A Chewable Cure “Kanna”: Biological and Pharmaceutical Properties of Sceletium tortuosum
Abstract
1. Introduction
2. Methodology Framework
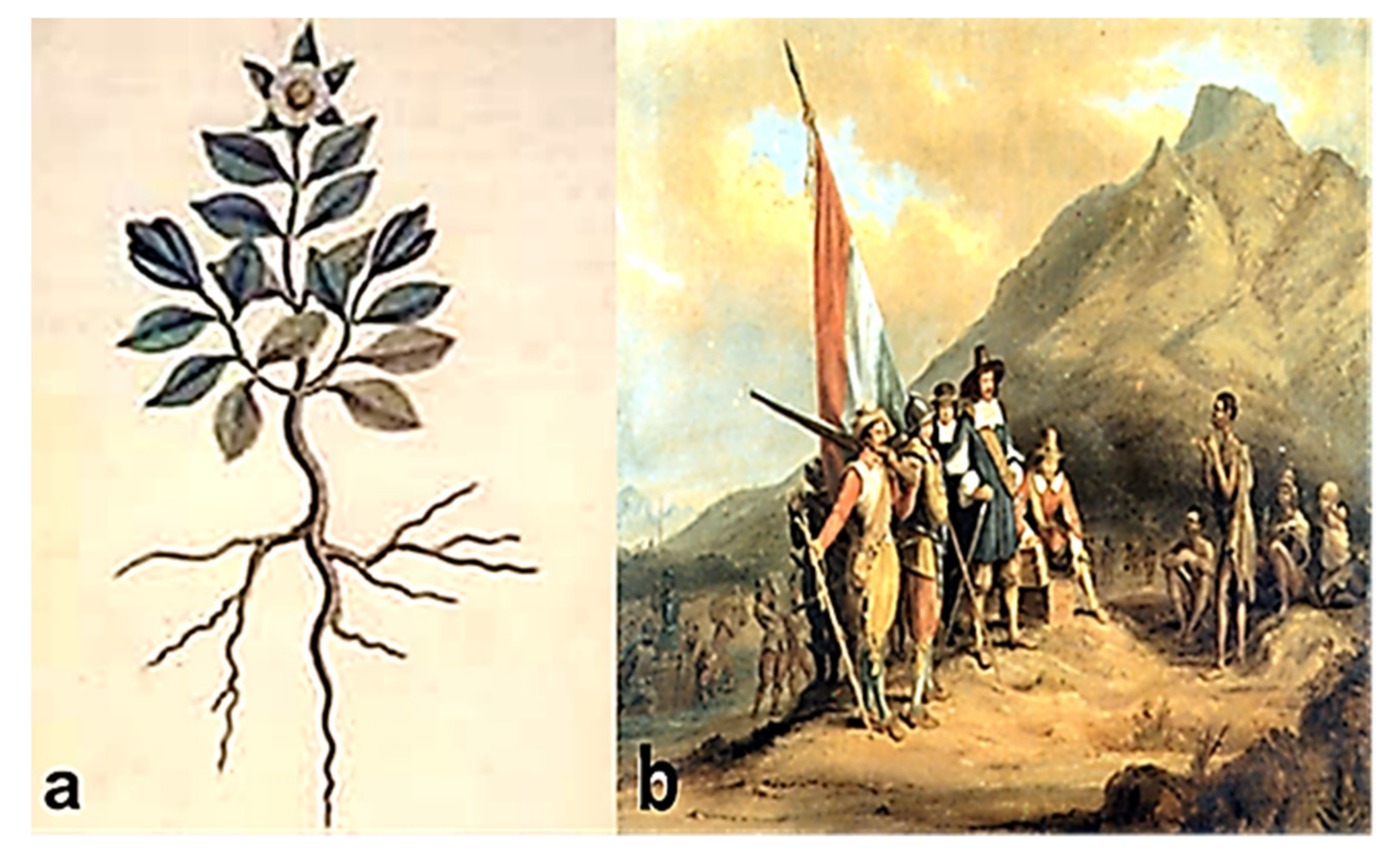
3. History, Description and Distribution of Sceletium
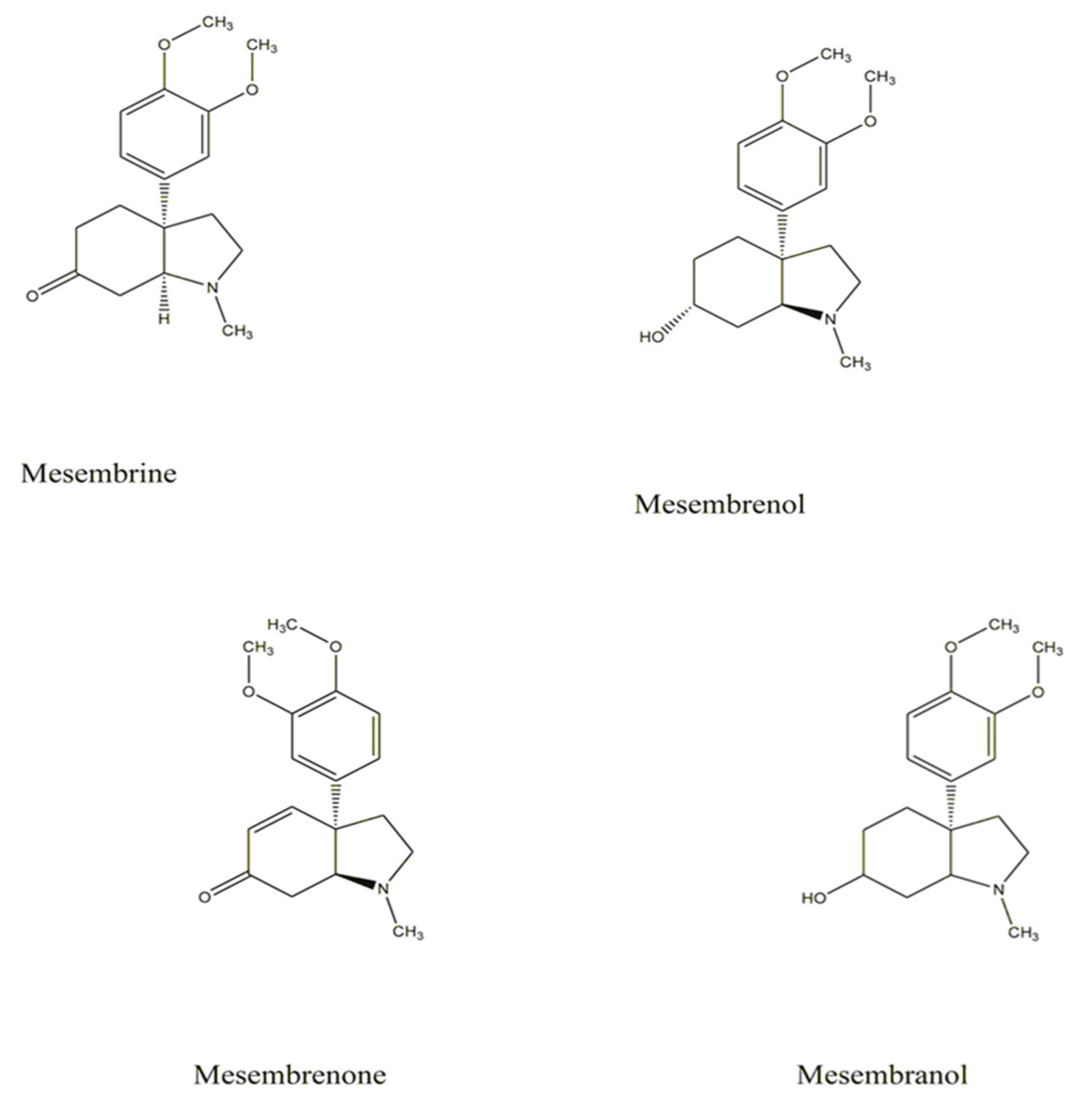
4. Phytochemical Constituents of Sceletium
5. Biological and Pharmaceutical Properties of Sceletium sp.
5.1. Antimicrobial Properties of Sceletium Plants
5.2. Anti-Stress Properties of Sceletium Plants
5.3. Anti-Depressant Properties of Sceletium Plants
5.4. Anxiolytic Properties of Sceletium Plants
5.5. Analgesic Properties of Sceletium Plants
5.6. Anti-Inflammatory Properties of Sceletium Plants
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gericke, N.; Viljoen, A.M. Sceletium—A review update. J. Ethnopharmacol. 2008, 119, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Stafford, G.I.; Pedersen, M.E.; van Staden, J.; Jäger, A.K. Review on plants with CNS-effects used in traditional South African medicine against mental diseases. J. Ethnopharmacol. 2008, 119, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Mothibe, M.E.; Sibanda, M. African Traditional Medicine: South African Perspective. Tradit. Complement. Med. 2019, 1–27. [Google Scholar] [CrossRef]
- Karunamoorthi, K.; Jegajeevanram, K.; Vijayalakshmi, J.; Mengistie, E. Traditional medicinal plants: A source of phytothera-peutic modality in resource-constrained health care settings. J. Evid. Based Complement. Altern. Med. 2013, 18, 67–74. [Google Scholar] [CrossRef]
- Street, A.; Prinsloo, G. Commercially important medicinal plants of South Africa: A review. J. Chem. 2013, 2013, 1–16. [Google Scholar] [CrossRef]
- Nandagoapalan, V.; Doss, A.; Marimuthu, C. Phytochemical analysis of some traditional medicinal plants. Biosci. Discov. 2016, 7, 17–20. [Google Scholar]
- Petrič, D.; Mravčáková, D.; Kucková, K.; Čobanová, K.; Kišidayová, S.; Cieslak, A.; Ślusarczyk, S.; Váradyová, Z. Effect of dry medicinal plants (wormwood, chamomile, fumitory and mallow) on in vitro ruminal antioxidant capacity and fermentation patterns of sheep. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1219–1232. [Google Scholar] [CrossRef]
- Murbach, T.S.; Hirka, G.; Szakonyine, I.P.; Gericke, N.; Endres, J.R. A toxicological safety assessment of a standardized extract of Sceletium tortuosum (Zembrin) in rats. Food Chem. Toxicol. 2014, 74, 190–199. [Google Scholar] [CrossRef]
