Cocrystal of Apixaban–Quercetin: Improving Solubility and Bioavailability of Drug Combination of Two Poorly Soluble Drugs
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Experimental Animals
2.3. Preparation of Apx–Que–2ACN
2.4. Preparation of Apx–Que
2.5. SXRD
2.6. PXRD
2.7. DSC
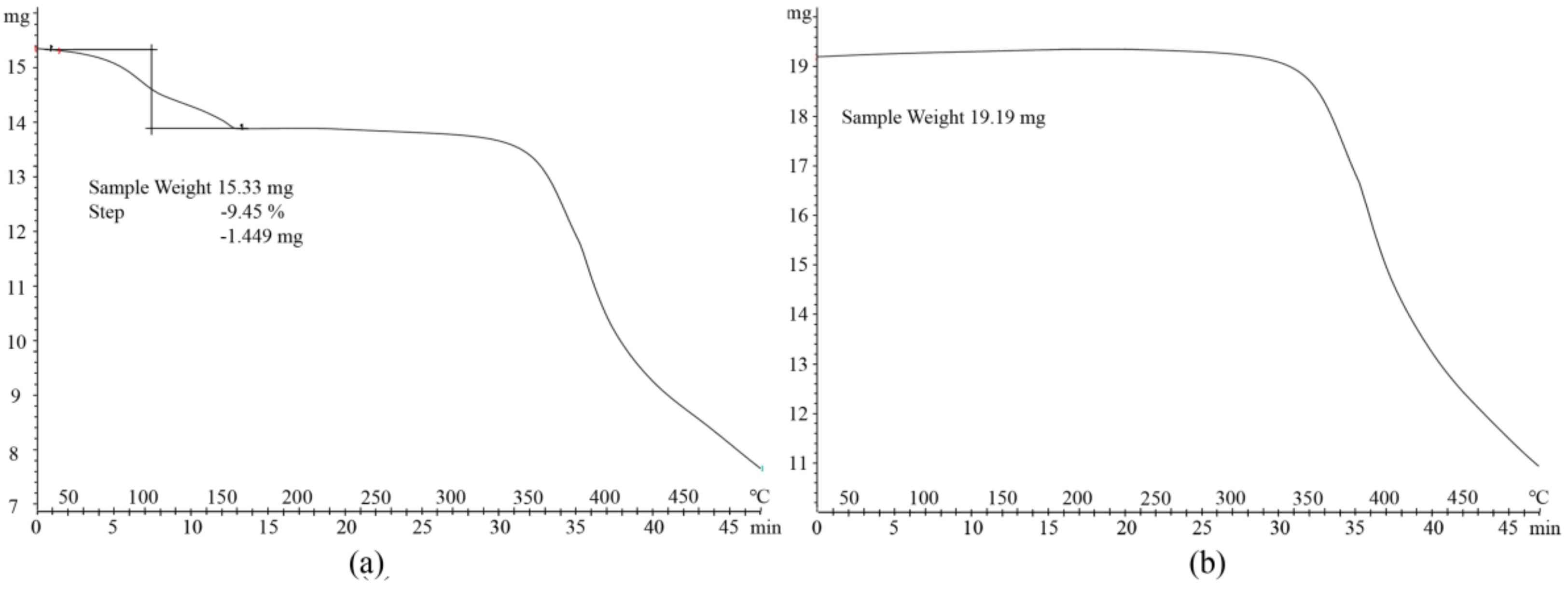
2.8. TGA
2.9. Stability Test
2.10. High Performance Liquid Chromatography (HPLC) Conditions
2.11. Powder Dissolution Measurements
2.12. Intrinsic Dissolution Measurements
2.13. Pharmacokinetic Study
2.13.1. Chromatographic Conditions
2.13.2. Method Validation
2.13.3. Pharmacokinetics
3. Results and Discussion
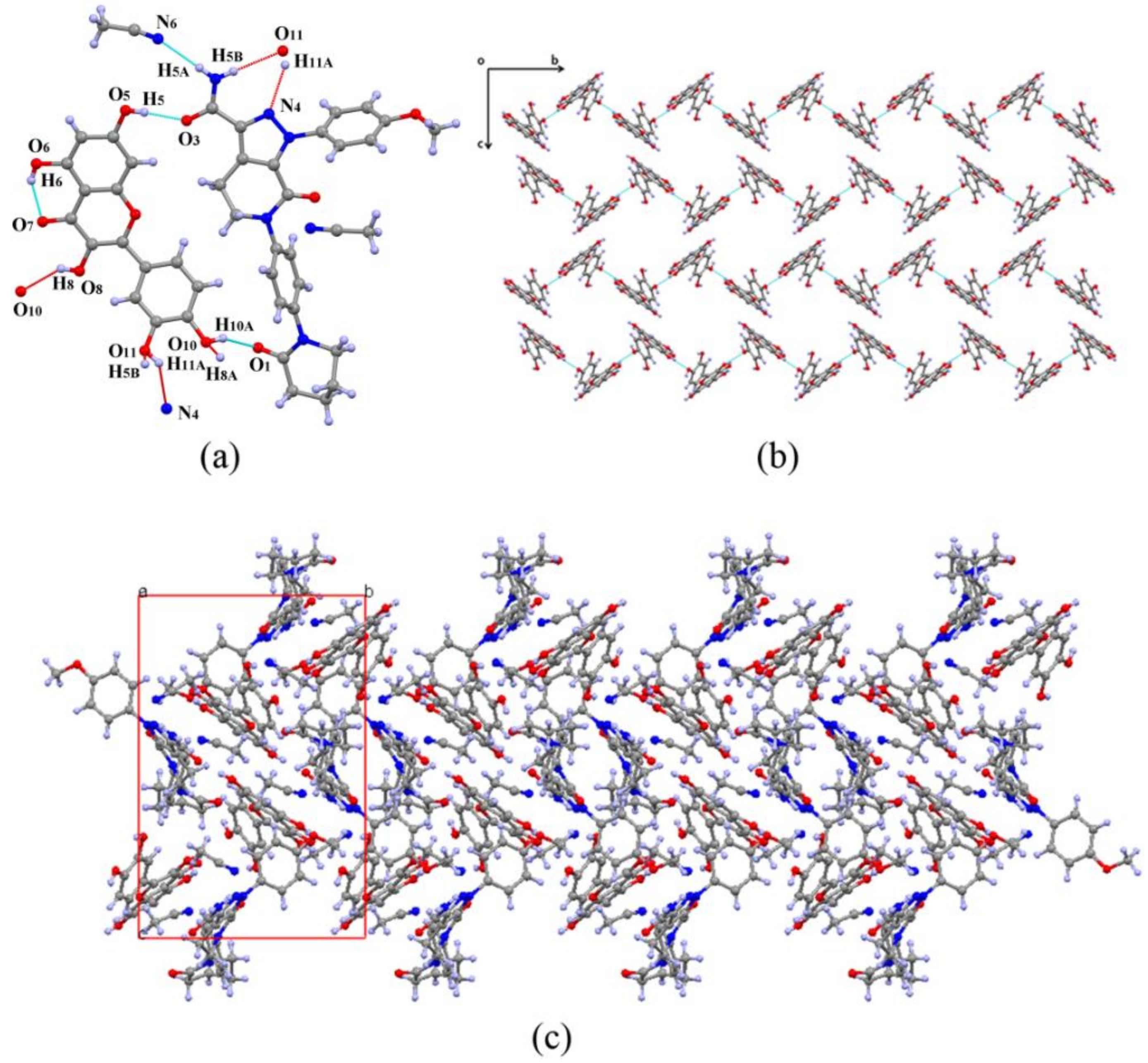
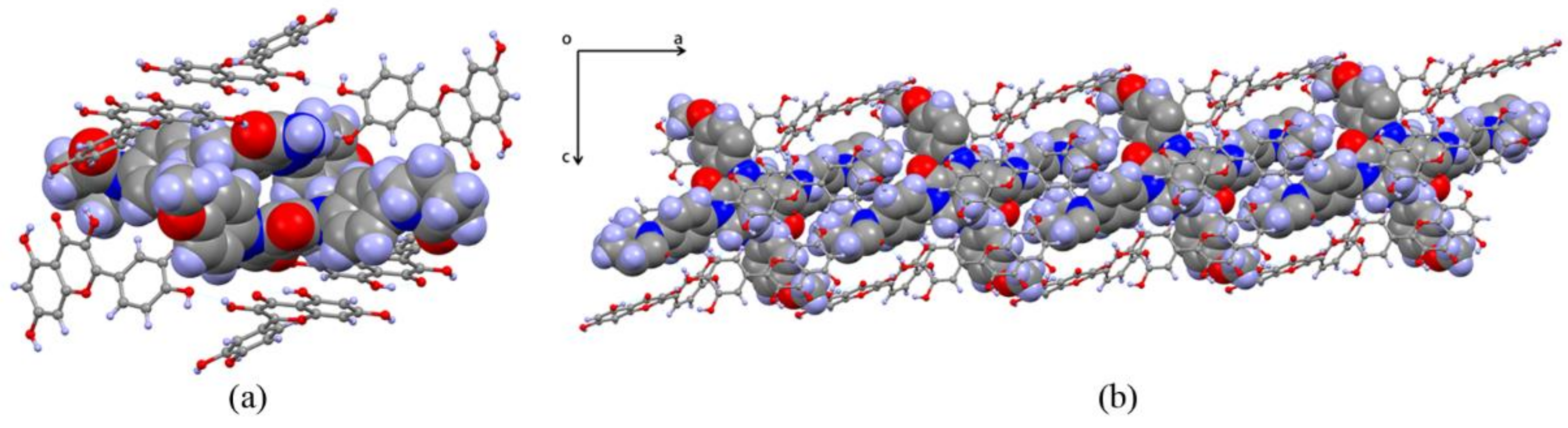
3.1. Crystal Structure Analysis
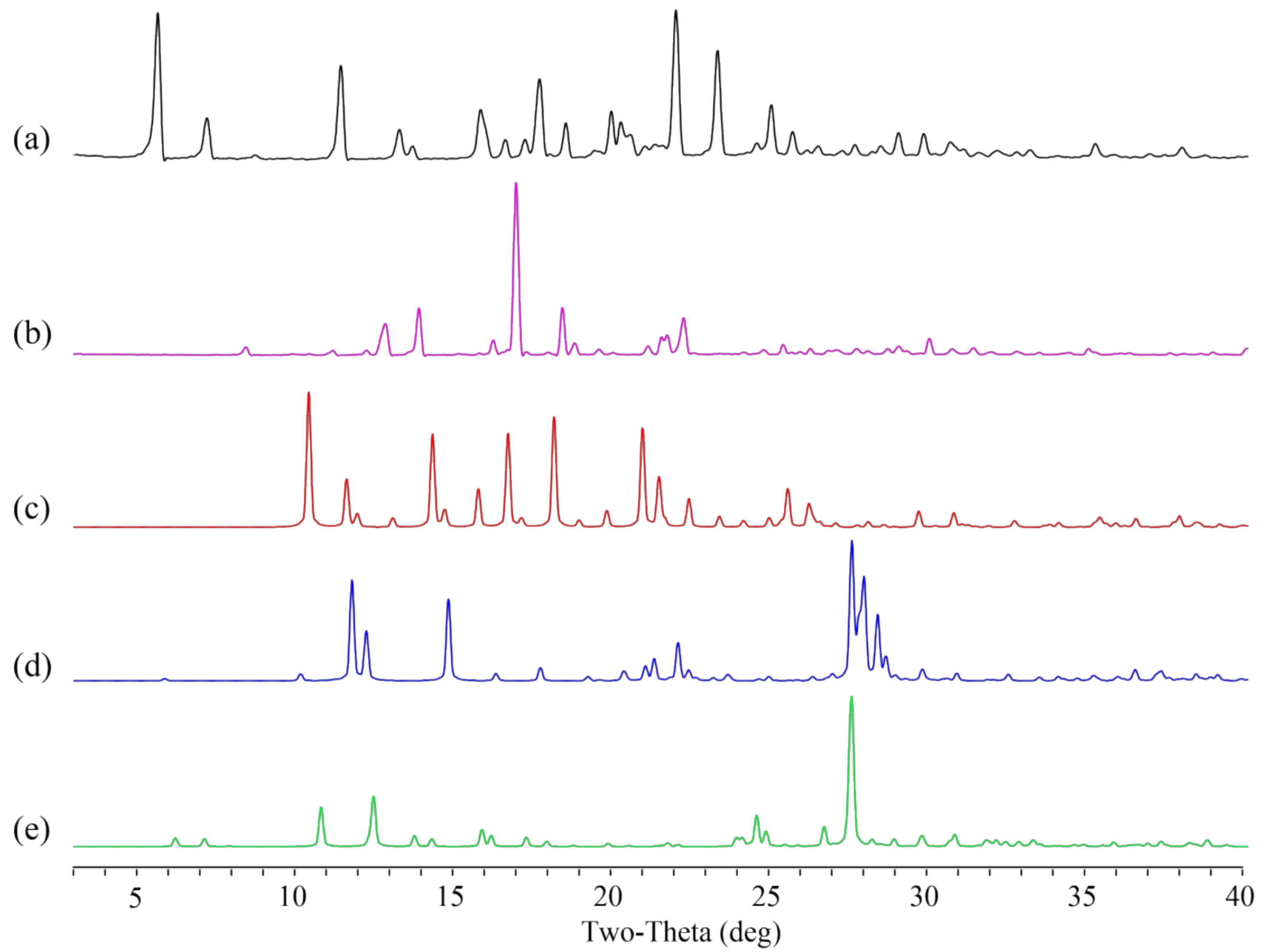
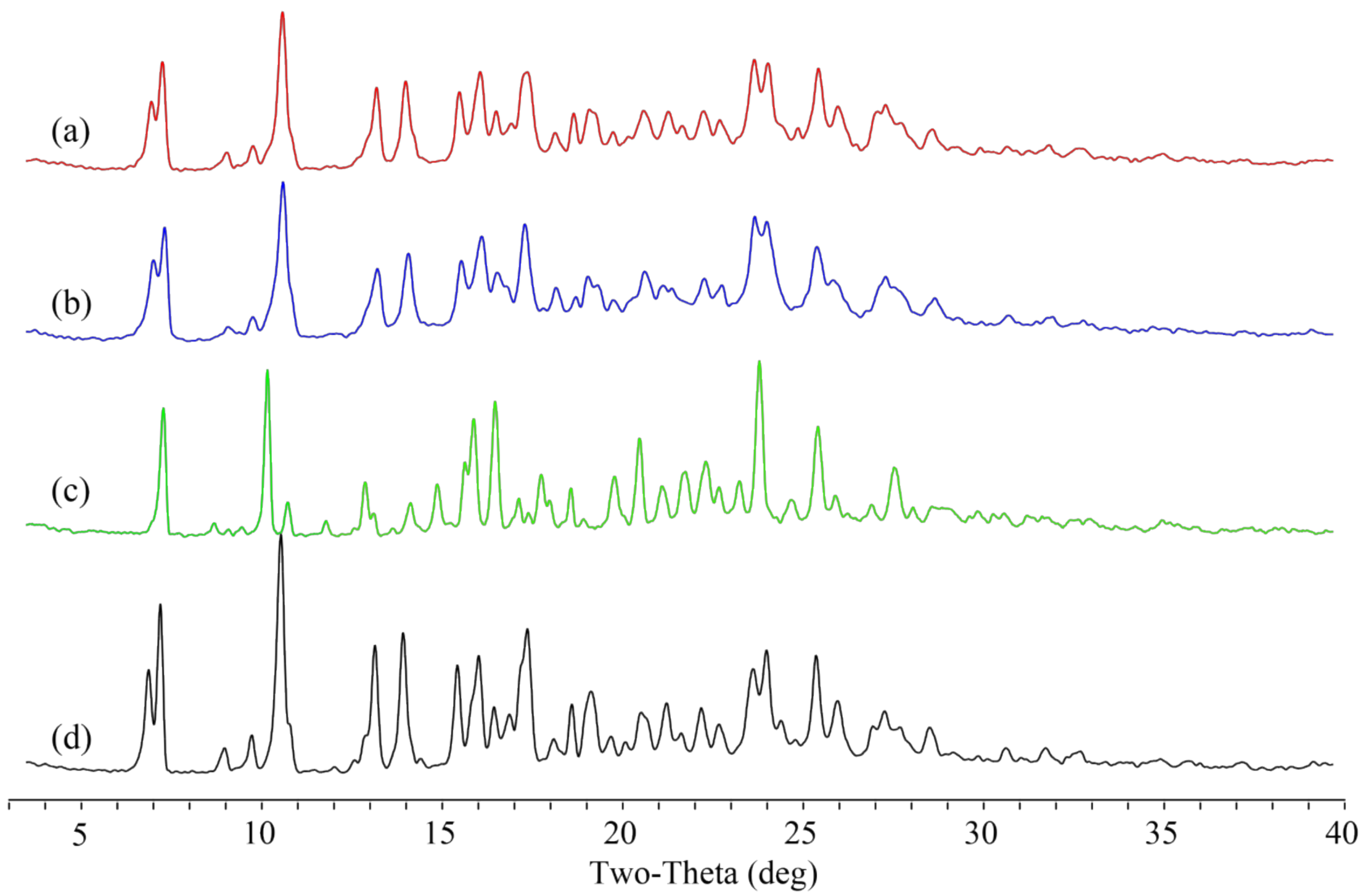
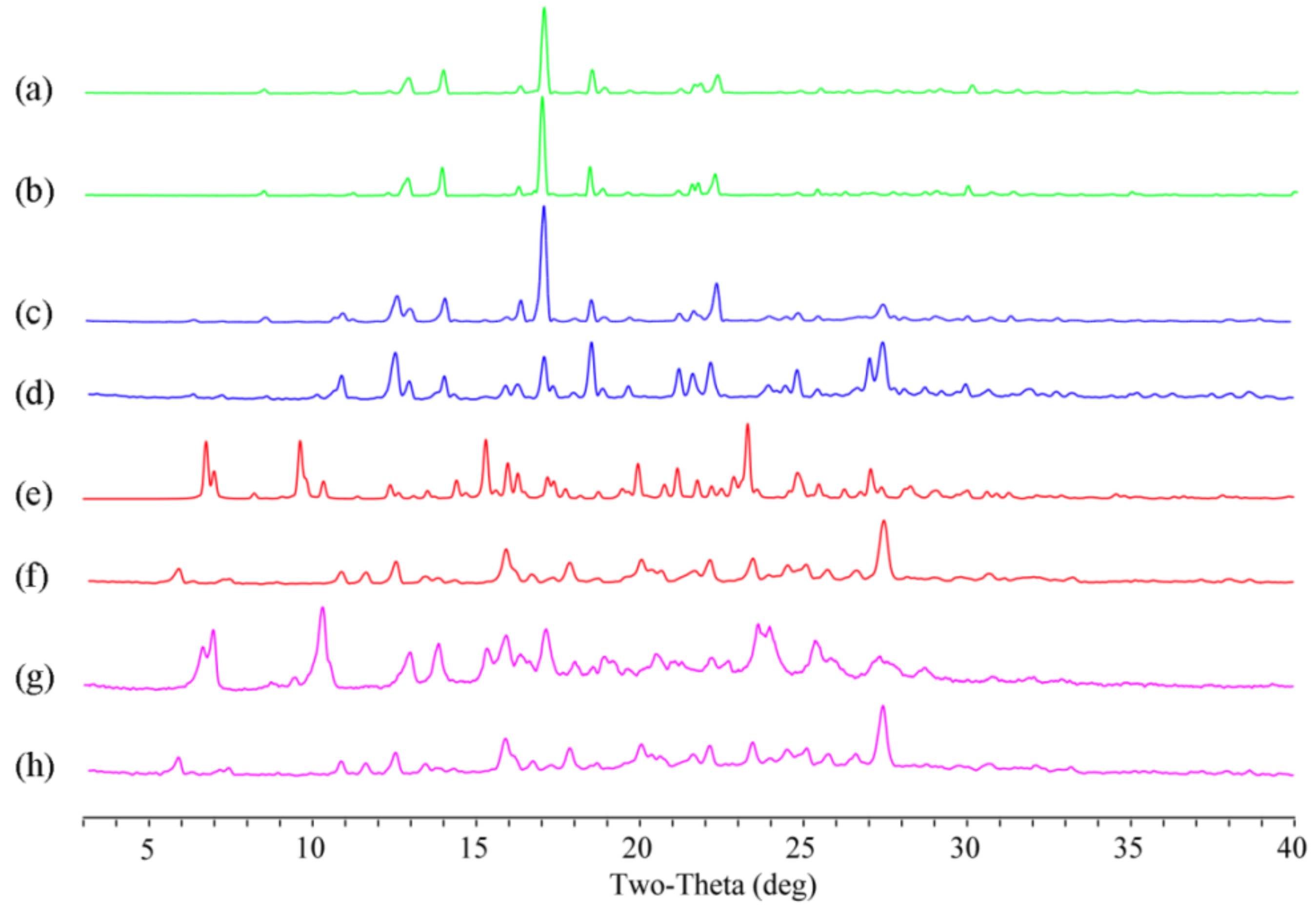
3.2. PXRD Analysis
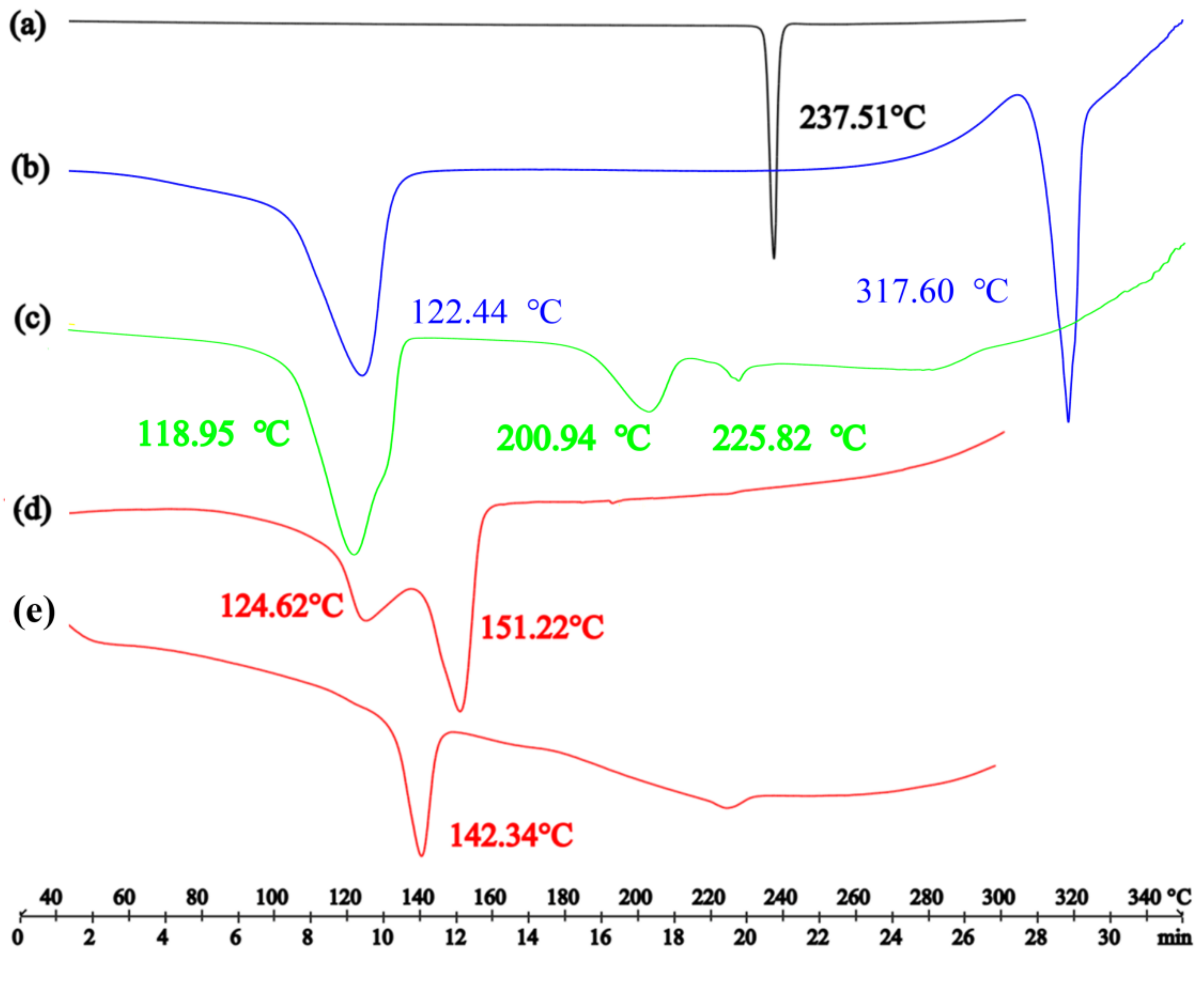
3.3. Thermal Analysis
3.4. Stability Analysis
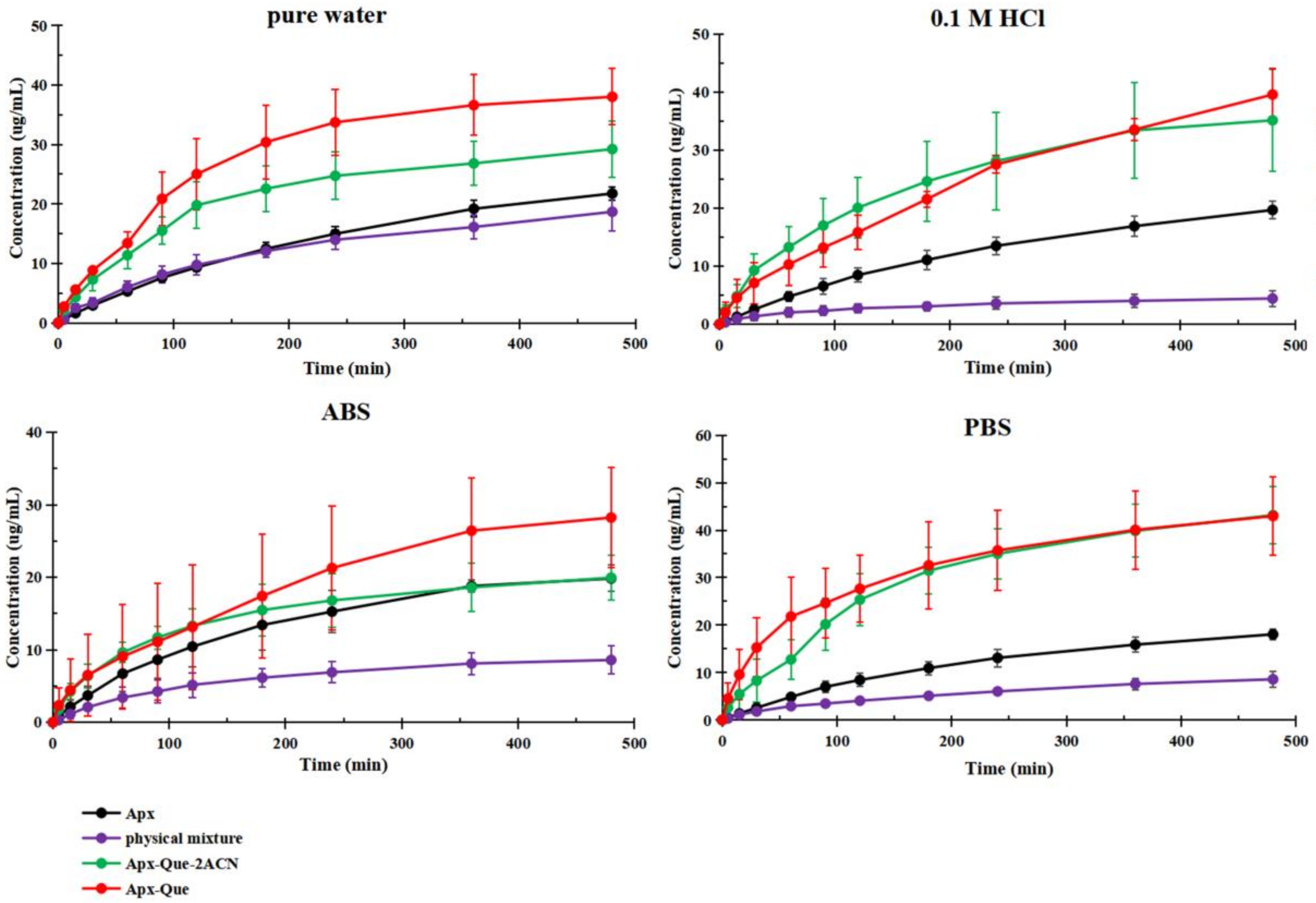
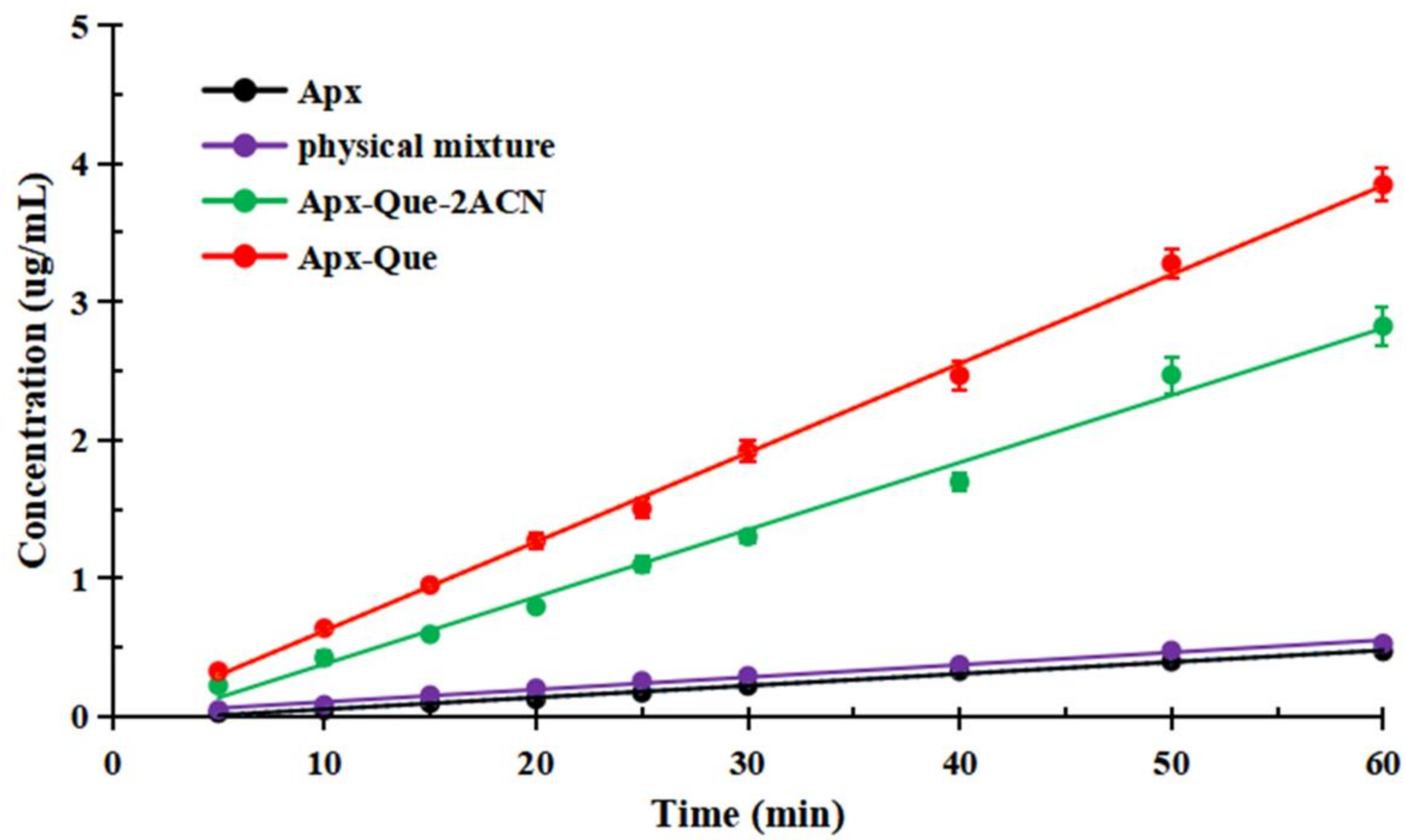
3.5. Powder Dissolution Measurements and IDR Studies
3.6. Pharmacokinetic Study
3.6.1. Method Validation
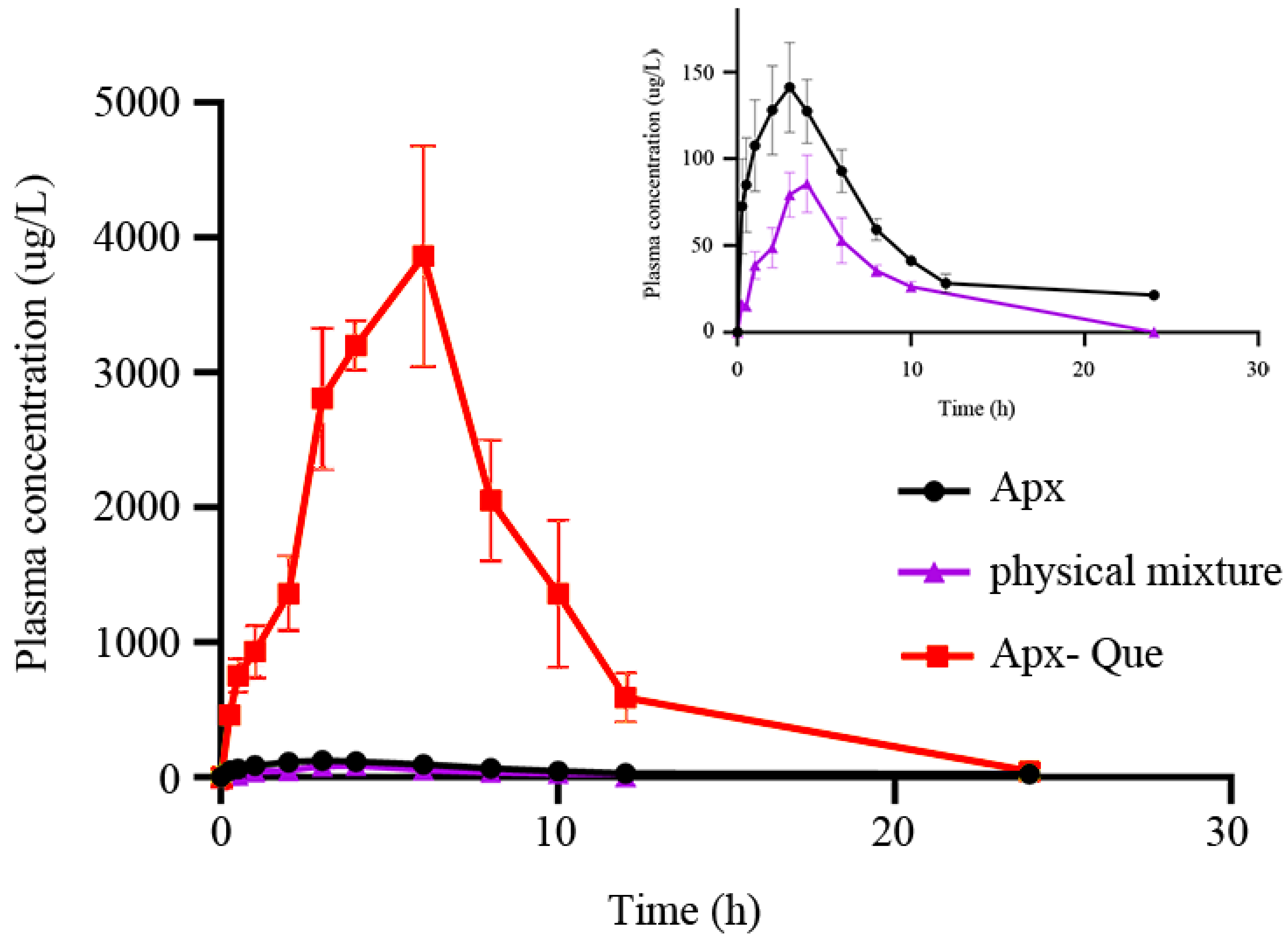
3.6.2. Pharmacokinetic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Competing Financial Interests:
Conflicts of Interest
Sample Availability
References
- Fleig, S.V.; Weger, B.; Haller, H.; Limbourg, F.P. Effectiveness of a Fixed-Dose, Single-Pill Combination of Perindopril and Amlodipine in Patients with Hypertension: A Non-Interventional Study. Adv. Ther. 2018, 35, 353–366. [Google Scholar] [CrossRef]
- Bakris, G.L.; A Sarafidis, P.; Weir, M.R.; Dahlöf, B.; Pitt, B.; Jamerson, K.; Velazquez, E.J.; Staikos-Byrne, L.; Kelly, R.Y.; Shi, V.; et al. Renal outcomes with different fixed-dose combination therapies in patients with hypertension at high risk for cardiovascular events (ACCOMPLISH): A prespecified secondary analysis of a randomised controlled trial. Lancet 2010, 375, 1173–1181. [Google Scholar] [CrossRef]
- Egan, C.G.; Pontremoli, R. Role of the fixed-dose combination lercanidipine-enalapril in renal protection. J. Nephrol. 2011, 24, 428–437. [Google Scholar] [CrossRef]
