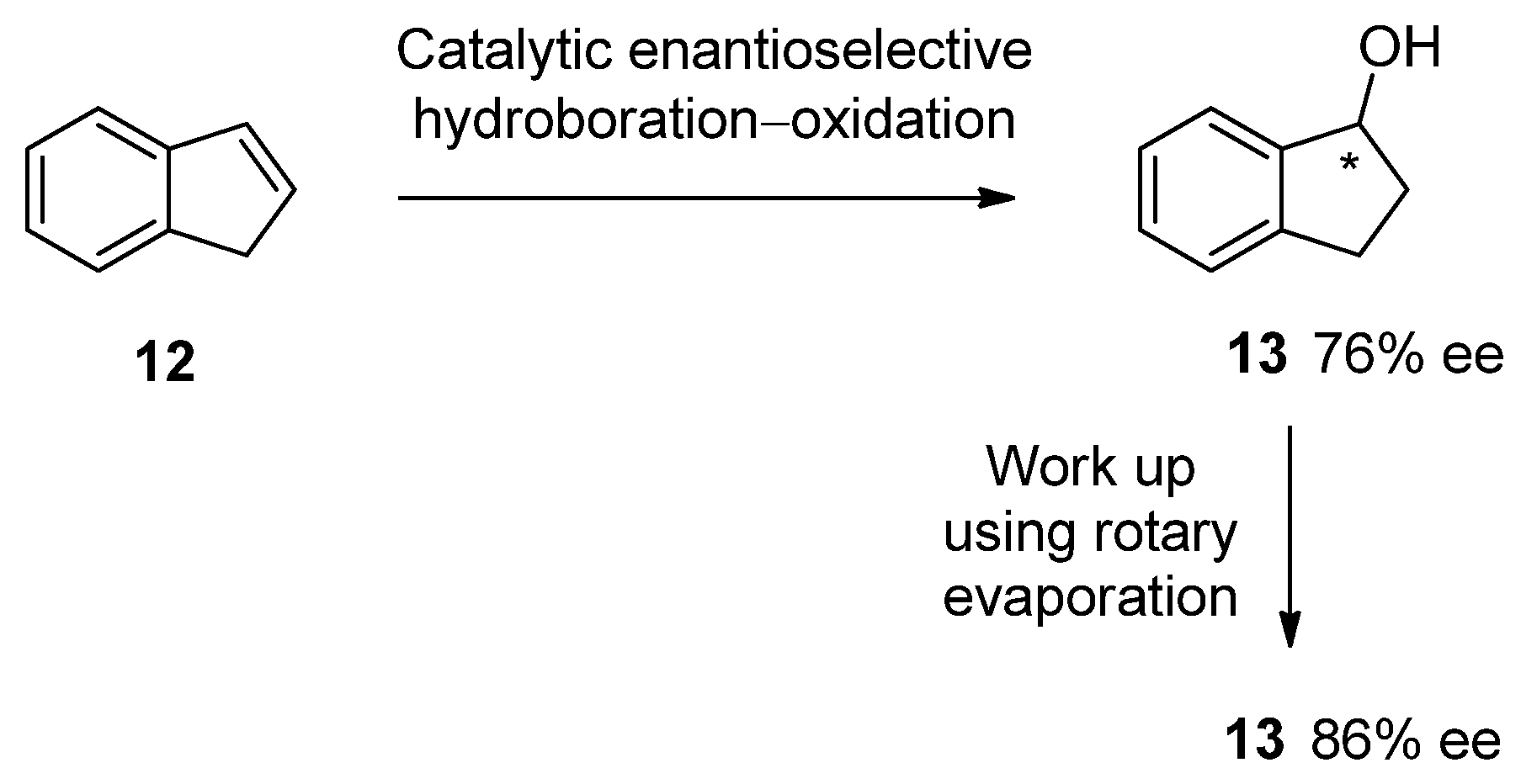
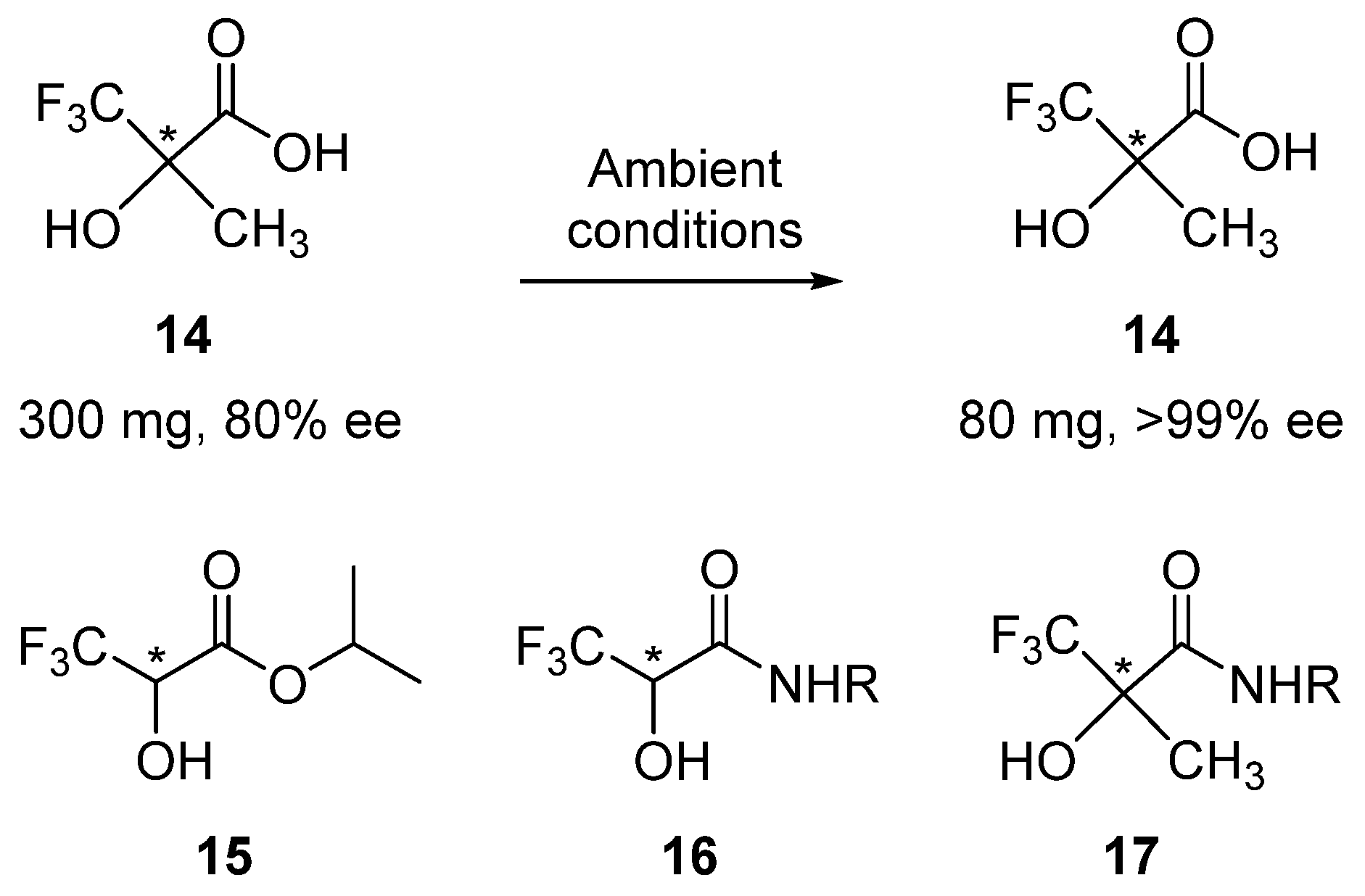
3. Pedagogic Examples from the Literature
3.1. SDEvS
3.2. SDEvC
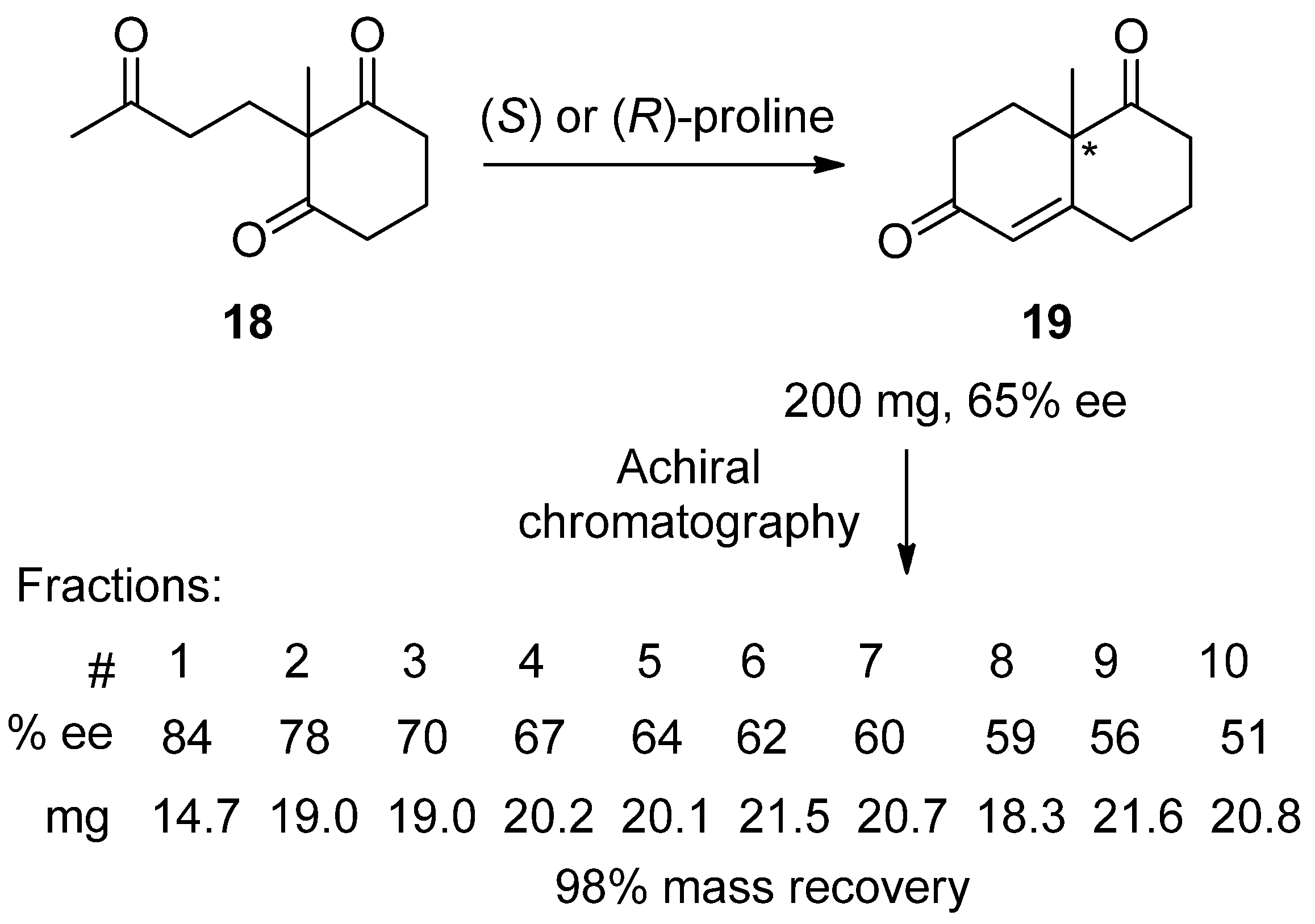
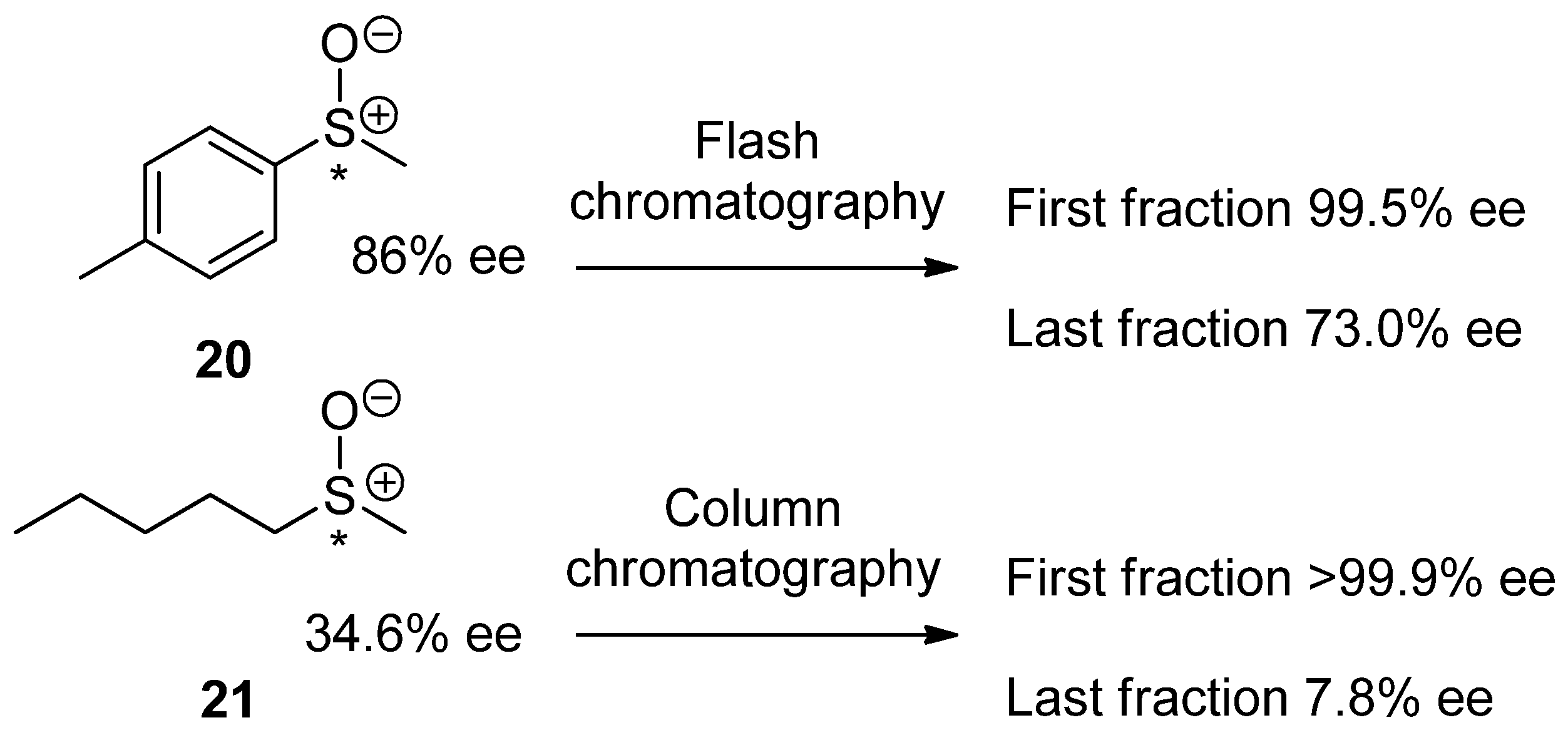
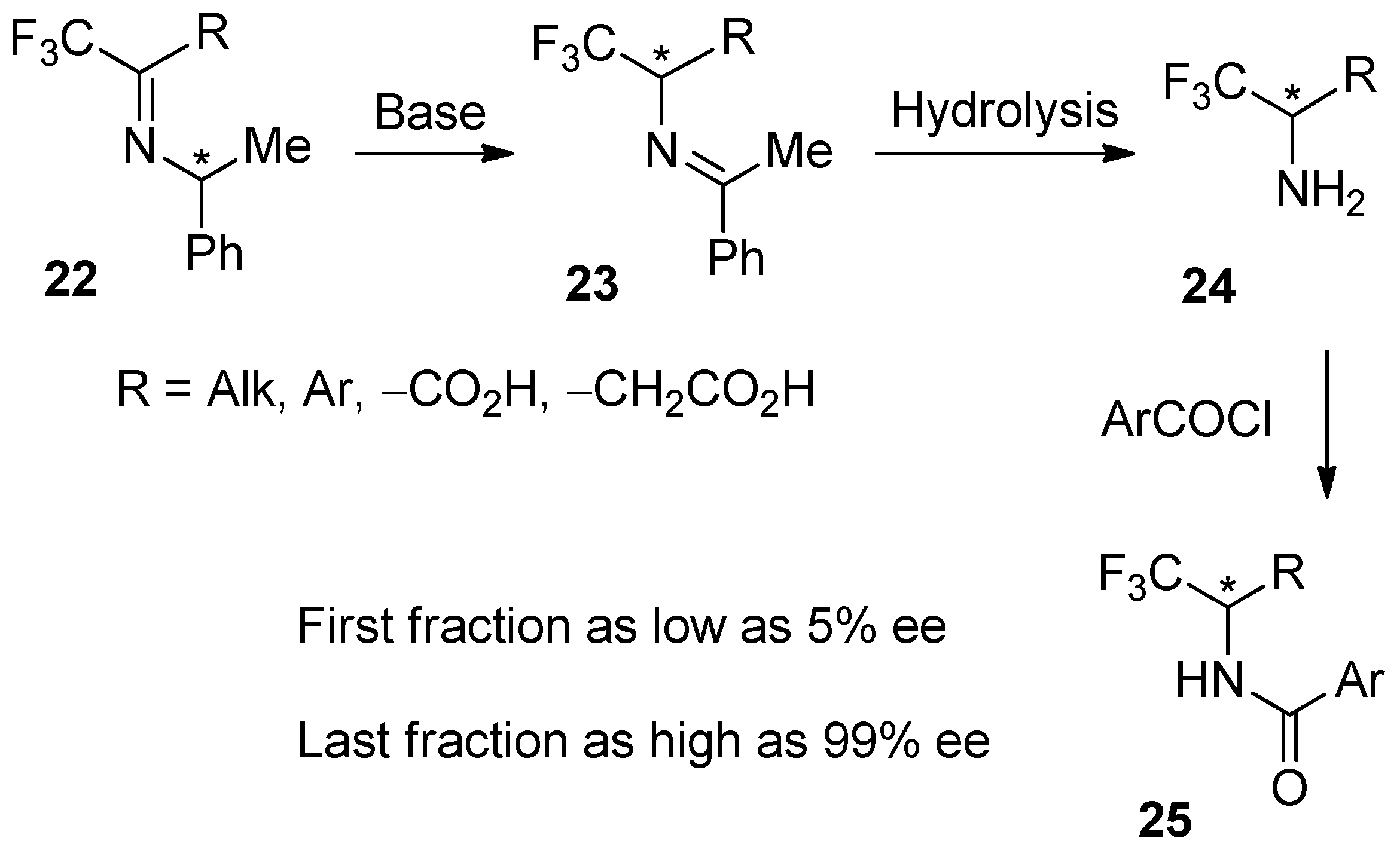
4. Recommended SDEvS/E and SDEvC Tests
4.1. General Considerations
4.2. SDEvS Test
4.3. SDEvC Test
5. Conclusions and Afterword
5.1. Conclusions
5.2. Afterword
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wernerova, M.; Hudlicky, T. On the practical limits of determining isolated product yields and ratios of stereoisomers: Reflections, analysis, and redemption. Synlett 2010, 2010, 2701–2707. [Google Scholar]
- Noyori, R.; Richmond, J.P. Ethical conduct in chemical research and publishing. Adv. Synth. Catal. 2013, 355, 3–8. [Google Scholar] [CrossRef]
- Carlson, R.; Hudlicky, T. On hype, malpractice, and scientific misconduct in organic synthesis. Helv. Chim. Acta 2012, 95, 2052–2062. [Google Scholar] [CrossRef]
- Payne, C.; Kass, S.R. How reliable are enantiomeric excess measurements obtained by chiral HPLC? ChemistrySelect 2020, 5, 1810–1817. [Google Scholar] [CrossRef]
- Han, J.; Kitagawa, O.; Wzorek, A.; Klika, K.D.; Soloshonok, V.A. The self-disproportionation of enantiomers (SDE): A menace or an opportunity? Chem. Sci. 2018, 9, 1718–1739. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wzorek, A.; Soloshonok, V.A.; Klika, K.D. The self-disproportionation of enantiomers (SDE): The effect of scaling down, potential problems versus prospective applications, possible new occurrences, and unrealized opportunities? Electrophoresis 2019, 40, 1869–1880. [Google Scholar] [CrossRef]
- Pietrusiewicz, K.M.; Borkowski, M.; Strzelecka, D.; Kielar, K.; Kicińska, W.; Karevych, S.; Jasiński, R.; Demchuk, O.M. A general phenomenon of spontaneous amplification of optical purity under achiral chromatographic conditions. Symmetry 2019, 11, 680. [Google Scholar] [CrossRef]
- Tokunaga, E.; Yamamoto, T.; Ito, E.; Shibata, N. Understanding the thalidomide chirality in biological processes by the self-disproportionation of enantiomers. Sci. Rep. 2018, 8, 17131. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Gellman, A.J. Enantiomer surface chemistry: Conglomerate versus racemate formation on surfaces. Chem. Soc. Rev. 2017, 46, 7787–7839. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, M.; Wzorek, A.; Kołbus, A.; Urbaniak, M.; Han, J.; Soloshonok, V.A.; Klika, K.D. Flurbiprofen: A study of the behavior of the scalemate by chromatography, sublimation, and NMR. Symmetry 2021, 13, 543. [Google Scholar] [CrossRef]
- Tsai, W.-L.; Hermann, K.; Hug, E.; Rohde, B.; Dreiding, A.S. Enantiomer-differentiation induced by an enantiomeric excess during chromatography with achiral phases. Helv. Chim. Acta 1985, 68, 2238–2243. [Google Scholar] [CrossRef]
- Dobashi, A.; Motoyama, Y.; Kinoshita, K.; Hara, S.; Fukasaku, N. Self-induced chiral recognition in the association of enantiomeric mixtures on silica gel chromatography. Anal. Chem. 1987, 59, 2209–2211. [Google Scholar] [CrossRef]
- Matusch, R.; Coors, C. Chromatographic separation of the excess enantiomer under achiral conditions. Angew. Chem. Int. Ed. 1989, 28, 626–627. [Google Scholar] [CrossRef]
- Loža, E.; Loļa, D.; Ķemme, A.; Freimanis, J. Enantiomeric enrichment of partially resolved 4-hydroxy-2-carboxymethylcyclopentanone derivatives by achiral phase chromatography. J. Chromatogr. A 1995, 708, 231–243. [Google Scholar] [CrossRef]
- Nicoud, R.-M.; Jaubert, J.-N.; Rupprecht, I.; Kinkel, J. Enantiomeric enrichment of non-racemic mixtures of binaphthol with non-chiral packings. Chirality 1996, 8, 234–243. [Google Scholar] [CrossRef]
- Monde, K.; Harada, N.; Takasugi, M.; Kutschy, P.; Suchy, M.; Dzurilla, M. Enantiomeric excess of a cruciferous phytoalexin, spirobrassinin, and its enantiomeric enrichment in an achiral HPLC system. J. Nat. Prod. 2000, 63, 1312–1314. [Google Scholar] [CrossRef]
- Suchý, M.; Kutschy, P.; Monde, K.; Goto, H.; Harada, N.; Takasugi, M.; Dzurilla, M.; Balentová, E. Synthesis, absolute configuration, and enantiomeric enrichment of a cruciferous oxindole phytoalexin, (S)-(−)-Spirobrassinin, and its oxazoline analog. J. Org. Chem. 2001, 66, 3940–3947. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, K.; Tsukagoshi, S.; Nakamura, T.; Watanabe, S.; Soloshonok, V.A.; Kitagawa, O. Chiral initiator-induces self-disproportionation of enantiomers via achiral chromatography: Application to enantiomer separation of racemate. Tetrahedron Lett. 2013, 54, 5220–5223. [Google Scholar] [CrossRef]
- Goto, M.; Tateishi, K.; Ebine, K.; Soloshonok, V.A.; Roussel, C.; Kitagawa, O. Chiral additive induced self-disproportionation of enantiomers under MPLC conditions: Preparation of enantiomerically pure samples of 1-(aryl)ethylamines from racemates. Tetrahedron Asymmetry 2016, 27, 317–321. [Google Scholar] [CrossRef]
- Nakamura, T.; Tateishi, K.; Tsukagoshi, S.; Hashimoto, S.; Watanabe, S.; Soloshonok, V.A.; Aceña, J.L.; Kitagawa, O. Self-disproportionation of enantiomers of non-racemic chiral amine derivatives through achiral chromatography. Tetrahedron 2012, 68, 4013–4017. [Google Scholar] [CrossRef]
- Kosugi, H.; Abe, M.; Hatsuda, R.; Uda, H.; Kato, M. A study of asymmetric protonation with chiral β-hydroxy sulfoxides. Asymmetric synthesis of (−)-epibatidine. Chem. Commun. 1997, 1857–1858. [Google Scholar] [CrossRef]