- Carpenter, J.M.; Jourdan, M.K.; Fountain, E.M.; Ali, Z.; Abe, N.; Khan, I.A.; Sufka, K.J. The effects of Sceletium tortuosum (L.) N.E. Br. extract fraction in the chick anxiety-depression model. J. Ethnopharmacol. 2016, 193, 329–332. [Google Scholar] [CrossRef]
- Smith, C. The effects of Sceletium tortuosum in an in vivo model of psychological stress. J. Ethnopharmacol. 2011, 133, 31–36. [Google Scholar] [CrossRef]
- Gericke, J. Evaluating the Antidepressant-Like Properties of Sceletium Tortuosum, Alone and as Adjunctive Treatment. Ph.D. Thesis, North-West University, Potchefstroom, South-Africa, 2019. [Google Scholar]
- Dimpfel, W.; Schombert, L.; Gericke, N. Electropharmacogram of Sceletium tortuosum extract based on spectral local field power in conscious freely moving rats. J. Ethnopharmacol. 2016, 177, 140–147. [Google Scholar] [CrossRef]
- Bennett, A.C.; Van Camp, A.; Lopez, V.; Smith, C. Sceletium tortuosum may delay chronic disease progression via alka-loid-dependent antioxidant or anti-inflammatory action. J. Physiol. Biochem. 2018, 74, 539–547. [Google Scholar] [CrossRef]
- Rusch, N. Controlled fermentation, honey, bees and alcohol: Archaeological and ethnohistorical evidence from southern Africa. S. Afr. Hum. 2020, 33, 1–31. [Google Scholar]
- Gericke, N. Kabbo’s! Kwaiń: The past, present and possible future of kanna. In The Ethnopharmacological Search for Psychoactive Drugs; Founding Director of HG&H Pharmaceuticals (Pty), Ltd.: Bryanston, South Africa, 2018; pp. 122–150. [Google Scholar]
- Scott, G.; Hewett, M. Pioneers in ethnopharmacology: The Dutch East India Company (VOC) at the Cape from 1650. J. Ethnopharmacol. 2008, 115, 339–360. [Google Scholar] [CrossRef]
- Setshedia, I.B.; Myera, M.; Dewara, J.; Foucheb, G. Isolation of a Pentacyclic Triterpenoid from Sceletium tortuosum. J. Appl. Sci. Technol. 2019, 1, 21–27. [Google Scholar]
- Chesselet, P. Sceletium tortuosum (L.) NE Br.(Mesembryanthemaceae). The South African National Biodiversity Institute (SANBI). 2005. Available online: http://pza.sanbi.org/sceletium-tortuosum (accessed on 12 February 2021).
- Cowling, R. Namaqualand: A Succulent Desert; Penguin Random House: Cape Town, South Africa, 1999; ISBN 9781928213314. [Google Scholar]