- German, P.; Mathias, A.; Brainard, D.M.; Kearney, B.P. Drug–Drug Interaction Profile of the Fixed-Dose Combination Tablet Regimen Ledipasvir/Sofosbuvir. Clin. Pharmacokinet. 2018, 57, 1369–1383. [Google Scholar] [CrossRef] [PubMed]
- Giles, T.D.; A Weber, M.; Basile, J.; Gradman, A.H.; Bharucha, D.B.; Chen, W.; Pattathil, M. Efficacy and safety of nebivolol and valsartan as fixed-dose combination in hypertension: A randomised, multicentre study. Lancet 2014, 383, 1889–1898. [Google Scholar] [CrossRef]
- Hair, P.I.; Scott, L.J.; Perry, C.M. Fixed-dose combination lercanidipine/enalapril. Drugs 2007, 67, 95–106. [Google Scholar] [CrossRef]
- Lawitz, E.; Poordad, F.F.; Pang, P.S.; Hyland, R.H.; Ding, X.; Mo, H.; Symonds, W.T.; McHutchison, J.G.; E Membreno, F. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): An open-label, randomised, phase 2 trial. Lancet 2014, 383, 515–523. [Google Scholar] [CrossRef]
- Poordad, F.; Sievert, W.; Mollison, L.; Bennett, M.; Tse, E.; Bräu, N.; Levin, J.; Sepe, T.; Lee, S.S.; Angus, V.; et al. Fixed-Dose Combination Therapy With Daclatasvir, Asunaprevir, and Beclabuvir for Noncirrhotic Patients With HCV Genotype 1 Infection. JAMA 2015, 313, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- Tshefu, A.K.; Gaye, O.; Kayentao, K.; Thompson, R.; Bhatt, K.M.; Sesay, S.S.S.; Bustos, D.G.; Tjitra, E.; Bedu-Addo, G.; Borghini-Fuhrer, I.; et al. Efficacy and safety of a fixed-dose oral combination of pyronaridine-artesunate compared with artemether-lumefantrine in children and adults with uncomplicated Plasmodium falciparum malaria: A randomised non-inferiority trial. Lancet 2010, 375, 1457–1467. [Google Scholar] [CrossRef]
- Figueirêdo, C.B.M.; Nadvorny, D.; Vieira, A.C.Q.D.M.; Schver, G.C.R.D.M.; Sobrinho, J.L.S.; Neto, P.J.R.; Lee, P.I.; Soares, M.F.D.L.R. Enhanced delivery of fixed-dose combination of synergistic antichagasic agents posaconazole-benznidazole based on amorphous solid dispersions. Eur. J. Pharm. Sci. 2018, 119, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Glass, S.A.-K.A.M.H.W.B.D.; Agatonovic-Kustrin, S.; Wisch, M.H. Artificial Neural Networks to Optimize Formulation Components of a Fixed- Dose Combination of Rifampicin, Isoniazid and Pyrazinamide in a Microemulsion. Curr. Drug Discov. Technol. 2005, 2, 195–201. [Google Scholar] [CrossRef]