- Suzuki, Y.; Han, J.; Kitagawa, O.; Aceña, J.L.; Klika, K.D.; Soloshonok, V.A. A comprehensive examination of the self-disproportionation of enantiomers (SDE) of chiral amides via achiral, laboratory-routine, gravity-driven column chromatography. RSC Adv. 2015, 5, 2988–2993. [Google Scholar] [CrossRef]
- Tanaka, K.; Osuga, H.; Suzuki, H.; Shogase, Y.; Kitahara, Y. Synthesis, enzymic resolution and enantiomeric enhancement of bis(hydroxymethyl)[7]thiaheterohelicenes. J. Chem. Soc. Perkin Trans. 1 1998, 935–940. [Google Scholar] [CrossRef]
- Ernholt, B.V.; Thomsen, I.B.; Lohse, A.; Plesner, I.W.; Jensen, K.B.; Hazell, R.G.; Liang, X.; Jacobsen, A.; Bols, M. Enantiospecific synthesis of 1-Azafagomine. Chem. Eur. J. 2000, 6, 278–287. [Google Scholar] [CrossRef]
- Charles, R.; Gil-Av, E. Self-amplification of optical activity by chromatography on an achiral adsorbent. J. Chromatogr. 1984, 298, 516–520. [Google Scholar] [CrossRef]
- Song, W.; Zhou, Y.; Fu, Y.; Xu, W. Self-disproportionation of enantiomers of prazoles via achiral, gravity-driven silica gel column chromatography. Tetrahedron Asymmetry 2013, 24, 909–912. [Google Scholar] [CrossRef]
- Zheng, J.; You, S.-L. Construction of axial chirality by rhodium-catalyzed asymmetric dehydrogenative heck coupling of biaryl compounds with alkenes. Angew. Chem. Int. Ed. 2014, 53, 13244–13247. [Google Scholar] [CrossRef] [PubMed]
- Maeno, M.; Kondo, H.; Tokunaga, E.; Shibata, N. Synthesis of fluorinated donepezil by palladium-catalyzed decarboxylative allylation of α-fluoro-β-keto ester with tri-substituted heterocyclic alkene and the self-disproportionation of its enantiomers. RSC Adv. 2016, 6, 85058–85062. [Google Scholar] [CrossRef]
- Soloshonok, V.A. Remarkable amplification of the self-disproportionation of enantiomers on achiral-phase chromatography columns. Angew. Chem. Int. Ed. 2006, 45, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Wzorek, A.; Sato, A.; Drabowicz, J.; Soloshonok, V.A.; Klika, K.D. Enantiomeric enrichments via the self-disproportionation of enantiomers (SDE) by achiral, gravity-driven column chromatography: A case study using N-(1-Phenylethyl)acetamide for optimizing the enantiomerically pure yield and magnitude of the SDE. Helv. Chim. Acta 2015, 98, 1147–1159. [Google Scholar] [CrossRef]
- Wzorek, A.; Sato, A.; Drabowicz, J.; Soloshonok, V.A.; Klika, K.D. Remarkable magnitude of the self-disproportionation of enantiomers (SDE) via achiral chromatography; application to the practical-scale enantiopurification of β-amino acid esters. Amino Acids 2016, 48, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, M.; Marcinkowska, M.; Wzorek, A.; Pajkert, R.; Han, J.; Klika, K.D.; Soloshonok, V.A.; Röschenthaler, G.-V. The self-disproportionation of enantiomers (SDE) via column chromatography of β-amino-α,α-difluorophosphonic acid derivatives. Amino Acids 2019, 51, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Soloshonok, V.A.; Berbasov, D.O. Self-Disproportionation of enantiomers of (R)-Ethyl 3-(3,5-Dinitrobenzamido)-4,4,4-trifluorobutanoate on achiral silica gel stationary phase. J. Fluorine Chem. 2006, 127, 597–603. [Google Scholar] [CrossRef]
- Maeno, M.; Tokunaga, E.; Yamamoto, T.; Suzuki, T.; Ogino, Y.; Ito, E.; Shiro, M.; Asahi, T.; Shibata, N. Self-disproportionation of enantiomers of thalidomide and its fluorinated analogue via gravity-driven achiral chromatography: Mechanistic rationale and implications. Chem. Sci. 2015, 6, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Nishimine, T.; Tokunaga, E.; Nakamura, S.; Shibata, N. Self-disproportionation of enantiomers of heterocyclic compounds having a tertiary trifluoromethyl alcohol center on chromatography with a non-chiral system. J. Fluorine Chem. 2010, 131, 521–524. [Google Scholar] [CrossRef]
- Hosaka, T.; Imai, T.; Wzorek, A.; Marcinkowska, M.; Kolbus, A.; Kitagawa, O.; Soloshonok, V.A.; Klika, K.D. The self-disproportionation of enantiomers (SDE) of α-amino acid derivatives; facets of steric and electronic properties. Amino Acids 2019, 51, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Mayani, V.J.; Abdi, S.H.R.; Kureshy, R.I.; Khan, N.H.; Agrawal, S.; Jasra, R.V. Enantiomer self-disproportionation of chiral compounds on achiral ordered mesoporous silica M41S and regular silica gel as a stationary phase. Chirality 2009, 21, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Diter, P.; Taudien, S.; Samuel, O.; Kagan, H.B. Enantiomeric enrichment of sulfoxides by preparative flash chromatography on an achiral phase. J. Org. Chem. 1994, 59, 370–378. [Google Scholar] [CrossRef]
- Sorochinsky, A.E.; Katagiri, T.; Ono, T.; Wzorek, A.; Aceña, J.L.; Soloshonok, V.A. Optical purifications via self-disproportionation of enantiomers by achiral chromatography: Case study of a series of α-CF3-containing secondary alcohols. Chirality 2013, 25, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Abás, S.; Arróniz, C.; Molins, E.; Escolano, C. Access to the enantiopure pyrrolobenzodiazepine (PBD) dilactam nucleus via self-disproportionation of enantiomers. Tetrahedron 2018, 74, 867–871. [Google Scholar] [CrossRef]
- Baumann, A.; Wzorek, A.; Soloshonok, V.A.; Klika, K.D.; Miller, A.K. Potentially mistaking enantiomers for different compounds due to the self-induced diastereomeric anisochronism (SIDA) phenomenon. Symmetry 2020, 12, 1106. [Google Scholar] [CrossRef]
- Aceña, J.L.; Sorochinsky, A.E.; Katagiri, T.; Soloshonok, V.A. Unconventional preparation of racemic crystals of isopropyl 3,3,3-trifluoro-2-hydroxypropanoate and their unusual crystallographic structure: The ultimate preference for homochiral intermolecular interactions. Chem. Commun. 2013, 49, 373–375. [Google Scholar] [CrossRef]