- Zwicky, E. Über Channa. ein Genussmittel der Hottentotten (Mesembrianthemum expansum L. und tortuosum L.). Wurde eins Doktors der Naturwissenschaften. Ph.D. Thesis, Eidgenossischen Technischen Hochschule in Zurich, Zurich, Switzerland, 1914. [Google Scholar]
- Popelak, A.; Lettenbauer, G. The mesembrine alkaloids. In The Alkaloids; Manske, R.H.F., Ed.; Academic Press: New York, NY, USA, 1967; Volume 9, pp. 467–482. [Google Scholar]
- Krstenansky, J.L. Mesembrine alkaloids: Review of their occurrence, chemistry, and pharmacology. J. Ethnopharmacol. 2017, 195, 10–19. [Google Scholar] [CrossRef]
- Schmid, R.; Van Wyk, B.-E.; Van Oudtshoorn, B.; Gericke, N. Medicinal Plants of South Africa. Taxon 1998, 47. [Google Scholar] [CrossRef]
- Gericke, N.P.; Van Wyk, B.E. Pharmaceutical Compositions Containing Mesembrine and Related Compounds. U.S. Patent 6,288,104, 11 September 2001. [Google Scholar]
- Patnala, S.; Kanfer, I. Chemotaxonomic studies of mesembrine-type alkaloids in Sceletium plant species. S. Afr. J. Sci. 2013, 109, 1–5. [Google Scholar] [CrossRef]
- Shikanga, E.A.; Viljoen, A.M.; Combrinck, S.; Marston, A.; Gericke, N. The chemotypic variation of Sceletium tortuosum alkaloids and commercial product formulations. Biochem. Syst. Ecol. 2012, 44, 364–373. [Google Scholar] [CrossRef]
- Zhao, J.; Khan, I.A.; Combrinck, S.; Sandasi, M.; Chen, W.; Viljoen, A.M. 1H-NMR and UPLC-MS metabolomics: Functional tools for exploring chemotypic variation in Sceletium tortuosum from two provinces in South Africa. Phytochemistry 2018, 152, 191–203. [Google Scholar] [CrossRef]
- Bennett, A.C.; Smith, C. Immunomodulatory effects of Sceletium tortuosum (Trimesemine™) elucidated in vitro: Implications for chronic disease. J. Ethnopharmacol. 2018, 214, 134–140. [Google Scholar] [CrossRef]
- Harvey, A.L.; Young, L.C.; Viljoen, A.M.; Gericke, N.P. Pharmacological actions of the South African medicinal and functional food plant Sceletium tortuosum and its principal alkaloids. J. Ethnopharmacol. 2011, 137, 1124–1129. [Google Scholar] [CrossRef]
- Sreekissoon, A.; Plačková, L.; Doležal, K.; Finnie, J.F.; Van Staden, J. In vitro and ex vivo vegetative propagation and cytokinin profiles of Sceletium tortuosum (L.) N. E. Br.: A South African medicinal plant. Plant Cell Tissue Organ Cult. (Pctoc.) 2021, 145, 191–202. [Google Scholar] [CrossRef]