- Mitra, A.; Wu, Y. Challenges and Opportunities in Achieving Bioequivalence for Fixed-Dose Combination Products. AAPS J. 2012, 14, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Shishoo, C.J.; A Shah, S.; Rathod, I.S.; Savale, S.S.; Vora, M.J. Impaired bioavailability of rifampicin in presence of isoniazid from fixed dose combination (FDC) formulation. Int. J. Pharm. 2001, 228, 53–67. [Google Scholar] [CrossRef]
- Singh, S.; Mariappan, T.; Shankar, R.; Sarda, N.; Singh, B. A critical review of the probable reasons for the poor variable bioavailability of rifampicin from anti-tubercular fixed-dose combination (FDC) products, and the likely solutions to the problem. Int. J. Pharm. 2001, 228, 5–17. [Google Scholar] [CrossRef]
- Taupitz, T.; Dressman, J.B.; Klein, S. New formulation approaches to improve solubility and drug release from fixed dose combinations: Case examples pioglitazone/glimepiride and ezetimibe/simvastatin. Eur. J. Pharm. Biopharm. 2013, 84, 208–218. [Google Scholar] [CrossRef]
- Bethune, S.J.; Huang, N.; Jayasankar, A.; Rodríguez-Hornedo, N. Understanding and Predicting the Effect of Cocrystal Components and pH on Cocrystal Solubility. Cryst. Growth Des. 2009, 9, 3976–3988. [Google Scholar] [CrossRef]
- Friščić, T.; Childs, S.L.; Rizvi, S.A.A.; Jones, W. The role of solvent in mechanochemical and sonochemical cocrystal formation: A solubility-based approach for predicting cocrystallisation outcome. CrystEngComm 2008, 11, 418–426. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Peng, B.; Zhu, B.; Wang, J.-R.; Mei, X. Improving Compliance and Decreasing Drug Accumulation of Diethylstilbestrol through Cocrystallization. Cryst. Growth Des. 2019, 19, 1942–1953. [Google Scholar] [CrossRef]
- Nicoli, S.; Bilzi, S.; Santi, P.; Caira, M.; Li, J.; Bettini, R. Ethyl-paraben and nicotinamide mixtures: Apparent solubility, thermal behavior and X-ray structure of the 1:1 co-crystal. J. Pharm. Sci. 2008, 97, 4830–4839. [Google Scholar] [CrossRef] [PubMed]
