Alkaloids of the Genus Datura: Review of a Rich Resource for Natural Product Discovery
Abstract
1. Introduction
2. History and Taxonomy
2.1. Medicine and Culture
2.2. Description and Taxonomy
3. Alkaloid Isolation and Purification, and Analytical Techniques for Detection, Quantification, and Identification
3.1. Extraction and Purification of Alkaloids
3.2. Crude Detection, Thin-Layer Chromatographic (TLC), Colorimetric, Densitometric, and Optical Methods
3.3. Gas Chromatography-Mass Spectrometry (GC-MS)
3.4. Liquid Chromatography-Mass Spectrometry (LC-MS) and LC-Tandem Mass Spectrometry (LC-MS/MS)
3.5. Other Mass Spectrometry Methods
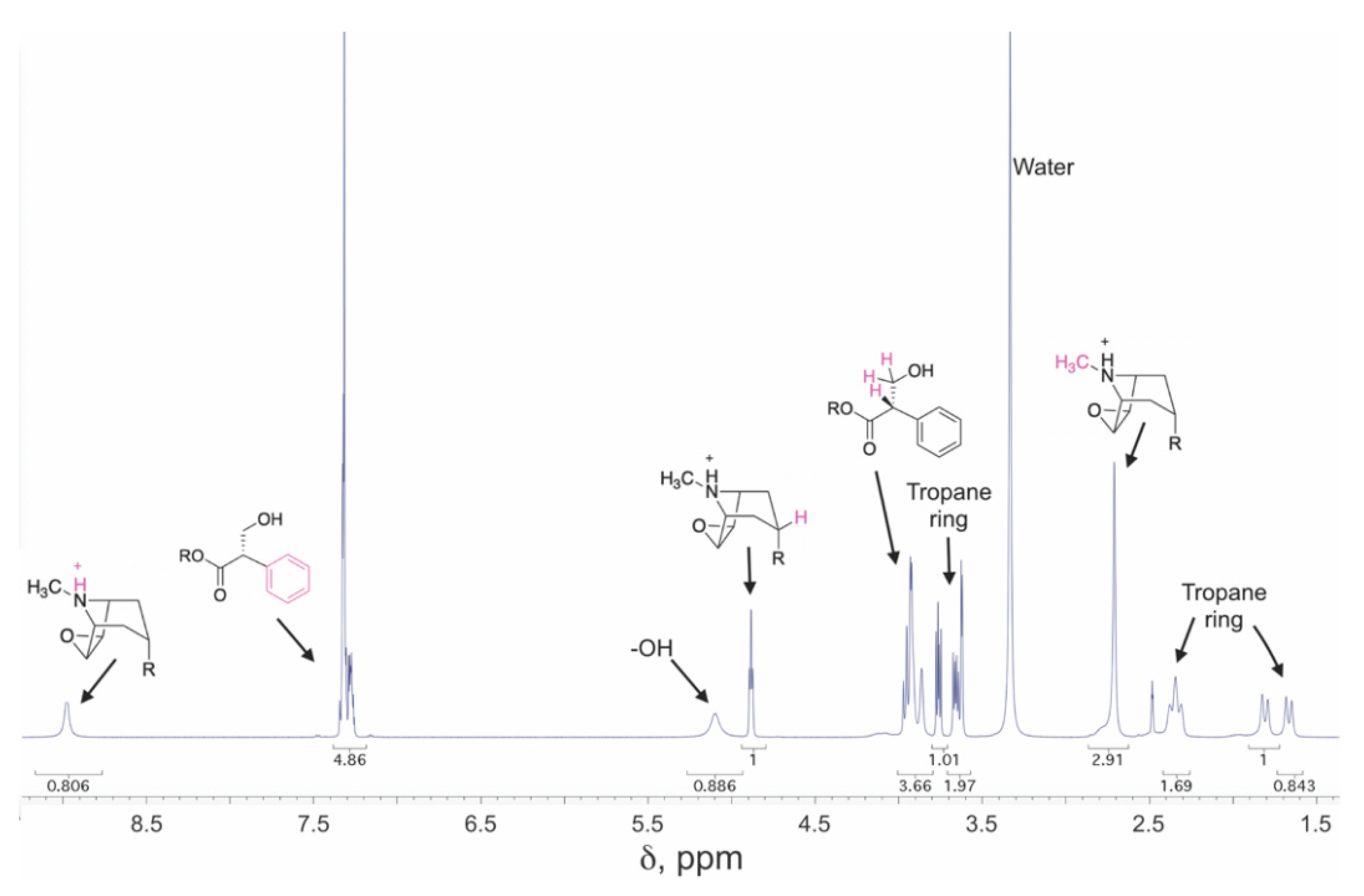
3.6. Nuclear Magnetic Resonance (NMR) Spectroscopy
4. Alkaloids of the Genus Datura—Tropane Alkaloids
4.1. History—And Some Troublesome Aspects of Identifying and Reporting Tropane Alkaloids
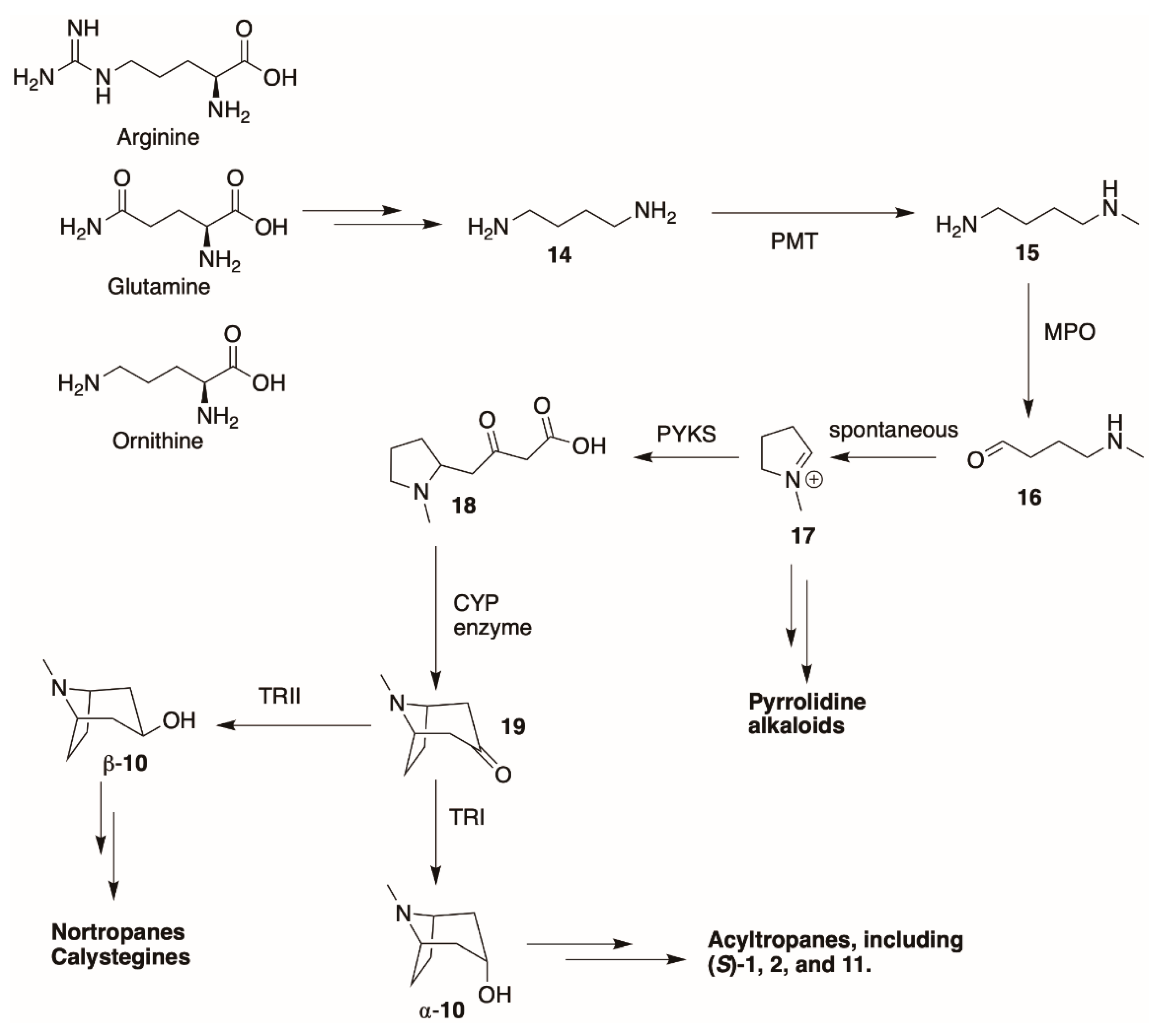
4.2. Biosynthesis of the Tropane Core
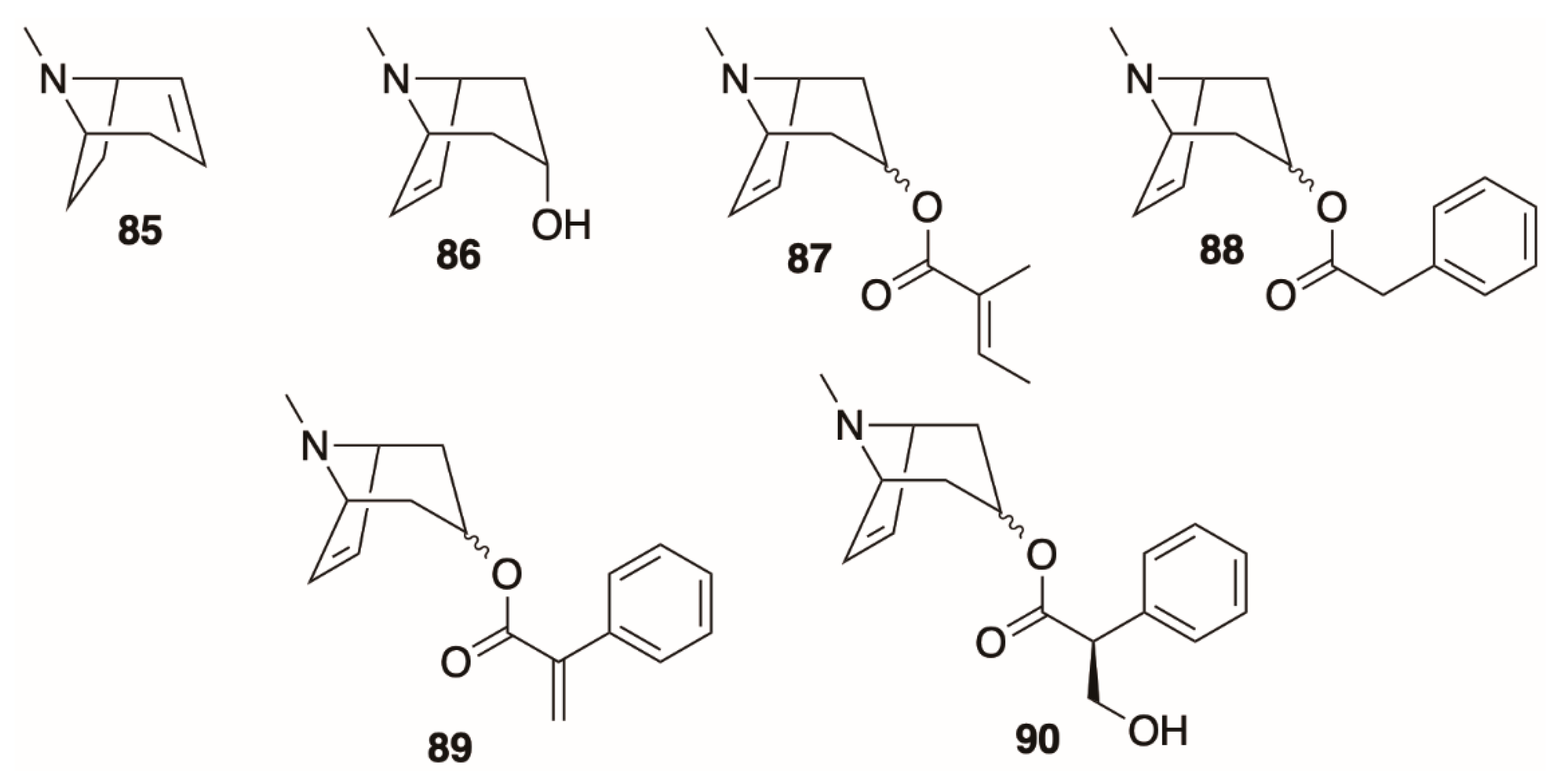
4.3. Monosubstituted Tropanes
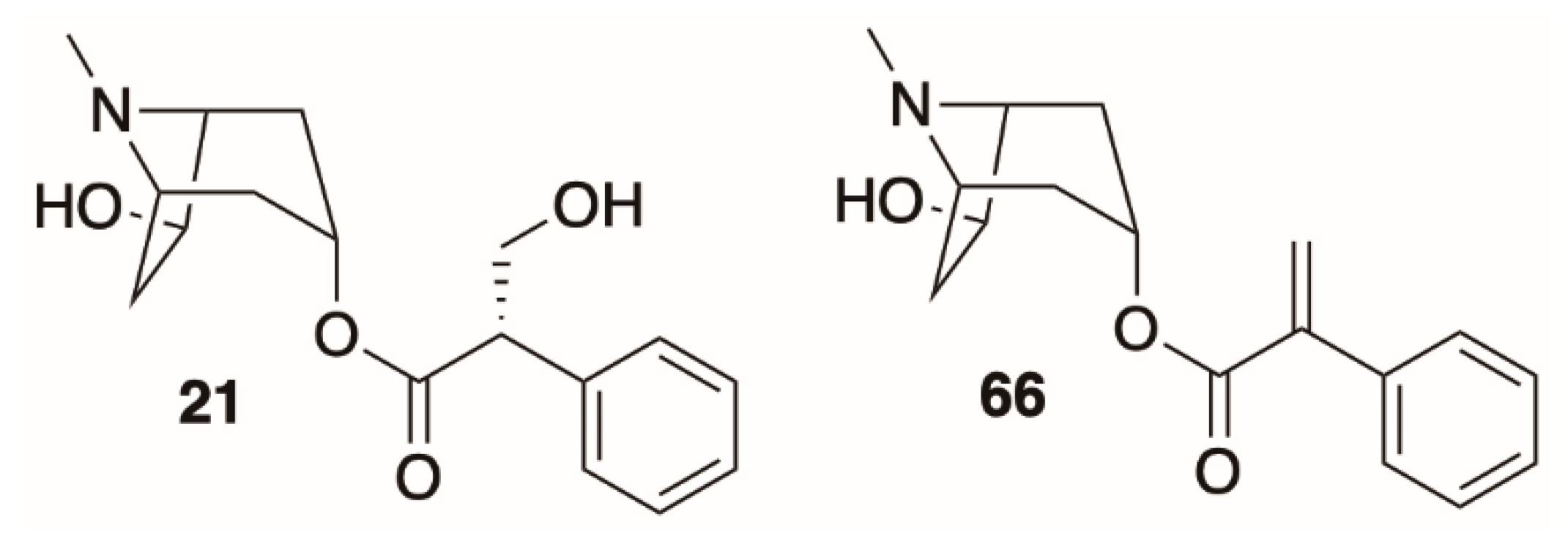
4.4. Disubstituted Tropanes
4.5. Trisubstituted and Epoxytropanes
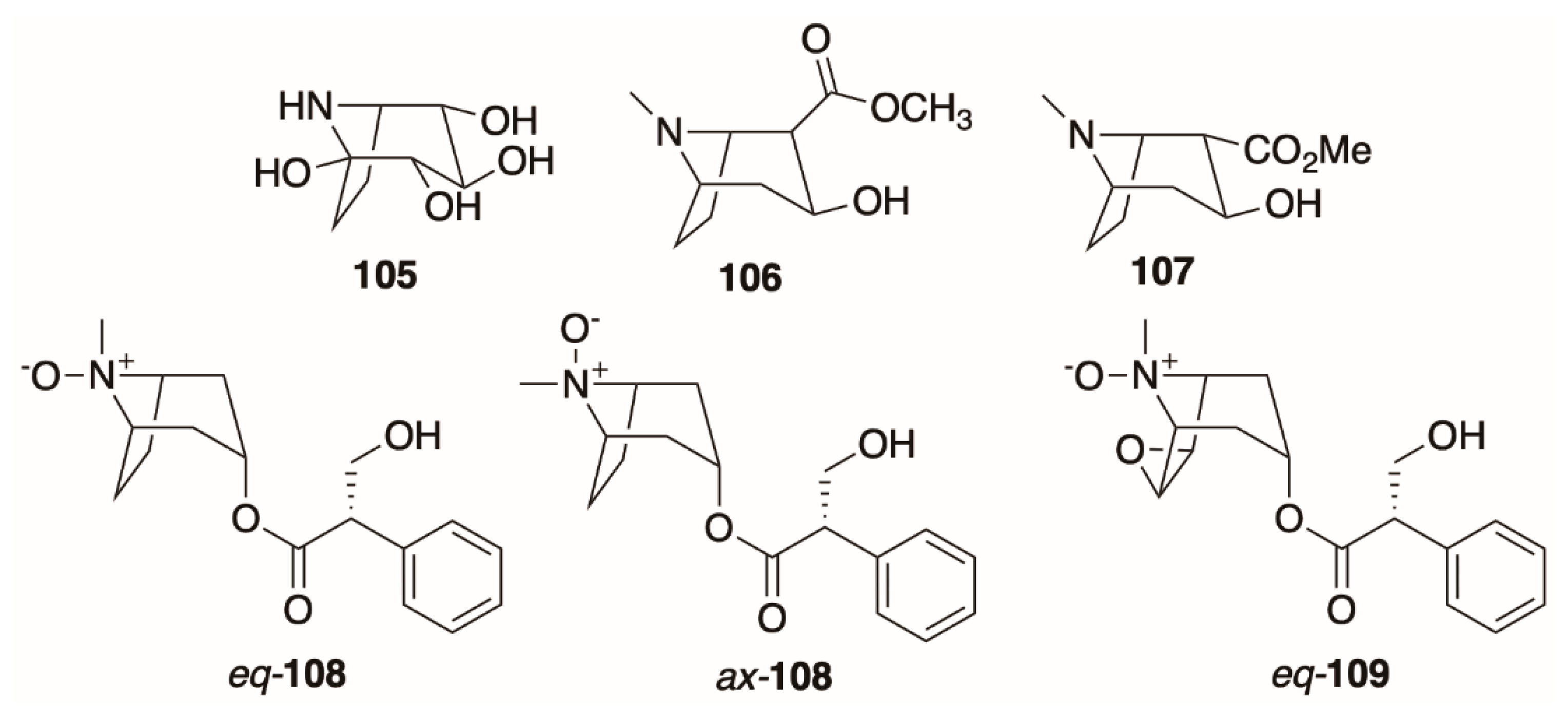
4.6. Dehydrotropanes, Nortropanes, and Calystegines
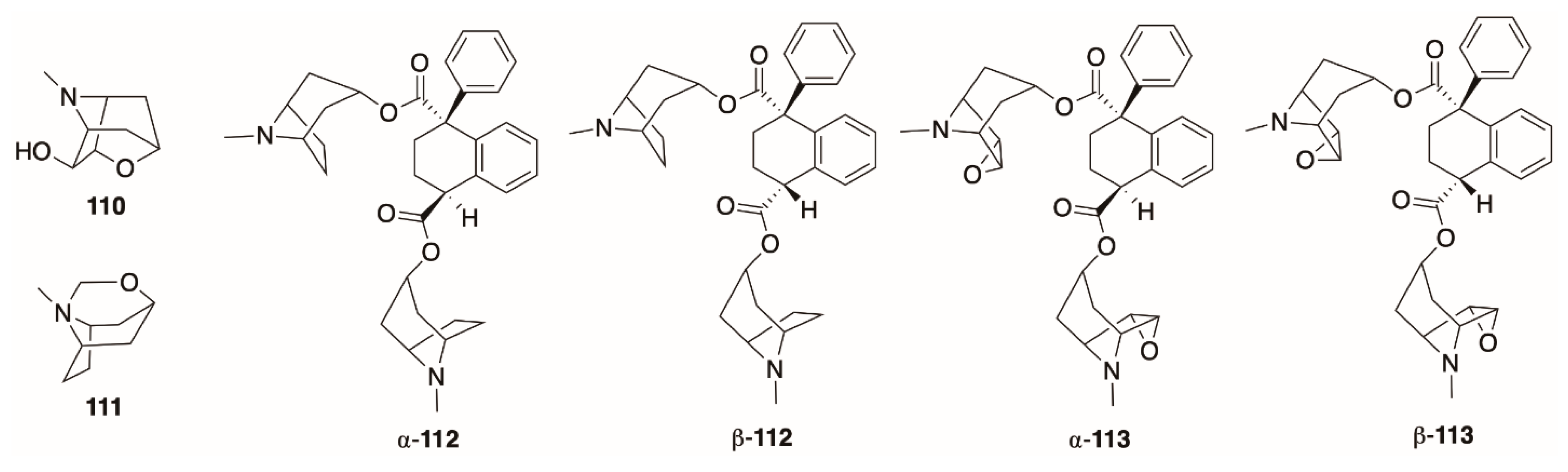
4.7. Ecgonine Derivatives, Tropane N-Oxides, and Cyclic and Dimeric Tropane Alkaloids
5. Alkaloids of the Genus Datura—Non-Tropane Alkaloids
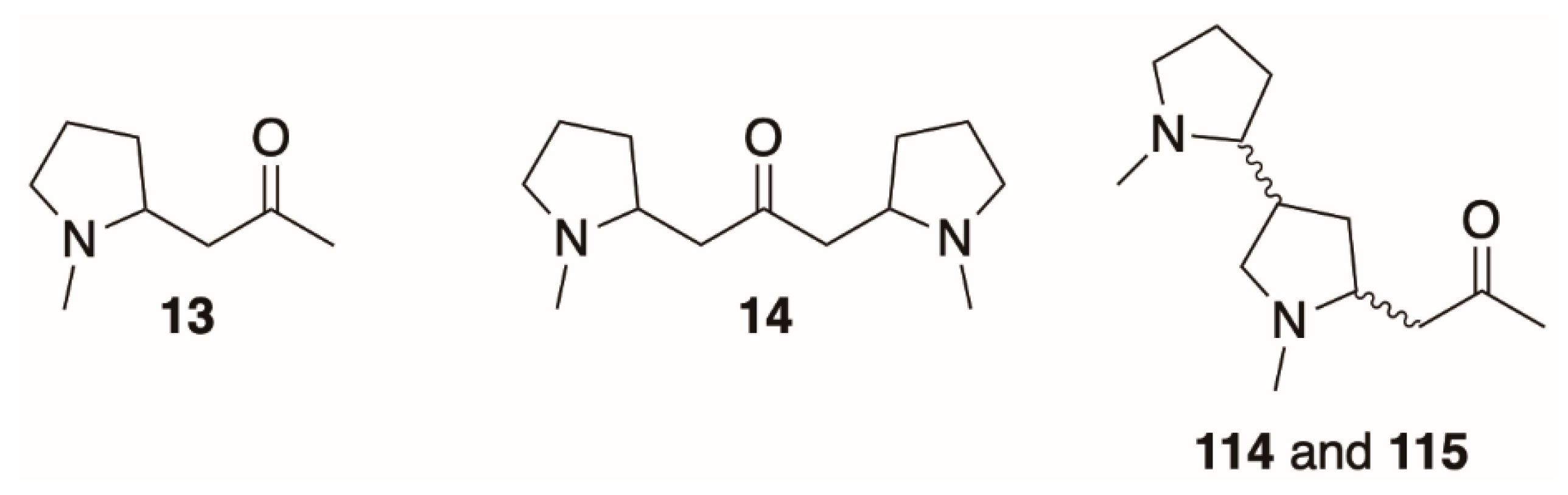
5.1. Pyrrolidines
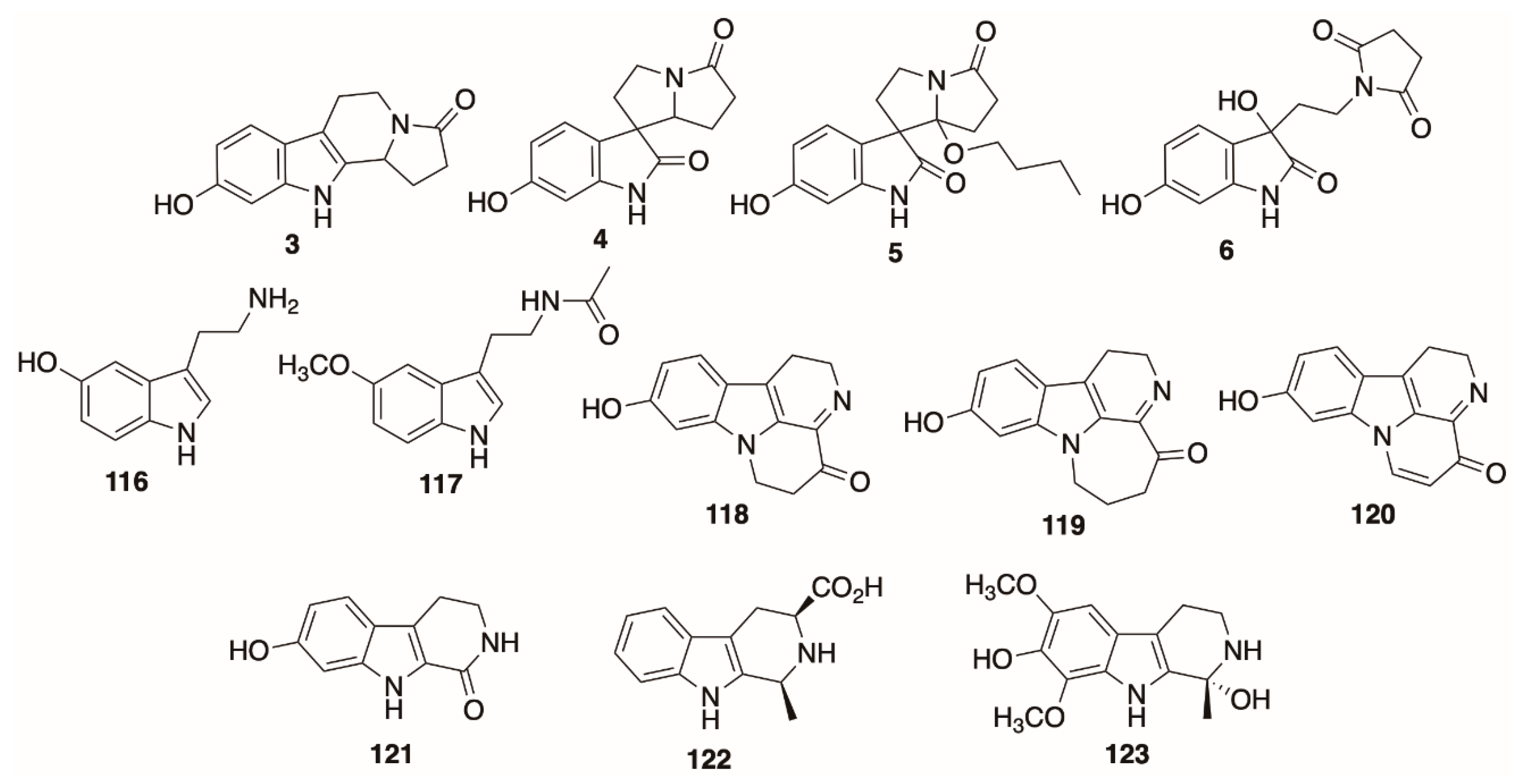
5.2. Indoles and Beta-Carbolines
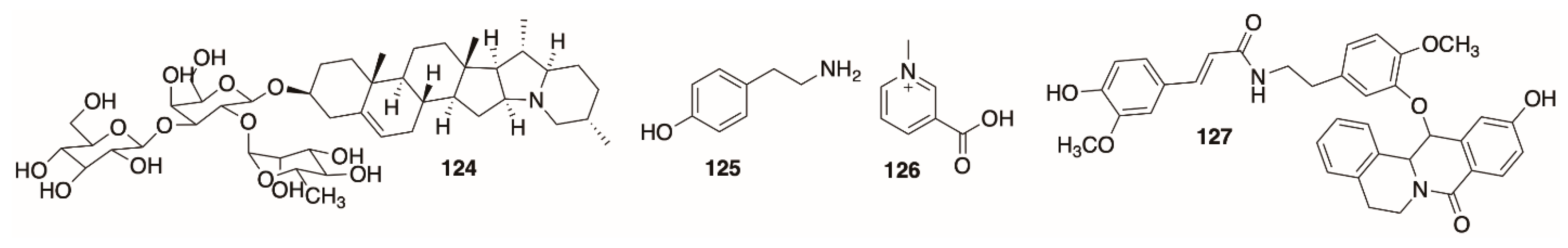
5.3. Miscellaneous Alkaloids
6. Factors Affecting Alkaloid Content and Composition
6.1. Location within the Plant
6.2. Plant Age
6.3. Ploidy of Plants
6.4. Light and Water Amount
6.5. Chemical Additives
6.6. Geography, Altitude, Climate, and Season
6.7. Insect Herbivory
7. Conclusions and Future Challenges: Stereochemistry, Dereplication, and Observer Bias
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Maheshwari, N.; Khan, A.; Chopade, B.A. Rediscovering the medicinal properties of Datura sp.: A review. J. Med. Plants Res. 2013, 7, 2885–2897. [Google Scholar]
- Benítez, G.; March-Salas, M.; Villa-Kamel, A.; Cháves-Jiménez, U.; Hernández, J.; Montes-Osuna, N.; Moreno-Chocano, J.; Cariñanos, P. The genus Datura L. (Solanaceae) in Mexico and Spain—Ethnobotanical perspective at the interface of medical and illicit uses. J. Ethnopharmacol. 2018, 218, 133–151. [Google Scholar] [CrossRef]
- Batool, A.; Batool, Z.; Qureshi, R.; Raja, N.I. Phytochemicals, pharmacological properties and biotechnological aspects of a highly medicinal plant: Datura stramonium. J. Plant Sci. 2020, 8, 29–40. [Google Scholar]
- Griffin, W.J.; Lin, G.D. Chemotaxonomy and geographical distribution of tropane alkaloids. Phytochemistry 2000, 53, 623–637. [Google Scholar] [CrossRef]
- WHO. World Health Organization Model List of Essential Medicines, 21st List, 2019; World Health Organization: Geneva, Switzerland, 2019; License: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Alexander, J.; Benford, D.; Cockburn, A.; Cravedi, J.-P.; Dogliotti, E.; Di Domenico, A.; Férnandez-Cruz, M.L.; Fürst, P.; Fink-Gremmels, J.; Galli, C.L.; et al. Scientific opinion of the panel on contaminants in the food chain on a request from the European Commission on tropane alkaloids (from Datura sp.) as undesirable substances in animal feed. EFSA J. 2008, 691, 1–55. [Google Scholar]
- Kerchner, A.; Farkas, A. Worldwide poisoning potential of Brugmansia and Datura. Forensic Toxicol. 2020, 38, 30–41. [Google Scholar] [CrossRef]
- Diker, D.; Markovitz, D.; Rothman, M.; Sendovski, U. Coma as a presenting sign of Datura stramonium seed tea poisoning. Eur. J. Intern. Med. 2007, 18, 336–338. [Google Scholar] [CrossRef]
- Kanchan, T.; Atreya, A. Datura: The Roadside Poison. Wilderness Environ. Med. 2016, 27, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Ricard, F.; Abe, E.; Duverneuil-Mayer, C.; Charlier, C.; de la Grandmaison, G.; Alvarez, J.C. Measurement of atropine and scopolamine in hair by LC-MS/MS after Datura stramonium chronic exposure. Forensic Sci. Int. 2012, 223, 256–260. [Google Scholar] [CrossRef]
- Hanna, J.P.; Schmidley, J.W.; Braselton, W.E. Datura Delirium. Clin. Neuropharmacol. 1992, 15, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Kohnen-Johannsen, K.L.; Kayser, O. Tropane alkaloids: Chemistry, pharmacology, biosynthesis, and production. Molecules 2019, 24, 796. [Google Scholar] [CrossRef] [PubMed]
- Marc, B.; Martis, A.; Moreau, C.; Arlie, G.; Kintz, P.; Leclerc, J. Acute Datura stramonium poisoning in an emergency department. Presse Med. 2007, 36, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Beverley, R.; Elkins, W.M. The History and Present State of Virginia, in Four Parts; R. Parker: London, UK, 1722; p. 24. [Google Scholar]
- Müller, J.L. Love potions and the ointment of witches: Historical aspects of the nightshade alkaloids. Clin. Toxicol. 1998, 36, 617–627. [Google Scholar] [CrossRef]
