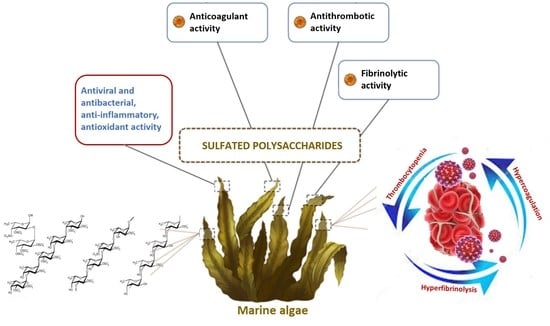
The Potency of Seaweed Sulfated Polysaccharides for the Correction of Hemostasis Disorders in COVID-19
Abstract
1. Introduction
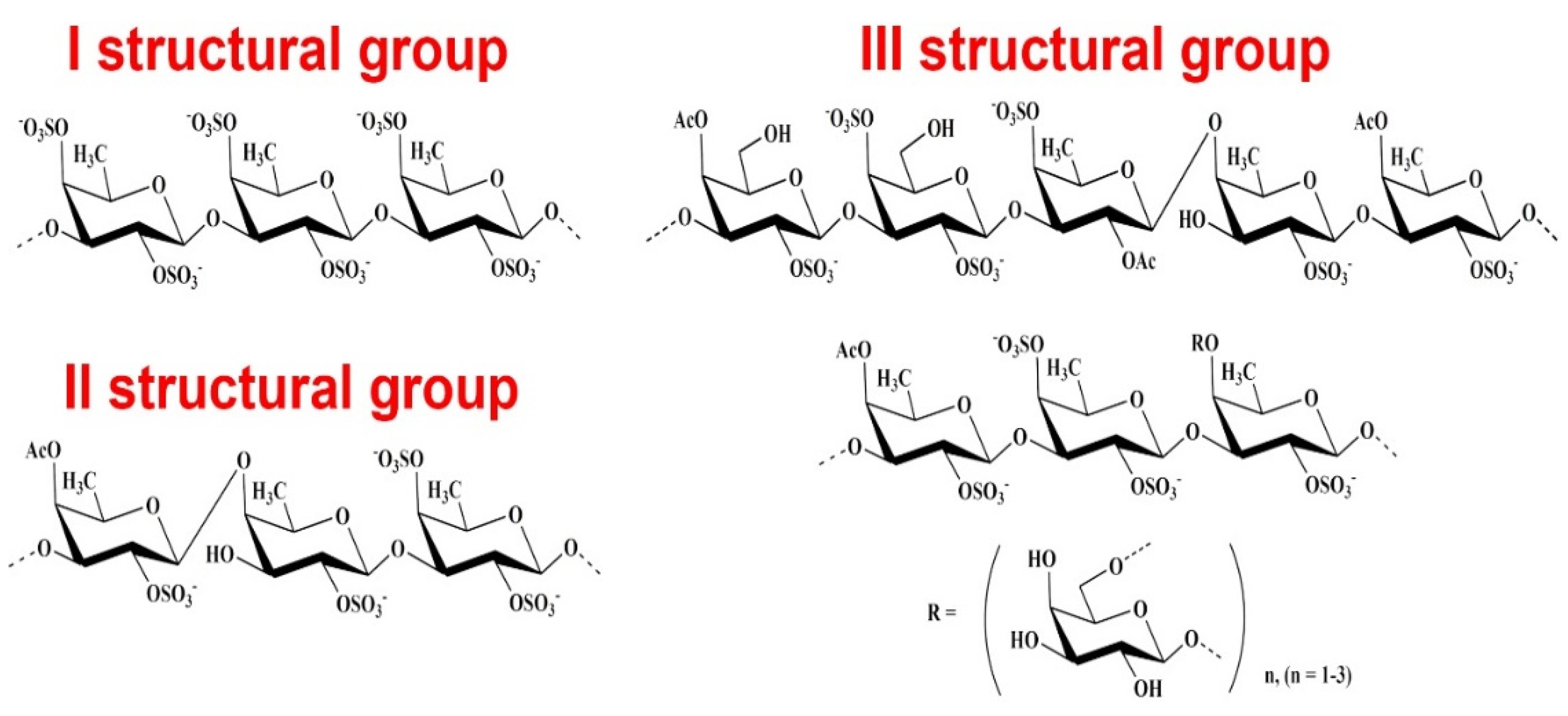
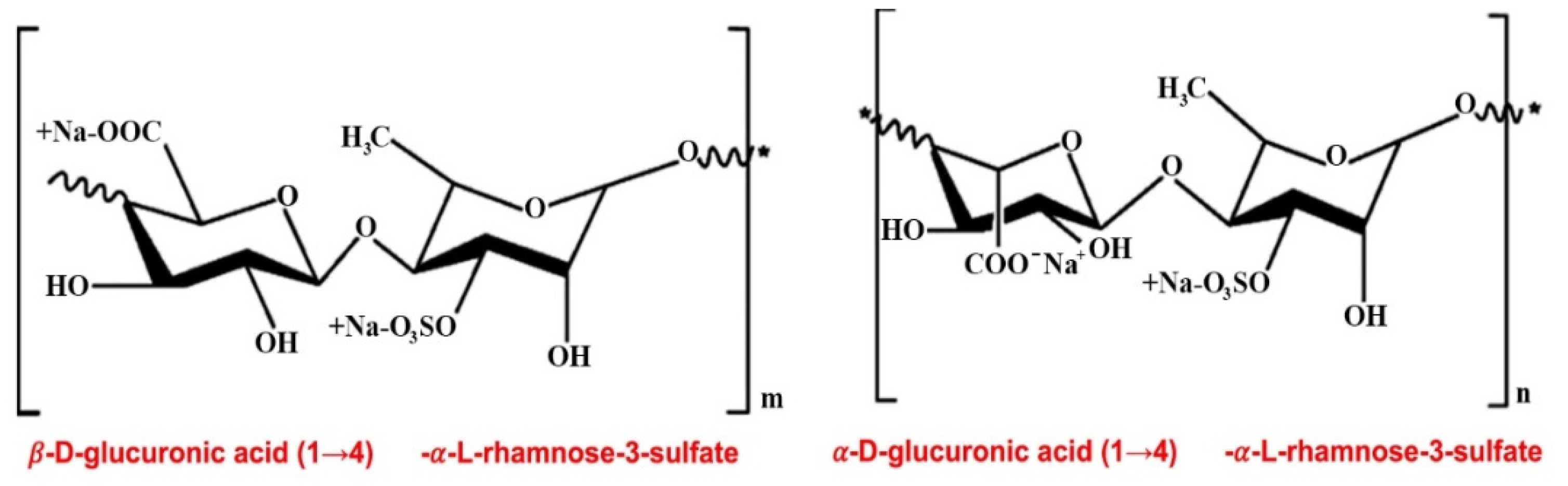
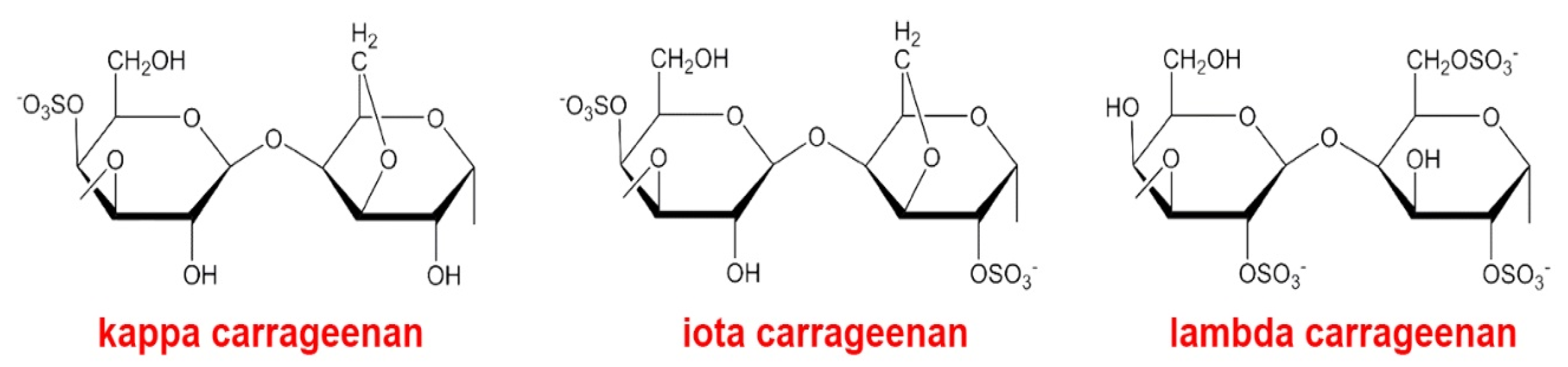
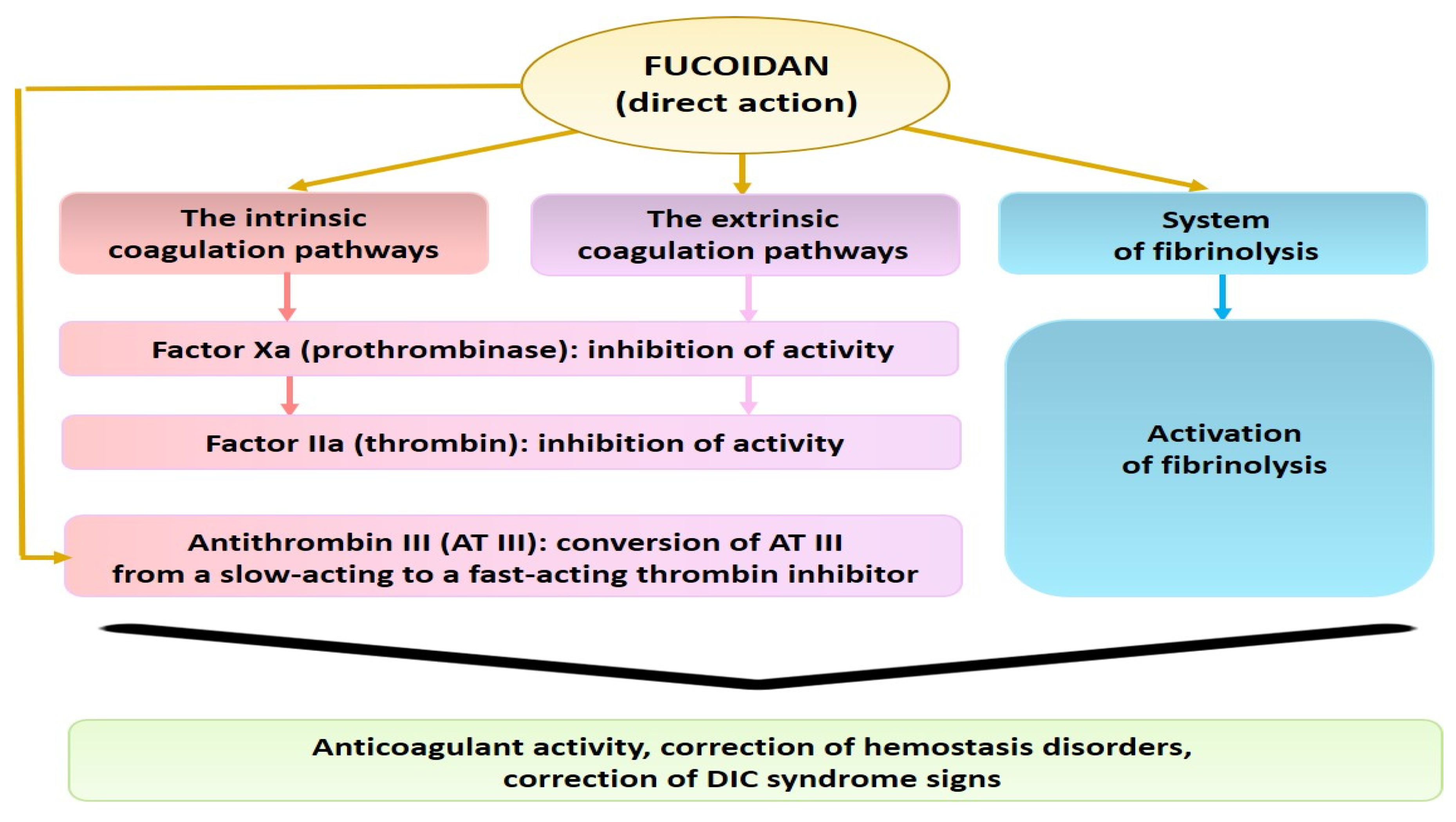
2. Anticoagulant, Antithrombotic, and Fibrinolytic Activities of Seaweed Sulfated Polysaccharides
3. Possible Pathophysiological Mechanisms Involved in the Hemostasis Disorders in the Pathological Progression of COVID-19
3.1. Systemic Endothelial Dysfunction
3.2. COVID-19-Associated Coagulopathy (CAC). Thrombotic Complications
3.3. Thrombocytopenia: The Role of Platelets in Thromboembolism
4. Treatment Strategies of COVID-19-Induced Hypercoagulation and the Potency of Seaweed Sulfated PSs for the Correction of Hemostasis Disorders
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Belen-Apak, F.B.; Sarıalioğlu, F. Pulmonary intravascular coagulation in COVID-19: Possible pathogenesis and recommendations on anticoagulant/thrombolytic therapy. J. Thromb. Thrombolysis 2020, 50, 278–280. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Levi, M.; Connors, J.M.; Thachil, J. Coagulopathy of coronavirus disease. Crit. Care Med. 2020, 48, 1358–1364. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Berkman, S.A.; Tapson, V.F. COVID-19 and Its Implications for Thrombosis and Anticoagulation. Semin Respir Crit. Care Med. 2021, 42, 316–326. [Google Scholar] [CrossRef]
- Klok, F.A.; Kruip, M.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.; Kant, K.M.; Kaptein, H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.K.; Mainbourg, S.; Friggeri, A.; Bertoletti, L.; Douplat, M.; Dargaud, Y.; Grange, C.; Lobbes, H.; Provencher, S.; Lega, J.C. Arterial and venous thromboembolism in COVID-19: A study-level meta-analysis. Thorax 2021, 23. [Google Scholar] [CrossRef]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Thachil, J.; Tang, N.; Gando, S.; Falanga, A.; Cattaneo, M.; Levi, M.; Clark, C.; Iba, T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 2020, 18, 1023–1026. [Google Scholar] [CrossRef]
- Kollias, A.; Kyriakoulis, K.G.; Dimakakos, E.; Poulakou, G.; Stergiou, G.S.; Syrigos, K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: Emerging evidence and call for action. Br. J. Haematol. 2020, 189, 846–847. [Google Scholar] [CrossRef] [PubMed]
- Jonmarker, S.; Hollenberg, J.; Dahlberg, M.; Stackelberg, O.; Litorell, J.; Everhov, Å.H.; Järnbert-Pettersson, H.; Söderberg, M.; Grip, J.; Schandl, A.; et al. Dosing of thromboprophylaxis and mortality in critically ill COVID-19 patients. Crit. Care 2020, 24, 653. [Google Scholar] [CrossRef] [PubMed]
- Kharma, N.; Roehrig, S.; Shible, A.A.; Elshafei, M.S.; Osman, D.; Elsaid, I.M.; Mustafa, S.F.; Aldabi, A.; Smain, O.A.M.; Lance, M.D. Anticoagulation in critically ill patients on mechanical ventilation suffering from COVID-19 disease, The ANTI-CO trial: A structured summary of a study protocol for a randomized controlled trial. Trials 2020, 21, 769. [Google Scholar] [CrossRef] [PubMed]
- Lemos, A.C.B.; do Espírito Santo, D.A.; Salvetti, M.C.; Gilio, R.N.; Agra, L.B.; Pazin-Filho, A.; Miranda, C.H. Therapeutic versus prophylactic anticoagulation for severe COVID-19: A randomized phase II clinical trial (HESACOVID). Thromb. Res. 2020, 196, 359–366. [Google Scholar] [CrossRef]