- Sishuba, A.; Leboko, J.; Ateba, C.N.; Manganyi, M.C. First Report: Diversity of Endophytic fungi Possessing Antifungal Activity Isolated from Native Kougoed (Sceletium tortuosum L.). Mycobiology 2021, 49, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.R. Amaryllidaceae, Sceletium, imidazole, oxazole, thiazole, peptide and miscellaneous alkaloids (July 1998 to June 1999). Nat. Prod. Rep. 2000, 18, 95–128. [Google Scholar] [CrossRef] [PubMed]
- Palmer-Young, E.C.; Sadd, B.M.; Irwin, R.E.; Adler, L.S. Synergistic effects of floral phytochemicals against a bumble bee par-asite. Ecol. Evol. 2017, 7, 1836–1849. [Google Scholar] [CrossRef]
- Sharma, K.; Guleria, S.; Razdan, V.K.; Babu, V. Synergistic antioxidant and antimicrobial activities of essential oils of some selected medicinal plants in combination and with synthetic compounds. Ind. Crop. Prod. 2020, 154. [Google Scholar] [CrossRef]
- Muszynska, B.; Łojewski, M.; Rojowski, J.; Opoka, W.; Sułkowska-Ziaja, K. Natural products of relevance in the prevention and supportive treatment of depression. Psychiatr. Pol. 2015, 49, 435–453. [Google Scholar] [CrossRef]
- Kapewangolo, P.; Tawha, T.; Nawinda, T.; Knott, M.; Hans, R. Sceletium tortuosum demonstrates in vitro anti-HIV and free radical scavenging activity. S. Afr. J. Bot. 2016, 106, 140–143. [Google Scholar] [CrossRef]
- Debora, M.S.J.; Baba, V.; Gomathi, S. Impact of stress on health. Narayana Nurs. J. 2018, 5, 11–14. [Google Scholar]
- Rai, D.; Bhatia, G.; Sen, T.; Palit, G. Anti-stress effects of Ginkgo biloba and Panax ginseng: A comparative study. J. Pharm. Sci. 2003, 93, 458–464. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Panahi, Y.; Sahraei, H.; Johnston, T.P.; Sahebkar, A. The impact of stress on body function: A review. EXCLI J. 2017, 16, 1057–1072. [Google Scholar]
- De Abreu, M.S.; Giacomini, A.C.; Genario, R.; dos Santos, B.E.; Marcon, L.; Demin, K.A.; Kalueff, A.V. The impact of housing environment color on zebrafish anxiety-like behavioral and physiological (cortisol) responses. Gen. Comp. Endocrinol. 2020, 294. [Google Scholar] [CrossRef]
- Daniels, T.E.; Olsen, E.M.; Tyrka, A.R. Stress and Psychiatric Disorders: The Role of Mitochondria. Annu. Rev. Clin. Psychol. 2020, 16, 165–186. [Google Scholar] [CrossRef]
- Global Organization for Stress, Stress Facts. Available online: http://www.gostress.com/stress-facts/ (accessed on 14 February 2021).
