Non-Invasive Evaluation of Acute Effects of Tubulin Binding Agents: A Review of Imaging Vascular Disruption in Tumors †
Abstract
1. Introduction
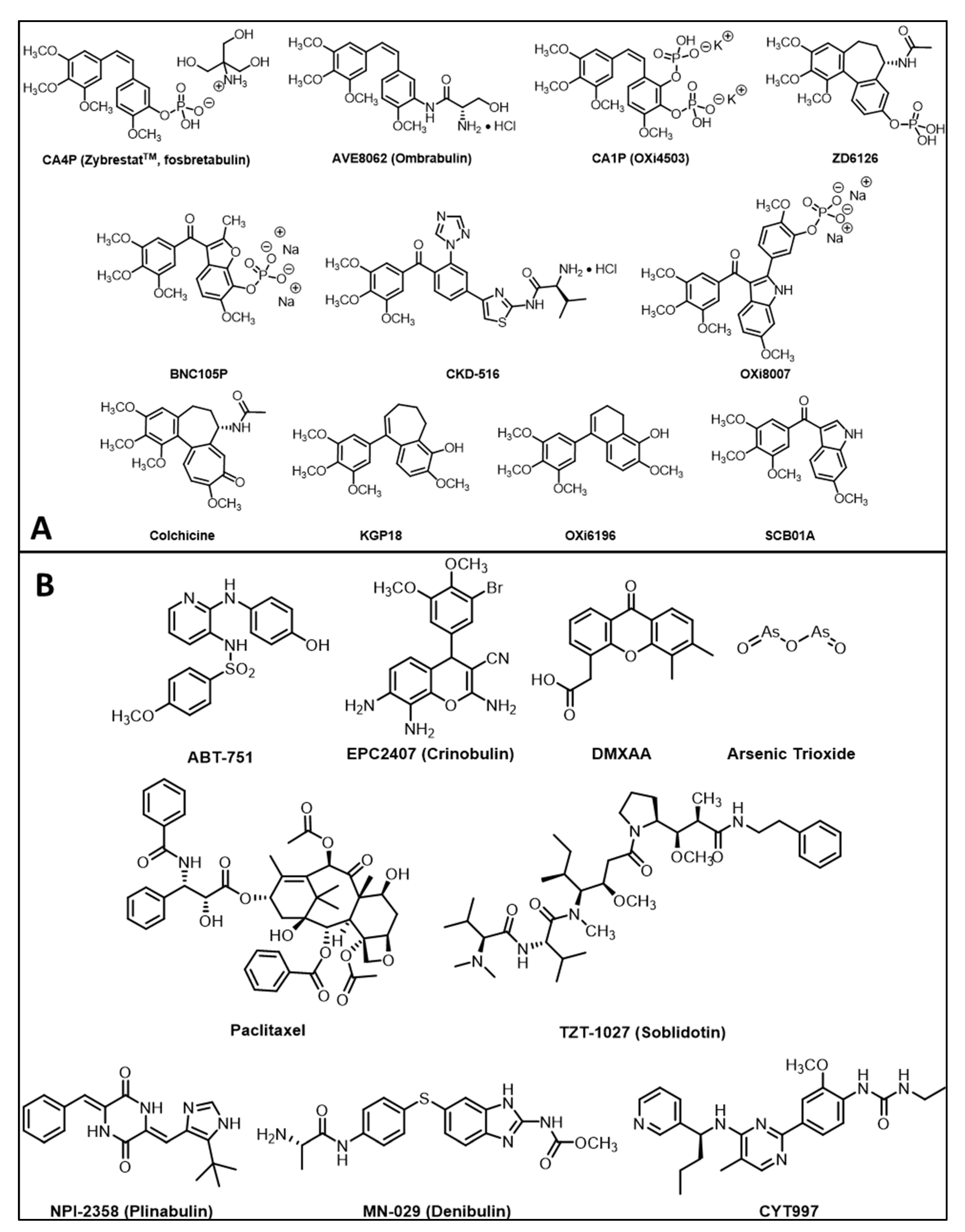
| Agent | Imaging Modality | Tumor Type | References |
|---|---|---|---|
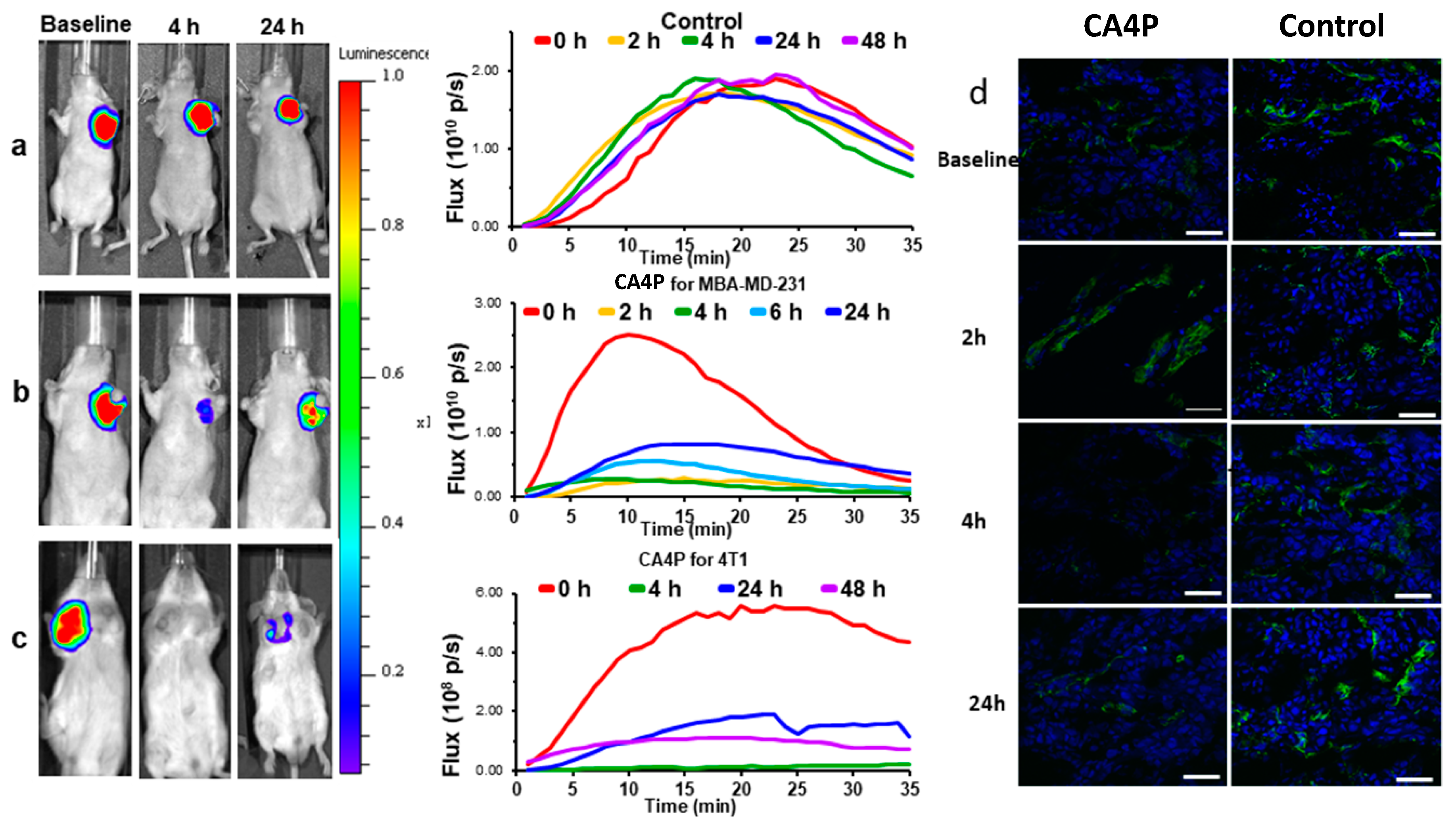
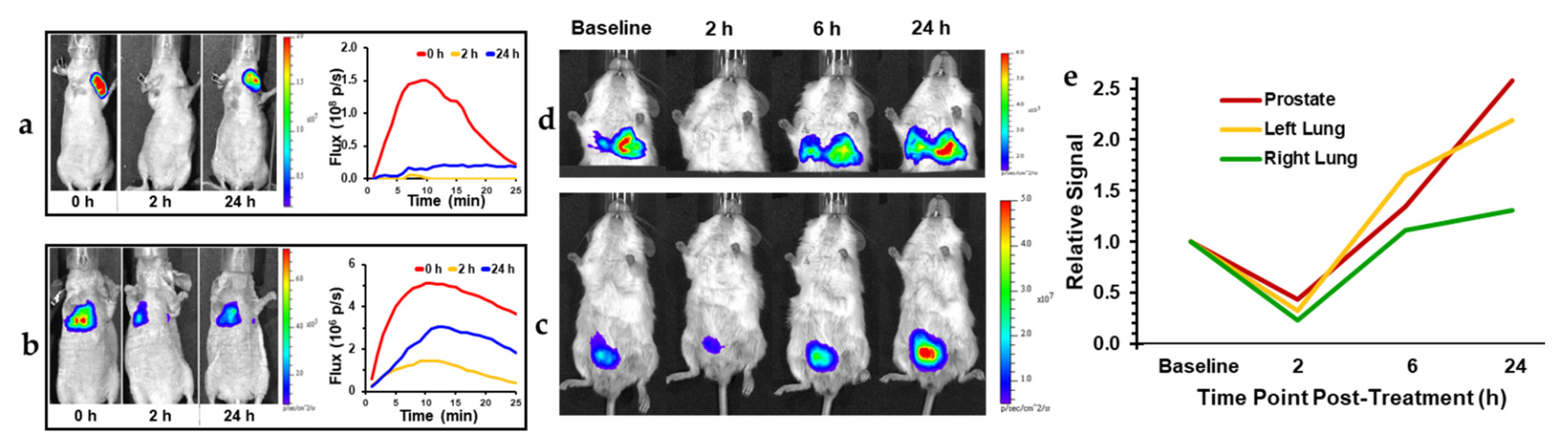
| CA4P | a BLI, b MRI, c MSOT/PAT, d PET/CT, e EPR, f US, g SPECT | Breast, liver, colorectal, bladder, pancreatic, prostate, lung, melanoma | a [5,89,90,91,92,93] cf. Figures 3 and 4 b [5,27,91,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107] c [92] cf. Figures 8 and 9 d [105,108], e [95] f [109,110,111,112], g [113] |
| CA1P | a BLI, b MRI, c MSOT, d PET/CT, f US | Colorectal, H&N, breast | a [5,114,115] b [103,105,114,116,117] c [118,119] d [105], f [5,91] |
| BNC105P | BLI | Kidney | [120] |
| AVE8062 | BLI, MRI, FDG-PET, CE-US | Colon, Ovarian, H&N | [121,122,123] |
| NPI2358 | DCE-MRI | Breast, sarcoma | [124] |
| ZD6126 | DCE-MRI, BOLD MRI | Colon, breast, prostate, fibrosarcoma | [11,37,125,126,127,128] |
| BPR0L075 | BLI | Breast | [129] |
| EPC2407 | BOLD MRI, DCE-MRI, MSOT, BLI, US | Head & Neck, glioma, prostate | [130,131,132] |
| DMXAA | a BLI, b MRI, c MSOT, d FDG-PET | Breast, colorectal, glioma, kidney, H&N | a [133,134], b [91,96,134,135,136,137,138], c [133,139,140] d [141] |
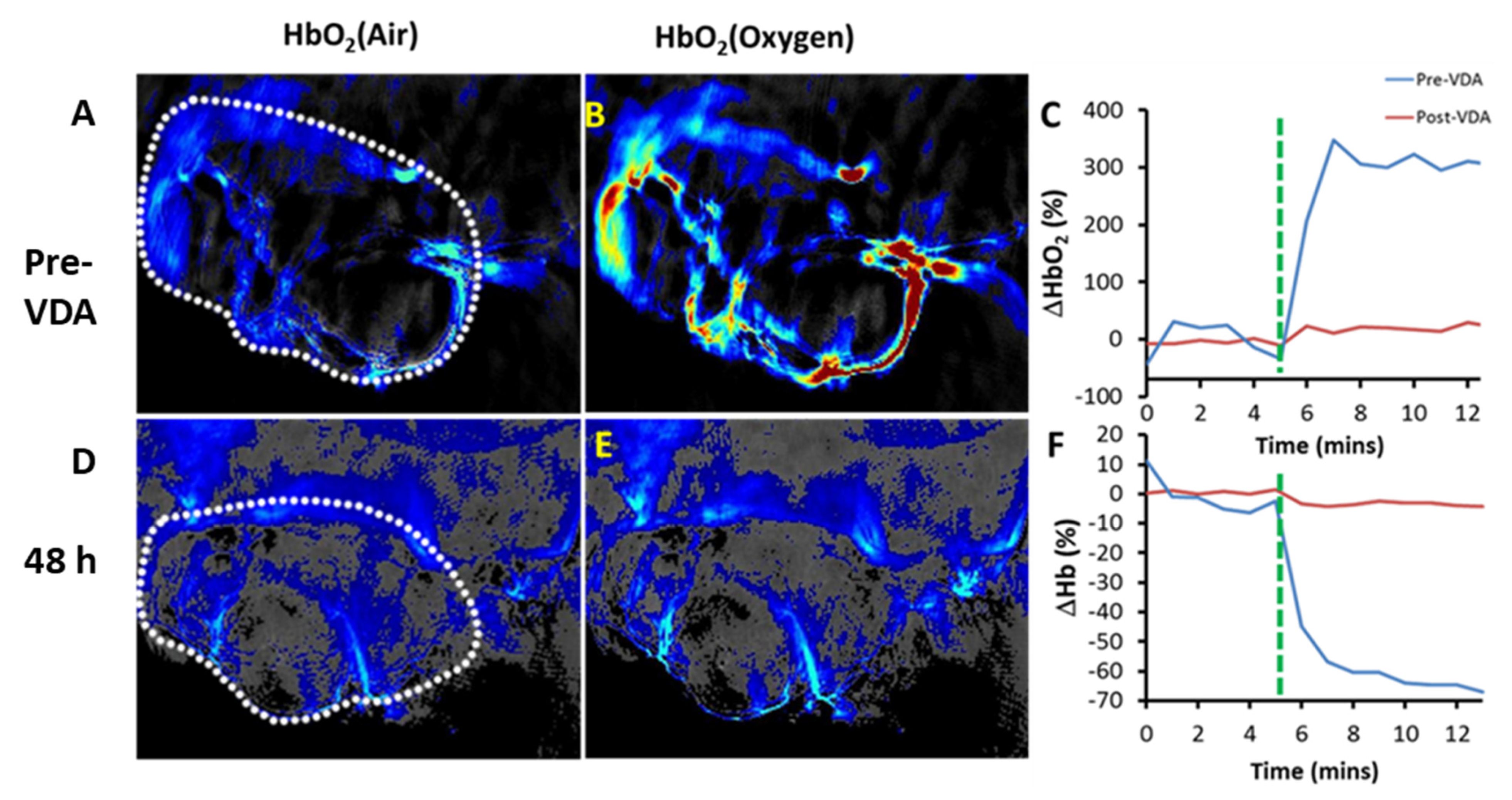
| OXi8007 | BLI, MRI, US | Breast, prostate | [17,74,93] cf. Figures 5, 9 and 11 |
| C118P | MRI | Liver (rabbit) | [142] |
| ABT-751 | MRI | Glioma (rat) | [143] |
| CA4P analogs | BLI, US | Breast, prostate, lung | [66,74,144,145,146,147] |
| Targeted prodrugs | BLI | 4T1 breast | [148] |
| Nanoparticles | MRI, optical, MSOT | 4T1, MCF-7 breast | [149,150,151,152,153] |
| Conjugates | MSOT | Colon | [154] |
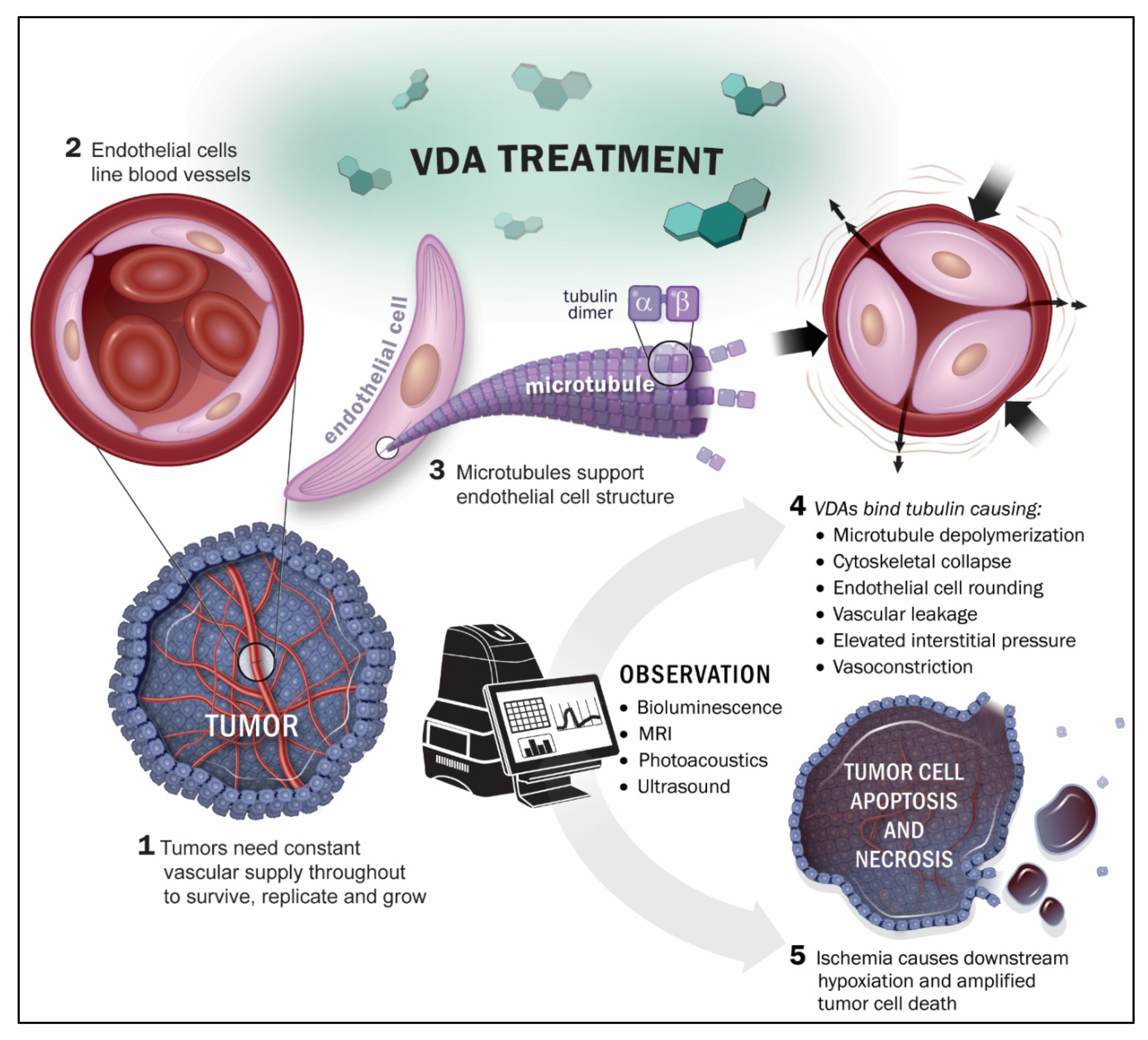
2. Imaging Technologies
2.1. Bioluminescence Imaging (BLI)
2.2. Magnetic Resonance Imaging (MRI)
| MRI a | VDA | Tumor Type | References |
|---|---|---|---|
| Perfusion/flow/vascular permeability DCE; DSC | CA4P, DMXAA, EPC2407, ZD6126, CKD-516 | Liver, colorectal, pancreatic, breast, glioma, prostate, carcinosarcoma, VX2, kidney, H&N | [5,27,37,96,97,100,102,103,104,105,106,117,124,125,126,127,128,131,132,135,136,137,138,219,220,221] |
| Diffusion DWI/IVIM | CA4P, CKD-516 | Liver, rhabdomyosarcoma, lung, VX2 | [98,102,104,117,219,222] |
| ASL | CA1P | Colorectal | [116] |
| BOLD, OE-MRI, 19F oximetry | ZD6126, CA4P, OXi8007 | Breast, bladder | [5,93,94,95,223] |
| CEST | TNF-α | SC colon tumors | [224] |
| pH | CA4P, DMXAA, ZD6126 | Breast | [91] |
| HP-pyruvate | CA1P, CA4P | Lymphoma, Breast | [105,225] |
2.3. Photoacoustics-Multispectral Optoacoustic Tomography (MSOT)
2.4. Other Modalities
2.5. Optimizing Combination Therapy
3. Discussion
4. Materials and Methods
5. Patents
- Kevin G. Pinney, Haichan Niu, Depoprosad Mondal, “Benzosuberene Analogues and Related Compounds with Activity as Anticancer Agents” (United States Patent: US 10,807,932 B2), issued 20 October 2020.