- Putra, O.D.; Furuishi, T.; Yonemochi, E.; Terada, K.; Uekusa, H. Drug–Drug Multicomponent Crystals as an Effective Technique to Overcome Weaknesses in Parent Drugs. Cryst. Growth Des. 2016, 16, 3577–3581. [Google Scholar] [CrossRef]
- Sa, R.; Zhang, Y.; Deng, Y.; Huang, Y.; Zhang, M.; Lou, B. Novel Salt Cocrystal of Chrysin with Berberine: Preparation, Characterization, and Oral Bioavailability. Cryst. Growth Des. 2018, 18, 4724–4730. [Google Scholar] [CrossRef]
- Almansa, C.; Mercè, R.; Tesson, N.; Farran, J.; Tomàs, J.; Plata-Salamán, C.R. Co-crystal of Tramadol Hydrochloride–Celecoxib (ctc): A Novel API–API Co-crystal for the Treatment of Pain. Cryst. Growth Des. 2017, 17, 1884–1892. [Google Scholar] [CrossRef]
- López-Cedrún, J.; Videla, S.; Burgueño, M.; Juárez, I.; Aboul-Hosn, S.; Martin-Granizo, R.; Grau, J.; Puche, M.; Gil-Diez, J.-L.; Hueto, J.-A.; et al. Co-crystal of Tramadol-Celecoxib in Patients with Moderate to Severe Acute Post-surgical Oral Pain: A Dose-Finding, Randomised, Double-Blind, Placebo- and Active-Controlled, Multicentre, Phase II Trial. Drugs R&D 2018, 18, 137–148. [Google Scholar] [CrossRef]
- Port, A.; Almansa, C.; Enrech, R.; Bordas, M.; Plata-Salamán, C.R. Differential Solution Behavior of the New API–API Co-Crystal of Tramadol–Celecoxib (CTC) versus Its Constituents and Their Combination. Cryst. Growth Des. 2019, 19, 3172–3182. [Google Scholar] [CrossRef]
- Videla, S.; Lahjou, M.; Vaqué, A.; Sust, M.; Escriche, M.; Soler, L.; Sans, A.; Sicard, E.; Gascón, N.; Encina, G.; et al. Pharmacokinetics of multiple doses of co-crystal of tramadol-celecoxib: Findings from a four-way randomized open-label phase I clinical trial. Br. J. Clin. Pharmacol. 2018, 84, 64–78. [Google Scholar] [CrossRef]
- Agnelli, G.; Buller, H.R.; Cohen, A.; Curto, M.; Gallus, A.S.; Johnson, M.; Porcari, A.; Raskob, G.E.; Weitz, J.I. Apixaban for extended treatment of venous thromboembolism. New Engl. J. Med. 2013, 368, 699–708. [Google Scholar] [CrossRef]