- Robinson, D.W.; Brown, K.; McMenemy, M.; Dennany, L.; Baker, M.J.; Allan, P.; Cartwright, C.; Bernard, J.; Sturt, F.; Kotoula, E.; et al. Datura quids at Pinwheel Cave, California, provide unambiguous confirmation of the ingestion of hallucinogens at a rock art site. Proc. Natl. Acad. Sci. USA 2020, 49, 31026–31037. [Google Scholar] [CrossRef]
- Litzinger, W.J. Ceramic evidence for prehistoric Datura use in North America. J. Ethnopharmacol. 1981, 4, 57–74. [Google Scholar] [CrossRef]
- Geeta, R.; Gharaibeh, W. Historical evidence for a pre-Columbian presence of Datura in the Old World and implications for a first millennium transfer from the New World. J. Biosci. 2007, 32, 1227–1244. [Google Scholar] [CrossRef]
- Seigler, D.S. Pyrrolidine, tropane, piperidine, and polyketide Alkaloids. In Plant Secondary Metabolism; Springer Science+Business Media: New York, NY, USA, 1998; pp. 531–545. [Google Scholar]
- Mace, E.S.; Gebhardt, C.G.; Lester, R.N. AFLP analysis of genetic relationships in the tribe Datureae (Solanaceae). Theor. Appl. Genet. 1999, 99, 634–641. [Google Scholar] [CrossRef]
- Gerlach, G.H. Datura Innoxia—A potential commercial source of scopolamine. Econ. Bot. 1948, 2, 436–454. [Google Scholar] [CrossRef]
- Berkov, S.; Zayed, R.; Doncheva, T. Alkaloid patterns in some varieties of Datura stramonium. Fitoterapia 2006, 77, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Verloove, F. Datura wrightii (Solanaceae), a neglected xenophyte, new to Spain. Bouteloua 2008, 4, 37–40. [Google Scholar]
- Hiraoka, N.; Tashimo, K.; Kinoshita, C.; Hiro’oka, M. Genotypes and alkaloid contents of Datura metel varieties. Biol. Pharm. Bull. 1995, 19, 1086–1089. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hammer, K.; Romeike, A.; Tittel, C. Vorarbeiten zur monographichen Darstellung von Wildpflanzensortimenten: Datura L., sections Dutra Bernh., Ceratocaulis Bernh. et Datura. Kulturpflanze 1983, 31, 13–75. [Google Scholar] [CrossRef]
- Haegi, L. Taxonomic account of Datura L. (Solanaceae) in Australia with a note on Brugmansia Pers. Aust. J. Bot. 1976, 24, 415–435. [Google Scholar] [CrossRef]
- Cavazos, M.L.; Jiao, M.; Bye, R. Phenetic analysis of Datura section Dutra (Solanaceae) in Mexico. J. Linn. Soc. Bot. 2000, 133, 493–507. [Google Scholar] [CrossRef][Green Version]
- Lockwood, T.E. Generic recognition of Brugmansia. Bot. Mus. Leaf. Harv. Univ. 1973, 23, 273–284. [Google Scholar]
- Christen, P.; Bieri, S.; Berkov, S. Methods of analysis: Tropane alkaloids from plant origin. In Natural Products; Ramawat, K.G., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1009–1048. [Google Scholar]
- Christen, P.; Cretton, S.; Humam, M.; Bieri Muñoz, O.; Joseph-Nathan, P. Chemistry and biological activity of alkaloids from the genus Schizanthus. Phytochem. Rev. 2020, 19, 615–641. [Google Scholar] [CrossRef]
- Petruczynik, A. Analysis of alkaloids from different chemical groups by different liquid chromatography methods. Cent. Eur. J. Chem. 2012, 10, 802–835. [Google Scholar] [CrossRef]
- Moreno-Pedraza, A.; Gabriel, J.; Treutler, H.; Winkler, R.; Vergara, F. Effects of water availability in the soil on tropane alkaloid production in cultivated Datura stramonium. Metabolites 2019, 9, 131. [Google Scholar] [CrossRef]
- Demeyer, K.; Dejaegere, R. Influence of the ion-balance in the growth medium on the yield and alkaloid content of Datura stramonium L. Plant Soil 1989, 114, 289–294. [Google Scholar] [CrossRef]
- Maltese, F.; Van der Kooy, F.; Verpoorte, R. Solvent derived artifacts in natural products chemistry. Nat. Prod. Commun. 2009, 4, 447–454. [Google Scholar]
- Hirano, I.; Iida, H.; Ito, Y.; Park, H.-D.; Takahashi, K. Effects of light conditions on growth and defense compound contents of Datura inoxia and D. stramonium. J. Plant. Res. 2019, 132, 473–480. [Google Scholar] [CrossRef]
- Hui, Y.S.; Yan, L.; Qi, W.; Ping, S.Y.; Wei, G.; Yuan, L.; You, Y.B.; Xue, K.H. Identification and quantification of alkaloid compounds from different parts and production areas of Datura metel L. Heterocycles 2020, 100, 468–584. [Google Scholar]
- Vitale, A.A.; Acher, A.; Pomilio, A.B. Alkaloids of Datura ferox from Argentina. J. Ethnopharmacol. 1995, 49, 81–89. [Google Scholar] [CrossRef]
- Sramska, P.; Maciejka, A.; Topolewska, A.; Stepnowski, P.; Halinski, L.P. Isolation of atropine and scopolamine from plant material using liquid-liquid extraction and EXtrelut® columns. J. Chromatogr. B 2017, 1043, 202–208. [Google Scholar] [CrossRef]
- Witte, L.; Müller, K.; Arfmann, H.-A. Investigation of the alkaloid pattern of Datura innoxia plants by capillary gas-liquid-chromatography-mass-spectrometry. Planta Med. 1987, 53, 192–197. [Google Scholar] [CrossRef]
- Doncheva, T.; Berkov, S.; Philipov, S. Comparative study of the alkaloids in the tribe Datureae and their chemosystematic significance. Biochem. Syst. Ecol. 2006, 34, 478–488. [Google Scholar] [CrossRef]
- Temerdashev, A.Z.; Kolychev, I.A.; Kiseleva, N.V. Chromatographic determination of some tropane alkaloids in Datura metel. J. Anal. Chem. 2012, 67, 960–966. [Google Scholar] [CrossRef]