- Paranjpe, I.; Fuster, V.; Lala, A.; Russak, A.J.; Glicksberg, B.S.; Levin, M.A.; Charney, A.W.; Narula, J.; Fayad, Z.A.; Bagiella, E.; et al. Association of treatment dose anticoagulation within-hospital survival among hospitalized patients with COVID-19. J. Am. Coll. Cardiol. 2020, 76, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Springer, G.F.; Wurzel, H.A.; McNeal, G.M.J.; Doughty, M.F. Isolation of anticoagulant fractions from crude fucoidan. Proc. Soc. Exp. Biol. Med. 1957, 94, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Hahn, T.; Lang, S.; Ulber, R.; Muffler, K. Novel procedures for the extraction of fucoidan from brown algae. Process Biochem. 2012, 47, 1691–1698. [Google Scholar] [CrossRef]
- Menshova, R.V.; Shevchenko, N.M.; Imbs, T.I.; Zvyagintseva, T.N.; Maluarenko, O.S.; Zaporoshets, T.S.; Besednova, N.N.; Ermakova, S.P. Fucoidans from brown alga Fucus evanescens: Structure and biological activity. Front. Mar. Sci. 2016, 3. [Google Scholar] [CrossRef]
- Ngo, D.H.; Kim, S.K. Sulfated polysaccharides as bioactive agents from marine algae. Int. J. Boil. Macromol. 2013, 62, 70–75. [Google Scholar] [CrossRef]
- Chi, Y.; Li, H.; Wang, P.; Du, C.; Ye, H.; Zuo, S.; Guan, H.; Wang, P. Structural characterization of ulvan extracted from Ulva clathRata assisted by an ulvan lyase. Carbohydr. Polym. 2020, 229, 115497. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.V.; Truong, H.B.; Tran, N.H.V.; Quach, T.M.T.; Nguyen, T.N.; Bui, M.L.; Yuguchi, Y.; Thanh, T.T.T. Structure, conformation in aqueous solution and antimicrobial activity of ulvan extracted from green seaweed Ulva reticulate. Nat. Prod. Res. 2018, 32, 2291–2296. [Google Scholar] [CrossRef]
- Tziveleka, L.A.; Ioannou, E.; Roussis, V. Ulvan, a bioactive marine sulphated polysaccharide as a key constituent of hybrid biomaterials: A review. Carbohydr. Polym. 2019, 218, 355–370. [Google Scholar] [CrossRef]
- Wang, S.; Wang, W.; Hou, L.; Qin, L.; He, M.; Li, W.; Mao, W. A sulfated glucuronorhamnan from the green seaweed Monostroma nitidum: Characteristics of its structure and antiviral activity. Carbohydr. Polym. 2020, 227, 115280. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Y.; Du, C.; Mou, H.; Wang, P. Compositional and structural characteristics of sulfated polysaccharide from Enteromorpha prolifera. Carbohydr. Polym. 2017, 165, 221–228. [Google Scholar] [CrossRef]
- Shen, Y.R.; Kuo, M.I. Effects of different carrageenan types on the rheological and water-holding properties of tofu. LWT 2017, 78, 122–128. [Google Scholar] [CrossRef]
- Torres, M.; Flórez-Fernández, N.; Domínguez, H. Integral Utilization of Red Seaweed for Bioactive Production. Mar. Drugs 2019, 17, 314. [Google Scholar] [CrossRef] [PubMed]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef]
- Athukorala, Y.; Jung, W.K.; Vasanthan, T.; Jeon, Y.J. An anticoagulative polysaccharide from an enzymatic hydrolysate of Ecklonia cava. Carbohydr. Polym. 2006, 66, 184–191. [Google Scholar] [CrossRef]
- Mestechkina, N.M.; Shcherbukhin, V.D. Sulfated polysaccharides and their anticoagulant activity: A review. Prikl. Biokhim. Mikrobiol. 2010, 46, 291–298. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Silva, T.M.A.; Alves, L.G.; Queiroz, K.C.S.; Santos, M.G.L.; Marques, C.T.; Chavante, S.F.; Rocha, H.A.O.; Leite, E.L. Partial characterization and anticoagulant activity of a heterofucan from the brown seaweed Padina gymnospora. Braz. J. Med. Biol. Res. 2005, 38, 523–533. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.B.; Zhang, Z.S.; Song, H.; Li, P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef]
- Minh Thu, Q.T.; Luong, D.V.; Nu, N.T.; Thanh Van, T.T.; Ly, B.M.; Thu Thuy, T.T. Effect of sulfation on the structure and anticoagulant activity of ulvan extracted from green seaweed Ulva reticulata. Vietnam J. Sci. Technol. 2016, 54, 373–379. [Google Scholar] [CrossRef][Green Version]