- Ishida, Y.; Aida, T. Homochiral supramolecular polymerization of an “S”-shaped chiral monomer: Translation of optical purity into molecular weight distribution. J. Am. Chem. Soc. 2002, 124, 14017–14019. [Google Scholar] [CrossRef] [PubMed]
- Doucet, H.; Fernandez, E.; Layzell, T.P.; Brown, J.M. the scope of catalytic asymmetric hydroboration/oxidation with rhodium complexes of 1,1’-(2-Diarylphosphino-1-naphthyl)isoquinolines. Chem. Eur. J. 1999, 5, 1320–1330. [Google Scholar] [CrossRef]
- Martens, J.; Bhushan, R. Purification of enantiomeric mixtures in enantioselective synthesis: Overlooked errors and scientific basis of separation in achiral environment. Helv. Chim. Acta 2014, 97, 161–187. [Google Scholar] [CrossRef]
- Martens, J.; Bhushan, R. Enantioseparations in achiral environments and chromatographic systems. Isr. J. Chem. 2016, 56, 990–1009. [Google Scholar] [CrossRef]
- Sorochinsky, A.E.; Soloshonok, V.A. Self-disproportionation of enantiomers of enantiomerically enriched compounds. In Differentiation of Enantiomers II; Schurig, V., Ed.; Springer: Cham, Switzerland, 2013; Volume 341, pp. 301–340. [Google Scholar]
- Soloshonok, V.A.; Klika, K.D. Terminology related to the phenomenon ‘self-disproportionation of enantiomers’ (SDE). Helv. Chem. Acta 2014, 97, 1583–1589. [Google Scholar] [CrossRef]
- Wallach, O. Zur kenntniss der terpene und der ätherischen oele. Ueber gebromte derivate der carvonreihe. Justus Liebigs Ann. Chem. 1895, 286, 119–143. [Google Scholar] [CrossRef]
- Pratt Brock, C.; Schweizer, W.B.; Dunitz, J.D. On the validity of Wallach’s rule: On the density and stability of racemic crystals compared with their chiral counterparts. J. Am. Chem. Soc. 1991, 113, 9811–9820. [Google Scholar] [CrossRef]
- Jacques, J.; Collet, A.; Wilen, S.H. Enantiomers, Racemates, and Resolutions; Wiley & Sons, Inc.: New York, NY, USA, 1981. [Google Scholar]
- O’Donnell, M.J.; Delgado, F. Enantiomeric enrichment of α-amino acid derivatives: Recrystallization of N-Fmoc α-amino acid tert-butyl esters. Tetrahedron 2001, 57, 6641–6650. [Google Scholar] [CrossRef]
- Cintas, P.; Viedma, C. On the physical basis of asymmetry and homochirality. Chirality 2012, 24, 894–908. [Google Scholar] [CrossRef]
- Han, J.; Nelson, D.J.; Sorochinsky, A.E.; Soloshonok, V.A. Self-disproportionation of enantiomers via sublimation; new and truly green dimension in optical purification. Curr. Org. Synth. 2011, 8, 310–317. [Google Scholar] [CrossRef]
- Yasumoto, M.; Ueki, H.; Ono, T.; Katagiri, T.; Soloshonok, V.A. Self-disproportionation of enantiomers of isopropyl 3,3,3-(trifluoro)lactate via sublimation: Sublimation rates vs. enantiomeric composition. J. Fluorine Chem. 2010, 131, 535–539. [Google Scholar] [CrossRef]
- Ueki, H.; Yasumoto, M.; Soloshonok, V.A. Rational application of self-disproportionation of enantiomers via sublimation—a novel methodological dimension for enantiomeric purifications. Tetrahedron Asymmetry 2010, 21, 1396–1400. [Google Scholar] [CrossRef]
- Tarasevych, A.V.; Sorochinsky, A.E.; Kukhar, V.P.; Chollet, A.; Daniellou, R.; Guillemin, J.-C. Partial sublimation of enantioenriched amino acids at low temperature. Is it coming from the formation of a euatmotic composition of the gaseous phase? J. Org. Chem. 2013, 78, 10530–10533. [Google Scholar] [CrossRef] [PubMed]
- Nanita, S.C.; Cooks, R.G. Serine octamers: Cluster formation, reactions, and implications for biomolecule homochirality. Angew. Chem. Int. Ed. 2006, 45, 554–569. [Google Scholar] [CrossRef]
- Katagiri, T.; Takahashi, S.; Tsuboi, A.; Suzaki, M.; Uneyama, K. Discrimination of enantiomeric excess of optically active trifluorolactate by distillation: Evidence for a multi-center hydrogen bonding network in the liquid state. J. Fluorine Chem. 2010, 131, 517–520. [Google Scholar] [CrossRef]
- Katagiri, T.; Uneyama, K. Chiral recognition by multicenter single proton hydrogen bonding of trifluorolactates. Chem. Lett. 2001, 30, 1330–1331. [Google Scholar] [CrossRef]
- Katagiri, T.; Yoda, C.; Furuhashi, K.; Ueki, K.; Kubota, T. Separation of an enantiomorph and its racemate by distillation: Strong chiral recognizing ability of trifluorolactates. Chem. Lett. 1996, 25, 115–116. [Google Scholar] [CrossRef]
- Koppenhoefer, B.; Trettin, U. Is it possible to affect the enantiomeric composition by a simple distillation process? Fresenius Z. Anal. Chem. 1989, 333, 750. [Google Scholar] [CrossRef]
- Zehnacker, A.; Suhm, M.A. Chirality recognition between neutral molecules in the gas phase. Angew. Chem. Int. Ed. 2008, 47, 6970–6992. [Google Scholar] [CrossRef]
- Albrecht, M.; Soloshonok, V.A.; Schrader, L.; Yasumoto, M.; Suhm, M.A. Chirality-dependent sublimation of α-(trifluoromethyl)-lactic acid: Relative vapor pressures of racemic, eutectic, and enantiomerically pure forms, and vibrational spectroscopy of isolated (S,S) and (S,R) dimers. J. Fluorine Chem. 2010, 131, 495–504. [Google Scholar] [CrossRef]
- Blackmond, D.G.; Klussmann, M. Spoilt for choice: Assessing phase behavior models for the evolution of homochirality. Chem. Commun. 2007, 3990–3996. [Google Scholar] [CrossRef] [PubMed]
- Soloshonok, V.A.; Ueki, H.; Yasumoto, M.; Mekala, S.; Hirschi, J.S.; Singleton, D.A. Phenomenon of optical self-purification of chiral non-racemic compounds. J. Am. Chem. Soc. 2007, 129, 12112–12113. [Google Scholar] [CrossRef]