- Djilani, A.; Legseir, B.; Soulimani, R.; Dicko, A.; Younos, C. New extraction technique for alkaloids. J. Braz. Chem. Soc. 2006, 17, 418–520. [Google Scholar] [CrossRef]
- Payne, J.; Hamill, J.D.; Robins, R.J.; Rhodes, M.J.C. Production of hyoscyamine by ‘hairy root’ cultures of Datura stramonium. Planta Med. 1987, 53, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Lanfranchi, D.A.; Tomi, F.; Casanova, J. Enantiomeric differentiation of atropine/hyoscyamine by 13C NMR spectroscopy and its application to Datura stramonium extract. Phytochem. Anal. 2010, 21, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.; Sultana, N.; Ahmed, S.S.; Haider, S. Isolation and structure of a new alkaloid datumetine from the leaves of Datura metel. I. J. Nat. Prod. 1986, 49, 511–513. [Google Scholar] [CrossRef]
- Ciechomska, M.; Wozniakiewicz, M.; Nowak, J.; Swiadek, K.; Bazylewicz, B.; Koscielniak, P. Development of a microwave-assisted extraction of atropine and scopolamine from Solanaceae family plants followed by a QuEChERS cleanup procedure. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 538–548. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, H.-B.; Liu, Y.; Algradi, A.M.; Naseem, A.; Zhou, Y.-Y.; She, X.; Li, L.; Yang, B.-Y.; Kuang, H.-X. New indole alkaloids from the seeds of Datura metel. L. Fitoterapia 2020, 146, 104726. [Google Scholar] [CrossRef] [PubMed]
- Welegergs, G.G.; Hulif, K.; Mulaw, S.; Gebretsadik, H.; Tekluu, B.; Temesgen, A. Isolation, structural elucidation and bioactivity studies of tropane derivatives of alkaloids from seeds extract of Datura stramonium. Sci. J. Chem. 2015, 3, 78–83. [Google Scholar] [CrossRef]
- Beresford, P.J.; Woolley, J.G. 6β-[2-methylbutanoyloxy]tropan-3α-ol, a new alkaloid from Datura ceratocaula, structure and biosynthesis. Phytochemistry 1974, 13, 2511–2513. [Google Scholar] [CrossRef]
- Fu, C.; Zhang, W.; Wu, Z.; Chen, P.; Hiu, A.; Zheng, Y.; Li, H.; Xu, K. A novel process for scopolamine separation from Hindu Datura extracts by liquid-liquid extraction, microporous resins, and crystallization. Sep. Sci. Technol. 2020, 55, 922–931. [Google Scholar] [CrossRef]
- Fuller, W.C.; Gibson, M.R. The Arginase-Alkaloid Relationship in Datura tatula L. J. Am. Pharm. Assoc. 1952, 41, 263–266. [Google Scholar] [CrossRef]
- Karnick, C.R.; Saxena, M.D. On the variability of alkaloid production in Datura species. Planta Med. 1970, 18, 266–269. [Google Scholar] [CrossRef] [PubMed]
- James, G.M.; Thewlis, B.H. The separation and identification of solanaceous alkaloids from normal and grafted plants. New Phytol. 1952, 51, 250–255. [Google Scholar] [CrossRef]
- Nash, R.J.; Rothschild, M.; Porter, E.A.; Watson, A.A.; Waigh, R.D.; Waterman, P.G. Calystegines in Solanum and Datura species and the death’s head hawk-moth (Acherontia atropus). Phytochemistry 1993, 34, 1281–1283. [Google Scholar] [CrossRef]
- Sharma, V.; Sharma, N.; Singh, B.; Gupta, R.C. Cytomorphological studies and HPTLC fingerprinting in different plant parts of three wild morphotypes of Datura metel L. “Thorn Apple” from North India. Int. J. Green. Pharm. 2009, 3, 40–46. [Google Scholar] [CrossRef]
- Malinowska, I.; Studzinski, M.; Niezabitowska, K.; Gadzikowska, M. Comparison of TLC and different micro TLC techniques in analysis of tropane alkaloids and their derivatives mixture from Datura Inoxia Mill. extract. Chromatographia 2013, 76, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, I.; Studzinski, M.; Niezabitowska, K.; Malinowski, H. Planar electrochromatography and thin-layer chromatography of tropane alkaloids from Datura Inoxia Mill. extract. in pseudo-reversed-phase systems. JPCJ Planar. Chromat. 2016, 29, 38–44. [Google Scholar] [CrossRef]
- Duez, P.; Chamart, S.; Hanocq, M.; Molle, L.; Vanhaelen, M.; Vanhaelen-Fastré, R. Comparison between thin-layer chromatography-densitometry and high-performance liquid chromatography for the determination of hyoscyamine and hyoscine in leaves, fruit and seeds of Datura (Datura spp.). J. Chromatogr. 1985, 329, 415–421. [Google Scholar] [CrossRef]
- Naumann, A.; Kurtze, L.; Krähmer, A.; Hagels, H.; Schulz, H. Discrimination of Solanaceae taxa and quantification of scopolamine and hyoscyamine by ATR-FTIR spectroscopy. Planta Med. 2014, 80, 1315–1320. [Google Scholar] [CrossRef]
- Li-Yi, H.; Guan-De, Z.; Yu-Yi, T.; Sagara, K.; Oshima, T.; Yoshida, T. Reversed-phase ion-pair high-performance liquid chromatographic separation and determination of tropane alkaloids in Chinese solanaceous plants. J. Chromatogr. 1989, 481, 428–433. [Google Scholar] [CrossRef]
- Ionkova, I.; Witte, L.; Alfermann, A.W. Spectrum of tropane alkaloids in transformed roots of Datura innoxia and Hyoscyamus x györffyi cultivated in vitro. Planta Med. 1994, 60, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Ford, Y.-Y.; Ratcliffe, R.G.; Robins, R.J. In vivo NMR analysis of tropane alkaloid metabolism in transformed root and de-differentiated cultures of Datura stramonium. Phytochemistry 1996, 43, 115–120. [Google Scholar] [CrossRef]