- Liu, X.; Du, P.; Liu, X.; Cao, S.; Qin, L.; He, M.; He, X.; Mao, W. Anticoagulant properties of a green algal rhamnan-type sulfated polysaccharide and its low-molecular-weight fragments prepared by mild acid degradation. Mar. Drugs 2018, 16, 445. [Google Scholar] [CrossRef] [PubMed]
- Adrien, A.; Bonnet, A.; Dufour, D.; Baudouin, S.; Maugard, T.; Bridiau, N. Anticoagulant activity of sulfated ulvan isolated from the green macroalga Ulva rigida. Mar. Drugs 2019, 17, 291. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Mao, W.; Zhang, X.; Qi, X.; Chen, Y.; Chen, Y.; Xu, J.; Zhao, C.; Hou, Y.; Yang, Y.; et al. Structural characterization of an anticoagulant-active sulfated polysaccharide isolated from green alga Monostroma latissimum. Carbohydr. Polym. 2011, 85, 394–400. [Google Scholar] [CrossRef]
- Synytsya, A.; Choi, D.J.; Pohl, R.; Na, Y.S.; Capek, P.; Lattová, E.; Taubner, T.; Choi, J.W.; Lee, C.W.; Park, J.K.; et al. Structural features and anti-coagulant activity of the sulphated polysaccharide SPS-CF from a green alga Capsosiphon fulvescens. Mar. Biotechnol. 2015, 17, 718–735. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Pyun, Y.R.; Hwang, J.K.; Mourão, P.A.S. A sulfated fucan from the brown alga Laminaria cichorioides has mainly heparin cofactor II-dependent anticoagulant activity. Carbohyd. Res. 2007, 342, 2326–2330. [Google Scholar] [CrossRef]
- Yang, C.; Chung, D.; Shin, I.S.; Lee, H.; Kim, J.; Lee, Y.; You, S. Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. Int. J. Biol. Macromol. 2008, 43, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H.; Pereira, M.S.; Valente, A.P.; Tollefsen, D.M.; Pavão, M.S.; Mourão, P.A. Selective cleavage and anticoagulant activity of a sulfated fucan: Stereospecific removal of a 2-sulfate ester from the polysaccharide by mild acid hydrolysis, preparation of oligosaccharides, and heparin cofactor II-dependent anticoagulant activity. Glycobiology 2005, 15, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Boisson-Vidal, C.; Chaubet, F.; Chevolot, L.; Sinquin, C.; Theveniaux, J.; Millet, J.; Sternberg, C.; Mulloy, B.; Fischer, A.M. Relationship between antithrombotic activities of fucans and their structure. Drug. Dev. Res. 2000, 51, 216–224. [Google Scholar] [CrossRef]
- Lahrsen, E.; Schoenfeld, A.K.; Alban, S. Size-dependent pharmacological activities of differently degraded fucoidan fractions from Fucus vesiculosus. Carbohyd. Polym. 2018, 189, 162–168. [Google Scholar] [CrossRef]
- Choi, Y.; Min, S.K.; Usoltseva, R.; Silchenko, A.; Zvyagintseva, T.; Ermakova, S.; Kim, J.K. Thrombolytic fucoidans inhibit the tPA-PAI1 complex, indicating activation of plasma tissue-type plasminogen activator is a mechanism of fucoidan-mediated thrombolysis in a mouse thrombosis model. Thromb. Res. 2018, 161, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Min, S.K.; Han, S.M.; Jang, J.S.; Kim, J.K. Stimulatory effect of an algal fucoidan on the release of vascular endothelial tissue-type plasminogen activator as a mechanism of fucoidan-mediated thrombolysis. Blood Coagul. Fibrinolysis 2016, 27, 594–596. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; He, X.; Qin, L.; He, M.; Yang, Y.; Liu, Z.; Mao, W. Anticoagulant and antithrombotic properties in vitro and in vivo of a novel sulfated polysaccharide from marine green alga Monostroma nitidum. Mar. Drugs 2019, 17, 247. [Google Scholar] [CrossRef] [PubMed]