- Kevin G. Pinney and Madhavi Sriram, “Combretastatin Analogs with Tubulin Binding Activity” (United States Patent: US 8,394,859), issued 12 March 2013.
- David J. Chaplin, Klaus Edvardsen, Kevin G. Pinney, Joseph Prezioso, Mark Wood, “Compositions and Methods with Enhanced Therapeutic Activity” (United States Patent: US 8,198,302), issued 12 June 2012.
- Kevin G. Pinney, Feng Wang, Maria Del Pilar Mejia, “Indole-Containing and Combretastatin-Related Anti-Mitotic and Anti-Tubulin Polymerization Agents” (EP 1 214 298 B1), issued 30 May 2012.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Sample Availability
Appendix A. Typical Procedure for BLI
Appendix B. Typical Procedure for MRI
Appendix C. Typical Procedure for MSOT
Appendix D. Typical Procedure for US for Dog and Mouse
References
- Folkman, J. Tumor angiogenesis. Adv. Cancer Res. 1985, 43, 175–203. [Google Scholar] [CrossRef]
- Siemann, D.W.; Bibby, M.C.; Dark, G.G.; Dicker, A.P.; Eskens, F.A.; Horsman, M.R.; Marme, D.; Lorusso, P.M. Differentiation and definition of vascular-targeted therapies. Clin. Cancer Res. 2005, 11, 416–420. [Google Scholar]
- Denekamp, J. Vascular Attack as a Therapeutic Strategy for Cancer. Cancer Metastasis Rev. 1990, 9, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Tozer, G.M.; Kanthou, C.; Baguley, B.C. Disrupting tumour blood vessels. Nat. Rev. Cancer 2005, 5, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Zhao, D.W.; Liu, L.; Trawick, M.L.; Pinney, K.G. A perspective on vascular disrupting agents that interact with tubulin: Preclinical tumor imaging and biological assessment. Integr. Biol. 2011, 3, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, P.E. Vascular targeting agents as cancer therapeutics. Clin. Cancer Res. 2004, 10, 415–427. [Google Scholar] [CrossRef]
- McDonald, D.M.; Choyke, P.L. Imaging of angiogenesis: From microscope to clinic. Nat. Med. 2003, 9, 713–725. [Google Scholar] [CrossRef]
- Kanthou, C.; Tozer, G.M. Microtubule depolymerizing vascular disrupting agents: Novel therapeutic agents for oncology and other pathologies. Int. J. Exp. Pathol. 2009, 90, 284–294. [Google Scholar] [CrossRef]
- Jayson, G.C.; Kerbel, R.; Ellis, L.M.; Harris, A.L. Antiangiogenic therapy in oncology: Current status and future directions. Lancet 2016, 388, 518–529. [Google Scholar] [CrossRef]
- Siemann, D.W. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by Tumor-Vascular Disrupting Agents. Cancer Treat. Rev. 2011, 37, 63–74. [Google Scholar] [CrossRef]
- Liang, W.; Ni, Y.; Chen, F. Tumor resistance to vascular disrupting agents: Mechanisms, imaging, and solutions. Oncotarget 2016, 7, 15444–15459. [Google Scholar] [CrossRef]
- Hill, S.A.; Lonergan, S.J.; Denekamp, J.; Chaplin, D.J. Vinca Alkaloids-Antivascular Effects in a Murine Tumor. Eur. J. Cancer 1993, 29, 1320–1324. [Google Scholar] [CrossRef]
- Bai, R.; Hamel, E. (-)-Rhazinilam and the diphenylpyridazinone NSC 613241: Two compounds inducing the formation of morphologically similar tubulin spirals but binding apparently to two distinct sites on tubulin. Arch. Biochem. Biophys. 2016, 604, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Singh, S.B.; Niven, M.L.; Hamel, E.; Schmidt, J.M. Isolation, structure, and synthesis of combretastatins A-1 and B-1, potent new inhibitors of microtubule assembly, derived from Combretum caffrum. J. Nat. Prod. 1987, 50, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Singh, S.B.; Hamel, E.; Lin, C.M.; Alberts, D.S.; Garcia-Kendall, D. Isolation and structure of the strong cell growth and tubulin inhibitor combretastatin A-4. Experientia 1989, 45, 209–211. [Google Scholar] [CrossRef]
- Kanthou, C.; Tozer, G.M. The tumor vascular targeting agent combretastatin A-4-phosphate induces reorganization of the actin cytoskeleton and early membrane blebbing in human endothelial cells. Blood 2002, 99, 2060–2069. [Google Scholar] [CrossRef]
- Strecker, T.E.; Odutola, S.O.; Lopez, R.; Cooper, M.S.; Tidmore, J.K.; Charlton-Sevcik, A.K.; Li, L.; MacDonough, M.T.; Hadimani, M.B.; Ghatak, A.; et al. The vascular disrupting activity of OXi8006 in endothelial cells and its phosphate prodrug OXi8007 in breast tumor xenografts. Cancer Lett. 2015, 369, 229–241. [Google Scholar] [CrossRef]
- Sheng, Y.; Hua, J.; Pinney, K.G.; Garner, C.M.; Kane, R.R.; Prezioso, J.A.; Chaplin, D.J.; Edvardsen, K. Combretastatin family member OXI4503 induces tumor vascular collapse through the induction of endothelial apoptosis. Int. J. Cancer 2004, 111, 604–610. [Google Scholar] [CrossRef]
- Nepali, K.; Ojha, R.; Lee, H.Y.; Liou, J.P. Early investigational tubulin inhibitors as novel cancer therapeutics. Expert Opin. Investig. Drugs 2016, 25, 917–936. [Google Scholar] [CrossRef] [PubMed]
- Chase, D.M.; Chaplin, D.J.; Monk, B.J. The development and use of vascular targeted therapy in ovarian cancer. Gynecol. Oncol. 2017, 145, 393–406. [Google Scholar] [CrossRef]
- Sosa, J.A.; Elisei, R.; Jarzab, B.; Bal, C.S.; Koussis, H.; Gramza, A.W.; Ben-Yosef, R.; Gitlitz, B.J.; Haugen, B.; Karandikar, S.M.; et al. A randomized phase II/III trial of a tumor vascular disrupting agent fosbretabulin tromethamine (CA4P) with carboplatin (C) and paclitaxel (P) in anaplastic thyroid cancer (ATC): Final survival analysis for the FACT trial. J. Clin. Oncol. 2011, 29, 5502. [Google Scholar] [CrossRef]
- Ya-Ting, J.; Yan-Na, L.; Zhao-Peng, L. Tubulin Colchicine Binding Site Inhibitors as Vascular Disrupting Agents in Clinical Developments. Curr. Med. Chem. 2015, 22, 1348–1360. [Google Scholar] [CrossRef]
- Blay, J.Y.; Pápai, Z.; Tolcher, A.W.; Italiano, A.; Cupissol, D.; López-Pousa, A.; Chawla, S.P.; Bompas, E.; Babovic, N.; Penel, N.; et al. Ombrabulin plus cisplatin versus placebo plus cisplatin in patients with advanced soft-tissue sarcomas after failure of anthracycline and ifosfamide chemotherapy: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015, 16, 531–540. [Google Scholar] [CrossRef]
- O’Connor, J.P.; Jackson, A.; Parker, G.J.; Roberts, C.; Jayson, G.C. Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies. Nat. Rev. Clin. Oncol. 2012, 9, 167–177. [Google Scholar] [CrossRef]
- Smolarczyk, R.; Czapla, J.; Jarosz-Biej, M.; Czerwinski, K.; Cichon, T. Vascular disrupting agents in cancer therapy. Eur. J. Pharmacol. 2021, 891, 173692. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.S.; Goh, V.; Carnell, D.; Meer, K.; Padhani, A.R.; Saunders, M.I.; Hoskin, P.J. Tumor antivascular effects of radiotherapy combined with combretastatin a4 phosphate in human non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 1375–1380. [Google Scholar] [CrossRef]
- Galbraith, S.M.; Maxwell, R.J.; Lodge, M.A.; Tozer, G.M.; Wilson, J.; Taylor, N.J.; Stirling, J.J.; Sena, L.; Padhani, A.R.; Rustin, G.J.S. Combretastatin A4 phosphate has tumor antivascular activity in rat and man as demonstrated by dynamic magnetic resonance imaging. J. Clin. Oncol. 2003, 21, 2831–2842. [Google Scholar] [CrossRef]
- Stevenson, J.P.; Rosen, M.; Sun, W.; Gallagher, M.; Haller, D.G.; Vaughn, D.; Giantonio, B.; Zimmer, R.; Petros, W.P.; Stratford, M.; et al. Phase I trial of the antivascular agent combretastatin A4 phosphate on a 5-day schedule to patients with cancer: Magnetic resonance imaging evidence for altered tumor blood flow. J. Clin. Oncol. 2003, 21, 4428–4438. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, A.; Robertson, K.; Cooney, M.; Petros, W.P.; Stratford, M.; Jesberger, J.; Rafie, N.; Overmoyer, B.; Makkar, V.; Stambler, B.; et al. A phase I pharmacokinetic and translational study of the novel vascular targeting agent combretastatin a-4 phosphate on a single-dose intravenous schedule in patients with advanced cancer. Cancer Res. 2002, 62, 3408–3416. [Google Scholar]
- Koh, D.M.; Blackledge, M.; Collins, D.J.; Padhani, A.R.; Wallace, T.; Wilton, B.; Taylor, N.J.; Stirling, J.J.; Sinha, R.; Walicke, P.; et al. Reproducibility and changes in the apparent diffusion coefficients of solid tumours treated with combretastatin A4 phosphate and bevacizumab in a two-centre phase I clinical trial. Eur. Radiol. 2009, 19, 2728–2738. [Google Scholar] [CrossRef]
- Anderson, H.L.; Yap, J.T.; Miller, M.P.; Robbins, A.; Jones, T.; Price, P.M. Assessment of pharmacodynamic vascular response in a phase I trial of combretastatin A4 phosphate. J. Clin. Oncol. 2003, 21, 2823–2830. [Google Scholar] [CrossRef]
- Patterson, D.M.; Zweifel, M.; Middleton, M.R.; Price, P.M.; Folkes, L.K.; Stratford, M.R.L.; Ross, P.; Halford, S.; Peters, J.; Balkissoon, J.; et al. Phase I Clinical and Pharmacokinetic Evaluation of the Vascular-Disrupting Agent OXi4503 in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2012, 18, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Rischin, D.; Bibby, D.C.; Chong, G.; Kremmidiotis, G.; Leske, A.F.; Matthews, C.A.; Wong, S.S.; Rosen, M.A.; Desai, J. Clinical, pharmacodynamic, and pharmacokinetic evaluation of BNC105P: A phase I trial of a novel vascular disrupting agent and inhibitor of cancer cell proliferation. Clin. Cancer Res. 2011, 17, 5152–5160. [Google Scholar] [CrossRef] [PubMed]
- Lickliter, J.D.; Francesconi, A.B.; Smith, G.; Burge, M.; Coulthard, A.; Rose, S.; Griffin, M.; Milne, R.; McCarron, J.; Yeadon, T.; et al. Phase I trial of CYT997, a novel cytotoxic and vascular-disrupting agent. Br. J. Cancer 2010, 103, 597–606. [Google Scholar] [CrossRef]
- Conte, G.D.; Bahleda, R.; Moreno, V.; Damian, S.; Perotti, A.; Lassau, N.; Farace, F.; Ong, M.; Stimpson, S.J.; Tunariu, N.; et al. A phase I study of ombrabulin (O) combined with bevacizumab (B) in patients with advanced solid tumors (NCT01193595). J. Clin. Oncol. 2012, 30, 3080. [Google Scholar] [CrossRef]
- Mita, M.M.; Spear, M.A.; Yee, L.K.; Mita, A.C.; Heath, E.I.; Papadopoulos, K.P.; Federico, K.C.; Reich, S.D.; Romero, O.; Malburg, L.; et al. Phase 1 first-in-human trial of the vascular disrupting agent plinabulin(NPI-2358) in patients with solid tumors or lymphomas. Clin. Cancer Res. 2010, 16, 5892–5899. [Google Scholar] [CrossRef]
- Evelhoch, J.L.; LoRusso, P.M.; He, Z.; DelProposto, Z.; Polin, L.; Corbett, T.H.; Langmuir, P.; Wheeler, C.; Stone, A.; Leadbetter, J.; et al. Magnetic resonance imaging measurements of the response of murine and human tumors to the vascular-targeting agent ZD6126. Clin. Cancer Res. 2004, 10, 3650–3657. [Google Scholar] [CrossRef] [PubMed]
- Lorza, A.M.A.; Ravi, H.; Philip, R.C.; Galons, J.P.; Trouard, T.P.; Parra, N.A.; Von Hoff, D.D.; Read, W.L.; Tibes, R.; Korn, R.L.; et al. Dose-response assessment by quantitative MRI in a phase 1 clinical study of the anti-cancer vascular disrupting agent crolibulin. Sci. Rep. 2020, 10, 14449. [Google Scholar] [CrossRef]
- Ricart, A.D.; Ashton, E.A.; Cooney, M.M.; Sarantopoulos, J.; Brell, J.M.; Feldman, M.A.; Ruby, K.E.; Matsuda, K.; Munsey, M.S.; Medina, G.; et al. A phase I study of MN-029 (denibulin), a novel vascular-disrupting agent, in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2011, 68, 959–970. [Google Scholar] [CrossRef]
- McKeage, M.; Fong, P.; Jeffery, M.; Baguley, B.C.; Kestell, P.; Ravic, M.; Jameson, M.B. 5,6-Dimethylxanthenone-4-acetic acid in the treatment of refractory tumors: A phase I safety study of a vascular disrupting agent. Clin. Cancer Res. 2006, 12, 1776–1784. [Google Scholar] [CrossRef]
- Horsman, M.R.; Siemann, D.W. Pathophysiologic effects of vascular-targeting agents and the implications for combination with conventional therapies. Cancer Res. 2006, 66, 11520–11539. [Google Scholar] [CrossRef]
- Clemenson, C.; Chargari, C.; Deutsch, E. Combination of vascular disrupting agents and ionizing radiation. Crit. Rev. Oncol. Hematol. 2013, 86, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Pedley, R.B.; Hill, S.A.; Boxer, G.M.; Flynn, A.A.; Boden, R.; Watson, R.; Dearling, J.; Chaplin, D.J.; Begent, R.H. Eradication of colorectal xenografts by combined radioimmunotherapy and combretastatin a-4 3-O-phosphate. Cancer Res. 2001, 61, 4716–4722. [Google Scholar] [PubMed]
- Kim, M.Y.; Shin, J.Y.; Kim, J.O.; Son, K.H.; Kim, Y.S.; Jung, C.K.; Kang, J.H. Anti-tumor efficacy of CKD-516 in combination with radiation in xenograft mouse model of lung squamous cell carcinoma. BMC Cancer 2020, 20, 1057. [Google Scholar] [CrossRef] [PubMed]
- Satterlee, A.B.; Rojas, J.D.; Dayton, P.A.; Huang, L. Enhancing Nanoparticle Accumulation and Retention in Desmoplastic Tumors via Vascular Disruption for Internal Radiation Therapy. Theranostics 2017, 7, 253–269. [Google Scholar] [CrossRef]