- Robins, R.J.; Abraham, T.W.; Parr, A.J.; Eagles, H.; Walton, N.J. The biosynthesis of tropane alkaloids in Datura stramonium: The identity of the intermediates between N-methylpyrrolinium salt and tropinone. J. Am. Chem. Soc. 1997, 119, 10929–10934. [Google Scholar] [CrossRef]
- El Bazaoui, A.; Bellimam, M.A.; Soulaymani, A. Nine new tropane alkaloids from Datura stramonium L. identified by GC/MS. Fitoterapia 2011, 82, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Miraldi, E.; Masti, A.; Ferri, S.; Comparini, I.B. Distribution of hyoscyamine and scopolamine in Datura stramonium. Fitoterapia 2001, 72, 644–648. [Google Scholar] [CrossRef]
- Patterson, S.; O’Hagan, D. Biosynthetic studies on the tropane alkaloid hyoscyamine in Datura stramonium; hyoscyamine is stable to in vivo oxidation and is not derived from littorine via a vicinal interchange process. Phytochemistry 2002, 61, 323–329. [Google Scholar] [CrossRef]
- Dupraz, J.-M.; Christen, P.; Kapetanidis, I. Tropane alkaloids in transformed roots of Datura quercifolia. Planta Med. 1994, 60, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Philipov, S.; Berkov, S.; Doncheva, T. GC-MS survey of Datura stramonium alkaloids. Compt. Rend. Acad. Bulg. Sci. 2007, 60, 239–250. [Google Scholar]
- Zhou, M.; Ma, X.; Sun, J.; Ding, G.; Ciu, Q.; Miao, Y.; Hou, Y.; Jiang, M.; Bai, G. Active fragments-guided drug discovery and design of selective tropane alkaloids using ultra-high performance liquid chromatography-quadrupole time-of-flight tandem mass spectrometry coupled with virtual calculation and biological evaluation. Anal. Bioanal. Chem. 2017, 409, 1145–1157. [Google Scholar] [CrossRef] [PubMed]
- Christen, P.; Roberts, M.; Phillipson, J.D.; Evans, W.C. Alkaloids of hairy root cultures of a Datura candida hybrid. Plant Cell Rep. 1990, 9, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, Y.; Wang, H.; Du, P.; Han, F.; Zhang, H. Analysis of scopolamine and its eighteen metabolites in rat urine by liquid chromatography-tandem mass spectrometry. Talanta 2005, 67, 984–991. [Google Scholar] [CrossRef]
- Berkov, S.; Doncheva, T.; Philipov, S.; Alexandrov, K. Ontogenetic variation of the tropane alkaloids in Datura stramonium. Biochem. Syst. Ecol. 2005, 33, 1017–1029. [Google Scholar] [CrossRef]
- Berkov, S. Alkaloids of Datura ceratocaula. Z. Naturforsch. 2003, 58c, 455–458. [Google Scholar] [CrossRef]
- Boros, B.; Farkas, A.; Jakabová, S.; Bacskay, I.; Kilár, F.; Felinger, A. LC-MS quantitative determination of atropine and scopolamine in the floral nectar of Datura species. Chromatographia 2010, 71, S43–S49. [Google Scholar] [CrossRef]
- De-la-Cruz, I.M.; Cruz, L.L.; Martínez-García, L.; Valverde, P.L.; Flores-Ortiz, C.M.; Hernández-Portilla, L.B.; Núñez-Farfán, J. Evolutionary response to herbivory: Population differentiation in microsatellite loci, tropane alkaloids and leaf trichome density in Datura stramonium. Arthropod Plant Interact. 2020, 14, 21–30. [Google Scholar] [CrossRef]
- Kariñho-Betancourt, E.; Agrawal, A.A.; Halitschke, R.; Núñez-Farfán, J. Phylogenetic correlations among chemical and physical plant defenses change with ontogeny. New Phytol. 2015, 206, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Liu, Y.; Qi, H.; Niu, L.; Zhu, Y.; Lu, W.; Xu, X.; Su, Y.; Yang, B.; Wang, Q. Characterization of the metabolic fate of Datura metel seed extract and its main constituents in rats. Front. Pharmacol. 2019, 10, 571. [Google Scholar] [CrossRef]
- Tapfuma, K.I.; Mekuto, L.; Makatini, M.M.; Mavumengwana, V. The LC-QTOF-MS/MS analysis data of detected metabolites from the crude extract of Datura stramonium leaves. Data Brief 2019, 25, 104094. [Google Scholar] [CrossRef] [PubMed]
- Satpute, S.B.; Vanmare, D.J. Chemical profile of Datura stramonium L. by using HPLC-MS spectra. Int. Res. J. Sci. Eng. 2018, 6, 231–235. [Google Scholar]
- Bell, D.S. and Jones, A.D. Solute attributes and molecular interactions contributing to “U-shaped” retention on a fluorinated high-performance liquid chromatography stationary phase. J. Chromatogr. A 2005, 1073, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.S.; Cramer, H.M.; Jones, A.D. Rational method development strategies on a fluorinated stationary phase: Mobile phase ionic concentration and temperature effects on the separation of ephedrine alkaloids. J. Chromatogr. A 2005, 1095, 113–118. [Google Scholar] [CrossRef]
- Papadoyannis, I.N. Instrumental analytical techniques used for Datura alkaloid analysis. Instrum. Sci. Technol. 1994, 22, 241–258. [Google Scholar] [CrossRef]
- El Bazaoui, A.; Stambouli, H.; Bellimam, M.A.; Soulaymani, A. Determination of tropane alkaloids in seeds of Datura stramonium L. by GC/MS and LC/MS. Ann. Toxicol. Anal. 2009, 21, 183–188. [Google Scholar]
- Gerber, R.; Naudé, T.W.; de Kock, S.S. Confirmed Datura poisoning in a horse most probably due to D. ferox in contaminated tef hay. Tydskr, S. Afr. Vet. Ver. 2006, 77, 86–89. [Google Scholar] [CrossRef][Green Version]