- Juenet, M.; Aid-Launais, R.; Li, B.; Berger, A.; Aerts, J.; Ollivier, V.; Nicoletti, A.; Letourneur, D.; Chauvierre, C. Thrombolytic therapy based on fucoidan-functionalized polymer nanoparticles targeting P-selectin. Biomaterials 2018, 156, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Hall, G.; Lang, D.; Qiu, X.; Doctor, V. Effect of native fucoidan, sulfated fucoidan, heparin and 6-aminohexanoic acid on the activation of glutamic-plasminogen by urokinase: Role of NaCl. Blood Coagul. Fibrinolysis 2006, 17, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Amarasekara, A.S.; Doctor, V.M. Effect of oversulfation on the chemical and biological properties of fucoidan. Carbohydr. Polym. 2006, 63, 224–228. [Google Scholar] [CrossRef]
- Alvarez, G.F.; Bihari, D.; Collins, D. Heparin-induced thrombosis with a normal platelet count. Crit. Care Resusc. 2007, 9, 51–53. Available online: https://pubmed.ncbi.nlm.nih.gov/17352667 (accessed on 23 April 2020). [PubMed]
- Castelli, R.; Cassinerio, E.; Cappellini, M.D.; Porro, F.; Graziadei, G.; Fabris, F. Heparin induced thrombocytopenia: Pathogenetic, clinical, diagnostic and therapeutic aspects. Cardiovasc. Hematol. Disord. Drug Targets 2007, 7, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Suleria, H.A.R.; Masci, P.P.; Zhao, K.N.; Addepalli, R.; Chen, W.; Osborne, S.A.; Gobe, G.C. Anti-coagulant and anti-thrombotic properties of blacklip abalone (Haliotis rubra): In Vitro and animal studies. Mar. Drugs 2017, 15, 240. [Google Scholar] [CrossRef]
- Ludwig, R.J. Therapeutic use of heparin beyond anticoagulation. Curr. Drug Discov. Technol. 2009, 6, 281–289. [Google Scholar] [CrossRef]
- Melo, F.R.; Pereira, M.S.; Foguel, D.; Mourão, P.A.S. Antithrombin-mediated anticoagulant activity of sulfated polysaccharides: Different mechanisms for heparin and sulfated galactans. J. Biol. Chem. 2004, 279, 20824–20835. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, T.A.; Besednova, N.N.; Mamaev, A.N.; Momot, A.P.; Shevchenko, N.M.; Zvyagintseva, T.N. Anticoagulant activity of fucoidan from brown algae Fucus evanescens of the Okhotsk Sea. Bull. Exp. Biol. Med. 2003, 136, 471–473. [Google Scholar] [CrossRef]
- Lapikova, E.S.; Drozd, N.N.; Tolstenkov, A.S.; Makarov, V.A.; Zvyagintseva, T.N.; Shevchenko, N.M.; Bakunina, I.U.; Besednova, N.N.; Kuznetsova, T.A. Inhibition of thrombin and factor Xa by Fucus evanescens fucoidan and its modified analogs. Bull. Exp. Biol. Med. 2008, 146, 328–333. [Google Scholar] [CrossRef]
- Jung, W.K.; Athukorala, Y.; Lee, Y.J.; Cha, S.H.; Lee, C.H.; Vasanthan, T.; Choi, K.S.; Yoo, S.H.; Kim, S.K.; Jeon, Y.J. Sulfated polysaccharide purified from Ecklonia cava accelerates antithrombin III-mediated plasma proteinase inhibition. J. Appl. Phycol. 2007, 19, 425–430. [Google Scholar] [CrossRef]
- Becker, C.F.; Guimarães, J.A.; Mourão, P.A.S.; Verli, H. Conformation of sulfated galactan and sulfated fucan in aqueous solutions: Implications to their anticoagulant activities. J. Mol. Graph. Model. 2007, 26, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological Activities of Fucoidan and the Factors Mediating Its Therapeutic Effects: A Review of Recent Studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Q.; Song, J. Toxicological evaluation of fucoidan extracted from Laminaria japonica in Wistar rats. Food Chem. Toxicol. 