- Horsman, M.R. Enhancing the radiation response of tumors but not early or late responding normal tissues using a vascular disrupting agent. Acta Oncol. 2017, 56, 1634–1638. [Google Scholar] [CrossRef] [PubMed]
- Siemann, D.W.; Chaplin, D.J.; Horsman, M.R. Realizing the Potential of Vascular Targeted Therapy: The Rationale for Combining Vascular Disrupting Agents and Anti-Angiogenic Agents to Treat Cancer. Cancer Investig. 2017, 35, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Tewari, K.S.; Sill, M.W.; Coleman, R.L.; Aghajanian, C.; Mannel, R.; DiSilvestro, P.A.; Powell, M.; Randall, L.M.; Farley, J.; Rubin, S.C.; et al. Bevacizumab plus fosbretabulin in recurrent ovarian cancer: Overall survival and exploratory analyses of a randomized phase II NRG oncology/gynecologic oncology group study. Gynecol. Oncol. 2020, 159, 79–87. [Google Scholar] [CrossRef]
- Martinelli, M.; Bonezzi, K.; Riccardi, E.; Kuhn, E.; Frapolli, R.; Zucchetti, M.; Ryan, A.J.; Taraboletti, G.; Giavazzi, R. Sequence dependent antitumour efficacy of the vascular disrupting agent ZD6126 in combination with paclitaxel. Br. J. Cancer 2007, 97, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Rustin, G.J.; Shreeves, G.; Nathan, P.D.; Gaya, A.; Ganesan, T.S.; Wang, D.; Boxall, J.; Poupard, L.; Chaplin, D.J.; Stratford, M.R.; et al. A Phase Ib trial of CA4P (combretastatin A-4 phosphate), carboplatin, and paclitaxel in patients with advanced cancer. Br. J. Cancer 2010, 102, 1355–1360. [Google Scholar] [CrossRef]
- Staflin, K.; Jarnum, S.; Hua, J.; Honeth, G.; Kannisto, P.; Lindvall, M. Combretastatin A-1 phosphate potentiates the antitumor activity of carboplatin and paclitaxel in a severe combined immunodeficiency disease (SCID) mouse model of human ovarian carcinoma. Int. J. Gynecol. Cancer 2006, 16, 1557–1564. [Google Scholar] [CrossRef]
- Yeung, S.C.; She, M.; Yang, H.; Pan, J.; Sun, L.; Chaplin, D. Combination chemotherapy including combretastatin A4 phosphate and paclitaxel is effective against anaplastic thyroid cancer in a nude mouse xenograft model. J. Clin. Endocrinol. Metab. 2007, 92, 2902–2909. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, D.J.; Yao, N.; Jin, Q.M.; Jiang, C.H.; Zhang, J.; Wang, F. Enhancing intratumoral biodistribution and antitumor activity of nab-paclitaxel through combination with a vascular disrupting agent, combretastatin A-4-phosphate. Cancer Chemother. Pharmacol. 2019, 84, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Azad, A.; Bhatia, S.; Drabkin, H.; Costello, B.; Sarantopoulos, J.; Kanesvaran, R.; Lauer, R.; Starodub, A.; Hauke, R.; et al. A Phase I/II Trial of BNC105P with Everolimus in Metastatic Renal Cell Carcinoma. Clin. Cancer Res. 2015, 21, 3420–3427. [Google Scholar] [CrossRef]
- Bellone, M.; Mondino, A.; Corti, A. Vascular targeting, chemotherapy and active immunotherapy: Teaming up to attack cancer. Trends Immunol. 2008, 29, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Horsman, M.R.; Wittenborn, T.R.; Nielsen, P.S.; Elming, P.B. Tumors Resistant to Checkpoint Inhibitors Can Become Sensitive after Treatment with Vascular Disrupting Agents. Int. J. Mol. Sci. 2020, 21, 4778. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.T.; Jiang, L.; Goto, M.; Hsu, P.L.; Li, L.; Zhang, Q.; Wei, L.; Yuan, S.J.; Hamel, E.; Morris-Natschke, S.L.; et al. In Vivo and Mechanistic Studies on Antitumor Lead 7-Methoxy-4-(2-methylquinazolin-4-yl)-3,4-dihydroquinoxalin-2(1H)-one and Its Modification as a Novel Class of Tubulin-Binding Tumor-Vascular Disrupting Agents. J. Med. Chem. 2017, 60, 5586–5598. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Lei, X.; Shi, C.; Huang, M.; Li, X.; Wu, B.; Li, Z.; Han, W.; Du, B.; Hu, J.; et al. Pericyte-targeting prodrug overcomes tumor resistance to vascular disrupting agents. J. Clin. Investig. 2017, 127, 3689–3701. [Google Scholar] [CrossRef]
- Rickardson, L.; Kutvonen, E.; Orasniemi, S.; Hogberg, M.; Kallio, M.J.; Rehnmark, S. Evaluation of the antitumor activity of NOV202, a novel microtubule targeting and vascular disrupting agent. Drug Des. Dev. Ther. 2017, 11, 1335–1351. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.W.; Holmes, T.; Fisher, M.; Tozer, G.M.; Harrity, J.P.A.; Kanthou, C. Evaluation of Sydnone-Based Analogues of Combretastatin A-4 Phosphate (CA4P) as Vascular Disrupting Agents for Use in Cancer Therapy. ChemMedChem 2018, 13, 2618–2626. [Google Scholar] [CrossRef]
- Zhai, X.; Wang, X.; Wang, J.; Liu, J.; Zuo, D.; Jiang, N.; Zeng, T.; Yang, X.; Jing, T.; Gong, P. Discovery and Optimization of Novel 5-Indolyl-7-arylimidazo[1,2-a]pyridine-8-carbonitrile Derivatives as Potent Antitubulin Agents Targeting Colchicine-binding Site. Sci. Rep. 2017, 7, 43398. [Google Scholar] [CrossRef]
- Han, F.; Wang, P.; Zhang, W.; Li, J.; Zhang, Q.; Qi, X.; Liu, M. CA-1H, a novel oxazole bearing analogue of combretastatin A-4, disrupts the tumor vasculatures and inhibits the tumor growth via inhibiting tubulin polymerization. Biomed. Pharmacother. 2016, 80, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Sriram, M.; Hall, J.J.; Grohmann, N.C.; Strecker, T.E.; Wootton, T.; Franken, A.; Trawick, M.L.; Pinney, K.G. Design, synthesis and biological evaluation of dihydronaphthalene and benzosuberene analogs of the combretastatins as inhibitors of tubulin polymerization in cancer chemotherapy. Bioorg. Med. Chem. 2008, 16, 8161–8171. [Google Scholar] [CrossRef] [PubMed]
- Tanpure, R.P.; George, C.S.; Sriram, M.; Strecker, T.E.; Tidmore, J.K.; Hamel, E.; Charlton-Sevcik, A.K.; Chaplin, D.J.; Trawick, M.L.; Pinney, K.G. An Amino-Benzosuberene Analogue That Inhibits Tubulin Assembly and Demonstrates Remarkable Cytotoxicity. Medchemcomm 2012, 3, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Maderna, A.; Sukuru, S.C.; Wagenaar, M.; O’Donnell, C.J.; Lam, M.H.; Musto, S.; Loganzo, F. New cytotoxic benzosuberene analogs. Synthesis, molecular modeling and biological evaluation. Bioorg. Med. Chem. Lett. 2013, 23, 6688–6694. [Google Scholar] [CrossRef] [PubMed]
- Devkota, L.; Lin, C.M.; Strecker, T.E.; Wang, Y.; Tidmore, J.K.; Chen, Z.; Guddneppanavar, R.; Jelinek, C.J.; Lopez, R.; Liu, L.; et al. Design, synthesis, and biological evaluation of water-soluble amino acid prodrug conjugates derived from combretastatin, dihydronaphthalene, and benzosuberene-based parent vascular disrupting agents. Bioorg. Med. Chem. 2016, 24, 938–956. [Google Scholar] [CrossRef] [PubMed]
- Perez-Perez, M.J.; Priego, E.M.; Bueno, O.; Martins, M.S.; Canela, M.D.; Liekens, S. Blocking Blood Flow to Solid Tumors by Destabilizing Tubulin: An Approach to Targeting Tumor Growth. J. Med. Chem. 2016, 59, 8685–8711. [Google Scholar] [CrossRef] [PubMed]
- Hura, N.; Sawant, A.V.; Kumari, A.; Guchhait, S.K.; Panda, D. Combretastatin-Inspired Heterocycles as Antitubulin Anticancer Agents. ACS Omega 2018, 3, 9754–9769. [Google Scholar] [CrossRef]
- Hamze, A.; Alami, M.; Provot, O. Developments of isoCombretastatin A-4 derivatives as highly cytotoxic agents. Eur. J. Med. Chem. 2020, 190, 112110. [Google Scholar] [CrossRef]
- Gracheva, I.A.; Shchegravina, E.S.; Schmalz, H.-G.; Beletskaya, I.P.; Fedorov, A.Y. Colchicine Alkaloids and Synthetic Analogues: Current Progress and Perspectives. J. Med. Chem. 2020, 63, 10618–10651. [Google Scholar] [CrossRef]
- Bukhari, S.N.A.; Kumar, G.B.; Revankar, H.M.; Qin, H.L. Development of combretastatins as potent tubulin polymerization inhibitors. Bioorg. Chem. 2017, 72, 130–147. [Google Scholar] [CrossRef]
- Agut, R.; Falomir, E.; Murga, J.; Martín-Beltrán, C.; Gil-Edo, R.; Pla, A.; Carda, M.; Marco, J.A. Synthesis of Combretastatin A-4 and 3′-Aminocombretastatin A-4 derivatives with Aminoacid Containing Pendants and Study of their Interaction with Tubulin and as Downregulators of the VEGF, hTERT and c-Myc Gene Expression. Molecules 2020, 25, 660. [Google Scholar] [CrossRef]
- Siemann, D.W.; Chaplin, D.J.; Walicke, P.A. A review and update of the current status of the vasculature-disabling agent combretastatin-A4 phosphate (CA4P). Expert Opin. Investig. Drugs 2009, 18, 189–197. [Google Scholar] [CrossRef]
- Hadimani, M.B.; Macdonough, M.T.; Ghatak, A.; Strecker, T.E.; Lopez, R.; Sriram, M.; Nguyen, B.L.; Hall, J.J.; Kessler, R.J.; Shirali, A.R.; et al. Synthesis of a 2-aryl-3-aroyl indole salt (OXi8007) resembling combretastatin A-4 with application as a vascular disrupting agent. J. Nat. Prod. 2013, 76, 1668–1678. [Google Scholar] [CrossRef]
- Tozer, G.M.; Prise, V.E.; Wilson, J.; Locke, R.J.; Vojnovic, B.; Stratford, M.R.L.; Dennis, M.F.; Chaplin, D.J. Combretastatin A-4 Phosphate as a Tumor Vascular-Targeting Agent. Cancer Res. 1999, 59, 1626–1634. [Google Scholar] [PubMed]
- Karatoprak, G.Ş.; Küpeli Akkol, E.; Genç, Y.; Bardakcı, H.; Yücel, Ç.; Sobarzo-Sánchez, E. Combretastatins: An Overview of Structure, Probable Mechanisms of Action and Potential Applications. Molecules 2020, 25, 2560. [Google Scholar] [CrossRef] [PubMed]
- Lew, Y.S.; Brown, S.L.; Griffin, R.J.; Song, C.W.; Kim, J.H. Arsenic trioxide causes selective necrosis in solid murine tumors by vascular shutdown. Cancer Res. 1999, 59, 6033–6037. [Google Scholar]
- Griffin, R.J.; Lee, S.H.; Rood, K.L.; Stewart, M.J.; Lyons, J.C.; Lew, Y.S.; Park, H.; Song, C.W. Use of arsenic trioxide as an antivascular and thermosensitizing agent in solid tumors. Neoplasia 2000, 2, 555–560. [Google Scholar] [CrossRef]
- Brown, S.L.; Kolozsvary, A.; Kim, J.H. Vascular targeting therapies for treatment of malignant disease. Cancer 2005, 104, 216–217; author reply 217. [Google Scholar] [CrossRef] [PubMed]
- Diepart, C.; Karroum, O.; Magat, J.; Feron, O.; Verrax, J.; Calderon, P.B.; Gregoire, V.; Leveque, P.; Stockis, J.; Dauguet, N.; et al. Arsenic trioxide treatment decreases the oxygen consumption rate of tumor cells and radiosensitizes solid tumors. Cancer Res. 2012, 72, 482–490. [Google Scholar] [CrossRef]
- Mason, R.P.; Ran, S.; Thorpe, P.E. Quantitative assessment of tumor oxygen dynamics: Molecular imaging for prognostic radiology. J. Cell Biochem. Suppl. 2002, 39, 45–53. [Google Scholar] [CrossRef]
- Chen, B.; Pogue, B.W.; Hoopes, P.J.; Hasan, T. Combining vascular and cellular targeting regimens enhances the efficacy of photodynamic therapy. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Neuschmelting, V.; Kim, K.; Malekzadeh-Najafabadi, J.; Jebiwott, S.; Prakash, J.; Scherz, A.; Coleman, J.A.; Kircher, M.F.; Ntziachristos, V. WST11 Vascular Targeted Photodynamic Therapy Effect Monitoring by Multispectral Optoacoustic Tomography (MSOT) in Mice. Theranostics 2018, 8, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Karwicka, M.; Pucelik, B.; Gonet, M.; Elas, M.; Dabrowski, J.M. Effects of Photodynamic Therapy with Redaporfin on Tumor Oxygenation and Blood Flow in a Lung Cancer Mouse Model. Sci. Rep. 2019, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Mallidi, S.; Watanabe, K.; Timerman, D.; Schoenfeld, D.; Hasan, T. Prediction of Tumor Recurrence and Therapy Monitoring Using Ultrasound-Guided Photoacoustic Imaging. Theranostics 2015, 5, 289–301. [Google Scholar] [CrossRef]
- Rennert, J.; Wiesinger, I.; Beyer, L.P.; Schicho, A.; Stroszczynski, C.; Wiggermann, P.; Jung, E.M. Color coded perfusion analysis and microcirculation imaging with contrast enhanced ultrasound (CEUS) for post-interventional success control following thermal ablative techniques of primary and secondary liver malignancies. Clin. Hemorheol. Microcirc. 2019, 73, 73–83. [Google Scholar] [CrossRef]
- Brown, S.L.; Nagaraja, T.N.; Aryal, M.P.; Panda, S.; Cabral, G.; Keenan, K.A.; Elmghirbi, R.; Mikkelsen, T.; Hearshen, D.; Knight, R.A.; et al. MRI-Tracked Tumor Vascular Changes in the Hours after Single-Fraction Irradiation. Radiat. Res. 2015, 183, 713–721. [Google Scholar] [CrossRef]
- Virani, N.A.; Kelada, O.J.; Kunjachan, S.; Detappe, A.; Kwon, J.; Hayashi, J.; Vazquez-Pagan, A.; Biancur, D.E.; Ireland, T.; Kumar, R.; et al. Noninvasive imaging of tumor hypoxia after nanoparticle-mediated tumor vascular disruption. PLoS ONE 2020, 15, e0236245. [Google Scholar] [CrossRef]
- Paroo, Z.; Bollinger, R.A.; Braasch, D.A.; Richer, E.; Corey, D.R.; Antich, P.P.; Mason, R.P. Validating Bioluminescence Imaging as a High-Throughput, Quantitative Modality for Assessing Tumor Burden. Mol. Imaging 2004, 3, 117–124. [Google Scholar] [CrossRef]
- Liu, Y.W.; De Keyzer, F.; Feng, Y.B.; Chen, F.; Song, S.L.; Swinnen, J.; Bormans, G.; Oyen, R.; Huang, G.; Ni, Y.C. Intra-individual comparison of therapeutic responses to vascular disrupting agent CA4P between rodent primary and secondary liver cancers. World J. Gastroenterol. 2018, 24, 2710–2721. [Google Scholar] [CrossRef]