- Luo, Y.; Wen, J.; Kanfer, I.; Yu, P.; Patnala, S. Sceletium tortuosum: Effects on Central Nervous System and Related Disease. J. Pharm. Biomed. Sci. 2020, 10, 151–160. [Google Scholar]
- Jahagirdar, A.Q.F.; Hugar, S.; Patil, V.; Nanjappaiah, A.K.H. Screening of Antistress activity of Ficus benghalensis Fruit extract. Res. J. Pharm. Technol. 2020, 13. [Google Scholar] [CrossRef]
- Sharma, A.; Anchariya, R.; Dubey, C. A Review on Anti-Stress Activity of Piper Methysticum. Asian J. Pharm. Res. Dev. 2020, 8, 130–136. [Google Scholar] [CrossRef]
- Darbar, S.D.; Saha, S.; Chattopadhyay, S.; Chattapadhyay, A. Anti-Stress Activity (in-vivo) of Multi Herbal Capsule-Trasina® in Experimental Murine Model. Asian J. Pharm. Res. Dev. 2020, 8, 52–58. [Google Scholar]
- Solati, K.; Heidari-Soureshjani, S.; Pocock, L. Effects and mechanisms of medicinal plants on stress hormone (cortisol): A sys-tematic review. World fam. Middle East. J. Fam. Med. 2017, 15, 117–123. [Google Scholar] [CrossRef]
- Terburg, D.; Syal, S.; A Rosenberger, L.; Heany, S.; Phillips, N.; Gericke, N.; Stein, D.J.; Van Honk, J. Acute effects of Sceletium tortuosum (Zembrin), a dual 5-HT reuptake and PDE4 inhibitor, in the human amygdala and its connection to the hypothalamus. Neuropsychopharmacology 2013, 38, 2708–2716. [Google Scholar] [CrossRef]
- Bennett, A.; López, V.; Van Camp, A.; Smith, C. Sceletium tortuosum and depression: Mechanisms elucidated. Planta Med. 2016, 81, S1–S381. [Google Scholar] [CrossRef]
- Hammen, C. Stress and Depression. Annu. Rev. Clin. Psychol. 2005, 1, 293–319. [Google Scholar] [CrossRef]
- World Health Organization (WHO). News Room. Fact Sheets. Depression. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 19 February 2021).
- Sasaki, K.; El Omri, A.; Kondo, S.; Han, J.; Isoda, H. Rosmarinus officinalis polyphenols produce anti-depressant like effect through monoaminergic and cholinergic functions modulation. Behav. Brain Res. 2013, 238, 86–94. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, J.-G. Health functions and structure–activity relationships of natural anthraquinones from plants. Food Funct. 2018, 9, 6063–6080. [Google Scholar] [CrossRef]
- Abe, N.; Ali, Z.; Khan, I. Structure of Novel Alkaloids from Sceletium tortuosum. Planta Med. 2013, 79. [Google Scholar] [CrossRef]
- Napoletano, M.; Fraire, C.; Santangelo, F.; Moriggi, E. Mesembrine is an inhibitor of PDE4 that follows structure-activity rela-tionship of rolipram. Chemistry 2001, 2001, 303–308, preprint archive. [Google Scholar]
- Perviz, S.; Khan, H.; Pervaiz, A. Plant Alkaloids as an Emerging Therapeutic Alternative for the Treatment of Depression. Front. Pharm. 2016, 7. [Google Scholar] [CrossRef]
- Khan, H. Therapeutic Potential of Plant Alkaloids as Antidepressant. Front. Clin. Drug Res. CNS Neurol. Disord. 2017, 5, 250–267. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Soleymani, A.; Cheng, Q. Traditional herbal medicines to overcome stress, anxiety and improve mental health in outbreaks of human coronaviruses. Phytother. Res. 2021, 35, 1237–1247. [Google Scholar] [CrossRef]
- Schell, R. Sceletium tortuosum and Mesembrine: A Potential Alternative Treatment for Depression. Bachelor’s Thesis, Scripps Colleges, Claremont, CA, USA, 2014. [Google Scholar]
- Villarreal, M.L.; Sharma, A.; Cardoso-Taketa, A.; Garcia, G. A systematic updated review of scientifically tested selected plants used for anxiety disorders. Bot. Targets 2012, 2, 21–39. [Google Scholar] [CrossRef][Green Version]
- Maphanga, V.B.; Skalicka-Woźniak, K.; Budzynska, B.; Enslin, G.M.; Viljoen, A.M. Screening selected medicinal plants for potential anxiolytic activity using an in vivo zebrafish model. Psychopharmacology 2020, 237, 3641–3652. [Google Scholar] [CrossRef] [PubMed]
- Kabli, N.; Nguyen, T.; Balboni, G.C.; Odowd, B.F.; George, S.R. Antidepressant-like and anxiolytic-like effects following activation of the μ-δ opioid receptor heteromer in the nucleus accumbens. Mol. Psychiatry 2014, 19, 986–994. [Google Scholar] [CrossRef]