2005, 43, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Lillicrap, D.; Labelle, A.; Knappe, S.; Keller, T.; Burnett, E.; Powell, S.; Johnsjn, K.W. Efficacy and safety of a new-class hemostatic drug candidate, AV513, in dogs with hemophilia A. Blood 2008, 111, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Gideon, T.P.; Rengasamy, R. Toxicological evaluation of fucoidan from Cladosiphon okamuranus. J. Med. Food. 2008, 11, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Guo, F.; Hu, J.; Zhang, L.; Xue, C.; Zhang, Z.; Li, B. Antithrombotic activity of oral administered low molecular weight fucoidan from Laminaria japonica. Thromb. Res. 2016, 144, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Bavishi, C.; Maddox, T.M.; Messerli, F.H. Coronavirus Disease 2019 (COVID-19) infection and renin angiotensin system blockers. JAMA Cardiol. 2020, 5, 745–747. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.M.; Levy, J.H. COVID‑19 and its implications for thrombosis and anticoagulation. Blood 2020, 135, 2033–2040. [Google Scholar] [CrossRef]
- Levi, M.; Thachil, J.; Iba, T.; Levy, J.H. Coagulation abnormalities and thrombosis in patients with COVID‑19. Lancet Haematol. 2020, 7, E438–E440. [Google Scholar] [CrossRef]
- Marongiu, F.; Grandone, E.; Barcellona, D. Pulmonary thrombosis in 2019-nCoV pneumonia? J. Tromb. Haemost. 2020, 18, 1511–1513. [Google Scholar] [CrossRef]
- Dhont, S.; Derom, E.; van Braeckel, E.; Depuydt, P.; Lambrecht, B.N. The pathophysiology of ‘happy’ hypoxemia in COVID-19. Respir. Res. 2020, 21, 198. [Google Scholar] [CrossRef]
- Serebrovska, Z.O.; Chong, E.Y.; Serebrovska, T.V.; Tumanovska, L.V.; Xi, L. Hypoxia, HIF-1α, and COVID-19: From pathogenic factors to potential therapeutic targets. Acta Pharmacol. Sin. 2020, 41, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Zubieta-Calleja, G.; Zubieta-DeUrioste, N. Pneumolysis and «Silent Hypoxemia» in COVID-19. Indian J. Clin. Biochem. 2021, 36, 112–116. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Vaninov, N. In the eye of the COVID-19 cytokine storm. Nat. Rev. Immunol. 2020, 20, 277. [Google Scholar] [CrossRef]
- Liu, B.; Li, M.; Zhou, Z.; Guan, X.; Xiang, Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020, 111, 102452. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Yang, L.; Liu, R.; Liu, F.; Wu, K.L.; Li, J.; Liu, X.H.; Zhu, C.L. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin. Chem. Lab. Med. 2020, 58, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cao, Y.; Chen, L.; Wu, D.; Yu, J.; Wang, H.; He, W.; Chen, L.; Dong, F.; Chen, W.; et al. Hematological features of persons with COVID-19. Leukemia 2020, 34, 2163–2172. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Q.; Wang, Y.; Wu, Y.; Xu, J.; Yu, Y.; Shang, Y. Thrombocytopenia and its association with mortality in patients with COVID-19. J. Thromb. Haemost. 2020, 18. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, W.; Guo, Y.; Chen, L.; Zhang, L.; Zhao, S.; Long, D.; Yu, L. Association between platelet parameters and mortality in coronavirus disease 2019: Retrospective cohort study. Platelets 2020, 31, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Middeldorp, S.; Coppens, M.; van Haaps, T.F.; Foppen, M.; Vlaar, A.P.; Müller, M.C.; Bouman, C.C.; Beenen, L.F.; Kootte, R.S.; Heijmans, J.; et al. Incidence of venous thromboembolism in hospitalized patients with COVID‑19. J. Thromb. Haemost. 2020, 18, 1995–2002. [Google Scholar] [CrossRef]
- Joob, B.; Wiwanitkit, V. Hemorrhagic Problem Among the Patients With COVID-19: Clinical Summary of 41 Thai Infected Patients. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620918308. [Google Scholar] [CrossRef] [PubMed]