- Marín-Sáez, J.; Romero-González, R.; Garrido Frenich, A. Multi-analysis determination of tropane alkaloids in cereals and solanaceaes seeds by liquid chromatography coupled to single stage Exactive-Orbitrap. J. Chromatogr. A 2017, 1518, 46–58. [Google Scholar] [CrossRef]
- Plumb, R.S.; Johnson, K.A.; Rainville, P.; Smith, B.W.; Wilson, I.D.; Castro-Perez, J.M.; Nicholson, J.K. UPLC/MSE; a new approach for generating molecular fragment information for biomarker structure elucidation. Rapid Commun. Mass Spectrom. 2006, 20, 1989–1994. [Google Scholar] [CrossRef]
- Marín-Sáez, J.; Romero-González, R.; Garrido Frenich, A. Enantiomeric determination and evalucation of the racemization process of atropine in Solanaceae seeds and contaminated samples by high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A. 2016, 1474, 79–84. [Google Scholar] [CrossRef]
- Lesiak, A.D.; Musah, R.A. Rapid high-throughput species identification of botanical material using direct analysis in real time high resolution mass spectrometry. J. Vis. Exp. 2016, 116, e54197. [Google Scholar] [CrossRef]
- Fowble, K.L.; Teramoto, K.; Cody, R.B.; Edwards, D.; Guarrera, D.; Musah, R.A. Development of “laser ablation direct analysis in real time imaging” mass spectrometry: Application to spatial distribution mapping of metabolites along the biosynthetic cascade leading to synthesis of atropine and scopolamine in plant tissue. Anal. Chem. 2017, 89, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Pedraza, A.; Rosas-Román, I.; Garcia-Rojas, N.S.; Guillén-Alonso, H.; Ovando-Vázquez, C.; Díaz-Ramírez, D.; Cuevas-Contreras, J.; Vergara, F.; Marsch-Martínez, N.; Molina-Torres, J.; et al. Elucidating the distribution of plant metabolites from native tissues with laser desorption low-temperature plasma mass spectrometry imaging. Anal. Chem. 2019, 91, 2734–2743. [Google Scholar] [CrossRef] [PubMed]
- Talaty, N.; Takáts, Z.; Cooks, R.G. Rapid in situ detection of alkaloids in plant tissue under ambient conditions using desorption electrospray ionization. Analyst 2005, 130, 1624–1633. [Google Scholar] [CrossRef]
- Humam, M.; Shoul, T.; Jeannerat, D.; Muñoz, O.; Christen, P. Chirality and numbering of substituted tropane alkaloids. Molecules 2011, 16, 7199–7209. [Google Scholar] [CrossRef] [PubMed]
- Fliniaux, O.; Mesnard, F.; Raynaud-Le Grandic, S.; Baltora-Rosset, S.; Bienaimé, C.; Robins, R.J.; Fliniaux, M.-A. Altered nitrogen metabolism associated with de-differentiated suspension cultures derived from root cultures of Datura stramonium studied by heteronuclear multiple bond coherence (HMBC) NMR spectroscopy. J. Exp. Bot. 2004, 55, 1053–1060. [Google Scholar] [CrossRef][Green Version]
- Sharp, G.; Edinburg, M.D. Our present knowledge of the mydriatic group. Pharm. Era 1897, 18, 587–588. [Google Scholar]
- Dunstan, W.R.; Brown, H.V. The alkaloid of Hyoscyamus muticus and of Datura stramonium grown in Egypt. J. Chem. Soc. Trans. 1901, 79, 71–74. [Google Scholar] [CrossRef]
- Lounasmaa, M.; Tamminen, T. The tropane alkaloids. In The Alkaloids; Bossi, A., Ed.; Academic Press: New York, NY, USA, 1993; Volume 44, pp. 1–114. [Google Scholar]
- James, W.O. Demonstration of alkaloids in Solanaceous meristems. Nature 1946, 4011, 377–378. [Google Scholar] [CrossRef] [PubMed]
- Walton, N.J.; Peerless, A.C.J.; Robins, R.J.; Rhodes, M.J.C.; Boswell, H.D.; Robins, D.J. Purification and properties of putrescine N-methyltransferase from transformed roots of Datura stramonium L. Planta 1994, 193, 9–15. [Google Scholar] [CrossRef]
- Bedewitz, M.A.; Jones, A.D.; D’Auria, J.C.; Barry, C.S. Tropinone synthesis via an atypical polyketide synthase and P450-mediated cyclization. Nat. Commun. 2018, 8, 5281. [Google Scholar] [CrossRef] [PubMed]
- Abraham, T.W.; Leete, E. New intermediate in the biosynthesis of the tropane alkaloids in Datura innoxia. J. Am. Chem. Soc. 1995, 117, 8100–8105. [Google Scholar] [CrossRef]
- Dräger, B. Tropinone reductase in root cultures of Hyoscyamus niger, Atropa belladonna, and Datura stramonium. Planta Med. 1990, 56, 499. [Google Scholar] [CrossRef]
- Robins, R.J.; Bachman, P.; Robinson, T.; Rhodes, M.J.C.; Yamada, Y. The formation of 3α- and 3β-acetoxytropanes by Datura stramonium transformed root cultures involves two acetyl-CoA-dependent acyltransferases. FEBS Lett. 1991, 292, 293–297. [Google Scholar] [PubMed]
- Rabot, S.; Peerless, A.C.J.; Robins, R.J. Tigloyl-CoA: Pseudtropine acyl transferase—an enzyme of tropane alkaloid biosynthesis. Phytochemistry 1995, 39, 315–322. [Google Scholar] [CrossRef]
- McGaw, B.A.; Woolley, J.G. Non-reversibility of the isoleucine-acetate pathway in Datura. Phytochemistry 1977, 16, 1711–1713. [Google Scholar] [CrossRef]
- Lucas, K.A.; Filley, J.R.; Erb, J.M.; Graybill, E.R.; Hawes, J.W. Peroxisomal metabolism of propionic acid and isobutyric acid in plants. J. Biol. Chem. 2007, 292, 24980–24989. [Google Scholar] [CrossRef]
- Ning, J.; Moghe, G.D.; Leong, B.; Kim, J.; Ofner, I.; Wang, Z.; Adams, C.; Jones, A.D.; Zamir, D.; Last, R.L. A feedback-insensitive isopropylmalate synthase affects acylsugar composition in cultivated and wild tomato. Plant Physiol. 2015, 169, 1821–1835. [Google Scholar] [CrossRef]