- Viedma, C.; Cintas, P. On the chiral homogeneity of nature: From atoms to small molecules. Isr. J. Chem. 2011, 51, 997–1006. [Google Scholar] [CrossRef]
- Mastai, Y.; Volkel, A.; Colfen, H. Separation of racemate from excess enantiomer of chiral nonracemic compounds via density gradient ultracentrifugation. J. Am. Chem. Soc. 2008, 130, 2426–2427. [Google Scholar] [CrossRef]
- Kozma, D.; Kassai, C.; Fogassy, E. Enantiomeric enrichment by the use of density differences between racemic compounds and optically active enantiomers. Tetrahedron Lett. 1995, 36, 3245–3246. [Google Scholar] [CrossRef]
- Yang, X.; Wong, S.Y.; Bwambok, D.K.; Atkinson, M.B.J.; Zhang, X.; Whitesides, G.M.; Myerson, A.S. Separation and enrichment of enantiopure from racemic compounds using magnetic levitation. Chem. Commun. 2014, 50, 7548–7551. [Google Scholar] [CrossRef]
- Han, J.; Soloshonok, V.A.; Klika, K.D.; Drabowicz, J.; Wzorek, A. Chiral sulfoxides: Advances in asymmetric synthesis and problems with the accurate determination of the stereochemical outcome. Chem. Soc. Rev. 2018, 47, 1307–1350. [Google Scholar] [CrossRef]
- Bánhegyi, D.F.; Pálovics, E. The stoichiometry, structure and possible formation of crystalline diastereomeric salts. Symmetry 2021, 13, 667. [Google Scholar] [CrossRef]
- Schurig, V. Elaborate treatment of retention in chemoselective chromatography—The retention increment approach and nonlinear effects. J. Chromatogr. A 2009, 1216, 1723–1736. [Google Scholar] [CrossRef]
- Jung, M.; Schurig, V. Computer simulation of three scenarios for the separation of non-racemic mixtures by chromatography on achiral stationary phases. J. Chromatogr. 1992, 605, 161–166. [Google Scholar] [CrossRef]
- Gil-Av, E.; Schurig, V. Resolution of non-racemic mixtures in achiral chromatographic systems: A model for the enantioselective effects observed. J. Chromatogr. A 1994, 666, 519–525. [Google Scholar] [CrossRef]
- Baciocchi, R.; Zenoni, G.; Mazzotti, M.; Morbidelli, M. Separation of binaphthol enantiomers through achiral chromatography. J. Chromatogr. A 2002, 944, 225–240. [Google Scholar] [CrossRef]
- Baciocchi, R.; Mazzotti, M.; Morbidelli, M. General model for the achiral chromatography of enantiomers forming dimers: Application to binaphthol. J. Chromatogr. A 2004, 1024, 15–20. [Google Scholar] [CrossRef]
- Soloshonok, V.A.; Roussel, C.; Kitagawa, O.; Sorochinsky, A.E. Self-disproportionation of enantiomers via achiral chromatography: A warning and extra dimension in optical purifications. Chem. Soc. Rev. 2012, 41, 4180–4188. [Google Scholar] [CrossRef]
- Carman, R.M.; Klika, K.D. Partially racemic compounds as brushtail possum urinary metabolites. Aust. J. Chem. 1992, 45, 651–657. [Google Scholar] [CrossRef]
- Flynn, A.J.; Ford, A.; Maguire, A.R. Localized partitioning of enantiomers in solid samples of sulfoxides: Importance of sampling method in determination of enantiopurity. J. Org. Chem. 2020, 85, 10216–10221. [Google Scholar] [CrossRef]
- Wzorek, A.; Sato, A.; Drabowicz, J.; Soloshonok, V.A. Self-disproportionation of enantiomers via achiral gravity-driven column chromatography: A case study of N-acyl-α-phenylethylamines. J. Chromatogr. A 2016, 1467, 270–278. [Google Scholar] [CrossRef]
- Terada, S.; Hirai, M.; Honzawa, A.; Kitagawa, O.; Kamizela, A.; Wzorek, A.; Soloshonok, V.A. Possible case of halogen bond-driven self-disproportionation of enantiomers (SDE) via achiral chromatography. Chem. Eur. J. 2017, 23, 14631–14638. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Niijima, E.; Terada, S.; Wzorek, A.; Soloshonok, V.A.; Horie, A.; Kitagawa, O. Chirality-dependent halogen bond in axially chiral quinazolin-4-one derivatives bearing ortho-halophenyl group. CrystEngComm 2019, 21, 3385–3389. [Google Scholar] [CrossRef]
- Takahashi, M.; Tanabe, H.; Nakamura, T.; Kuribara, D.; Yamazaki, T.; Kitagawa, O. Atropisomeric lactam chemistry: Catalytic enantioselective synthesis, application to asymmetric enolate chemistry and synthesis of key intermediates for NET inhibitors. Tetrahedron 2010, 66, 288–296. [Google Scholar] [CrossRef]
- Wzorek, A.; Klika, K.D.; Drabowicz, J.; Sato, A.; Aceña, J.L.; Soloshonok, V.A. The self-disproportionation of the enantiomers (SDE) of methyl n-pentyl sulfoxide via achiral, gravity-driven column chromatography: A case study. Org. Biomol. Chem. 2014, 12, 4738–4746. [Google Scholar] [CrossRef]
- Soloshonok, V.A.; Berbasov, D.O. Self-disproportionation of enantiomers on achiral phase chromatography. One more example of fluorine’s magic powers. Chim. Oggi Chem. Today 2006, 24, 44–47. [Google Scholar]
- Sorochinsky, A.E.; Aceña, J.L.; Soloshonok, V.A. Self-disproportionation of enantiomers of chiral, non-racemic fluoroorganic compounds: Role of fluorine as enabling element. Synthesis 2013, 45, 141–152. [Google Scholar] [CrossRef]
- Yasumoto, M.; Ueki, H.; Soloshonok, V.A. Self-disproportionation of enantiomers of 3,3,3-trifluorolactic acid amides via sublimation. J. Fluorine Chem. 2010, 131, 266–269. [Google Scholar] [CrossRef]
- Yasumoto, M.; Ueki, H.; Soloshonok, V.A. Self-disproportionation of enantiomers of α-trifluoromethyl lactic acid amides via sublimation. J. Fluorine Chem. 2010, 131, 540–544. [Google Scholar] [CrossRef]
- Mei, H.; Han, J.; Fustero, S.; Medio-Simon, M.; Sedgwick, D.M.; Santi, C.; Ruzziconi, R.; Soloshonok, V.A. Fluorine-containing drugs approved by the FDA in 2018. Chem. Eur. J. 2019, 25, 11797–11819. [Google Scholar] [CrossRef]