- Breidahl, T.; Nielsen, F.U.; Stodkilde-Jorgensen, H.; Maxwell, R.J.; Horsman, M.R. The effects of the vascular disrupting agents combretastatin A-4 disodium phosphate, 5,6-dimethylxanthenone-4-acetic acid and ZD6126 in a murine tumour: A comparative assessment using MRI and MRS. Acta Oncol. 2006, 45, 306–316. [Google Scholar] [CrossRef]
- Dey, S.; Kumari, S.; Kalainayakan, S.P.; Campbell, J., 3rd; Ghosh, P.; Zhou, H.; FitzGerald, K.E.; Li, M.; Mason, R.P.; Zhang, L.; et al. The vascular disrupting agent combretastatin A-4 phosphate causes prolonged elevation of proteins involved in heme flux and function in resistant tumor cells. Oncotarget 2018, 9, 4090–4101. [Google Scholar] [CrossRef]
- Zhou, H.; Hallac, R.R.; Lopez, R.R.; Denney, R.; MacDonough, M.T.; Li, L.; Liu, L.; Graves, E.E.; Trawick, M.L.; Pinney, K.G.; et al. Evaluation of tumor ischemia in response to an indole-based vascular disrupting agent using BLI and 19F MRI. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 143–153. [Google Scholar]
- Thomas, C.D.; Walczak, C.; Kaffy, J.; Pontikis, R.; Jouanneau, J.; Volk, A. Early effects of combretastatin A4 phosphate assessed by anatomic and carbogen-based functional magnetic resonance imaging on rat bladder tumors implanted in nude mice. Neoplasia 2006, 8, 587–595. [Google Scholar] [CrossRef]
- Colliez, F.; Fruytier, A.C.; Magat, J.; Neveu, M.A.; Cani, P.D.; Gallez, B.; Jordan, B.F. Monitoring Combretastatin A4-induced tumor hypoxia and hemodynamic changes using endogenous MR contrast and DCE-MRI. Magn. Reson. Med. 2016, 75, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Beauregard, D.A.; Pedley, R.B.; Hill, S.A.; Brindle, K.M. Differential sensitivity of two adenocarcinoma xenografts to the anti-vascular drugs combretastatin A4 phosphate and 5,6-dimethylxanthenone-4-acetic acid, assessed using MRI and MRS. NMR Biomed. 2002, 15, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Liu, Y.; Peeters, R.; Feng, Y.; Yu, J.; Himmelreich, U.; Oyen, R.; Ni, Y. Vascular disrupting agent in pancreatic and hepatic tumour allografts: Observations of location-dependent efficacy by MRI, microangiography and histomorphology. Br. J. Cancer 2017, 117, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Thoeny, H.C.; De Keyzer, F.; Chen, F.; Vandecaveye, V.; Verbeken, E.K.; Ahmed, B.; Sun, X.; Ni, Y.; Bosmans, H.; Hermans, R.; et al. Diffusion-weighted magnetic resonance imaging allows noninvasive in vivo monitoring of the effects of combretastatin a-4 phosphate after repeated administration. Neoplasia 2005, 7, 779–787. [Google Scholar] [CrossRef]
- Song, W.T.; Tang, Z.H.; Zhang, D.W.; Yu, H.Y.; Chen, X.S. Coadministration of Vascular Disrupting Agents and Nanomedicines to Eradicate Tumors from Peripheral and Central Regions. Small 2015, 11, 3755–3761. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; De Keyzer, F.; Wang, Y.X.; Wang, F.N.; Feng, Y.B.; Chen, F.; Yu, J.; Liu, J.J.; Song, S.L.; Swinnen, J.; et al. The first study on therapeutic efficacies of a vascular disrupting agent CA4P among primary hepatocellular carcinomas with a full spectrum of differentiation and vascularity: Correlation of MRI-microangiography-histopathology in rats. Int. J. Cancer 2018, 143, 1817–1828. [Google Scholar] [CrossRef]
- Nielsen, T.; Bentzen, L.; Pedersen, M.; Tramm, T.; Rijken, P.F.J.W.; Bussink, J.; Horsman, M.R.; Ostergaard, L. Combretastatin A-4 Phosphate Affects Tumor Vessel Volume and Size Distribution as Assessed Using MRI-Based Vessel Size Imaging. Clin. Cancer Res. 2012, 18, 6469–6477. [Google Scholar] [CrossRef]
- Wang, H.J.; Sun, X.H.; Chen, F.; De Keyzer, F.; Yu, J.; Landuyt, W.; Vandecaveye, V.; Peeters, R.; Bosmans, H.; Hermans, R.; et al. Treatment of Rodent Liver Tumor with Combretastatin A4 Phosphate Noninvasive Therapeutic Evaluation Using Multiparametric Magnetic Resonance Imaging in Correlation With Microangiography and Histology. Investig. Radiol. 2009, 44, 44–53. [Google Scholar] [CrossRef]
- Salmon, H.W.; Siemann, D.W. Effect of the second-generation vascular disrupting agent OXi4503 on tumor vascularity. Clin. Cancer Res. 2006, 12, 4090–4094. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.Z.; Liu, D.X.; Xiao, Z.Y.; Zhang, D.; Liu, G.F.; Liu, G.S.; Chen, H.W.; Luo, L.P. Monitoring Tumor Response to Antivascular Therapy Using Non-Contrast Intravoxel Incoherent Motion Diffusion-Weighted MRI. Cancer Res. 2017, 77, 3491–3501. [Google Scholar] [CrossRef] [PubMed]
- Iversen, A.B.; Busk, M.; Bertelsen, L.B.; Laustsen, C.; Munk, O.L.; Nielsen, T.; Wittenborn, T.R.; Bussink, J.; Lok, J.; Stodkilde-Jorgensen, H.; et al. The potential of hyperpolarized C-13 magnetic resonance spectroscopy to monitor the effect of combretastatin based vascular disrupting agents. Acta Oncol. 2017, 56, 1626–1633. [Google Scholar] [CrossRef] [PubMed]
- Fruytier, A.C.; Magat, J.; Neveu, M.A.; Karroum, O.; Bouzin, C.; Feron, O.; Jordan, B.; Cron, G.O.; Gallez, B. Dynamic contrast-enhanced MRI in mouse tumors at 11.7T: Comparison of three contrast agents with different molecular weights to assess the early effects of combretastatin A4. NMR Biomed. 2014, 27, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Fruytier, A.C.; Le Duff, C.S.; Po, C.; Magat, J.; Bouzin, C.; Neveu, M.A.; Feron, O.; Jordan, B.F.; Gallez, B. The Blood Flow Shutdown Induced by Combretastatin A4 Impairs Gemcitabine Delivery in a Mouse Hepatocarcinoma. Front. Pharmacol. 2016, 7, 8. [Google Scholar] [CrossRef]
- Li, J.J.; Zhou, M.; Liu, F.Y.; Xiong, C.Y.; Wang, W.Q.; Cao, Q.Z.; Wen, X.X.; Robertson, J.D.; Ji, X.; Wang, Y.A.; et al. Hepatocellular Carcinoma: Intra-arterial Delivery of Doxorubicin-loaded Hollow Gold Nanospheres for Photothermal Ablation-Chemoembolization Therapy in Rats. Radiology 2016, 281, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Abma, E.; Stock, E.; De Spiegelaere, W.; Van Brantegem, L.; Vanderperren, K.; Ni, Y.; Vynck, M.; Daminet, S.; De Clercq, K.; de Rooster, H. Power Doppler ultrasound and contrast-enhanced ultrasound demonstrate non-invasive tumour vascular response to anti-vascular therapy in canine cancer patients. Sci. Rep. 2019, 9, 9262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chen, Y.; Liu, J.; Yang, Y.; Lv, Q.; Wang, J.; Zhang, L.; Xie, M. Quantitative Evaluation of Combretastatin A4 Phosphate Early Efficacy in a Tumor Model with Dynamic Contrast-Enhanced Ultrasound. Ultrasound Med. Biol. 2018, 44, 840–852. [Google Scholar] [CrossRef]
- Sunar, U.; Makonnen, S.; Zhou, C.; Durduran, T.; Yu, G.; Wang, H.W.; Lee, W.M.; Yodh, A.G. Hemodynamic responses to antivascular therapy and ionizing radiation assessed by diffuse optical spectroscopies. Opt. Express 2007, 15, 15507–15516. [Google Scholar] [CrossRef]
- Seguin, J.; Mignet, N.; Latorre Ossa, H.; Tanter, M.; Gennisson, J.-L. Evaluation of Antivascular Combretastatin A4 P Efficacy Using Supersonic Shear Imaging Technique of Ectopic Colon Carcinoma CT26. Ultrasound Med. Biol. 2017, 43, 2352–2361. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, M.; Yao, N.; Jiang, C.; Liu, W.; Li, T.; Song, S.; Huang, D.; Yin, Z.; Qiu, Y.; et al. Preclinical Evaluation of Radioiodinated Hoechst 33258 for Early Prediction of Tumor Response to Treatment of Vascular-Disrupting Agents. Contrast Media Mol. Imaging 2018, 2018, 5237950. [Google Scholar] [CrossRef]
- Bothwell, K.D.; Folaron, M.; Seshadri, M. Preclinical Activity of the Vascular Disrupting Agent OXi4503 against Head and Neck Cancer. Cancers 2016, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Benezra, M.; Phillips, E.; Tilki, D.; Ding, B.S.; Butler, J.; Dobrenkov, K.; Siim, B.; Chaplin, D.; Rafii, S.; Rabbany, S.; et al. Serial monitoring of human systemic and xenograft models of leukemia using a novel vascular disrupting agent. Leukemia 2012, 26, 1771–1778. [Google Scholar] [CrossRef]
- Johnson, S.P.; Ramasawmy, R.; Campbell-Washburn, A.E.; Wells, J.A.; Robson, M.; Rajkumar, V.; Lythgoe, M.F.; Pedley, R.B.; Walker-Samuel, S. Acute changes in liver tumour perfusion measured non-invasively with arterial spin labelling. Br. J. Cancer 2016, 114, 897–904. [Google Scholar] [CrossRef]
- Shao, H.B.; Ni, Y.C.; Zhang, J.; Chen, F.; Dai, X.; Fan, G.G.; Sun, Z.P.; Xu, K. Dynamic Contrast-Enhanced and Diffusion-Weighted Magnetic Resonance Imaging Noninvasive Evaluation of Vascular Disrupting Treatment on Rabbit Liver Tumors. PLoS ONE 2013, 8, e82649. [Google Scholar] [CrossRef] [PubMed]
- Laufer, J.; Johnson, P.; Zhang, E.; Treeby, B.; Cox, B.; Pedley, B.; Beard, P. In vivo preclinical photoacoustic imaging of tumor vasculature development and therapy. J. Biomed. Opt. 2012, 17, 056016. [Google Scholar] [CrossRef]
- Johnson, S.P.; Ogunlade, O.; Lythgoe, M.F.; Beard, P.; Pedley, R.B. Longitudinal Photoacoustic Imaging of the Pharmacodynamic Effect of Vascular Targeted Therapy on Tumors. Clin. Cancer Res. 2019, 25, 7436–7447. [Google Scholar] [CrossRef] [PubMed]
- Lavranos, T.; Leske, A.; Beaumont, D.; Gasic, J.; Kremmidiotis, G. Evaluation of the anti-cancer effects of the tumor selective Vascular Disruption Agent BNC105 in preclinical renal cancer models. In Proceedings of the 102nd Annual Meeting of AACR, Orlando, FL, USA, 2–6 April 2011; p. 662. [Google Scholar]
- Ravoori, M.K.; Margalit, O.; Singh, S.; Kim, S.-H.; Wei, W.; Menter, D.G.; DuBois, R.N.; Kundra, V. Magnetic Resonance Imaging and Bioluminescence Imaging for Evaluating Tumor Burden in Orthotopic Colon Cancer. Sci. Rep. 2019, 9, 6100. [Google Scholar] [CrossRef]
- Kim, T.J.; Ravoori, M.; Landen, C.N.; Kamat, A.A.; Han, L.Y.; Lu, C.; Lin, Y.G.; Merritt, W.M.; Jennings, N.; Spannuth, W.A.; et al. Antitumor and Antivascular Effects of AVE8062 in Ovarian Carcinoma. Cancer Res. 2007, 67, 9337–9345. [Google Scholar] [CrossRef]
- Clemenson, C.; Jouannot, E.; Merino-Trigo, A.; Rubin-Carrez, C.; Deutsch, E. The vascular disrupting agent ombrabulin (AVE8062) enhances the efficacy of standard therapies in head and neck squamous cell carcinoma xenograft models. Investig. New Drugs 2013, 31, 273–284. [Google Scholar] [CrossRef]
- Bertelsen, L.B.; Shen, Y.Y.; Nielsen, T.; Stodkilde-Jorgensen, H.; Lloyd, G.K.; Siemann, D.W.; Horsman, M.R. Vascular effects of plinabulin (NPI-2358) and the influence on tumour response when given alone or combined with radiation. Int. J. Radiat. Biol. 2011, 87, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, D.J.O.; Robinson, S.P.; Howe, F.A.; Griffiths, J.R.; Ryan, A.J.; Blakey, D.C.; Peers, I.S.; Waterton, J.C. Single dose of the antivascular agent, ZD6126 (N-acetylcoichinol-O-phosphate), reduces perfusion for at least 96 h in the GH3 prolactinoma rat tumor model. Neoplasia 2004, 6, 150–157. [Google Scholar] [CrossRef]
- Robinson, S.P.; McIntyre, D.J.; Checkley, D.; Tessier, J.J.; Howe, F.A.; Griffiths, J.R.; Ashton, S.E.; Ryan, A.J.; Blakey, D.C.; Waterton, J.C. Tumour dose response to the antivascular agent ZD6126 assessed by magnetic resonance imaging. Br. J. Cancer 2003, 88, 1592–1597. [Google Scholar] [CrossRef] [PubMed]
- Burrell, J.S.; Bradley, R.S.; Walker-Samuel, S.; Jamin, Y.; Baker, L.C.J.; Boult, J.K.R.; Withers, P.J.; Halliday, J.; Waterton, J.C.; Robinson, S.P. MRI measurements of vessel calibre in tumour xenografts: Comparison with vascular corrosion casting. Microvasc. Res. 2012, 84, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Walker-Samuel, S.; Boult, J.K.R.; McPhail, L.D.; Box, G.; Eccles, S.A.; Robinson, S.P. Non-invasive in vivo imaging of vessel calibre in orthotopic prostate tumour xenografts. Int. J. Cancer 2012, 130, 1284–1293. [Google Scholar] [CrossRef]
- Liu, L.; Beck, H.; Wang, X.; Hsieh, H.P.; Mason, R.P.; Liu, X. Tubulin-destabilizing agent BPR0L075 induces vascular-disruption in human breast cancer mammary fat pad xenografts. PLoS ONE 2012, 7, e43314. [Google Scholar] [CrossRef] [PubMed]
- Rich, L.J.; Seshadri, M. Photoacoustic imaging of vascular hemodynamics: Validation with blood oxygenation level-dependent MR imaging. Radiology 2015, 275, 110–118. [Google Scholar] [CrossRef]
- Folaron, M.; Seshadri, M. Bioluminescence and MR Imaging of the Safety and Efficacy of Vascular Disruption in Gliomas. Mol. Imaging Biol. Mib Off. Publ. Acad. Mol. Imaging 2016, 18, 860–869. [Google Scholar] [CrossRef]
- Kalmuk, J.; Folaron, M.; Buchinger, J.; Pili, R.; Seshadri, M. Multimodal imaging guided preclinical trials of vascular targeting in prostate cancer. Oncotarget 2015, 6, 24376–24392. [Google Scholar] [CrossRef]