- Hafeez, Z.; Benoit, S.; Cakir-Kiefer, C.; Dary, A.; Miclo, L. Food protein-derived anxiolytic peptides: Their potential role in anxiety management. Food Funct. 2021, 12, 1415–1431. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Nieman, A.N.; Mian, Y.; Zahn, N.M.; Mikulsky, B.N.; Poe, M.M.; Methuku, K.R.; Liu, Y.; Cook, J.M.; Stafford, D.C.; et al. A Structure-Activity Relationship Comparison of Imidazodiazepines Binding at Kappa, Mu, and Delta Opioid Receptors and the GABAA Receptor. Molecules 2020, 25, 3864. [Google Scholar] [CrossRef] [PubMed]
- Manda, V.K.; Avula, B.; Ashfaq, M.K.; Abe, N.; Khan, I.A.; Khan, S.I. Quantification of mesembrine and mesembrenone in mouse plasma using UHPLC-QToF-MS: Application to a pharmacokinetic study. Biomed. Chromatogr. 2017, 31, e3815. [Google Scholar] [CrossRef] [PubMed]
- Loria, M.J.; Ali, Z.; Abe, N.; Sufka, K.J.; Khan, I.A. Effects of Sceletium tortuosum in rats. J. Ethnopharmacol. 2014, 155, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Perrine, S.A.; Hoshaw, B.A. Unterwald EM. Delta opioid receptor ligands modulate anxiety-like behaviors in the rat. Br. J. Pharm. 2006, 147, 864–872. [Google Scholar] [CrossRef]
- Reay, J.; Wetherell, M.A.; Morton, E.; Lillis, J.; Badmaev, V. Sceletium tortuosum (Zembrin®) ameliorates experimentally induced anxiety in healthy volunteers. Hum. Psychopharmacol. Clin. Exp. 2020, 35, 1–7. [Google Scholar] [CrossRef]
- Fountain, E.M. The Effects of Sceletium tortuosum in the Chick Anxiety-Depression Model. Undergraduate Thesis, University of Mississippi, Sally McDonnell Barksdale Honors College, Oxford, MS, USA, 2016. [Google Scholar]
- Smith, M.T.; Crouch, N.R.; Gericke, N.; Hirst, M. Psychoactive constituents of the genus Sceletium NE Br. and other Mesem-bryanthemaceae: A review. J. Ethnopharmacol. 1996, 50, 119–130. [Google Scholar] [CrossRef]
- Brunetti, P.; Faro, A.F.L.; Tini, A.; Busardò, F.P.; Carlier, J. Pharmacology of Herbal Sexual Enhancers: A Review of Psychiatric and Neurological Adverse Effects. Pharmacology 2020, 13, 309. [Google Scholar] [CrossRef]
- Bakalov, D.; Hadjiolova, R.; Pechlivanova, D. Pathophysiology of Depression and Novel Sources of Phytochemicals for its Treatment—A Systematic Review. Acta Med. Bulg. 2020, 47, 69–74. [Google Scholar] [CrossRef]
- Saura, C.A.; Valero, J. The role of CREB signaling in Alzheimer’s disease and other cognitive disorders. Rev. Neurosci. 2011, 22, 153–169. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A. Nonresolving Inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Setshedi, I.B. Phytochemical Isolation of Compounds from the Plant Selenium tortuosum. Ph.D. Thesis, University of South Africa (Unisa), Pretoria, South Africa, 2012. [Google Scholar]
- Setshed, I.I.; Peter, X.K.; Fouché, G.; Myer, M.; Dewar, J. Isolation of compounds from Sceletium tortuosum and the detection of antimalarial activity of the isolates and extracts. In Proceedings of the CSIR Conference 2010, Pretoria, South Africa, October 2010; Available online: http://hdl.handle.net/10204/4316 (accessed on 23 February 2021).
- Setshedi, I.I.; Fouche, G.; Dewar, J.; Maharaj, V.; Myer, M.S. Phytochemical isolation of compounds from Sceletium tortuosum and activity testing against Plasmodium falciparum. Onderstepoort J. Vet. Res. 2012, 79. [Google Scholar] [CrossRef]
- Swart, A.C.; Smith, C. Modulation of glucocorticoid, mineralocorticoid and androgen production in H295 cells by Trimesem-ine™, a mesembrine-rich Sceletium extract. J. Ethnopharmacol. 2016, 177, 35–45. [Google Scholar] [CrossRef]
- Bennett, A.C. Neuro- and Immunomodulatory Effects of Sceletium tortuosum. Master’s Thesis, University of Stellenbosch, Stellenbosch, South Africa, 2018. [Google Scholar]
- Chiu, S.; Gericke, N.; Farina-Woodbury, M.; Badmaev, V.; Raheb, H.; Terpstra, K.; Antongiorgi, J.; Bureau, Y.; Cernovsky, Z.; Hou, J.; et al. Proof-of-Concept Randomized Controlled Study of Cognition Effects of the Proprietary Extract Sceletium tortuosum (Zembrin) Targeting Phosphodiesterase-4 in Cognitively Healthy Subjects: Implications for Alzheimer’s Dementia. Evid. Based Complement. Altern. Med. 2014, 68, 25389443. [Google Scholar]
- Nell, H.; Siebert, M.; Chellan, P.; Gericke, N. A Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Trial of Extract Sceletium tortuosum (Zembrin) in Healthy Adults. J. Altern. Complement. Med. 2013, 19, 898–904. [Google Scholar] [CrossRef]