- Mei, H.; Remete, A.M.; Zou, Y.; Moriwaki, H.; Fustero, S.; Kiss, L.; Soloshonok, V.A.; Han, J. Fluorine-containing drugs approved by the FDA in 2019. Chin. Chem. Lett. 2020, 31, 2401–2413. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, J.; Wang, S.; Gu, Z.; Aceña, J.L.; Izawa, K.; Liu, H.; Soloshonok, V.A. Recent advances in the trifluoromethylation methodology and new CF3-containing drugs. J. Fluorine Chem. 2014, 167, 37–54. [Google Scholar] [CrossRef]
- Han, J.; Kiss, L.; Mei, H.; Remete, A.M.; Ponikvar-Svet, M.; Sedgwick, D.M.; Roman, R.; Fustero, S.; Moriwaki, H.; Soloshonok, V.A. Chemical aspects of human and environmental overload with fluorine. Chem. Rev. 2021, 121, 4678–4742. [Google Scholar] [CrossRef]
- Mei, H.; Han, J.; White, S.; Graham, D.J.; Izawa, K.; Sato, T.; Fustero, S.; Meanwell, N.A.; Soloshonok, V.A. Tailor-made amino acids and fluorinated motifs as prominent traits in the modern pharmaceuticals. Chem. Eur. J. 2020, 26, 11349–11390. [Google Scholar] [CrossRef]
- Mei, H.; Han, J.; Klika, K.D.; Izawa, K.; Sato, T.; Meanwell, N.A.; Soloshonok, V.A. Applications of fluorine-containing amino acids for drug design. Eur. J. Med. Chem. 2020, 186, 111826. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Remete, A.M.; Dobson, L.S.; Kiss, L.; Izawa, K.; Moriwaki, H.; Soloshonok, V.A.; O’Hagan, D. Next generation organofluorine containing blockbuster drugs. J. Fluorine Chem. 2020, 239, 109639. [Google Scholar] [CrossRef]
- Fujiwara, T.; O’Hagan, D. Successful fluorine-containing herbicide agrochemicals. J. Fluorine Chem. 2014, 167, 16–29. [Google Scholar] [CrossRef]
- Jeschke, P. The unique role of halogen substituents in the design of modern agrochemicals. Pest Manag. Sci. 2010, 66, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P. The unique role of fluorine in the design of active ingredients for modern crop protection. ChemBioChem 2004, 5, 570–589. [Google Scholar] [CrossRef]
- Jeschke, P. Latest Generation of Halogen-Containing Pesticides. Pest Manag. Sci. 2017, 73, 1053–1066. [Google Scholar] [CrossRef]
- Bravo, P.; Guidetti, M.; Viani, F.; Zanda, M.; Markovsky, A.L.; Sorochinsky, A.E.; Soloshonok, I.V.; Soloshonok, V.A. Chiral sulfoxide controlled asymmetric additions to CN double bond. An efficient approach to stereochemically defined α-fluoroalkyl amino compounds. Tetrahedron 1998, 54, 12789–12806. [Google Scholar] [CrossRef]
- Drabowicz, J.; Jasiak, A.; Wzorek, A.; Sato, A.; Soloshonok, V.A. Self-disproportionation of enantiomers (SDE) of chiral sulfoxides, amides and thioamides via achiral chromatography. Arkivoc 2017, 2017, 557–578. [Google Scholar] [CrossRef]
- Richardson, P.; Hawkey, C.J.; Stack, W.A. Proton pump inhibitors. Pharmacology and rationale for use in gastrointestinal disorders. Drugs 1998, 56, 307–335. [Google Scholar] [CrossRef]
- Wzorek, A.; Kamizela, A.; Sato, A.; Soloshonok, V.A. Self-disproportionation of enantiomers (SDE) via achiral gravity-driven column chromatography of N-fluoroacyl-1-phenylethylamines. J. Fluorine Chem. 2017, 196, 37–43. [Google Scholar] [CrossRef]
- Han, J.; Wzorek, A.; Kwiatkowska, M.; Soloshonok, V.A.; Klika, K.D. The self-disproportionation of enantiomers (SDE) of amino acids and their derivatives. Amino Acids 2019, 51, 865–889. [Google Scholar] [CrossRef]
- Soloshonok, V.A.; Izawa, K. Asymmetric Synthesis and Application of Alpha-Amino Acids; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Liu, J.; Han, J.; Izawa, K.; Sato, T.; White, S.; Meanwell, N.A.; Soloshonok, V.A. Cyclic tailor-made amino acids in the design of modern pharmaceuticals. Eur. J. Med. Chem. 2020, 208, 112736. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Konno, H.; Sato, T.; Soloshonok, V.A.; Izawa, K. Tailor-made amino acids in the design of small-molecule blockbuster drug. Eur. J. Med. Chem. 2021, 220, 113448. [Google Scholar] [CrossRef]
- Yin, Z.; Hu, W.; Zhang, W.; Konno, H.; Moriwaki, H.; Izawa, K.; Soloshonok, V.A.; Han, J. Tailor-made amino acid-derived pharmaceuticals approved by the FDA in 2019. Amino Acids 2020, 52, 1227–1261. [Google Scholar] [CrossRef] [PubMed]
- Takeda, R.; Kawamura, A.; Kawashima, A.; Sato, T.; Moriwaki, H.; Izawa, K.; Akaji, K.; Wang, S.; Liu, H.; Aceña, J.L.; et al. Chemical dynamic kinetic resolution and (S)/(R)-interconversion of unprotected α-amino acids. Angew. Chem. Int. Ed. 2014, 53, 12214–12217. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Gu, X.; Soloshonok, V.A.; Carducci, M.D.; Hruby, V.J. Stereoselective synthesis of conformationally constrained reverse turn dipeptide mimetics. Tetrahedron Lett. 2001, 42, 145–148. [Google Scholar] [CrossRef]
- Klika, K.D.; Budovská, M.; Kutschy, P. Enantiodifferentiation of phytoalexin spirobrassinin derivatives using the chiral solvating agent (R)-(+)-1,1′-bi-2-naphthol in conjunction with molecular modeling. Tetrahedron Asymmetry 2010, 21, 647–658. [Google Scholar] [CrossRef]
- Nakajima, M.; Kanayama, K.; Miyoshi, I.; Hashimoto, S.-I. Catalytic asymmetric synthesis of binaphthol derivatives by aerobic oxidative coupling of 3-Hydroxy-2-naphthoates with chiral diamine-copper complex. Tetrahedron Lett. 1995, 36, 9519–9520. [Google Scholar] [CrossRef]
- Nakajima, M.; Miyoshi, I.; Kanayama, K.; Hashimoto, S.-I.; Noji, M.; Koga, K. Enantioselective synthesis of binaphthol derivatives by oxidative coupling of naphthol derivatives catalyzed by chiral diamine copper complexes. J. Org. Chem. 1999, 64, 2264–2271. [Google Scholar] [CrossRef]