- Hong, S.; Zheng, D.W.; Zhang, C.; Huang, Q.X.; Cheng, S.X.; Zhang, X.Z. Vascular disrupting agent induced aggregation of gold nanoparticles for photothermally enhanced tumor vascular disruption. Sci. Adv. 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Folaron, M.; Kalmuk, J.; Lockwood, J.; Frangou, C.; Vokes, J.; Turowski, S.G.; Merzianu, M.; Rigual, N.R.; Sullivan-Nasca, M.; Kuriakose, M.A.; et al. Vascular priming enhances chemotherapeutic efficacy against head and neck cancer. Oral Oncol. 2013, 49, 893–902. [Google Scholar] [CrossRef] [PubMed]
- McPhail, L.D.; McIntyre, D.J.O.; Ludwig, C.; Kestell, P.; Griffiths, J.R.; Kelland, L.R.; Robinson, S.P. Rat tumor response to the vascular-disrupting agent 5,6-dimethylxanthenone-4-acetic acid as measured by dynamic contrast-enhanced magnetic resonance imaging, plasma 5-hydroxyindoleacetic acid levels, and tumor necrosis. Neoplasia 2006, 8, 199–206. [Google Scholar] [CrossRef]
- Seshadri, M.; Ciesielski, M.J. MRI-based characterization of vascular disruption by 5,6-dimethylxanthenone-acetic acid in gliomas. J. Cereb. Blood Flow Metab. 2009, 29, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Hectors, S.J.; Jacobs, I.; Lok, J.; Peters, J.; Bussink, J.; Hoeben, F.J.; Keizer, H.M.; Janssen, H.M.; Nicolay, K.; Schabel, M.C.; et al. Improved Evaluation of Antivascular Cancer Therapy Using Constrained Tracer-Kinetic Modeling for Multiagent Dynamic Contrast-Enhanced MRI. Cancer Res. 2018, 78, 1561–1570. [Google Scholar] [CrossRef]
- Ellis, L.; Shah, P.; Hammers, H.; Lehet, K.; Sotomayor, P.; Azabdaftari, G.; Seshadri, M.; Pili, R. Vascular disruption in combination with mTOR inhibition in renal cell carcinoma. Mol. Cancer Ther. 2012, 11, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Fadhel, M.N.; Baskoy, S.A.; Wang, Y.J.; Hysi, E.; Kolios, M.C. Use of photoacoustic imaging for monitoring vascular disrupting cancer treatments. J. Biophotonics 2020. [Google Scholar] [CrossRef]
- Song, W.T.; Tang, Z.H.; Zhang, D.W.; Li, M.Q.; Gu, J.K.; Chen, X.S. A cooperative polymeric platform for tumor-targeted drug delivery. Chem. Sci. 2016, 7, 728–736. [Google Scholar] [CrossRef]
- Milanović, D.; Braun, F.; Weber, W.; Grosu, A.L.; Behe, M.; Niedermann, G. The influence of the combined treatment with Vadimezan (ASA404) and taxol on the growth of U251 glioblastoma xenografts. BMC Cancer 2012, 12, 242. [Google Scholar] [CrossRef]
- He, J.; Liu, C.; Li, T.; Liu, Y.; Wang, S.; Zhang, J.; Chen, L.; Wang, C.; Feng, Y.; Floris, G.; et al. Pictorial Imaging-Histopathology Correlation in a Rabbit with Hepatic VX2 Tumor Treated by Transarterial Vascular Disrupting Agent Administration. Int. J. Med. Sci. 2020, 17, 2269–2275. [Google Scholar] [CrossRef]
- Luo, Y.; Hradil, V.P.; Frost, D.J.; Rosenberg, S.H.; Gordon, G.B.; Morgan, S.J.; Gagne, G.D.; Cox, B.F.; Tahir, S.K.; Fox, G.B. ABT-751, a novel tubulin-binding agent, decreases tumor perfusion and disrupts tumor vasculature. Anticancer Drugs 2009, 20, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Herdman, C.A.; Devkota, L.; Lin, C.M.; Niu, H.; Strecker, T.E.; Lopez, R.; Liu, L.; George, C.S.; Tanpure, R.P.; Hamel, E.; et al. Structural interrogation of benzosuberene-based inhibitors of tubulin polymerization. Bioorg. Med. Chem. 2015, 23, 7497–7520. [Google Scholar] [CrossRef]
- Niu, H.C.; Strecker, T.E.; Gerberich, J.L.; Campbell, J.W.; Saha, D.; Mondal, D.; Hamel, E.; Chaplin, D.J.; Mason, R.P.; Trawick, M.L.; et al. Structure Guided Design, Synthesis, and Biological Evaluation of Novel Benzosuberene Analogues as Inhibitors of Tubulin Polymerization. J. Med. Chem. 2019, 62, 5594–5615. [Google Scholar] [CrossRef]
- Herdman, C.A.; Strecker, T.E.; Tanpure, R.P.; Chen, Z.; Winters, A.; Gerberich, J.; Liu, L.; Hamel, E.; Mason, R.P.; Chaplin, D.J.; et al. Synthesis and Biological Evaluation of Benzocyclooctene-based and Indene-based Anticancer Agents that Function as Inhibitors of Tubulin Polymerization. Medchemcomm 2016, 7, 2418–2427. [Google Scholar] [CrossRef] [PubMed]
- Maguire, C.J.; Chen, Z.; Mocharla, V.P.; Sriram, M.; Strecker, T.E.; Hamel, E.; Zhou, H.; Lopez, R.; Wang, Y.; Mason, R.P.; et al. Synthesis of dihydronaphthalene analogues inspired by combretastatin A-4 and their biological evaluation as anticancer agents. Medchemcomm 2018, 9, 1649–1662. [Google Scholar] [CrossRef]
- Winn, B.A.; Devkota, L.; Kuch, B.; MacDonough, M.T.; Strecker, T.E.; Wang, Y.; Shi, Z.; Gerberich, J.L.; Mondal, D.; Ramirez, A.J.; et al. Bioreductively Activatable Prodrug Conjugates of Combretastatin A-1 and Combretastatin A-4 as Anticancer Agents Targeted toward Tumor-Associated Hypoxia. J. Nat. Prod. 2020, 83, 937–954. [Google Scholar] [CrossRef] [PubMed]
- Thebault, C.J.; Ramniceanu, G.; Boumati, S.; Michel, A.; Seguin, J.; Larrat, B.; Mignet, N.; Menager, C.; Doan, B.T. Theranostic MRI liposomes for magnetic targeting and ultrasound triggered release of the antivascular CA4P. J. Control. Release 2020, 322, 137–148. [Google Scholar] [CrossRef]
- Paris, J.L.; Villaverde, G.; Gomez-Grana, S.; Vallet-Regi, M. Nanoparticles for multimodal antivascular therapeutics: Dual drug release, photothermal and photodynamic therapy. Acta Biomater. 2020, 101, 459–468. [Google Scholar] [CrossRef]
- Hao, Y.; Peng, J.R.; Zhang, Y.G.; Chen, L.J.; Luo, F.; Wang, C.; Qian, Z.Y. Tumor Neovasculature-Targeted APRPG-PEG-PDLLA/MPEG-PDLLA Mixed Micelle Loading Combretastatin A-4 for Breast Cancer Therapy. ACS Biomater. Sci. Eng. 2018, 4, 1986–1999. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Tang, Z.; Song, W.; Zhang, D.; Li, M.; Liu, H.; Cheng, J.; Zhong, W.; Chen, X. Inhibiting Solid Tumor Growth In Vivo by Non-Tumor-Penetrating Nanomedicine. Small 2017, 13, 1600954. [Google Scholar] [CrossRef]
- Fentahun Darge, H.; Yibru Hanurry, E.; Simegniew Birhan, Y.; Worku Mekonnen, T.; Tizazu Andrgie, A.; Chou, H.-Y.; Lai, J.-Y.; Tsai, H.-C. Multifunctional drug-loaded micelles encapsulated in thermo-sensitive hydrogel for in vivo local cancer treatment: Synergistic effects of anti-vascular and immuno-chemotherapy. Chem. Eng. J. 2021, 406, 126879. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, D.; Song, W.; Tang, Z.; Zhu, J.; Ma, Z.; Wang, X.; Chen, X.; Tong, T. A poly(l-glutamic acid)-combretastatin A4 conjugate for solid tumor therapy: Markedly improved therapeutic efficiency through its low tissue penetration in solid tumor. Acta Biomater. 2017, 53, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Saito, S.; Sato, Y.; Akita, H.; Kawaguchi, T.; Sugiyama, K.; Sato, H. Differential relationship between changes in tumour size and microcirculatory functions induced by therapy with an antivascular drug and with cytotoxic drugs: Implications for the evaluation of therapeutic efficacy of AC7700 (AVE8062). Eur. J. Cancer 2003, 39, 1957–1966. [Google Scholar] [CrossRef]
- Davis, P.D.; Dougherty, G.J.; Blakey, D.C.; Galbraith, S.M.; Tozer, G.M.; Holder, A.L.; Naylor, M.A.; Nolan, J.; Stratford, M.R.L.; Chaplin, D.J.; et al. ZD6126. Nov. Vasc.-Target. Agent That Causes Sel. Destr. Tumor Vasc. 2002, 62, 7247–7253. [Google Scholar]
- Inglis, D.J.; Lavranos, T.C.; Beaumont, D.M.; Leske, A.F.; Brown, C.K.; Hall, A.J.; Kremmidiotis, G. The vascular disrupting agent BNC105 potentiates the efficacy of VEGF and mTOR inhibitors in renal and breast cancer. Cancer Biol. Ther. 2014, 15, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Lee, J.M.; Jeon, Y.S.; Lee, I.J.; Choi, Y.; Park, J.; Kiefer, B.; Kim, C.; Han, J.K.; Choi, B.I. Vascular disrupting effect of CKD-516: Preclinical study using DCE-MRI. Investig. New Drugs 2013, 31, 1097–1106. [Google Scholar] [CrossRef]
- Zhou, H.L.; Campbell, J.; Lopez, R.; Zhang, Z.; Saha, D.; Denney, R.; Twawick, M.L.; Pinney, K.; Mason, R. Monitoring Tumor Response to Vascular Disrupting Agent Using Photoacoustic Tomography and Multiparametric MRI. J. Nucl. Med. 2016, 57, 1408. [Google Scholar]
- Kuo, C.C.; Hsieh, H.P.; Pan, W.Y.; Chen, C.P.; Liou, J.P.; Lee, S.J.; Chang, Y.L.; Chen, L.T.; Chen, C.T.; Chang, J.Y. BPR0L075, a novel synthetic indole compound with antimitotic activity in human cancer cells, exerts effective antitumoral activity in vivo. Cancer Res. 2004, 64, 4621–4628. [Google Scholar] [CrossRef] [PubMed]
- Alhasan, M.K.; Liu, L.; Lewis, M.A.; Magnusson, J.; Mason, R.P. Comparison of Optical and Power Doppler Ultrasound Imaging for Non-Invasive Evaluation of Arsenic Trioxide as a Vascular Disrupting Agent in Tumors. PLoS ONE 2012, 7, e46106. [Google Scholar] [CrossRef]
- Cesca, M.; Bizzaro, F.; Zucchetti, M.; Giavazzi, R. Tumor delivery of chemotherapy combined with inhibitors of angiogenesis and vascular targeting agents. Front. Oncol. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Natsume, T.; Watanabe, J.; Ogawa, K.; Yasumura, K.; Kobayashi, M. Tumor-specific antivascular effect of TZT-1027 (Soblidotin) elucidated by magnetic resonance imaging and confocal laser scanning microscopy. Cancer Sci. 2007, 98, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Glowa, C.; Karger, C.P.; Brons, S.; Zhao, D.; Mason, R.P.; Huber, P.E.; Debus, J.; Peschke, P. Carbon ion radiotherapy decreases the impact of tumor heterogeneity on radiation response in experimental prostate tumors. Cancer Lett. 2016, 378, 97–103. [Google Scholar] [CrossRef]
- Corliss, B.A.; Mathews, C.; Doty, R.; Rohde, G.; Peirce, S.M. Methods to label, image, and analyze the complex structural architectures of microvascular networks. Microcirculation 2019, 26, e12520. [Google Scholar] [CrossRef]
- Chaplin, D.J.; Olive, P.L.; Durand, R.E. Intermittent blood flow in a murine tumor: Radiobiological effects. Cancer Res. 1987, 47, 597–601. [Google Scholar]
- Trotter, M.J.; Chaplin, D.J.; Durand, R.E.; Olive, P.L. The Use of Fluorescent-Probes to Identify Regions of Transient Perfusion in Murine Tumors. Int. J. Radiat. Oncol. Biol. Phys. 1989, 16, 931–934. [Google Scholar] [CrossRef]
- Prinzen, F.W.; Bassingthwaighte, J.B. Blood flow distributions by microsphere deposition methods. Cardiovasc. Res. 2000, 45, 13–21. [Google Scholar] [CrossRef]
- Hodeige, D.; De Pauw, M.; Eechaute, W.; Weyne, J.; Heyndrickx, G.R. On the validity of blood flow measurement using colored microspheres. Am. J. Physiol.-Heart Circ. Physiol. 1999, 276, H1150–H1158. [Google Scholar] [CrossRef]
- Robertson, R.T.; Levine, S.T.; Haynes, S.M.; Gutierrez, P.; Baratta, J.L.; Tan, Z.; Longmuir, K.J. Use of labeled tomato lectin for imaging vasculature structures. Histochem. Cell Biol. 2015, 143, 225–234. [Google Scholar] [CrossRef]
- Lippert, J.W., 3rd. Vascular disrupting agents. Bioorg. Med. Chem. 2007, 15, 605–615. [Google Scholar] [CrossRef]
- McLoughlin, E.C.; O’Boyle, N.M. Colchicine-Binding Site Inhibitors from Chemistry to Clinic: A Review. Pharmaceuticals 2020, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- McPhail, L.D.; Robinson, S.P. Lessons from Animal Imaging in Preclinical Models; Springer: New York, NY, USA, 2010; pp. 95–116. [Google Scholar]
- Zweifel, M.; Padhani, A.R. MRI to Assess. Vascular Disruptive Agents; Springer: New York, NY, USA, 2010; pp. 137–163. [Google Scholar]
- Serkova, N.J.; Glunde, K.; Haney, C.R.; Farhoud, M.; DeLille, A.; Redente, E.F.; Simberg, D.; Westerly, D.C.; Griffin, L.; Mason, R.P. Preclinical Applications of Multi-Platform Imaging in Animal Models of Cancer. Cancer Res. 2020, 81, 1189–1200. [Google Scholar] [CrossRef]
- Gill, J.H.; Rockley, K.L.; De Santis, C.; Mohamed, A.K. Vascular Disrupting Agents in cancer treatment: Cardiovascular toxicity and implications for co-administration with other cancer chemotherapeutics. Pharmacol. Ther. 2019, 202, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Hormuth, D.A., 2nd; Sorace, A.G.; Virostko, J.; Abramson, R.G.; Bhujwalla, Z.M.; Enriquez-Navas, P.; Gillies, R.; Hazle, J.D.; Mason, R.P.; Quarles, C.C.; et al. Translating preclinical MRI methods to clinical oncology. J. Magn. Reson. Imaging 2019, 50, 1377–1392. [Google Scholar] [CrossRef] [PubMed]
- Contag, C.H.; Ross, B.D. It’s not just about anatomy: In vivo bioluminescence imaging as an eyepiece into biology. J. Magn. Reson. Imaging 2002, 16, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Alsawaftah, N.; Farooq, A.; Dhou, S.; Majdalawieh, A.F. Bioluminescence Imaging Applications in Cancer: A Comprehensive Review. IEEE Rev. Biomed. Eng. 2021, 14, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Contero, A.; Richer, E.; Gondim, A.; Mason, R.P. High-throughput quantitative bioluminescence imaging for assessing tumor burden. Methods Mol. Biol. 2009, 574, 37–45. [Google Scholar] [CrossRef]
- PerkinElmer. In Vivo Imaging. Available online: https://www.perkinelmer.com/category/in-vivo-imaging-instruments (accessed on 22 April 2021).