- Alexander, J.H.; Lopes, R.D.; James, S.; Kilaru, R.; He, Y.; Mohan, P.; Bhatt, D.L.; Goodman, S.; Verheugt, F.W.; Flather, M.; et al. Apixaban with Antiplatelet Therapy after Acute Coronary Syndrome. New Engl. J. Med. 2011, 365, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, N.; Frost, C.E.; Yu, Z.; He, K.; Zhang, H.; Humphreys, W.G.; Pinto, D.; Chen, S.; Bonacorsi, S.; Wong, P.C.; et al. Apixaban Metabolism and Pharmacokinetics after Oral Administration to Humans. Drug Metab. Dispos. 2008, 37, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.C.; Crain, E.J.; Xin, B.; Wexler, R.R.; Lam, P.Y.S.; Pinto, D.J.; Luettgen, J.M.; Knabb, R.M. Apixaban, an oral, direct and highly selective factor Xa inhibitor:in vitro, antithrombotic and antihemostatic studies. J. Thromb. Haemost. 2008, 6, 820–829. [Google Scholar] [CrossRef]
- Beretz, A.; Cazenave, J.-P.; Anton, R. Inhibition of aggregation and secretion of human platelets by quercetin and other flavonoids: Structure-activity relationships. Inflamm. Res. 1982, 12, 382–387. [Google Scholar] [CrossRef]
- Beretz, A.; Stierle, A.; Anton, R.; Cazenave, J.-P. Role of cyclic AMP in the inhibition of human platelet aggregation by quercetin, a flavonoid that potentiates the effect of prostacyclin. Biochem. Pharmacol. 1982, 31, 3597–3600. [Google Scholar] [CrossRef]
- Duan, Y.; Sun, N.; Xue, M.; Wang, X.; Yang, H. Synthesis of regioselectively acylated quercetin analogues with improved antiplatelet activity. Mol. Med. Rep. 2017, 16, 9735–9740. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, G.P.; Wolffram, S.; De Vos, R.; Bovy, A.; Gibbins, J.M.; A Lovegrove, J. Ingestion of onion soup high in quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in man: A pilot study. Br. J. Nutr. 2006, 96, 482–488. [Google Scholar] [PubMed]
- Kobzar, G.; Mardla, V.; Samel, N. Effects of α-tocopherol, l-arginine, and quercetin on aggregation of human platelets. Nutr. Res. 2005, 25, 569–575. [Google Scholar] [CrossRef]
- Oh, W.J.; Endale, M.; Park, S.-C.; Cho, J.Y.; Rhee, M.H. Dual Roles of Quercetin in Platelets: Phosphoinositide-3-Kinase and MAP Kinases Inhibition, and cAMP-Dependent Vasodilator-Stimulated Phosphoprotein Stimulation. Evidence-Based Complement. Altern. Med. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, L.; Yao, J.; Ma, Y.-Y.; Chen, J.-M.; Lu, T.-B. Improving the Solubility and Bioavailability of Apixaban via Apixaban–Oxalic Acid Cocrystal. Cryst. Growth Des. 2016, 16, 2923–2930. [Google Scholar] [CrossRef]