- Meyer, G.M.; Wink, C.S.; Zapp, J.; Maurer, H.H. GC-MS, LC-MS n, LC-high resolution-MS n, and NMR studies on the metab-olism and toxicological detection of mesembrine and mesembrenone, the main alkaloids of the legal high “Kanna” isolated from Sceletium tortuosum. Anal. Bioanal. Chem. 2015, 407, 761–778. [Google Scholar] [CrossRef]
- Fitzgerald, M.; Heinrich, M.; Booker, A. Medicinal Plant Analysis: A Historical and Regional Discussion of Emergent Complex Techniques. Front. Pharm. 2020, 10. [Google Scholar] [CrossRef]
- Hunter, E.; Stander, M.; Kossmann, J.; Chakraborty, S.; Prince, S.; Peters, S.; Loedolff, B. Toward the identification of a phy-tocannabinoid-like compound in the flowers of a South African medicinal plant (Leonotis leonurus). BMC Res. Notes 2020, 13, 1–6. [Google Scholar] [CrossRef]
- Van Wyk, A.; Prinsloo, G. Medicinal plant harvesting, sustainability and cultivation in South Africa. Biol. Conserv. 2018, 227, 335–342. [Google Scholar] [CrossRef]
- Ozioma, E.-O.J.; Chinwe, O.A.N. Herbal Medicines in African Traditional Medicine. Herb. Med. 2019, 10, 191–214. [Google Scholar] [CrossRef]
- Fokunang, E.T.; Fonmboh, D.J.; Mballa, R.N.; Nyuyki, A.B.; Fokunang, L.B.; Kaba, N.; Abong, T.B.; Duerr, R.; Richard, E.; Ondoua, M.-T.A.; et al. Pharmacovigilance of Natural Herbal Medicines Research for Efficacy, Safety and Quality Assurance of Phytomedicine Products. J. Complement. Altern. Med. Res. 2020, 21–37. [Google Scholar] [CrossRef]
- Chinsamy, M. South African Triple Heritage and Public Healthcare. PULA: Botswana J. Afr. Stud. 2017, 31, 1–15. [Google Scholar]
- Sankaramourthy, D.; Subramanian, K.; Sadras, S.R. Safety and Regulatory Issues on Traditional Medicine Entrusted Drug Discovery. In Evidence Based Validation of Traditional Medicines; J.B. Metzler: Stuttgart, Germany, 2021; pp. 589–603. [Google Scholar]
- World Health Organization. General Guidelines for Methodologies on Research and Evaluation of Traditional Medicine; No. WHO/EDM/TRM/2000.1; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Liu, X.; Jiang, W.; Su, M.; Sun, Y.; Liu, H.; Nie, L.; Zang, H. Quality evaluation of traditional Chinese medicines based on fin-gerprinting. J. Sep. Sci. 2020, 43, 6–17. [Google Scholar] [CrossRef]
- Street, R.; Stirk, W.; Van Staden, J. South African traditional medicinal plant trade—Challenges in regulating quality, safety and efficacy. J. Ethnopharmacol. 2008, 119, 705–710. [Google Scholar] [CrossRef]
- Ayeleso, T.B.; Ramachela, K.; Mukwevho, E. A review of therapeutic potentials of sweet potato: Pharmacological activities and influence of the cultivar. Trop. J. Pharm. Res. 2017, 15. [Google Scholar] [CrossRef]
- Rinschen, M.M.; Ivanisevic, J.; Giera, M.; Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 2019, 20, 353–367. [Google Scholar] [CrossRef]
- Sotenjwa, V.Z.; Chen, W.; Veale, C.G.; Anokwuru, C.P.; Tankeu, S.Y.; Combrinck, S.; Kamatou, G.P.; Viljoen, A.M. Chemotypic variation of non-volatile constituents of Artemisia afra (African wormwood) from South Africa. Fitoterapia 2020, 147. [Google Scholar] [CrossRef]
- Djokam, M.; Sandasi, M.; Chen, W.; Viljoen, A.; Vermaak, I. Hyperspectral Imaging as a Rapid Quality Control Method for Herbal Tea Blends. Appl. Sci. 2017, 7, 268. [Google Scholar] [CrossRef]
- Sandasi, M.; Chen, W.; Vermaak, I.; Viljoen, A. Non-destructive quality assessment of herbal tea blends using hyperspectral imaging. Phytochem. Lett. 2018, 24, 94–101. [Google Scholar] [CrossRef]
- Pudumo, J.; Chaudhary, S.; Chen, W.; Viljoen, A.; Vermaak, I.; Veale, C. HPTLC fingerprinting of Croton gratissimus leaf extract with Preparative HPLC-MS-isolated marker compounds. S. Afr. J. Bot. 2018, 114, 32–36. [Google Scholar] [CrossRef]
- Nsuala, B.N.; Kamatou, G.P.; Sandasi, M.; Enslin, G.; Viljoen, A. Variation in essential oil composition of Leonotis leonurus, an important medicinal plant in South Africa. Biochem. Syst. Ecol. 2017, 70, 155–161. [Google Scholar] [CrossRef]

| Biological Activity | Part Used | Chemical Composition | Ref. |
|---|---|---|---|
| Antidepressant-like properties, anxiolytic, mood elevator | Not specified | Alkaloids | [1] |
| Toxicological tests | Sceletium tortuosum (Zembrin®) | Mesembrenone, mesembrenol and mesembrin | [8] |