- McFadyen, J.D.; Stevens, H.; Peter, K. The Emerging Threat of (Micro) Thrombosis in COVID-19 and Its Therapeutic Implications. Circ. Res. 2020, 127, 571–587. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhou, Q.; Xu, J. Mechanism of thrombocytopenia in COVID-19 patients. Ann. Hematol. 2020, 99, 1205–1208. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, X.; Jiao, Y.; Li, Z.; Liu, Q.; Ye, J.; Yang, M. Mechanisms involved in the development of thrombocytopenia in patients with COVID-19. Thromb. Res. 2020, 193, 110–115. [Google Scholar] [CrossRef]
- Lippi, G.; Plebani, M.; Henry, B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID‑19) infections: A meta‑analysis. Clin. Chim. Acta 2020, 506, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Amgalan, A.; Othman, M. Exploring possible mechanisms for COVID-19 induced thrombocytopenia: Unanswered Questions. J. Thromb. Haemost. 2020, 18, 1514–1516. [Google Scholar] [CrossRef]
- Spadarella, G.; Di Minno, A.; Donati, M.B.; Mormile, M.; Ventre, I.; Di Minno, G. From unfractionated heparin to pentasaccharide: Paradigm of rigorous science growing in the understanding of the in vivo thrombin generation. Blood Rev. 2020, 39, 10061. [Google Scholar] [CrossRef]
- Hirsh, J.; Raschke, R. Heparin and low-molecular-weight heparin: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004, 126, 188S–203S. [Google Scholar] [CrossRef]
- Rezaie, A.R. Prothrombin protects factor Xa in the prothrombinase complex from inhibition by the heparin-antithrombin complex. Blood 2001, 97, 2308–2313. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, P.; Liverani, L.; Spelta, F.; Mascellani, G.; Parma, B. Variability of Heparins and Heterogeneity of Low Molecular Weight Heparins. Semin Thromb Hemost. 2007, 33, 496–502. [Google Scholar] [CrossRef]
- Navarese, E.P.; De Luca, G.; Castriota, F.; Kozinski, M.; Gurbel, P.A.; Gibson, C.M.; Andreotti, F.; Buffon, A.; Siller-Matula, J.M.; Sukiennik, A.; et al. Low-molecular-weight heparins vs. unfractionated heparin in the setting of percutaneous coronary intervention for ST-elevation myocardial infarction: A meta-analysis. J. Thromb. Haemost. 2011, 9, 1902–1915. [Google Scholar] [CrossRef] [PubMed]
- Padilla, A.; Gray, E.; Pepper, D.S.; Barrowcliffe, T.W. Inhibition of thrombin generation by heparin and low molecular weight (LMW) heparins in the absence and presence of platelet factor 4 (PF4). Br. J. Haematol. 1992, 82, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Xie, H.; Tian, R.; Yu, K.; Wang, R. Efficacy of low molecular weight heparin in patients with acute exacerbation of chronic obstructive pulmonary disease receiving ventilatory support. COPD 2014, 11, 171–176. [Google Scholar] [CrossRef]
- Cohen, A.T.; Davidson, B.L.; Gallus, A.S.; Lassen, M.R.; Prins, M.H.; Tomkowski, W.; Turpie, A.G.; Egberts, J.F.; Lensing, A.W.; ARTEMIS Investigators. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: Randomised placebo controlled trial. BMJ 2006, 332, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, F.; Chehade, S.; Lazo-Langner, A. Thrombosis risk associated with COVID-19 infection. A scoping review. Thromb. Res. 2020, 192, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Testa, S.; Prandoni, P.; Paoletti, O.; Morandini, R.; Tala, M.; Dellanoce, C.; Giorgi-Pierfranceschi, M.; Betti, M.; Danz, G.B.; Pan, A.; et al. Direct oral anticoagulant plasma levels’ striking increase in severe COVID-19 respiratory syndrome patients treated with antiviral agents: The Cremona experience. J. Thromb. Haemost. 2020, 18, 1320–1323. [Google Scholar] [CrossRef]
- University of Liverpool. COVID-19 Drug Interactions. Available online: https://www.covid19-druginteractions.org/prescribing-resources (accessed on 23 April 2020).