- SpectralInstruments. In Vivo, Optimized. Available online: https://spectralinvivo.com/imaging-systems/ (accessed on 22 April 2021).
- Scintica. Newton 7.0. Available online: https://www.scintica.com/products/vilber-imaging/vilber-newton-7/ (accessed on 22 April 2021).
- Sonovol. Molecular Imaging with Soft Tissue Contrast. Available online: https://sonovol.com/products/strata/ (accessed on 22 April 2021).
- Medilume. Prism In Vivo Imaging System. Available online: https://www.medilumine.com/product/prism-in-vivo-imaging-system/ (accessed on 22 April 2021).
- Vieworks. Bioimaging. Available online: https://bioimaging.vieworks.com/?_ga=2.75796496.489309006.1613065635-1361123655.1613065635 (accessed on 20 March 2021).
- Lewis, M.A.; Richer, E.; Slavine, N.V.; Kodibagkar, V.D.; Soesbe, T.C.; Antich, P.P.; Mason, R.P. A Multi-Camera System for Bioluminescence Tomography in Preclinical Oncology Research. Diagnostics 2013, 3, 325–343. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, K.K.-H.; Yu, J.; Eslami, S.; Iordachita, I.; Reyes, J.; Malek, R.; Tran, P.T.; Patterson, M.S.; Wong, J.W. Bioluminescence Tomography-Guided Radiation Therapy for Preclinical Research. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 1144–1153. [Google Scholar] [CrossRef]
- Deng, Z.; Xu, X.; Garzon-Muvdi, T.; Xia, Y.; Kim, E.; Belcaid, Z.; Luksik, A.; Maxwell, R.; Choi, J.; Wang, H.; et al. In Vivo Bioluminescence Tomography Center of Mass-Guided Conformal Irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 612–620. [Google Scholar] [CrossRef]
- Kuo, C.; Coquoz, O.; Troy, T.L.; Xu, H.; Rice, B.W. Three-dimensional reconstruction of in vivo bioluminescent sources based on multispectral imaging. J. Biomed. Opt. 2007, 12, 024007. [Google Scholar] [CrossRef]
- Yamashita, S.; Katsumi, H.; Hibino, N.; Isobe, Y.; Yagi, Y.; Tanaka, Y.; Yamada, S.; Naito, C.; Yamamoto, A. Development of PEGylated aspartic acid-modified liposome as a bone-targeting carrier for the delivery of paclitaxel and treatment of bone metastasis. Biomaterials 2018, 154, 74–85. [Google Scholar] [CrossRef]
- Bollinger, R.A. Evaluation of the Light Emission Kinetics in Luciferin/Luciferase-Based In Vivo Bioluminescence Imaging for Guidance in the Development of Small Animal Imaging Study Design. Ph.D. Thesis, UT Southwestern, Dallas, TX, USA, 2006. [Google Scholar]
- Dikmen, Z.G.; Gellert, G.; Dogan, P.; Mason, R.; Antich, P.; Richer, E.; Wright, W.E.; Shay, J.E. A New Diagnostic System in Cancer Research: Bioluminescent Imaging (BLI). Turk. J. Med. Sci. 2005, 35, 65–70. [Google Scholar]
- Dikmen, Z.G.; Gellert, G.C.; Jackson, S.; Gryaznov, S.; Tressler, R.; Dogan, P.; Wright, W.E.; Shay, J.W. In vivo inhibition of lung cancer by GRN163L: A novel human telomerase inhibitor. Cancer Res. 2005, 65, 7866–7873. [Google Scholar] [CrossRef]
- Ghosh, P.; Guo, Y.; Ashrafi, A.; Chen, J.; Dey, S.; Zhong, S.; Liu, J.; Campbell, J.; Konduri, P.C.; Gerberich, J.; et al. Oxygen-enhanced optoacoustic tomography reveals the effectiveness of targeting heme and oxidative phosphorylation at normalizing tumor vascular oxygenation. Cancer Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Zhang, Y.; Tang, S.N.; Qin, C.T.; Karelia, D.; Sharma, A.; Jiang, C.; Lu, J.X. Optimizing live-animal bioluminescence imaging prediction of tumor burden in human prostate cancer xenograft models in SCID-NSG mice. Prostate 2019, 79, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Xu, N.; Guo, K.; Xu, A.; Takenaka, F.; Matsuura, E.; Liu, C.; Kumon, H.; Huang, P. Real-time monitoring of tumor progression and drug responses in a preclinical mouse model of prostate cancer. Oncotarget 2016, 7, 33025–33034. [Google Scholar] [CrossRef]
- Tumati, V.; Mathur, S.; Song, K.; Hsieh, J.T.; Zhao, D.; Takahashi, M.; Dobin, T.; Gandee, L.; Solberg, T.D.; Habib, A.A.; et al. Development of a locally advanced orthotopic prostate tumor model in rats for assessment of combined modality therapy. Int. J. Oncol. 2013, 42, 1613–1619. [Google Scholar] [CrossRef]
- Zhou, H.L.; Zhao, D.W. Ultrasound Imaging-guided Intracardiac Injection to Develop a Mouse Model of Breast Cancer Brain Metastases Followed by Longitudinal MRI. J. Vis. Exp. 2014. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.Y.; Davis, C.M.; Lan, X.Y.; Hienz, R.D.; Jablonska, A.; Thomas, A.M.; Velarde, E.; Li, S.; Janowski, M.; Kai, M.; et al. Neuroinflammation After Stereotactic Radiosurgery-Induced Brain Tumor Disintegration Is Linked to Persistent Cognitive Decline in a Mouse Model of Metastatic Disease. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Kimbrough, C.W.; Hudson, S.; Khanal, A.; Egger, M.E.; McNally, L.R. Orthotopic pancreatic tumors detected by optoacoustic tomography using Syndecan-1. J. Surg. Res. 2015, 193, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Poeschinger, T.; Renner, A.; Weber, T.; Scheuer, W. Bioluminescence Imaging Correlates with Tumor Serum Marker, Organ Weights, Histology, and Human DNA Levels during Treatment of Orthotopic Tumor Xenografts with Antibodies. Mol. Imaging Biol. 2013, 15, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, J.; Saito, R.; Hayashi, Y.; Kitada, N.; Tamaki, S.; Han, Y.X.; Semba, K.; Maki, S.A. High Sensitivity In Vivo Imaging of Cancer Metastasis Using a Near-Infrared Luciferin Analogue seMpai. Int. J. Mol. Sci. 2020, 21, 7896. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Dai, J.J.; Yu, Y.; Zhang, Q.; Liu, S.; Huang, G.M.; Zhang, Z.; Chen, T.K.; Pan, R.L.; Lu, L.T.; et al. Non-invasive Bioluminescence Monitoring of Hepatocellular Carcinoma Therapy in an HCR Mouse Model. Front. Oncol. 2019, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Labitzky, V.; Baranowsky, A.; Maar, H.; Hanika, S.; Starzonek, S.; Ahlers, A.K.; Stubke, K.; Koziolek, E.J.; Heine, M.; Schafer, P.; et al. Modeling Spontaneous Bone Metastasis Formation of Solid Human Tumor Xenografts in Mice. Cancers 2020, 12, 385. [Google Scholar] [CrossRef]
- Wang, W.D.; Belosay, A.; Yang, X.J.; Hartman, J.A.; Song, H.X.; Iwaniec, U.T.; Turner, R.T.; Churchwell, M.I.; Doerge, D.R.; Helferich, W.G. Effects of letrozole on breast cancer micro-metastatic tumor growth in bone and lung in mice inoculated with murine 4T1 cells. Clin. Exp. Metastasis 2016, 33, 475–485. [Google Scholar] [CrossRef]
- Kato, Y.; Yoshimura, K.; Shin, T.; Verheul, H.; Hammers, H.; Sanni, T.B.; Salumbides, B.C.; Van Erp, K.; Schulick, R.; Pili, R. Synergistic in vivo antitumor effect of the histone deacetylase inhibitor MS-275 in combination with interleukin 2 in a murine model of renal cell carcinoma. Clin. Cancer Res. 2007, 13, 4538–4546. [Google Scholar] [CrossRef]
- Sarraf-Yazdi, S.; Mi, J.; Dewhirst, M.W.; Clary, B.M. Use of in vivo bioluminescence imaging to predict hepatic tumor burden in mice. J. Surg. Res. 2004, 120, 249–255. [Google Scholar] [CrossRef]
- Zhao, D.; Richer, E.; Antich, P.P.; Mason, R.P. Antivascular effects of combretastatin A4 phosphate in breast cancer xenograft assessed using dynamic bioluminescence imaging (BLI) and confirmed by magnetic resonance imaging (MRI). FASEB J. 2008, 22, 2445–2451. [Google Scholar] [CrossRef]
- Cecic, I.; Chan, D.A.; Sutphin, P.D.; Ray, P.; Gambhir, S.S.; Giaccia, A.J.; Graves, E.E. Oxygen sensitivity of reporter genes: Implications for preclinical imaging of tumor hypoxia. Mol. Imaging 2007, 6, 219–228. [Google Scholar] [CrossRef]
- Liu, L.; Trawick, M.L.; Pinney, K.; Mason, R.P. Abstract 4194: Assessment of novel benzosuberene-based vascular disrupting agents (VDA) on diverse tumor lines. Cancer Res. 2016, 76, 4194. [Google Scholar] [CrossRef]
- Mura, S.; Zouhiri, F.; Lerondel, S.; Maksimenko, A.; Mougin, J.; Gueutin, C.; Brambilla, D.; Caron, J.; Sliwinski, E.; Lepape, A.; et al. Novel isoprenoyl nanoassembled prodrug for paclitaxel delivery. Bioconjug. Chem. 2013, 24, 1840–1849. [Google Scholar] [CrossRef]
- Mowday, A.M.; Lieuwes, N.G.; Biemans, R.; Marcus, D.; Rezaeifar, B.; Reniers, B.; Verhaegen, F.; Theys, J.; Dubois, L.J. Use of a Luciferase-Expressing Orthotopic Rat Brain Tumor Model to Optimize a Targeted Irradiation Strategy for Efficacy Testing with Temozolomide. Cancers 2020, 12, 1585. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Belzile, O.; Zhang, Z.; Wagner, J.; Ahn, C.; Richardson, J.A.; Saha, D.; Brekken, R.A.; Mason, R.P. The effect of flow on blood oxygen level dependent (R(*) 2) MRI of orthotopic lung tumors. Magn. Reson. Med. 2019, 81, 3787–3797. [Google Scholar] [CrossRef] [PubMed]
- Bruker. Imaging in Life Sciences. Available online: https://www.bruker.com/en/applications/academia-life-science/imaging.html (accessed on 22 April 2021).
- MRSolutions. Magnetic Resonance Imaging. Available online: https://www.mrsolutions.com/mr-imaging-main/mr-imaging/ (accessed on 22 April 2021).[Green Version]
- Yankeelov, T.E.; Luci, J.J.; Lepage, M.; Li, R.; Debusk, L.; Lin, P.C.; Price, R.R.; Gore, J.C. Quantitative pharmacokinetic analysis of DCE-MRI data without an arterial input function: A reference region model. Magn. Reson. Imaging 2005, 23, 519–529. [Google Scholar] [CrossRef]
- Padhani, A.R. Dynamic contrast-enhanced MRI in clinical oncology: Current status and future directions. J. Magn. Reson. Imaging 2002, 16, 407–422. [Google Scholar] [CrossRef]
- Liu, Y.W.; Wang, S.C.; Zhao, X.H.; Feng, Y.B.; Bormans, G.; Swinnen, J.; Oyen, R.; Huang, G.; Ni, Y.C.; Li, Y. Predicting Clinical Efficacy of Vascular Disrupting Agents in Rodent Models of Primary and Secondary Liver Cancers: An Overview with Imaging-Histopathology Correlation. Diagnostics 2020, 10, 78. [Google Scholar] [CrossRef]
- Shao, H.; Ni, Y.; Dai, X.; Zhang, J.; Chen, F.; Fan, G.; Sun, Z.; Li, Y.; Zhou, H.; Xu, K. Diffusion-weighted MR imaging allows monitoring the effect of combretastatin A4 phosphate on rabbit implanted VX2 tumor model: 12-day dynamic results. Eur. J. Radiol. 2012, 81, 578–583. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Goo, J.M.; Lee, K.H.; Ha, S.; Paeng, J.C. Monitoring tumor response to the vascular disrupting agent CKD-516 in a rabbit VX2 intramuscular tumor model using PET/MRI: Simultaneous evaluation of vascular and metabolic parameters. PLoS ONE 2018, 13, e0192706. [Google Scholar] [CrossRef] [PubMed]
- Joo, I.; Lee, J.M.; Grimm, R.; Han, J.K.; Choi, B.I. Monitoring Vascular Disrupting Therapy in a Rabbit Liver Tumor Model: Relationship between Tumor Perfusion Parameters at IVIM Diffusion-weighted MR Imaging and Those at Dynamic Contrast-enhanced MR Imaging. Radiology 2016, 278, 104–113. [Google Scholar] [CrossRef]
- Zhao, D.; Jiang, L.; Hahn, E.W.; Mason, R.P. Tumor physiological response to combretastatin A4 phosphate assessed by MRI. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Liu, D.X.; Li, Y.G.; Xu, X.; Xu, J.D.; Yadav, N.N.; Zhou, S.B.; van Zijl, P.C.M.; Liu, G.S. CEST MRI monitoring of tumor response to vascular disrupting therapy using high molecular weight dextrans. Magn. Reson. Med. 2019, 82, 1471–1479. [Google Scholar] [CrossRef]
- Bohndiek, S.E.; Kettunen, M.I.; Hu, D.E.; Witney, T.H.; Kennedy, B.W.C.; Gallagher, F.A.; Brindle, K.M. Detection of Tumor Response to a Vascular Disrupting Agent by Hyperpolarized C-13 Magnetic Resonance Spectroscopy. Mol. Cancer Ther. 2010, 9, 3278–3288. [Google Scholar] [CrossRef] [PubMed]
- Rofsky, N.M.; Sherry, A.D.; Lenkinski, R.E. Nephrogenic systemic fibrosis: A chemical perspective. Radiology 2008, 247, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Fraum, T.J.; Ludwig, D.R.; Bashir, M.R.; Fowler, K.J. Gadolinium-based contrast agents: A comprehensive risk assessment. J. Magn. Reson. Imaging 2017, 46, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jamin, Y.; Boult, J.K.R.; Cummings, C.; Waterton, J.C.; Ulloa, J.; Sinkus, R.; Bamber, J.C.; Robinson, S.P. Tumour biomechanical response to the vascular disrupting agent ZD6126 in vivo assessed by magnetic resonance elastography. Br. J. Cancer 2014, 110, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.P.; Boult, J.K.; Jamin, Y.; Babur, M.; Finegan, K.G.; Williams, K.J.; Little, R.A.; Jackson, A.; Parker, G.J.; Reynolds, A.R.; et al. Oxygen-Enhanced MRI Accurately Identifies, Quantifies, and Maps Tumor Hypoxia in Preclinical Cancer Models. Cancer Res. 2016, 76, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Beeman, S.C.; Shui, Y.B.; Perez-Torres, C.J.; Engelbach, J.A.; Ackerman, J.J.; Garbow, J.R. O2 -sensitive MRI distinguishes brain tumor versus radiation necrosis in murine models. Magn. Reson. Med. 2016, 75, 2442–2447. [Google Scholar] [CrossRef]
- Hallac, R.R.; Zhou, H.; Pidikiti, R.; Song, K.; Stojadinovic, S.; Zhao, D.; Solberg, T.; Peschke, P.; Mason, R.P. Correlations of noninvasive BOLD and TOLD MRI with pO2and relevance to tumor radiation response. Magn. Reson. Med. 2014, 71, 1863–1873. [Google Scholar] [CrossRef]
- Matsumoto, K.; Bernardo, M.; Subramanian, S.; Choyke, P.; Mitchell, J.B.; Krishna, M.C.; Lizak, M.J. MR assessment of changes of tumor in response to hyperbaric oxygen treatment. Magn. Reson. Med. 2006, 56, 240–246. [Google Scholar] [CrossRef]
- Yang, D.M.; Arai, T.J.; Campbell, J.W., 3rd; Gerberich, J.L.; Zhou, H.; Mason, R.P. Oxygen-sensitive MRI assessment of tumor response to hypoxic gas breathing challenge. NMR Biomed. 2019, 32, e4101. [Google Scholar] [CrossRef] [PubMed]
- Remmele, S.; Mason, R.P.; O’Connor, J.P.B. MRI Hypoxia Measurements. In Functional Imaging in Oncology; Luna, A., Ed.; Springer: Heidelberg, Germany, 2014; pp. 269–289. [Google Scholar]
- Madhu, B.; Waterton, J.C.; Griffiths, J.R.; Ryan, A.J.; Robinson, S.P. Response of RIF-1 fibrosarcomas to the vascular-disrupting agent ZD6126 assessed by in vivo and ex vivo H-1 magnetic resonance spectroscopy. Neoplasia 2006, 8, 560–567. [Google Scholar] [CrossRef] [PubMed]
- McPhail, L.D.; Chung, Y.L.; Madhu, B.; Clark, S.; Griffiths, J.R.; Kelland, L.R.; Robinson, S.P. Tumor dose response to the vascular disrupting agent, 5,6-dimethylxanthenone-4-acetic acid, using in vivo magnetic resonance spectroscopy. Clin. Cancer Res. 2005, 11, 3705–3713. [Google Scholar] [CrossRef] [PubMed]
- iThera. Listening to Molecules. Available online: https://www.ithera-medical.com/ (accessed on 22 April 2021).