| Anxiety, depression | Whole plant | Alkaloids | [9] |
| Antioxidant and anti-inflammatory, neuroprotective effects | Leaves | Mesembrine | [13] |
| Immunomodulatory effects, depression, inflammation | Sceletium tortuosum (Trimesemine™) | Alkaloids | [28] |
| Antiviral, antioxidant activity | Whole plant | Anthraquinones, terpenes, polyphenols, anthocyanin, tannins, alkaloids, glycosides, carbohydrates and coumarins | [36] |
| Sleep function, improves memory and enhances cognitive function | Whole plant | Alkaloids | [43] |
| Anxiolytic effect | Sceletium tortuosum (Zembrin®) | Alkaloids | [48] |
| Central nervous system activity | Whole plant | Alkaloids | [61] |
| Antimalarial activity | Whole plant | Mesembrine | [75,76,77] |
| Stress-related illnesses | Whole plant | Δ7-mesembrenone, mesembrenone and mesembrine. | [78] |
| Immunomodulatory effects | leaves | Δ7-mesembrenone | [79] |
| Neurocognitive effects | Sceletium tortuosum (Zembrin®) | Alkaloids | [80] |
| Antidepressant | Not specified | Mesembrines, Mesembrenol, mesembrenone | [59] |
| Safety, tolerability, promotes coping with stress and sleep. | Sceletium tortuosum (Zembrin®) | Mesembrine | [81] |
| Toxicological tests | Whole plant | Mesembrine, mainly the O- and N demethyl-dihydro, hydroxy, and bis-demethyl-dihydro metabolites | [82] |
| Aim of the Study | Methodology Used | Ref. | ||
|---|---|---|---|---|
| Plant Preparation | Biological Techniques | Phytochemical Screening | ||
| To determine psychological effects of Sceletium tortuosum in an in vivo model | S. tortuosum parts were ground into powder form. | In vivo model, using Male Wistar rats in a double placebo study. Oral administration of extract for 17 days and stress was induced. Behavior was monitored. | N/A | [10] |
| To investigate anti-HIV and free radical scavenging activity of crude extracts of S. tortuosum. | S. tortuosum parts were ground into powder form. Crude extracts of S. tortuosum prepared using ethanol and ethyl acetate. | Anti-HIV assays FRET-based Sensolyte HIV-1 kit used. Fluorogenic substrate mixed with extracts in a 96 well plate. Fluorescence intensity measured. DPPH assay Extract mixed in ethanol and the absorbance measured. | Total phenolic content Total flavonoids content Total tannin content | [36] |
| To determine the acute effects of Sceletium tortuosum (Zembrin) in human amygdala | Sceletium (Zembrin) | Sceletium (Zembrin) administrated to humans in a placebo double-blind, cross-over study. | N/A | [48] |
| To screen for anxiolytic activity of Sceletium tortuosum, using an in vivo zebrafish model | S. tortuosum parts ground into powder form. Crude extracts of S. tortuosum prepared in various solvents. | MTC assay used in different concentrations in the zebrafish model study. Larvae placed in a 48-well plate with the control. MTC plates incubated for 18 h at 28.5 °C. | N/A | [60] |
| To determine the effects of Sceletium tortuosum in the Chick Anxiety-Depression Model | S. tortuosum parts ground into powder form and water used as a solvent. | Preclinical model assay on Male Silver Laced Wyandotte chick administrated S. tortuosum in various concentrations and induced with stress. Distress vocalizations captured the data. | N/A | [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manganyi, M.C.; Bezuidenhout, C.C.; Regnier, T.; Ateba, C.N. A Chewable Cure “Kanna”: Biological and Pharmaceutical Properties of Sceletium tortuosum. Molecules 2021, 26, 2557. https://doi.org/10.3390/molecules26092557
Manganyi MC, Bezuidenhout CC, Regnier T, Ateba CN. A Chewable Cure “Kanna”: Biological and Pharmaceutical Properties of Sceletium tortuosum. Molecules. 2021; 26(9):2557. https://doi.org/10.3390/molecules26092557
Chicago/Turabian StyleManganyi, Madira Coutlyne, Cornelius Carlos Bezuidenhout, Thierry Regnier, and Collins Njie Ateba. 2021. "A Chewable Cure “Kanna”: Biological and Pharmaceutical Properties of Sceletium tortuosum" Molecules 26, no. 9: 2557. https://doi.org/10.3390/molecules26092557
APA StyleManganyi, M. C., Bezuidenhout, C. C., Regnier, T., & Ateba, C. N. (2021). A Chewable Cure “Kanna”: Biological and Pharmaceutical Properties of Sceletium tortuosum. Molecules, 26(9), 2557. https://doi.org/10.3390/molecules26092557