- Marietta, M.; Ageno, W.; Artoni, A.; De Candia, E.; Gresele, P.; Marchetti, M.; Marcucci, R.; Tripodi, A. COVID-19 and haemostasis: A position paper from Italian society on thrombosis and haemostasis (SISET). Blood Transfus. 2020, 18, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Young, E. The anti-inflammatory effects of heparin and related compounds. Thromb. Res. 2008, 122, 743–752. [Google Scholar] [CrossRef]
- Thachil, J. The versatile heparin in COVID-19. J. Thromb. Haemost. 2020, 18, 1020–1022. [Google Scholar] [CrossRef]
- Tichelaar, Y.I.; Kluin-Nelemans, H.J.; Meijer, K. Infections and inflammatory diseases as risk factors for venous thrombosis. A systematic review. Thromb. Haemost. 2012, 107, 827–837. [Google Scholar] [CrossRef]
- Poterucha, T.J.; Libby, P.; Goldhaber, S.Z. More than an anticoagulant: Do heparins have direct anti-inflammatory effects? Thromb. Haemost. 2017, 117, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Atallah, B.; Mallah, S.I.; AlMahmeed, W. Anticoagulation in COVID-19. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 260–261. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, W.B. Thromboinflammation in COVID-19 acute lung injury. Paediatr. Respir. Rev. 2020, 35, 20–24. [Google Scholar] [CrossRef]
- Mycroft-West, C.; Su, D.; Elli, S.; Guimond, S.; Miller, G.; Turnbull, J.; Yates, E.; Guerrini, M.; Fernig, D.; Lima, M.; et al. The 2019 coronavirus (SARS-CoV-2) surface protein (Spike) S1 receptor binding domain undergoes conformational change upon heparin binding. BioRxiv 2020. [Google Scholar] [CrossRef]
- Thomas, W.; Varley, J.; Johnston, A.; Symington, E.; Robinson, M.; Sheares, K.; Lavinio, A.; Besser, M. Thrombotic complications of patients admitted to intensive care with COVID-19 at a teaching hospital in the United Kingdom. Thromb. Res. 2020, 191, 76–77. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Fagot Gandet, F.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- White, D.; MacDonald, S.; Bull, T.; Hayman, M.; de Monteverde-Robb, R.; Sapsford, D.; Lavinio, A.; Varley, J.; Johnston, A.; Besser, M.; et al. Heparin resistance in COVID-19 patients in the intensive care unit. J. Thromb. Thrombolysis 2020, 50, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Bulanov, A.Y.; Roitman, E.V. New coronavirus infection, hemostasis and heparin dosing problems: It is important to say now. Tromboz Gemostaz i Reologiya 2020, 2, 11–18. (In Russian) [Google Scholar] [CrossRef]
- Cuker, A.; Tseng, E.K.; Nieuwlaat, R.; Angchaisuksiri, P.; Blair, C.; Dane, K.; Davila, J.; DeSancho, M.T.; Diuguid, D.; Griffin, D.O.; et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021, 5, 872–888. [Google Scholar] [CrossRef]
- Fox, S.E.; Akmatbekov, A.; Harbert, J.L.; Li, G.; Quincy Brown, J.; Vander Heide, R.S. Pulmonary and cardiac pathology in Covid-19: The first autopsy series from New Orleans. Lancet Respir. Med. 2020, 8, 681–686. [Google Scholar] [CrossRef]
- Kow, C.S.; Hasan, S.S. The use of antiplatelet agents for arterial thromboprophylaxis in COVID-19. Rev. Esp. Cardiol. 2021, 74, 114–115. [Google Scholar] [CrossRef]
- Ranucci, M.; Ballotta, A.; Di Dedda, U.; Bayshnikova, E.; Dei Poli, M.; Resta, M.; Falco, M.; Albano, G.; Menicanti, L. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J. Thromb. Haemost. 2020, 18, 1747–1751. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Liu, S.; Sun, J.; Chen, Z.; Jiang, M.; Zhang, Q.; Wei, Y.; Wang, X.; Huang, Y.Y.; et al. Potential therapeutic effects of dipyridamole in the severely ill patients with COVID-19. Acta Pharm. Sin. B 2020, 10, 1205–1215. [Google Scholar] [CrossRef]
- Panka, B.A.; de Grooth, H.J.; Spoelstra-de Man, A.M.; Looney, M.R.; Tuinman, P.R. Prevention or treatment of ARDS with aspirin: A review of preclinical models and meta-analysis of clinical studies. Shock 2017, 47, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.P.; Christie, J.D. Linking genetics to ARDS pathogenesis: The role of the platelet. Chest 2015, 147, 585–586. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bikdeli, B.; Madhavan, M.V.; Gupta, A.; Jimenez, D.; Burton, J.R.; Der Nigoghossian, C.; Chuich, T.; Nouri, S.N.; Dreyfus, I.; Driggin, E.; et al. Global COVID-19 Thrombosis Collaborative Group. Pharmacological Agents Targeting Thromboinflammation in COVID-19: Review and Implications for Future Research. Thromb. Haemost. 2020, 120, 1004–1024. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, T.; Han, M.; Li, X.; Wu, D.; Xu, Y.; Zhu, Y.; Liu, Y.; Wang, X.; Wang, L. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. 2020, 92, 791–796. [Google Scholar] [CrossRef]