- Nieminen, V.; Murzin, D.Y.; Klika, K.D. NMR and molecular modeling of the dimeric self-association of the enantiomers of 1,1′-bi-2-naphthol and 1-phenyl-2,2,2-trifluoroethanol in the solution state and their relevance to enantiomer self-disproportionation on achiral-phase chromatography (ESDAC). Org. Biomol. Chem. 2009, 7, 537–542. [Google Scholar] [CrossRef]
- Baciocchi, R.; Zenoni, G.; Valentini, M.; Mazzotti, M.; Morbidelli, M. Measurement of the dimerization equilibrium constants of enantiomers. J. Phys. Chem. A 2002, 106, 10461–10469. [Google Scholar] [CrossRef]
- Brussee, J.; Jansen, A.C.A. A highly stereoselective synthesis of S-(−)-[1,1′-binaphthalene]-2,2′-diol. Tetrahedron Lett. 1983, 24, 3261–3262. [Google Scholar] [CrossRef]
- Ishii, A.; Soloshonok, V.A.; Mikami, K. Asymmetric catalysis of the friedel-crafts reaction with fluoral by chiral binaphthol-derived titanium catalysts through asymmetric activation. J. Org. Chem. 2000, 65, 1597–1599. [Google Scholar] [CrossRef] [PubMed]
- Girard, C.; Kagan, H.B. Nonlinear effects in asymmetric synthesis and stereoselective reactions: Ten years of investigation. Angew. Chem. Int. Ed. 1998, 37, 2922–2959. [Google Scholar] [CrossRef]
- Girard, C.; Kagan, H.B. On diastereomeric perturbations. Can. J. Chem. 2000, 78, 816–828. [Google Scholar] [CrossRef]
- Noyori, R.; Suga, S.; Oka, H.; Kitamura, M. Self and nonself recognition of chiral catalysts: The origin of nonlinear effects in the amino-alcohol catalyzed asymmetric addition of diorganozincs to aldehydes. Chem. Rec. 2001, 1, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Martens, J.; Bhushan, R. Resolution of enantiomers with achiral phase chromatography. J. Liq. Chromatogr. Relat. Technol. 1992, 15, 1–27. [Google Scholar] [CrossRef]
- Han, J.; Wzorek, A.; Klika, K.D.; Soloshonok, V.A. Fluorine-Containing Pharmaceuticals and the Phenomenon of the Self-Disproportionation of Enantiomers in Late-Stage Fluorination of Bioactive Molecules and Biologically-Relevant Substrates; Postigo, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 321–355. [Google Scholar]
- Han, J.; Wzorek, A.; Klika, K.D.; Soloshonok, V.A. The Role of Fluorine in the Self-Disproportionation of Enantiomers (SDE) Phenomenon of Scalemic Samples of Fluoroorganics in Frontiers of Organofluorine Chemistry; Ojima, I., Ed.; World Scientific Publishing Co.: London, UK, 2020; pp. 283–340. [Google Scholar]
- Han, J.; Fustero, S.; Moriwaki, H.; Wzorek, A.; Soloshonok, V.A.; Klika, K.D. The self-disproportionation of enantiomers (SDE): Fluorine as an SDE-phoric substituent. In Organofluorine Chemistry: Synthesis, Modeling, and Applications; Szabó, K.J., Selander, N., Eds.; Wiley-VCH: Weinheim, Germany, 2021; pp. 281–306. [Google Scholar]
- Wzorek, A.; Sato, A.; Drabowicz, J.; Soloshonok, V.A. Self-disproportionation of enantiomers (SDE) of chiral nonracemic amides via achiral chromatography. Isr. J. Chem. 2016, 56, 977–989. [Google Scholar] [CrossRef]
- Pracejus, G. Optische aktivierung von N-Phthalyl-α-aminosäure-derivaten durch tert-basen-katalyse. Liebigs Ann. Chem. 1959, 622, 10–22. [Google Scholar] [CrossRef]
- Kwart, H.; Hoster, D.P. Separation of an enantiomorph and its racemate by sublimation. J. Org. Chem. 1967, 32, 1867–1870. [Google Scholar] [CrossRef]
- Garin, D.L.; Cooke Greco, D.J.; Kelly, L. Enhancement of optical activity by fractional sublimation. An alternative to fractional crystallization and a warning. J. Org. Chem. 1977, 42, 1249–1251. [Google Scholar] [CrossRef]
- Tsuzuki, S.; Orita, H.; Ueki, H.; Soloshonok, V.A. First principle lattice energy calculations for enantiopure and racemic crystals of α-(trifluoromethyl)lactic acid: Is self-disproportionation of enantiomers controlled by thermodynamic stability of crystals? J. Fluorine Chem. 2010, 131, 461–466. [Google Scholar] [CrossRef]
- Tonner, R.; Soloshonok, V.A.; Schwerdtfeger, P. Theoretical investigations into the enantiomeric and racemic forms of α-(trifluoromethyl)lactic acid. Phys. Chem. Chem. Phys. 2011, 13, 811–817. [Google Scholar] [CrossRef]
- Wang, J.; Sánchez-Roselló, M.; Aceña, J.L.; del Pozo, C.; Sorochinsky, A.E.; Fustero, S.; Soloshonok, V.A.; Liu, H. Fluorine in pharmaceutical industry: Fluorine-containing drugs introduced to the market in the last decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J.L.; Soloshonok, V.A.; Izawa, K.; Liu, H. Next generation of fluorine-containing pharmaceuticals, compounds currently in phase II–III clinical trials of major pharmaceutical companies: New structural trends and therapeutic areas. Chem. Rev. 2016, 116, 422–518. [Google Scholar] [CrossRef] [PubMed]
- Cundy, K.C.; Crooks, P.A. Unexpected phenomenon in the high-performance liquid chromatographic analysis of racemic 14C-labelled nicotine: Separation of enantiomers in a totally achiral system. J. Chromatogr. 1983, 281, 17–33. [Google Scholar] [CrossRef]
- Reyes-Rangel, G.; Vargas-Caporali, J.; Juaristi, E. Asymmetric Michael addition reaction organocatalyzed by stereoisomeric pyrrolidine sulfinamides under neat conditions. A brief study of self-disproportionation of enantiomers. Tetrahedron 2017, 73, 4707–4718. [Google Scholar] [CrossRef]
- Li, J.; Mo, Y.; Yan, L.; Feng, X.; Su, Z.; Liu, X. Organocatalytic stereoselective [8+2] cycloaddition of tropones with azlactones. CCS Chem. 2021, 3, 784–793. [Google Scholar] [CrossRef]
- Kotora, M.; Cadart, T.; Nečas, D.; Kaiser, R.P.; Favereau, L.; Císařová, I.; Gyepes, R.; Hodačová, J.; Kalíková, K.; Bednárová, L.; et al. Rhodium catalyzed enantioselective synthesis of highly fluorescent and CPL active Dispiroindeno[2,1-c]fluorenes. Chem. Eur. J. 2021. [Google Scholar] [CrossRef]