- Liu, F.; Wang, L.-Y.; Yu, M.-C.; Li, Y.-T.; Wu, Z.-Y.; Yan, C.-W. A new cocrystal of isoniazid-quercetin with hepatoprotective effect: The design, structure, and in vitro/in vivo performance evaluation. Eur. J. Pharm. Sci. 2020, 144, 105216. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.-T.; Yang, D.-Z.; Zhang, L.; Yang, S.-Y.; Du, G.-H.; Lu, Y. Salt solvates of quinolones and oxicams: Theoretical computation, structural characterization and dissolution studies. J. Mol. Struct. 2021, 1223, 128865. [Google Scholar] [CrossRef]
- Liu, X.; Michalchuk, A.A.L.; Pulham, C.R.; Boldyreva, E.V. An acetonitrile-solvated cocrystal of piroxicam and succinic acid with co-existing zwitterionic and non-ionized piroxicam molecules. Acta Crystallogr. Sect. C Struct. Chem. 2019, 75, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cheng, Y.; Xie, Q.; Xiao, B.; Wang, Z.; Liu, J.; Wu, G. Enhanced breakdown strength of aligned-sodium-titanate- nanowire/epoxy nanocomposites and their anisotropic dielectric properties. Compos. Part. A: Appl. Sci. Manuf. 2019, 120, 84–94. [Google Scholar] [CrossRef]
- Chen, S.; Meng, G.; Kong, B.; Xiao, B.; Wang, Z.; Jing, Z.; Gao, Y.; Wu, G.; Wang, H.; Cheng, Y. Asymmetric alicyclic amine-polyether amine molecular chain structure for improved energy storage density of high-temperature crosslinked polymer capacitor. Chem. Eng. J. 2020, 387, 123662. [Google Scholar] [CrossRef]
- Cui, J.; Zhou, Z.; Jia, M.; Chen, X.; Shi, C.; Zhao, N.; Guo, X. Solid Polymer Electrolytes with Flexible Framework of SiO2 Nanofibers for Highly Safe Solid Lithium Batteries. Polymers 2020, 12, 1324. [Google Scholar] [CrossRef]
- Pan, C.; Kou, K.; Zhang, Y.; Li, Z.; Wu, G. Enhanced through-plane thermal conductivity of PTFE composites with hybrid fillers of hexagonal boron nitride platelets and aluminum nitride particles. Compos. Part. B: Eng. 2018, 153, 1–8. [Google Scholar] [CrossRef]
- Pan, C.; Zhang, J.; Kou, K.; Zhang, Y.; Wu, G. Investigation of the through-plane thermal conductivity of polymer composites with in-plane oriented hexagonal boron nitride. Int. J. Heat Mass Transf. 2018, 120, 1–8. [Google Scholar] [CrossRef]