- Bedewitz, M.A.; Góngora-Castillo, E.; Uebler, J.B.; Gonzales-Vigil, E.; Wiegert-Rininger, K.E.; Childs, K.L.; Hamilton, J.P.; Vaillancourt, B.; Yeo, Y.-S.; Chappell, J.; et al. A root-expressed L-phenylalanine: 4-hydroxyphenylpyruvate aminotransferase is required for tropane alkaloid biosynthesis in Atropa belladonna. Plant Cell 2014, 26, 3745–3762. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Zeng, J.; Wang, J.; Huang, J.-P.; Zhou, W.; Yang, C.; Lan, X.; Chen, M.; Huang, S.-X.; Kai, G.; et al. Functional genomics analysis reveals two novel genes required for littorine biosynthesis. New Phytol. 2020, 225, 1906–1914. [Google Scholar] [CrossRef]
- Lanoue, A.; Boitel-Conti, M.; Portais, J.-C.; Laberche, J.-C.; Barbotin, J.-N.; Christen, P.; Sangwan-Norreel, S. Kinetic study of littorine rearrangement in Datura innoxia hairy roots by 13C NMR Spectroscopy. J. Nat. Prod. 2002, 65, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Sandala, G.M.; Smith, D.M.; Radom, L. The carbon-skeleton rearrangement in tropane alkaloid biosynthesis. J. Am. Chem. Soc. 2008, 130, 10684–10690. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, T.; Zhang, R.; Bu, Q.; Yin, Q.; Zhang, L.; Chen, W. Molecular cloning and functional analysis of hyoscyamine 6β-hydroxylase (H6H) in the poisonous and medicinal plant Datura innoxia mill. Plant Physiol. Biochem. 2020, 153, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Pramod, K.K.; Singh, S.; Jayabaskaran, C. Biochemical and structural characterization of recombinant hyoscyamine 6β-hydroxylase from Datura metel L. Plant Physiol. Biochem. 2011, 48, 966–970. [Google Scholar] [CrossRef]
- El Bazaoui, A.; Bellimam, M.A.; Soulaymani, A. Tropane alkaloids of Datura innoxia from Morocco. Z. Naturforsch. 2012, 67c, 8–14. [Google Scholar] [CrossRef]
- Berkov, S.; Zayed, R. Comparison of tropane alkaloid spectra between Datura innoxia grown in Egypt and Bulgaria. Z. Naturforsch. 2004, 59c, 184–186. [Google Scholar] [CrossRef]
- Harfi, B.; Khelifi, L.; Khelifi-Slaoui, M.; Assaf-Ducrocq, C.; Gontier, E. Tropane alkaloids GC/MS analysis and low dose elicitors’ effects on hyoscyamine biosynthetic pathway in hairy roots of Algerian Datura species. Sci. Rep. 2018, 8, 17951. [Google Scholar] [CrossRef]
- El-Iman, Y.M.A.; Evans, W.C.; Grout, R.J.; Ramsey, K.P.A. Alkaloids of Erythroxylum zambesiacum root-bark. Phytochemistry 1987, 26, 2385–2389. [Google Scholar] [CrossRef]
- Ushimaru, R.; Ruszczycky, M.; Chang, W.-C.; Yan, F.; Liu, Y.-N.; Liu, H.W. Substrate conformation correlates with the outcome of hyoscyamine 6β-hydroxylase catalyzed oxidation reactions. J. Am. Chem. Soc. 2018, 140, 7433–7436. [Google Scholar] [CrossRef]
- Pavlov, A.; Berkov, S.; Weber, J.; Bley, T. Hyoscyamine biosynthesis in Datura stramonium hairy root in vitro systems with different ploidy levels. Appl. Biochem. Biotechnol. 2009, 157, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Dräger, B. Chemistry and biology of calystegines. Nat. Prod. Rep. 2004, 21, 211–223. [Google Scholar] [CrossRef]
- Dräger, B.; van Almsick, A.; Mrachatz, G. Distribution of calystegines in several Solanaceae. Planta Med. 1995, 61, 577–579. [Google Scholar] [CrossRef]
- Dräger, B.; Funck, C.; Höhler, A.; Mrachatz, G.; Nahrstedt, A.; Portsteffen, A.; Schaal, A.; Schmidt, R. Calystegines as a new group of tropane alkaloids in Solanaceae. Plant Cell, Tissue Organ Cult. 1994, 38, 235–240. [Google Scholar] [CrossRef]
- Jenett-Siems, K.; Weigl, R.; Böhm, A.; Mann, P.; Tofern-Reblin, B.; Ott, S.C.; Ghomian, A.; Kaloga, M.; Siems, K.; Witte, L.; et al. Chemotaxonomy of the pantropical genus Merremia (Convolvulaceae) based on the distribution of tropane alkaloids. Phytochemistry 2005, 66, 1448–1464. [Google Scholar] [CrossRef]
- Phillipson, J.D.; Handa, S.S. N-oxides of hyoscyamine and hyoscine in the Solanaceae. Phytochemistry 1975, 14, 999–1003. [Google Scholar] [CrossRef]
- Heusner, A. Notiz zur Stereochemie von Scopin and Scopolin. Chem. Ber. 1954, 7, 1063–1067. [Google Scholar] [CrossRef]
- Aripova, S.F.; Yunusov, S.S. Dimeric tropane alkaloids. Chem. Nat. Compd. 1991, 27, 389–395. [Google Scholar] [CrossRef]
- Aripova, S.F.; Tashkhodzhaev, B. α-and β-scopadonnines from Datura inoxia seeds. Chem. Nat. Compd. 2004, 27, 464–468. [Google Scholar] [CrossRef]
- Yang, B.-Y.; Xia, Y.-G.; Wang, Q.-H.; Dou, D.-Q.; Kuang, H.-X. Two new amide alkaloids from the flower of Datura metel L. Fitoterapia 2010, 81, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Parr, A.J.; Payne, J.; Eagles, J.; Chapman, B.T.; Robins, R.J.; Rhodes, M.J.C. Variation in tropane alkaloid accumulation within the Solanaceae and strategies for its exploitation. Phytochemistry 1990, 29, 2545–2550. [Google Scholar] [CrossRef]
- McGaw, B.A.; Woolley, J.G. The separate roles of hygrine enantiomers in the biosynthesis of tropane alkaloids. J. Pharm. Pharmacol. 1997, 29, 16. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.C.; Somanabandhu, A. Alkaloids of Datura discolor. Phytochemistry 1974, 13, 304–305. [Google Scholar] [CrossRef]