- Robak, M.A.; Herbage, M.A.; Ellman, J.A. Synthesis and applications of tert-Butanesulfinamide. Chem. Rev. 2010, 110, 3600–3740. [Google Scholar] [CrossRef]
- Fioravanti, S. Trifluoromethyl aldimines: An overview in the last ten years. Tetrahedron 2016, 72, 4449–4489. [Google Scholar] [CrossRef]
- Mei, H.; Xie, C.; Han, J.; Soloshonok, V.A. N-tert-Butanesulfinyl-(3,3,3)-trifluoroacetaldimine: Versatile reagent for asymmetric synthesis of trifluoromethyl-containing amines and amino acids of pharmaceutical importance. Eur. J. Org. Chem. 2016, 2016, 5917–5932. [Google Scholar] [CrossRef]
- Mei, H.; Han, J.; Fustero, S.; Román, R.; Ruzziconi, R.; Soloshonok, V.A. Recent progress in the application of fluorinated chiral sulfinimine reagents. J. Fluorine Chem. 2018, 216, 57–70. [Google Scholar] [CrossRef]
- Agranat, I.; Caner, H. Intellectual property and chirality of drugs. Drug Discov. Today 1999, 4, 313–321. [Google Scholar] [CrossRef]
- Bentley, R. Role of sulfur chirality in the chemical processes of biology. Chem. Soc. Rev. 2005, 34, 609–624. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, G.E.; Kelly, P.; Lawrence, S.E.; Maguire, A.R. Synthesis of enantioenriched sulfoxides. Arkivoc 2011, 2011, 1–110. [Google Scholar] [CrossRef]
- Wojaczynska, E.; Wojaczynski, J. Enantioselective synthesis of sulfoxides: 2000–2009. Chem. Rev. 2010, 110, 4303–4356. [Google Scholar] [CrossRef]
- O’Mahony, G.E.; Ford, A.; Maguire, A.R. Asymmetric oxidation of sulfides. J. Sulfur Chem. 2013, 34, 301–341. [Google Scholar] [CrossRef]
- Sipos, G.; Drinkel, E.E.; Dorta, R. The emergence of sulfoxides as efficient ligands in transition metal catalysis. Chem. Soc. Rev. 2015, 44, 3834–3860. [Google Scholar] [CrossRef]
- Otocka, S.; Kwiatkowska, M.; Madalińska, L.; Kiełbasiński, P. Chiral organosulfur ligands/catalysts with a stereogenic sulfur atom: Applications in asymmetric synthesis. Chem. Rev. 2017, 117, 4147–4181. [Google Scholar] [CrossRef]
- Brunel, J.-M.; Kagan, H.B. Catalytic enantioselective oxidation of sulfides with a chiral titanium complex. Bull. Soc. Chim. Fr. 1996, 133, 1109–1115. [Google Scholar]
- Kagan, H.B.; Riant, O. Advances in Asymmetric Synthesis; Elsevier: Amsterdam, The Netherlands, 1997; Volume 2, pp. 189–235. [Google Scholar]
- Brunel, J.-M.; Luukas, T.O.; Kagan, H.B. Nonlinear effects as ‘indicators’ in the tuning of asymmetric catalysts. Tetrahedron Asymmetry 1998, 9, 1941–1946. [Google Scholar] [CrossRef]
- Fenwick, D.R.; Kagan, H.B. Asymmetric amplification. In Top Stereochem; Denmark, S.E., Ed.; Wiley: Hoboken, NJ, USA, 1999; Volume 22, pp. 257–296. [Google Scholar]
- Mata, E.G. Recent advances in the synthesis of sulfoxides from sulfides. Phosphorus Sulfur Silicon Relat. Elem. 1996, 117, 231–286. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, C.; Dai, Z.; Yang, M.; Pan, Y.; Hu, H. Efficient asymmetric oxidation of sulfides and kinetic resolution of sulfoxides catalyzed by a vanadium–salan system. J. Org. Chem. 2004, 69, 8500–8503. [Google Scholar] [CrossRef] [PubMed]
- Sprout, C.M.; Seto, C.T. Using enzyme inhibition as a high throughput method to measure the enantiomeric excess of a chiral sulfoxide. Org. Lett. 2005, 7, 5099–5102. [Google Scholar] [CrossRef]
- Legros, J.; Bolm, C. Investigations on the iron-catalyzed asymmetric sulfide oxidation. Chem. Eur. J. 2005, 11, 1086–1092. [Google Scholar] [CrossRef]
- Tanaka, H.; Nishikawa, H.; Uchida, T.; Katsuki, T. Photopromoted ru-catalyzed asymmetric aerobic sulfide oxidation and epoxidation using water as a proton transfer mediator. J. Am. Chem. Soc. 2010, 132, 12034–12041. [Google Scholar] [CrossRef]
- Han, J.; Sorochinsky, A.E.; Ono, T.; Soloshonok, V.A. Biomimetic transamination–A metal-free alternative to the reductive amination. Application for generalized preparation of fluorine-containing amines and amino acids. Curr. Org. Synth. 2011, 8, 281–294. [Google Scholar] [CrossRef]
- Soloshonok, V.A.; Ono, T.; Soloshonok, I.V. Enantioselective biomimetic transamination of β-Keto carboxylic acid derivatives. An efficient asymmetric synthesis of β-Fluoroalkyl-β-amino acids. J. Org. Chem. 1997, 62, 7538–7539. [Google Scholar] [CrossRef]
- Soloshonok, V.A.; Kirilenko, A.G.; Fokina, N.A.; Galushko, S.V.; Kukhar, V.P.; Svedas, V.K.; Resnati, G. Chemo-enzymatic approach to the synthesis of each of the four isomers of α-Alkyl-β-Fluoroalkyl-substituted β-amino acids. Tetrahedron Asymmetry 1994, 5, 1225–1228. [Google Scholar] [CrossRef]
- Soloshonok, V.A.; Yasumoto, M. Catalytic asymmetric synthesis of α-(Trifluoromethyl)benzylamine via cinchonidine derived base-catalyzed biomimetic 1,3-proton shift reaction. J. Fluorine Chem. 2007, 128, 170–173. [Google Scholar] [CrossRef]
- Soloshonok, V.A.; Kirilenko, A.G.; Galushko, S.V.; Kukhar, V.P. Catalytic asymmetric synthesis of β-Fluoroalkyl-β-amino acids via biomimetic [1,3]-proton shift reaction. Tetrahedron Lett. 1994, 35, 5063–5064. [Google Scholar] [CrossRef]
- Soloshonok, V.A.; Kukhar, V.P. Biomimetic transamination of α-Keto perfluorocarboxylic esters. An efficient preparative synthesis of β,β,β-Trifluoroalanine. Tetrahedron 1997, 53, 8307–8314. [Google Scholar] [CrossRef]
- Soloshonok, V.A.; Gerus, I.I.; Yagupolskii, Y.L.; Kukhar, V.P. Fluorine-containing amino acids. III. α-Trifluoromethyl amino acids. Zh. Org. Khim. 1987, 23, 2308–2313. [Google Scholar]