- FujiVisualsonics. Our Latest, Most Advanced Multi-Modal Imaging Platform. Available online: https://www.visualsonics.com/product/imaging-systems/vevo-lazr-x (accessed on 22 April 2021).
- Balasundaram, G.; Ding, L.; Li, X.T.; Attia, A.B.E.; Dean-Ben, X.L.; Ho, C.J.H.; Chandrasekharan, P.; Tay, H.C.; Lim, H.Q.; Ong, C.B.; et al. Noninvasive Anatomical and Functional Imaging of Orthotopic Glioblastoma Development and Therapy using Multispectral Optoacoustic Tomography. Transl. Oncol. 2018, 11, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewski, M.R.; Gehrung, M.; Joseph, J.; Quiros-Gonzalez, I.; Disselhorst, J.A.; Bohndiek, S.E. Oxygen-Enhanced and Dynamic Contrast-Enhanced Optoacoustic Tomography Provide Surrogate Biomarkers of Tumor Vascular Function, Hypoxia, and Necrosis. Cancer Res. 2018, 78, 5980–5991. [Google Scholar] [CrossRef]
- Ron, A.; Deán-Ben, X.L.; Gottschalk, S.; Razansky, D. Volumetric Optoacoustic Imaging Unveils High-Resolution Patterns of Acute and Cyclic Hypoxia in a Murine Model of Breast Cancer. Cancer Res. 2019, 79, 4767–4775. [Google Scholar] [CrossRef]
- Hupple, C.W.; Morscher, S.; Burton, N.C.; Pagel, M.D.; McNally, L.R.; Cardenas-Rodriguez, J. A light-fluence-independent method for the quantitative analysis of dynamic contrast-enhanced multispectral optoacoustic tomography (DCE MSOT). Photoacoustics 2018, 10, 54–64. [Google Scholar] [CrossRef]
- Zhou, H.L.; Campbell, J.; O’Kelly, D.; Gerberich, J.; Mason, R. Exploring a fluorescent blood pool agent in photoacoustic imaging. J. Nucl. Med. 2016, 57, 1214. [Google Scholar]
- Tomaszewski, M.R.; Gonzalez, I.Q.; O’Connor, J.P.; Abeyakoon, O.; Parker, G.J.; Williams, K.J.; Gilbert, F.J.; Bohndiek, S.E. Oxygen Enhanced Optoacoustic Tomography (OE-OT) Reveals Vascular Dynamics in Murine Models of Prostate Cancer. Theranostics 2017, 7, 2900–2913. [Google Scholar] [CrossRef]
- Mason, R.P. Oxygen breathing challenge- the simplest theranostic. Theranostics 2017, 7, 3873–3875. [Google Scholar] [CrossRef]
- Zhou, H.L.; Zhang, Z.; Denney, R.; Williams, J.S.; Gerberich, J.; Stojadinovic, S.; Saha, D.; Shelton, J.M.; Mason, R.P. Tumor physiological changes during hypofractionated stereotactic body radiation therapy assessed using multi-parametric magnetic resonance imaging. Oncotarget 2017, 8, 37464–37477. [Google Scholar] [CrossRef]
- O’Kelly, D.; Zhou, H.; Mason, R.P. Tomographic breathing detection: A method to noninvasively assess in situ respiratory dynamics. J. Biomed. Opt. 2018, 23, 1–6. [Google Scholar] [CrossRef]
- O’Kelly, D.; Guo, Y.; Mason, R. Evaluating Online Filtering Algorithms to Enhance Dynamic Multispectral Optoacoustic Tomography. Photoacoustics 2020, 100184. [Google Scholar] [CrossRef]
- Zhao, D.; Pacheco-Torres, J.; Hallac, R.R.; White, D.; Peschke, P.; Cerdan, S.; Mason, R.P. Dynamic oxygen challenge evaluated by NMR T1 and T2*--insights into tumor oxygenation. NMR Biomed. 2015, 28, 937–947. [Google Scholar] [CrossRef] [PubMed]
- White, D.A.; Zhang, Z.; Li, L.; Gerberich, J.; Stojadinovic, S.; Peschke, P.; Mason, R.P. Developing oxygen-enhanced magnetic resonance imaging as a prognostic biomarker of radiation response. Cancer Lett. 2016, 380, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Tomaszewski, M.R.; Quiros-Gonzalez, I.; Weber, J.; Brunker, J.; Bohndiek, S.E. Evaluation of Precision in Optoacoustic Tomography for Preclinical Imaging in Living Subjects. J. Nucl. Med. 2017, 58, 807–814. [Google Scholar] [CrossRef] [PubMed]
- McNally, L.R.; Mezera, M.; Morgan, D.E.; Frederick, P.J.; Yang, E.S.; Eltoum, I.E.; Grizzle, W.E. Current and Emerging Clinical Applications of Multispectral Optoacoustic Tomography (MSOT) in Oncology. Clin. Cancer Res. 2016, 22, 3432–3439. [Google Scholar] [CrossRef]
- Weber, J.; Beard, P.C.; Bohndiek, S.E. Contrast agents for molecular photoacoustic imaging. Nat. Methods 2016, 13, 639–650. [Google Scholar] [CrossRef]
- Abma, E.; De Spiegelaere, W.; Vanderperren, K.; Stock, E.; Van Brantegem, L.; Cornelis, I.; Daminet, S.; Ni, Y.; Vynck, M.; Verstraete, G.; et al. A single dose of intravenous combretastatin A4-phosphate is reasonably well tolerated and significantly reduces tumour vascularization in canine spontaneous cancers. Vet. Comp. Oncol. 2018, 16, 467–477. [Google Scholar] [CrossRef]
- Siim, B.G.; Laux, W.T.; Rutland, M.D.; Palmer, B.N.; Wilson, W.R. Scintigraphic imaging of the hypoxia marker (99m)technetium-labeled 2,2 ‘-(1,4-diaminobutane)bis(2-methyl-3-butanone) dioxime (Tc-99m-labeled HL-91; Prognox): Noninvasive detection of tumor response to the antivascular agent 5,6-dimethylxanthenone-4-acetic acid. Cancer Res. 2000, 60, 4582–4588. [Google Scholar]
- Liu, L.; Mason, R.P.; Gimi, B. Dynamic bioluminescence and fluorescence imaging of the effects of the antivascular agent Combretastatin-A4P (CA4P) on brain tumor xenografts. Cancer Lett. 2015, 356, 462–469. [Google Scholar] [CrossRef]
- Lee, J.A.; Biel, N.M.; Kozikowski, R.T.; Siemann, D.W.; Sorg, B.S. In vivo spectral and fluorescence microscopy comparison of microvascular function after treatment with OXi4503, Sunitinib and their combination in Caki-2 tumors. Biomed. Opt. Express 2014, 5, 1965–1979. [Google Scholar] [CrossRef] [PubMed]
- Mita, M.M.; Sargsyan, L.; Mita, A.C.; Spear, M. Vascular-disrupting agents in oncology. Expert Opin. Investig. Drugs 2013, 22, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Masunaga, S.; Nagasawa, H.; Nagata, K.; Suzuki, M.; Uto, Y.; Hori, H.; Kinashi, Y.; Ono, K. Dependency of the effect of a vascular disrupting agent on sensitivity to tirapazamine and gamma-ray irradiation upon the timing of its administration and tumor size, with reference to the effect on intratumor quiescent cells. J. Cancer Res. Clin. Oncol. 2007, 133, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Chang, C.-H.; Kim, J.G.; Liu, H.; Mason, R.P. In vivo near-infrared spectroscopy and MRI monitoring of tumor response to Combretastatin A4 phosphate correlated with therapeutic outcome. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wu, J.; Yang, C.G.; Yu, H.Y.; Yang, S.C.; Li, T.T.; Chen, J.T.; Tang, Z.H.; Chen, X.S. Combretastatin A4 Nanoparticles Combined with Hypoxia-Sensitive Imiquimod: A New Paradigm for the Modulation of Host Immunological Responses during Cancer Treatment. Nano Lett. 2019, 19, 8021–8031. [Google Scholar] [CrossRef] [PubMed]
- Welford, A.F.; Biziato, D.; Coffelt, S.B.; Nucera, S.; Fisher, M.; Pucci, F.; Di Serio, C.; Naldini, L.; De Palma, M.; Tozer, G.M.; et al. TIE2-expressing macrophages limit the therapeutic efficacy of the vascular-disrupting agent combretastatin A4 phosphate in mice. J. Clin. Investig. 2011, 121, 1969–1973. [Google Scholar] [CrossRef]

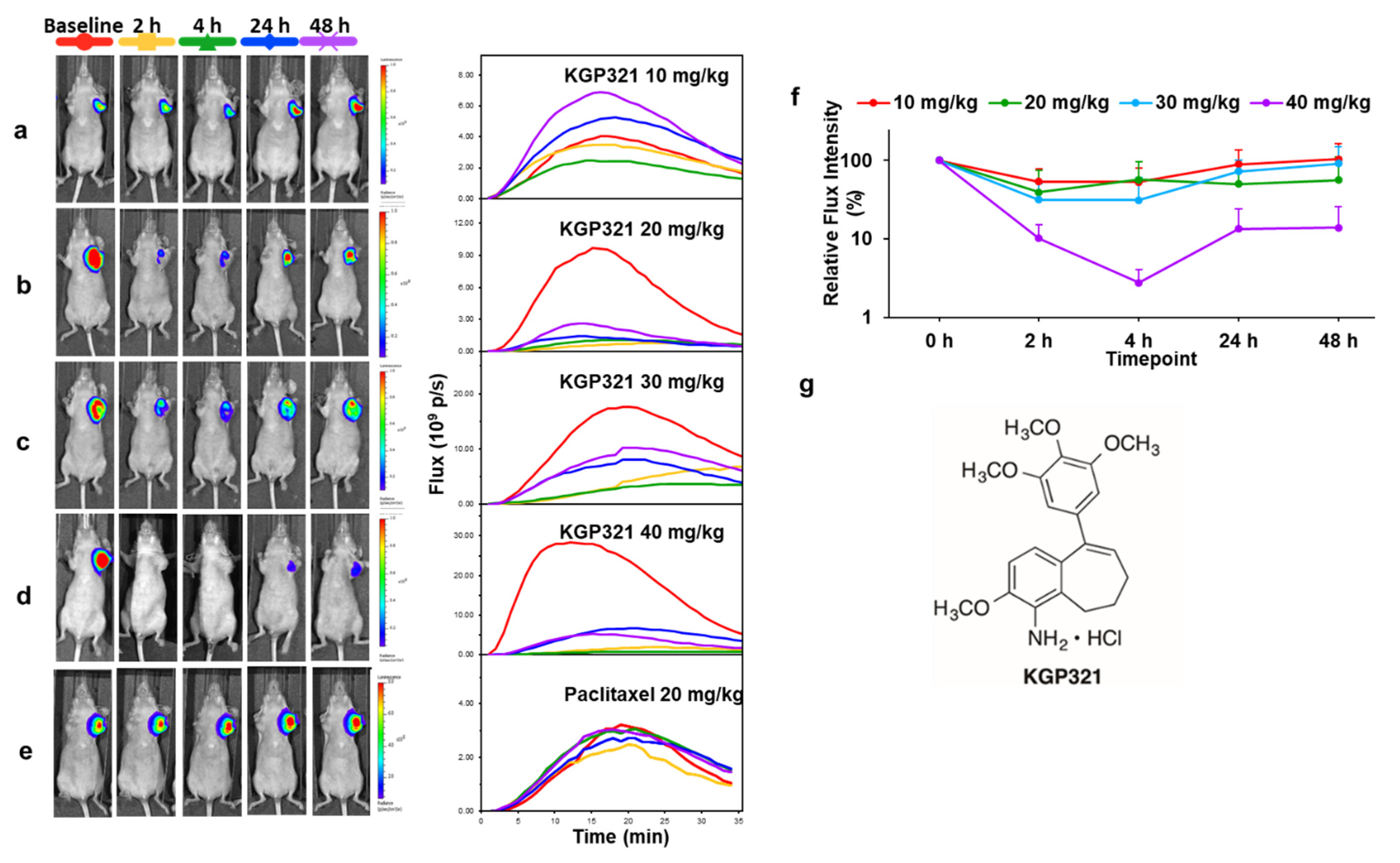
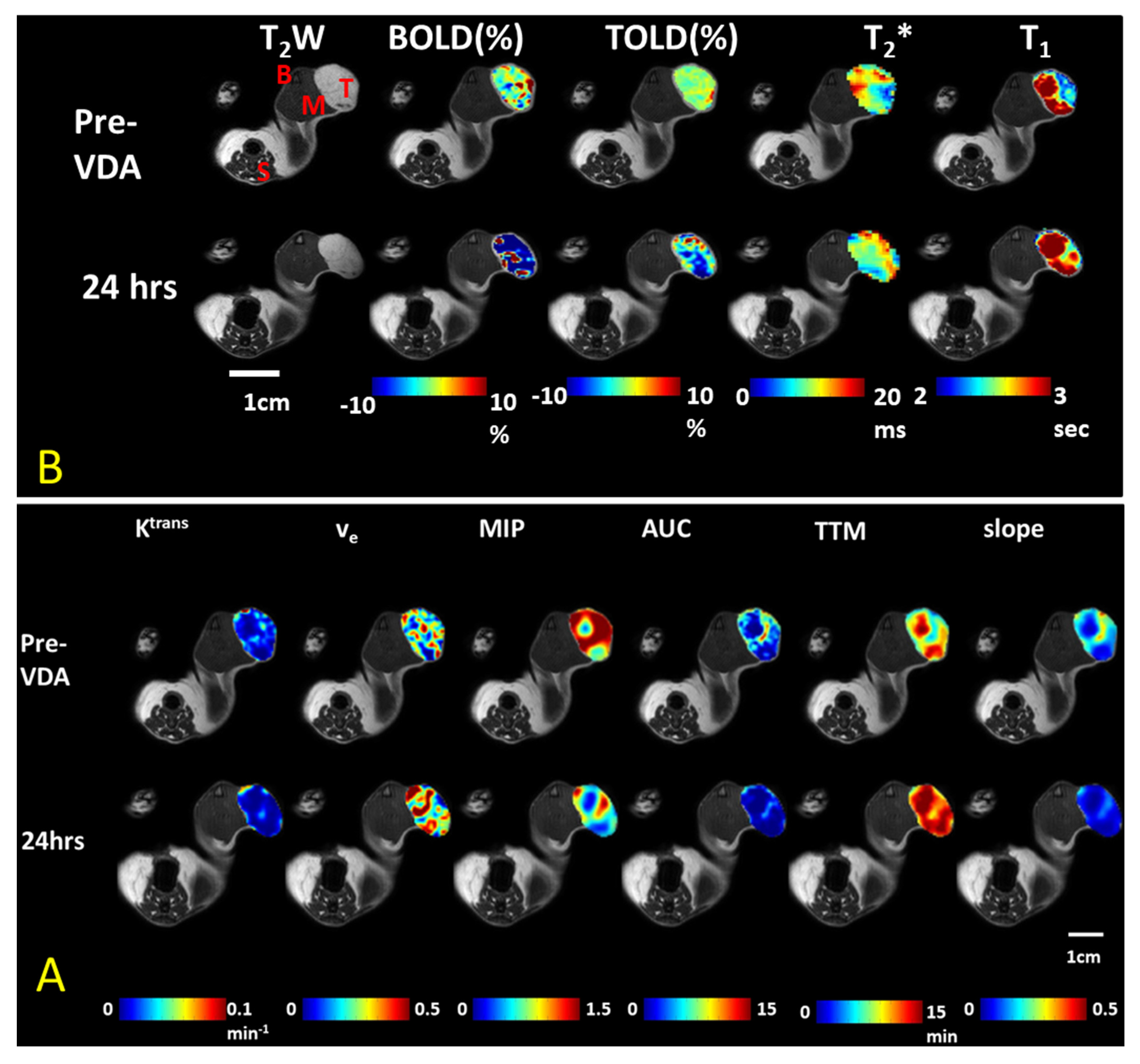