- El-Shazly, A.; Tei, A.; Witte, L.; El-Domiaty, M.; Wink, M. Tropane alkaloids of Hyoscyamus boveanus, H. muticus, and H. albus from Egypt. Z. Naturforsch. 1997, 52c, 529–539. [Google Scholar] [CrossRef]
- Murch, S.J.; Alan, A.R.; Cao, J.; Saxena, P.K. Melatonin and serotonin in flowers and fruits of Datura metel L. J. Pineal Res. 2009, 47, 277–283. [Google Scholar] [CrossRef]
- Maier, I.; Jurenitsch, J.; Heresch, F.; Haslinger, E.; Schulz, G.; Pöhm, M.; Jentzsch, K. Fluorodaturatin und Homofluorodaturatin—zwei neue β-carbolinderivate in Samen von Datura stramonium L. var. stramonium. Monatsh. Chem. 1981, 112, 1425–1439. [Google Scholar] [CrossRef]
- Jurenitsch, J.; Piccardi, K.; Pöhm, M. 1,2-Dehydrofluorodaturatine—A further β-carboline derivative from seeds of Datura stramonium L. var. stramonium. Sci. Pharm. 1984, 52, 301–309. [Google Scholar]
- Okwu, D.E.; Igara, E.C. Isolation, characterization, and antibacterial activity screening of a new β-carboline alkaloid from Datura metel Linn. Der Chemica Sinica 2011, 2, 261–267. [Google Scholar]
- Karmakar, U.K.; Toume, K.; Ishikawa, N.; Arai, M.A.; Sadhu, S.K.; Ahmed, F.; Ishibashi, M. Bioassay-guided isolation of compounds from Datura stramonium with TRAIL-resistance overcoming activity. Nat. Prod. Commun. 2016, 11, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Nuhu, H.; Ghani, A. Alkaloid content of the leaves of three Nigerian Datura species. Nig. J. Nat. Prod. And Med. 2002, 6, 15–18. [Google Scholar]
- Houmani, Z.; Cosson, L.; Houmani, M. Datura ferox L. and D. quercifolia Kunth (Solanaceae) in Algeria. Flora Mediterr. 1999, 9, 57–60. [Google Scholar]
- Jakabová, S.; Vincze, L.; Farkas, A.; Kilár, F.; Boros, B.; Felinger, A. Determination of tropane alkaloids atropine and scopolamine by liquid chromatography-mass spectrometry in plant organs of Datura species. J. Chromatogr. A 2012, 1232, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Berkov, S.; Philipov, S. Alkaloid production in diploid and autotetraploid plants of Datura stramonium. Pharm. Biol. 2002, 40, 617–621. [Google Scholar] [CrossRef]
- Cosson, L. Influence de l’éclairement sur les variations ontogéniques de teneurs en scopolamine et en hyoscyamine des feuilles de Datura metel. Phytochemistry 1969, 8, 2227–2233. [Google Scholar] [CrossRef]
- Demeyer, K.; Dejaegere, R. The influence of the Ca2+/K+ balance and light energy on alkaloid content and partitioning in Datura stramonium. Aust. J. Bot. 1997, 45, 81–101. [Google Scholar] [CrossRef]
- Brachet, J.; Cosson, L. Changes in the total alkaloid content of Datura innoxia Mill. subjected to salt stress. J. Exp. Bot. 1986, 37, 650–656. [Google Scholar] [CrossRef]
- Demeyer, K.; Van de Velde, H.; Dejaegere, R. Influence of IAA and DMAA on hyoscyamine and scopolamine production in Datura stramonium. Planta Med. 1989, 55, 693. [Google Scholar] [CrossRef]
- Gupta, S.; Madan, C.L. Effect of growth retardants on growth and alkaloid formation in Datura metel var. fastuosa. Planta Med. 1975, 28, 193–200. [Google Scholar] [CrossRef]
- Jousse, C.; Vu, T.D.; Tran, T.L.M.; Al Balkhi, M.H.; Molinié, R.; Boitel-Conti, M.; Pilard, S.; Mathiron, D.; Hehn, A.; Bourgaud, F.; et al. Tropane alkaloid profiling of hydroponic Datura innoxia Mill. plants inoculated with Agrobacterium rhizogenes. Phytochem. Anal. 2010, 21, 118–127. [Google Scholar] [CrossRef]
- Padula, L.Z.; Bandoni, A.L.; Rondina, R.V.D.; Coussio, J.D. Quantitative determination of total alkaloids and scopolamine in Datura ferox growing in Argentina. Planta. Med. 1976, 29, 357–360. [Google Scholar] [CrossRef]
- Shonle, I.; Bergelson, J. Evolutionary ecology of the tropane alkaloids of Datura stramonium L. (Solanaceae). Evolution 2000, 54, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Castillo, G.; Cruz, L.L.; Tapia-López, R.; Olmedo-Vicente, E.; Carmona, D.; Anaya-Lang, A.L.; Fornoni, J.; Andraca-Gómez, G.; Valverde, P.L.; Núñez-Farfán, J. Selection mosaic exerted by specialist and generalist herbivores on chemical and physical defense of Datura stramonium. PLoS ONE 2014, 9, e102478. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.K.; Tseng, A.S.; Potter, K.A.; Davidowitz, G.; Hildebrand, J.G. The effects of the alkaloid scopolamine on the performance and behavior of two caterpillar species. Arthropod Plant Interact. 2018, 12, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Philipov, S.; Berkov, S. GC-MS investigation of tropane alkaloids in Datura Stramonium. Z. Naturforsch. 2002, 57c, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.C.; Ghani, A.; Woolley, V.A. Distribution of littorine and other alkaloids in the roots of Datura species. Phytochemistry 1972, 11, 2527–2529. [Google Scholar] [CrossRef]
- Reina, M.; Burgueño-Tapia, E.; Bucio, M.A.; Joseph-Nathan, P. Absolute configuration of tropane alkaloids from Schizanthus species by vibrational circular dichroism. Phytochemistry 2010, 71, 810–815. [Google Scholar] [CrossRef]