- Wan, L.; Zhang, X.; Wu, G.; Feng, A. Thermal conductivity and dielectric properties of bismaleimide/cyanate ester copolymer. High. Volt. 2017, 2, 167–171. [Google Scholar] [CrossRef]
- Machado, T.C.; Kuminek, G.; Cardoso, S.G.; Rodríguez-Hornedo, N. The role of pH and dose/solubility ratio on cocrystal dissolution, drug supersaturation and precipitation. Eur. J. Pharm. Sci. 2020, 152, 105422. [Google Scholar] [CrossRef]
- Shimpi, M.R.; Alhayali, A.; Cavanagh, K.L.; Rodríguez-Hornedo, N.; Velaga, S.P.J.C.G. Tadalafil–malonic acid cocrystal: Physicochemical characterization, pH-solubility, and supersaturation studies. Cryst. Growth Des. 2018, 18, 4378–4387. [Google Scholar]
- Yadav, B.; Gunnam, A.; Thipparaboina, R.; Nangia, A.K.; Shastri, N.R. Hepatoprotective Cocrystals of Isoniazid: Synthesis, Solid State Characterization, and Hepatotoxicity Studies. Cryst. Growth Des. 2019, 19, 5161–5172. [Google Scholar] [CrossRef]
- Ren, S.; Liu, M.; Hong, C.; Li, G.; Sun, J.; Wang, J.; Zhang, L.; Xie, Y. The effects of pH, surfactant, ion concentration, coformer, and molecular arrangement on the solubility behavior of myricetin cocrystals. Acta Pharm. Sin. B 2019, 9, 59–73. [Google Scholar] [CrossRef] [PubMed]
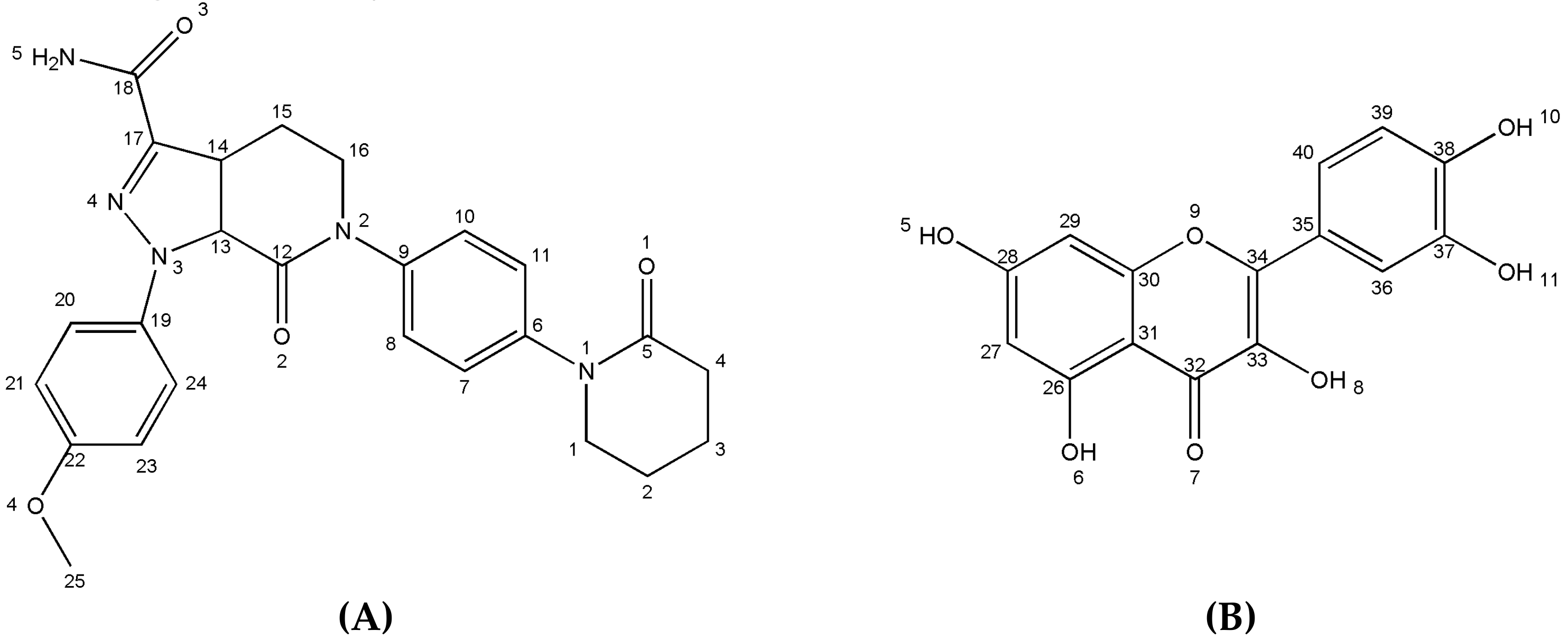


| Compound | Apx–Que–2ACN |
|---|---|
| Empirical formula | C44H41N7O11 |
| T (K) | 293 |
| Crystal system | monoclinic |
| Space group | P21/n |
| a (Å) | 17.115 |
| b (Å) | 12.888 |
| c (Å) | 19.500 |
| α (deg) | 90.00 |
| β (deg) | 92.06 |
| γ (deg) | 90.00 |
| Volume (Å3) | 4298 |
| Z | 4 |
| Calculated density (g/cm3) | 1.304 |
| Absorption coeff. (mm−1) | 0.794 |
| F (000) | 1768 |
| Crystal size (mm) | 0.2×0.2×0.2 |
| Rint | 0.0710 |
| R1 [I>2σ(I)] | 0.0712 |
| wR2 (all data) | 0.2170 |
| GOF | 1.087 |
| D-H···A | d (D···A) (Å) | ∠ (DHA) (deg) | Symmetry Code |
|---|---|---|---|
| N5-H5A···N6 | 3.053 | 172.33 | [-x+1, -y, -z+1] |
| N5-H5B···O11 | 3.180 | 165.31 | [x+1/2, -y+1/2, z+1/2] |
| O5-H5···O3 | 2.698 | 173.81 | [x, y+1, z] |
| O6-H6···O7 | 2.648 | 148.68 | |
| O8-H8A···O10 | 2.786 | 141.29 | [-x+1/2, y-1/2, -z+1/2] |
| O11-H11A···N4 | 2.878 | 135.87 | [x-1/2, -y+1/2, z-1/2] |
| O10-H10A···O1 | 2.615 | 160.38 | [x, y+1, z ] |
| Samples | Original Sample | Apx | Physical Mixture | Apx–Que–2ACN | Apx–Que |