- Wool, G.D.; Miller, J.L. Review Article. The Impact of COVID-19 Disease on platelets and coagulation. Pathobiology 2021, 88, 15–27. [Google Scholar] [CrossRef]
- Ahmadi, A.; Moghadamtousi, S.Z.; Abubakar, S.; Zandi, K. Antiviral Potential of Algae Polysaccharides Isolated from Marine Sources: A Review. BioMed. Res. Int. 2015, 10. [Google Scholar] [CrossRef]
- Damonte, E.B.; Matulewicz, M.C.; Cerezo, A.S. Sulfated seaweed polysaccharides as antiviral agents. Curr. Med. Chem. 2004, 11, 2399–2419. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.X.; Guan, H.S. The antiviral activities and mechanisms of marine polysaccharides: An overview. Mar. Drugs 2012, 10, 2795–2816. [Google Scholar] [CrossRef] [PubMed]
- Zaporozhets, T.S.; Besednova, N.N. Biologically active compounds from marine organisms in the strategies for combating coronaviruses. Review. AIMS Microbiol. 2020, 6, 470–494. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immuno-modulatory and anti-inflammatory effects of fucoidan: A Review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chen, Y.; Chan, W.Y. Marine natural products with inflammatory activity. Appl. Microbiol. Biotechnol. 2016, 100, 1645–1666. [Google Scholar] [CrossRef]
- Cui, M.; Wu, J.; Wang, S.; Shu, H.; Zhang, M.; Liu, K.; Liu, K. Characterization and anti-inflammatory effects of sulfated polysaccharide from the red seaweed Gelidium pacificum Okamura. Int. J. Biol. Macromol. 2019, 129, 377–385. [Google Scholar] [CrossRef]
- Majee, S.B.; Avlani, D.; Biswas, G.R. Pharmacological, pharmaceutical, cosmetic and diagnostic applications of sulfated polysaccharides from marine algae and bacteria. Afr. J. Pharm. Pharmacol. 2017, 11, 68–77. [Google Scholar] [CrossRef]
- Giriwono, P.E.; Iskandriati, D.; Tan, C.P.; Andarwulan, N. Sargassum seaweed as a source of anti-inflammatory substances and the potential insight of the tropical species: A review. Mar. Drugs 2019, 17, 590. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jin, W.; Sood, A.; Montgomery, D.W.; Grant, O.C.; Fuster, M.M.; Fu, L.; Dordick, J.S.; Woods, R.J.; Zhang, F.; et al. Characterization of heparin and severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) spike glycoprotein binding interactions. Antiviral Res. 2020, 181, 104873. [Google Scholar] [CrossRef]
- Kwon, P.S.; Oh, H.; Kwon, S.J.; Jin, W.; Zhang, F.; Fraser, K.; Hong, J.J.; Linhardt, R.J.; Dordick, J.S. Sulfated polysaccharides effectively inhibit SARS-CoV-2 in vitro. Cell Discov. 2020, 6, 50. [Google Scholar] [CrossRef]
- Hans, N.; Malik, A.; Naik, S. Antiviral activity of sulfated polysaccharides from marine algae and its application in combating COVID-19: Mini review. Biores. Technol. Rep. 2021, 13, 100623. [Google Scholar] [CrossRef]
- Pagarete, A.; Ramos, A.S.; Puntervoll, P.; Allen, M.J.; Verdelho, V. Antiviral Potential of Algal Metabolites—A Comprehensive Review. Mar. Drugs 2021, 19, 94. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsova, T.A.; Andryukov, B.G.; Makarenkova, I.D.; Zaporozhets, T.S.; Besednova, N.N.; Fedyanina, L.N.; Kryzhanovsky, S.P.; Shchelkanov, M.Y. The Potency of Seaweed Sulfated Polysaccharides for the Correction of Hemostasis Disorders in COVID-19. Molecules 2021, 26, 2618. https://doi.org/10.3390/molecules26092618
Kuznetsova TA, Andryukov BG, Makarenkova ID, Zaporozhets TS, Besednova NN, Fedyanina LN, Kryzhanovsky SP, Shchelkanov MY. The Potency of Seaweed Sulfated Polysaccharides for the Correction of Hemostasis Disorders in COVID-19. Molecules. 2021; 26(9):2618. https://doi.org/10.3390/molecules26092618
Chicago/Turabian StyleKuznetsova, Tatyana A., Boris G. Andryukov, Ilona D. Makarenkova, Tatyana S. Zaporozhets, Natalya N. Besednova, Ludmila N. Fedyanina, Sergey P. Kryzhanovsky, and Mikhail Yu. Shchelkanov. 2021. "The Potency of Seaweed Sulfated Polysaccharides for the Correction of Hemostasis Disorders in COVID-19" Molecules 26, no. 9: 2618. https://doi.org/10.3390/molecules26092618
APA StyleKuznetsova, T. A., Andryukov, B. G., Makarenkova, I. D., Zaporozhets, T. S., Besednova, N. N., Fedyanina, L. N., Kryzhanovsky, S. P., & Shchelkanov, M. Y. (2021). The Potency of Seaweed Sulfated Polysaccharides for the Correction of Hemostasis Disorders in COVID-19. Molecules, 26(9), 2618. https://doi.org/10.3390/molecules26092618