- Soloshonok, V.A.; Kirilenko, A.G.; Kukhar, V.P.; Resnati, G. Transamination of fluorinated β-Keto carboxylic esters. A biomimetic approach to β-Polyfluoroalkyl-β-amino acids. Tetrahedron Lett. 1993, 34, 3621–3624. [Google Scholar] [CrossRef]
- Soloshonok, V.A.; Kukhar, V.P. Biomimetic base-catalyzed [1,3]-proton shift reaction. A practical synthesis of β-Fluoroalkyl-β-amino acids. Tetrahedron 1996, 52, 6953–6964. [Google Scholar] [CrossRef]
- Zhu, Y.; Mao, Y.; Mei, H.; Pan, Y.; Han, J.; Soloshonok, V.A.; Hayashi, T. Palladium-catalyzed asymmetric allylic alkylations of colby pro-enolates with MBH carbonates: Enantioselective access to quaternary C-F oxindoles. Chem. Eur. J. 2018, 24, 8994–8998. [Google Scholar] [CrossRef] [PubMed]
- Sha, W.; Zhu, Y.; Mei, H.; Han, J.; Soloshonok, V.A.; Pan, Y. Catalytic enantioselective cyano-trifluoromethylation of styrenes. ChemistrySelect 2017, 2, 1129–1132. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.; Mei, H.; Han, J.; Soloshonok, V.A.; Pan, Y. Catalytic asymmetric aldol addition reactions of 3-fluoro-indolinone derived enolates. Org. Biomol. Chem. 2017, 15, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, W.; Mei, H.; Han, J.; Soloshonok, V.A.; Pan, Y. Catalytic enantioselective Michael addition reactions of tertiary enolates generated by detrifluoroacetylation. Chem. Eur. J. 2017, 23, 11221–11225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, C.; Dai, Y.; Mei, H.; Han, J.; Soloshonok, V.A.; Pan, Y. Catalytic asymmetric detrifluoroacetylative aldol reactions of aliphatic aldehydes for construction of C-F quaternary stereogenic centers. J. Fluorine Chem. 2016, 184, 28–35. [Google Scholar] [CrossRef]
- Sha, W.; Zhang, W.; Mei, H.; Soloshonok, V.A.; Han, J.; Pan, Y. Catalytic cascade aldol-cyclization of tertiary ketone enolates for enantioselective synthesis of keto-esters with a C-F quaternary stereogenic center. Org. Biomol. Chem. 2016, 14, 7295–7303. [Google Scholar] [CrossRef]
- Xie, C.; Wu, L.; Han, J.; Soloshonok, V.A.; Pan, Y. Assembly of fluorinated quaternary stereogenic centers via catalytic enantioselective detrifluoroacetylative aldol reactions. Angew. Chem. Int. Ed. 2015, 54, 6019–6023. [Google Scholar] [CrossRef]
- Soloshonok, V.A.; Wzorek, A.; Klika, K.D. A question of policy: Should tests for the self-disproportionation of enantiomers (SDE) be mandatory for reports involving scalemates? Tetrahedron Asymmetry 2017, 28, 1430–1434. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.; Sha, W.; Mei, H.; Han, J.; Soloshonok, V.A. Detrifluoroacetylative generation and chemistry of fluorine containing tertiary enolates. J. Fluorine Chem. 2017, 198, 2–9. [Google Scholar] [CrossRef]
- Shibatomi, K.; Narayama, A.; Abe, Y.; Iwasa, S. Practical synthesis of 4,4,4-trifluorocrotonaldehyde: A versatile precursor for the enantioselective formation of trifluoromethylated stereogenic centers via organocatalytic 1,4-additions. Chem. Commun. 2012, 48, 7380–7382. [Google Scholar] [CrossRef]
- Shibatomi, K.; Narayama, A.; Soga, Y.; Muto, T.; Iwasa, S. Enantioselective gem-chlorofluorination of active methylene compounds using a chiral spiro oxazoline ligand. Org. Lett. 2011, 13, 2944–2947. [Google Scholar] [CrossRef]
- Shibatomi, K.; Soga, Y.; Narayama, A.; Fujisawa, I.; Iwasa, S. Highly enantioselective chlorination of β-Keto esters and subsequent SN2 displacement of tertiary chlorides: A flexible method for the construction of quaternary stereogenic centers. J. Am. Chem. Soc. 2012, 134, 9836–9839. [Google Scholar] [CrossRef]
- Shibatomi, K.; Futatsugi, K.; Kobayashi, F.; Iwasa, S.; Yamamoto, H. Stereoselective construction of halogenated quaternary stereogenic centers via catalytic asymmetric diels−alder reaction. J. Am. Chem. Soc. 2010, 132, 5625–5627. [Google Scholar] [CrossRef]
- Huang, G.; Yang, J.; Zhang, X. Highly enantioselective zinc/BINOL-catalyzed alkynylation of α-ketoimine ester: A new entry to optically active quaternary α-CF3 α-amino acids. Chem. Commun. 2011, 47, 5587–5589. [Google Scholar] [CrossRef] [PubMed]
- Walęcka-Kurczyk, A.; Walczak, K.; Kuźnik, A.; Stecko, S.; Październiok-Holewa, A. The synthesis of α-Aminophosphonates via enantioselective organocatalytic reaction of 1-(N-Acylamino)alkylphosphonium salts with dimethyl phosphite. Molecules 2020, 25, 405. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Wzorek, A.; Klika, K.D.; Soloshonok, V.A. Recommended Tests for the Self-Disproportionation of Enantiomers (SDE) to Ensure Accurate Reporting of the Stereochemical Outcome of Enantioselective Reactions. Molecules 2021, 26, 2757. https://doi.org/10.3390/molecules26092757
Han J, Wzorek A, Klika KD, Soloshonok VA. Recommended Tests for the Self-Disproportionation of Enantiomers (SDE) to Ensure Accurate Reporting of the Stereochemical Outcome of Enantioselective Reactions. Molecules. 2021; 26(9):2757. https://doi.org/10.3390/molecules26092757
Chicago/Turabian StyleHan, Jianlin, Alicja Wzorek, Karel D. Klika, and Vadim A. Soloshonok. 2021. "Recommended Tests for the Self-Disproportionation of Enantiomers (SDE) to Ensure Accurate Reporting of the Stereochemical Outcome of Enantioselective Reactions" Molecules 26, no. 9: 2757. https://doi.org/10.3390/molecules26092757
APA StyleHan, J., Wzorek, A., Klika, K. D., & Soloshonok, V. A. (2021). Recommended Tests for the Self-Disproportionation of Enantiomers (SDE) to Ensure Accurate Reporting of the Stereochemical Outcome of Enantioselective Reactions. Molecules, 26(9), 2757. https://doi.org/10.3390/molecules26092757