|---|---|---|---|---|---|
| Pure water | 7 | 6.5 | 6.1 | 6.2 | 6.0 |
| 0.1 M HCl | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 |
| ABS | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 |
| PBS | 6.8 | 6.8 | 6.8 | 6.8 | 6.8 |
| Parameter | Apx | Physical Mixture | Apx–Que |
|---|---|---|---|
| AUC0-t (μg·L−1·h) | 1164.92 ± 314.64 | 485.77 ± 146.96 | 28,994.69 ± 7555.7 |
| AUC0-∞ (μg·L−1·h) | 1363.98 ± 316.70 | 677.07 ± 134.62 | 29,173.15 ± 7646.13 |
| MRT0-t (h) | 6.26 ± 1.8 | 4.69 ± 0.64 | 6.41 ± 1.22 |
| MRT0-∞ (h) | 10.33 ± 5.89 | 7.36 ± 2.03 | 6.54 ± 1.234 |
| t1/2 (h) | 6.89 ± 4.51 | 3.92 ± 2.11 | 3.02 ± 0.46 |
| Tmax (h) | 2.80 ± 1.30 | 4.17 ± 1.94 | 5.40 ± 1.34 |
| CLz/F (L·h−1·kg−1) | 46.24 ± 12.20 | 91.22 ± 15.84 | 2.19 ± 10.66 |
| Vz/F (L·kg−1) | 0.428 ± 0.234 | 0.494 ± 0.239 | 0.009 ± 0.004 |
| Cmax (μg·L−1) | 141.18 ± 47.78 | 95.67 ± 39.14 | 4551.72 ± 932.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Kong, D.; Wang, H.; Jiao, L.; Zhao, X.; Song, J.; Yang, D.; Yang, H.; Yang, S.; Du, G.; et al. Cocrystal of Apixaban–Quercetin: Improving Solubility and Bioavailability of Drug Combination of Two Poorly Soluble Drugs. Molecules 2021, 26, 2677. https://doi.org/10.3390/molecules26092677
Zhang L, Kong D, Wang H, Jiao L, Zhao X, Song J, Yang D, Yang H, Yang S, Du G, et al. Cocrystal of Apixaban–Quercetin: Improving Solubility and Bioavailability of Drug Combination of Two Poorly Soluble Drugs. Molecules. 2021; 26(9):2677. https://doi.org/10.3390/molecules26092677
Chicago/Turabian StyleZhang, Li, Dewen Kong, Hongjuan Wang, Lingtai Jiao, Xiaoyue Zhao, Junke Song, Dezhi Yang, Haiguang Yang, Shiying Yang, Guanhua Du, and et al. 2021. "Cocrystal of Apixaban–Quercetin: Improving Solubility and Bioavailability of Drug Combination of Two Poorly Soluble Drugs" Molecules 26, no. 9: 2677. https://doi.org/10.3390/molecules26092677
APA StyleZhang, L., Kong, D., Wang, H., Jiao, L., Zhao, X., Song, J., Yang, D., Yang, H., Yang, S., Du, G., & Lu, Y. (2021). Cocrystal of Apixaban–Quercetin: Improving Solubility and Bioavailability of Drug Combination of Two Poorly Soluble Drugs. Molecules, 26(9), 2677. https://doi.org/10.3390/molecules26092677