- Muñoz, M.A.; Muñoz, O.; Joseph-Nathan, P. Absolute configuration determination and conformational Analysis of (–)-(3S,6S)-3α,6β-diacetoxytropane using vibrational circular dichroism and DFT techniques. Chirality 2010, 22, 234–241. [Google Scholar] [CrossRef]
- Humam, M.; Christen, P.; Muñoz, O.; Hostettmann, K.; Jeannerat, D. Absolute configuration of tropane alkaloids bearing two α,β-unsaturated ester functions using electronic CD spectroscopy: Application to (R,R)-trans-3-hydroxysenecioyloxy-6-senecioloxytropane. Chirality 2008, 20, 20–25. [Google Scholar] [CrossRef]
- Hubert, J.; Nuzillard, J.-M.; Renault, J.-H. Dereplication strategies in natural product research: How many tools and methodologies behind the same concept? Phytochem. Rev. 2017, 16, 55–95. [Google Scholar] [CrossRef]
- Wang, Z.; Kang, D.; Jia, X.; Zhang, H.; Guo, J.; Liu, C.; Meng, Q.; Liu, W. Analysis of alkaloids from Peganum harmala L. sequential extracts by liquid chromatography coupled to ion mobility spectrometry. J. Chromatogr. B 2018, 1096, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Schrimpe-Rutletdge, A.C.; Sherrod, S.D.; McLean, J.A. Improving the discovery of secondary metabolite natural products using ion mobility-mass spectrometry. Curr. Opin. Chem. Biol. 2018, 42, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2016, 14, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Chávez, D.; Cui, B.; Chai, H.-B.; García, R.; Mejía, M.; Farnsworth, N.R.; Cordell, G.A.; Pezzuto, J.M.; Kinghorn, A.D. Reversal of multidrug resistance by tropane alkaloids from the stems of Erythroxylum rotundifolium. J. Nat. Prod. 2002, 65, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Özçelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral, and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef]


| Class | Representative Structure, Where R = Acyl or Hydroxyl | Diagnostic GC-MS (EI) Fragmentation (m/z, M+) | Diagnostic LC-MS (ESI) Fragmentation (m/z) | References |
|---|---|---|---|---|
| Monosubstituted tropane | 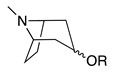 | 141, 140, 124 (usually base peak), 96, 94 (N-methylpyridinium cation), 83, 82 (base peak when no ester at C-3), 42 | 142 (loss of anhydro-acid), 124 (loss of neutral acid), 93, 91 (loss of methylamine, dehydrogenation), 77 | [37,68] |
| Disubstituted tropane |  | 156, 140, 138, 122, 113 (prominent if 3-hydroxyl), 96 (3-hydroxyl), 95, 94 (usually base peak if not 3-hydroxyl), 55, 42 | 158 (sometimes, dihydroxytropane), 140 (loss of anhydro-acid), 122 (loss of neutral acid), 91 (loss of methylamine) | [39,40,68,69,70] |
| Trisubstituted tropane (non-epoxy) |  | 154, 138, 113 (if 3-hydroxyl) 94 (often base peak) | 156, 138, 120, sometimes 94, 93 or 91 | [37,39,40,64,68,69] |
| Epoxytropane | 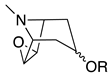 | 154, 94 (often base peak), 55, 42 | 156, 138, generally little fragmentation beyond that. | [37,69] |
| 3-Monosubstituted Nortropane |  | 156, 138, 110 (base peak), 80, 68 | 128 (loss of anhydro-acid), 110 (loss of neutral acid), 93, 91 | [40,61,68] |
| 3-substituted 6,7-Epoxynortropane | 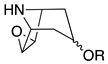 | 122 (base peak), 124, 94, 80 | 142, 124 (norscopine) | [61,68,71,72] |
| 3-substituted 6,7-Dehydrotropane | 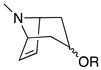 | 94 (base peak) | 140, (loss of anhydro acid) 122 (loss of neutral acid), 93, 91 | [64] |
| Tigloyl esters | 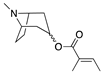 | Loss of 99 | Loss of 100 | [37] |
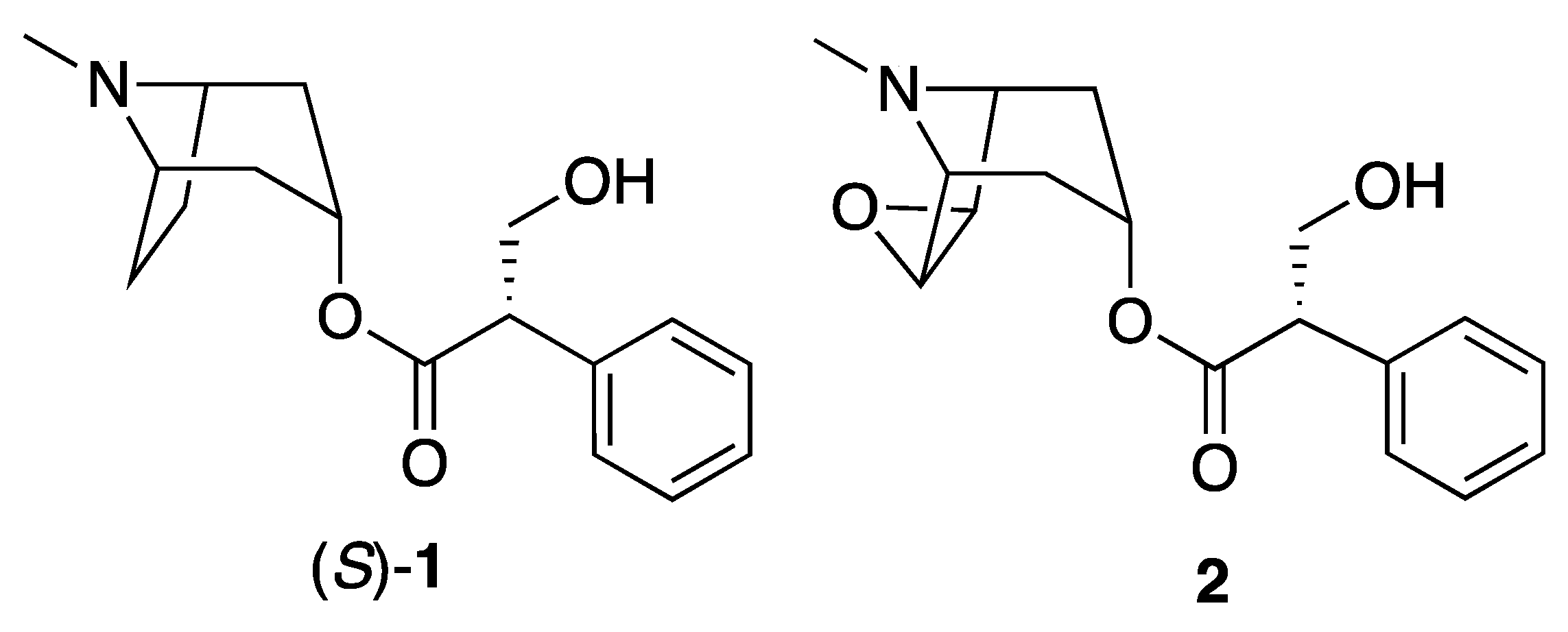
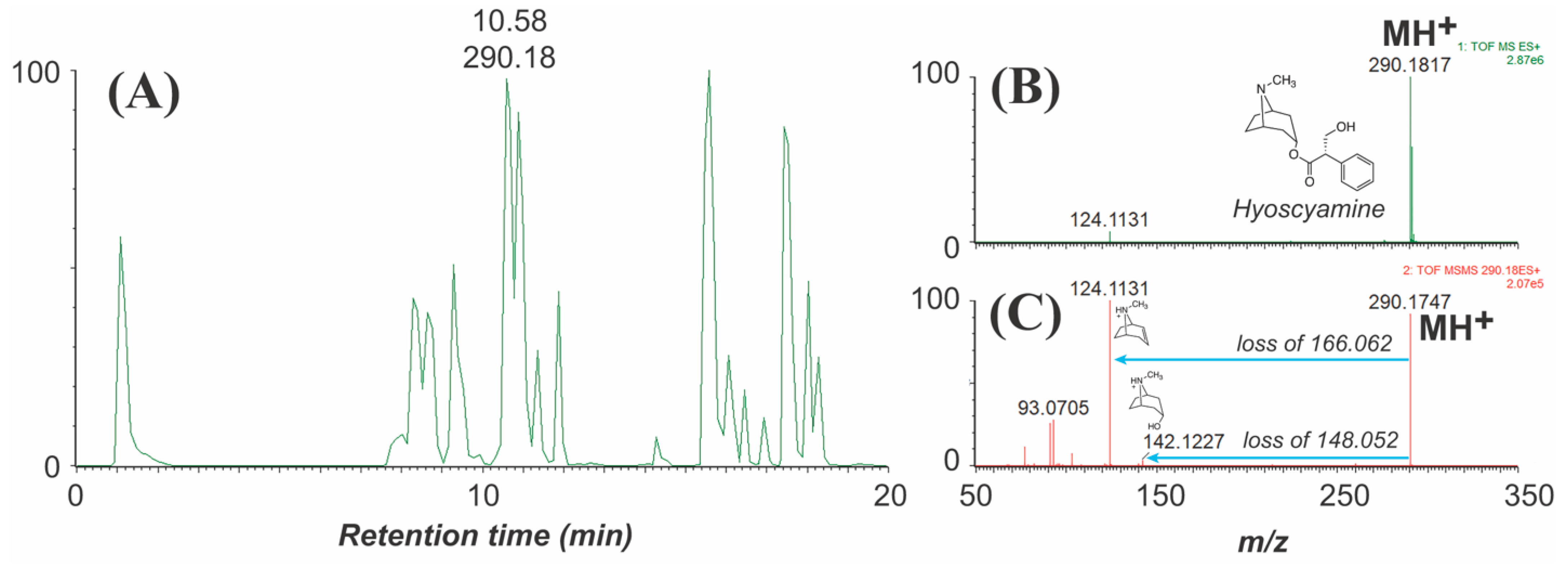
| Tropic acid derivative [(S)-1 or substituted on tropic acid] | 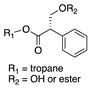 | 271 (dehydration or elimination of substituent), loss of 30 (formaldehyde via McLafferty rearrangement), 133, 121, 103 (derived from apotropic acid) | 272 (dehydration or elimination of substituent), 103, loss of 166.064 or 148.044) | [37,68,69,73] |
| Phenylacetate esters | 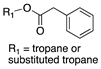 | 91 (derived from benzyl group) | Loss of 136.05 (neutral acid) | [37,61] |
| Pyrrolidine |  | Often low-intensity M+; 85, 84 (usually base peak), 83, 82, 70, 55, 32 | 84 (N-methylpyrrolinium ion), loss of 83. | [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cinelli, M.A.; Jones, A.D. Alkaloids of the Genus Datura: Review of a Rich Resource for Natural Product Discovery. Molecules 2021, 26, 2629. https://doi.org/10.3390/molecules26092629
Cinelli MA, Jones AD. Alkaloids of the Genus Datura: Review of a Rich Resource for Natural Product Discovery. Molecules. 2021; 26(9):2629. https://doi.org/10.3390/molecules26092629
Chicago/Turabian StyleCinelli, Maris A., and A. Daniel Jones. 2021. "Alkaloids of the Genus Datura: Review of a Rich Resource for Natural Product Discovery" Molecules 26, no. 9: 2629. https://doi.org/10.3390/molecules26092629
APA StyleCinelli, M. A., & Jones, A. D. (2021). Alkaloids of the Genus Datura: Review of a Rich Resource for Natural Product Discovery. Molecules, 26(9), 2629. https://doi.org/10.3390/molecules26092629