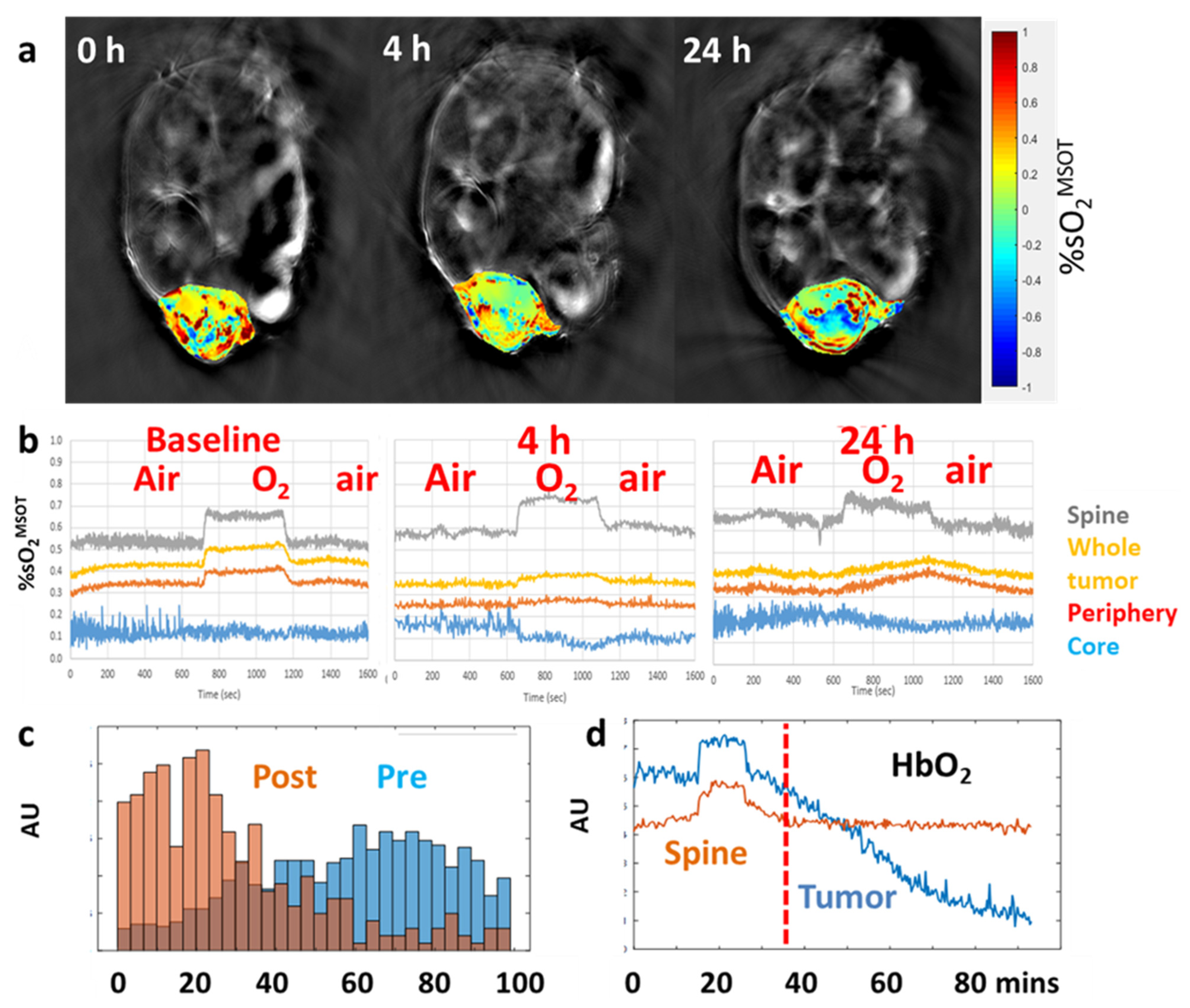
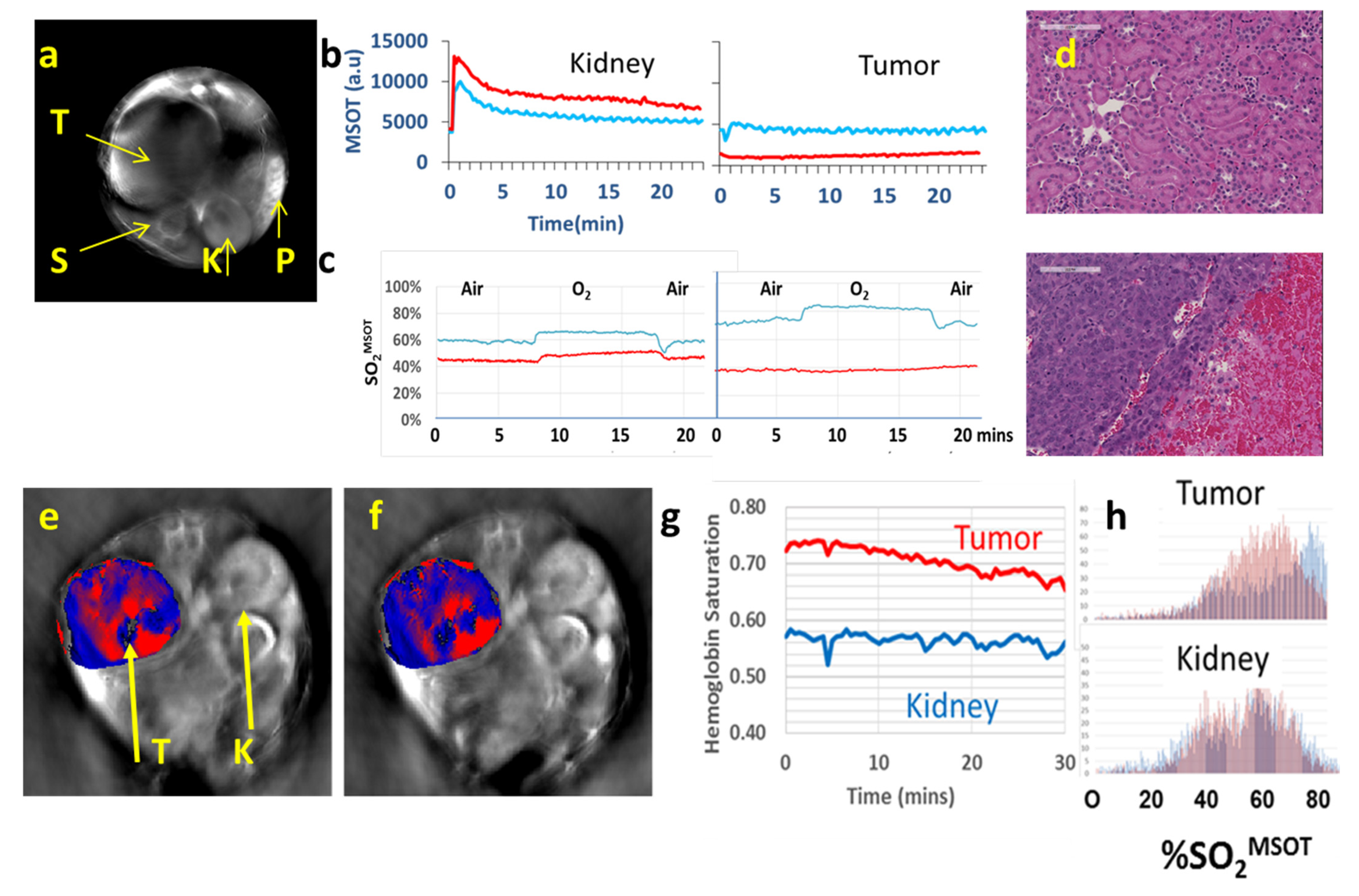

| Agent | Imaging Modality | Tumor Type | Trial | References |
|---|---|---|---|---|
| CA4P (fosbretabulin; Zybrestat) | DCE-MRI or -CT a DWI-MRI b 15O-PET | Lung cancer, ovarian, renal, breast | Phase 1 | [26,27,28,29] a [30] b [31] |
| CA1P (OXi4503) | DCE-MRI 15O-PET | Various | Phase 1 | [32] |
| BNC105P | DCE MRI | Various | Phase 1 21 patients; now in Phase 2 | [33] |
| CYT997 | DCE-MRI | Various | 31 patients | [34] |
| AVE8062 (Ombrabulin) | DCE-US | Various, mostly ovarian | 25 patients | [35] |
| NPI2358 (Plinabulin) | DCE-MRI | Various | 38 patients | [36] |
| ZD6126 | DCE-MRI | Colon | [37] | |
| EPC2407 (Crolibulin) | DCE- & DWI-MRI | Various | 11 subjects, Phase 1 | [38] |
| MN-029 (Denibulin) | DCE-MRI | Various | 34 subjects, Phase 1 | [39] |
| DMXAA (ASA404; 5,6-dimethylxanthenone-4-acetic acid; vadimezan) | DCE-MRI | Various | Phase 1 | [40] |
| Modality | Cost | Throughput | Spatial Resolution | Temporal Resolution | Need for Contrast Agent | Ease of Use |
|---|---|---|---|---|---|---|
| BLI | $ | High 5–10 mice | Surface planar | 1 s | Yes | Easy |
| MRI | $$$$$ | Usually single subject | 200 µm in plane × 2 mm | s-mins | Typically, yes | Sophisticated methods available |
| MSOT | $$$ | Single subject | 120 µm in plane by 200 µm | s | No | Image quality very sensitive to meticulous setup |
| US | $$ | Single subject | 100 to 1000 µm | Sub second | Often | Fairly easy |
| PET/CT | $$$$$ | Typically, 1–4 mice or single larger subject | 3 mm isotropic | mins | Yes | Issues of radioactivity: expense/safety |
Short Biography of Authors
 | Li Liu has served on the faculty of UT Southwestern Medical Center in the department of Radiology since 2008 after completing her Ph.D. from Nankai University in Tianjin, China, and postdoctoral fellowship training in molecular pharmacology at the Max Planck Chemical and Ecology Institute in Jena, Germany, in genetics and microbiology at the University of Florida, and in molecular imaging at UT Southwestern. Dr. Liu’s research focuses on development and application of reporter genes and molecular gene reporters for multimodality imaging, particularly related to cancer in small animal models. Dynamic imaging provides effective insight into tumor progression and response to therapy, particularly as applied to the development of vascular disputing agents in vivo. Most recently, she has explored multispectral optoacoustic tomography (MSOT) to provide real-time non-invasive insight into the vasculature of malignant tumors with respect to experimental drugs. |
 | Devin O’Kelly is a Computational Scientist at the BioHPC high-performance computing facility, located at the University of Texas Southwestern Medical Center at Dallas. He earned his Ph.D. in biomedical and molecular imaging with a supplement in computational and systems biology, developing models and methods for interpreting MSOT data in the context of dynamic vascular imaging. His primary research interests focus on improving and enabling the effective analysis of high-dimensional imaging data, as well as relating acquired data to models of the underlying biological systems. |
 | Regan J. Schuetze is a research technician in the Prognostic Imaging Research Lab. He joined the lab at UT Southwestern in 2020 after graduating from Furman University with a degree in neuroscience. His previous research investigated the role of dopamine in post-traumatic stress disorder and comorbid substance abuse and his current research represents a new interest in broader physiology. |
 | Graham J. Carlson is a Ph.D. candidate at Baylor University carrying out research under the mentorship of Dr. Pinney. He joined the Pinney Laboratory in 2014 after graduating from Hope College with a degree in chemistry. His current research focuses on the design and synthesis of new inhibitors of tubulin polymerization together with corresponding vascular disrupting agents (VDAs) and various associated prodrugs, designed to enhance selectivity, efficacy and aqueous solubility of promising lead molecules. |
 | Heling Zhou received her undergraduate degree in biomedical engineering at Sun Yet-sen University and her doctorate degree in radiological sciences at UT Southwestern Medical Center. She developed imaging analytical techniques to assess tumor microenvironment and its relationship to treatment outcomes. She is now pursuing a career as a data engineer, building scalable data management and analytics solutions, and is currently a data engineer lead in a health-tech company developing artificial intelligence for radiologists. |
 | Mary Lynn Trawick (formerly Dr. Mary Lynn Fink) is Associate Professor of Biochemistry in the Department of Chemistry and Biochemistry at Baylor University where she participates in the Institute for Biomedical Studies, the Molecular Biosciences Center, and the Center for Drug Discovery, and is a member of the Institutional Biosafety Committee. Her major research interests include the design, evaluation, and mechanism of action of novel anticancer agents with selectivity for the tumor microenvironment, and strategies to inhibit cancer metastasis. |
 | Kevin G. Pinney has served on the faculty of Baylor University in the Department of Chemistry and Biochemistry since 1993 after completing his Ph.D. at the University of Illinois, Urbana-Champaign and postdoctoral studies at the University of South Carolina. He is a Professor of Chemistry leading a dynamic research group focused on the design and synthesis of structurally diverse and biologically interesting small-molecule analogues, many of which are inspired by natural products. Well-established, diverse research projects include vascular disrupting agents (VDAs), inhibitors of tubulin polymerization, inhibitors of cathepsin L (and/or K), hypoxia-activated prodrugs and related drug-linker constructs. He teaches classes at both the undergraduate and graduate level in the area of organic chemistry. Research in his laboratory has been funded over the years by various grants from the National Institutes of Health, the Cancer Prevention and Research Institute of Texas (CPRIT), and a pharmaceutical company. He continues to enjoy productive research collaborations including those with the Mason Group (UT Southwestern Medical Center, Dallas, TX) and the Trawick Group (Baylor University). |
 | Ralph P. Mason is Professor of Radiology and Fellow of the Royal Society of Chemistry. He serves as the Director of the Small Animal Imaging Shared Resource at Simmons Comprehensive Cancer Center. He joined UT Southwestern 35 years ago after completing undergraduate and postgraduate studies at the University of Cambridge. His primary research interest is prognostic radiology: developing and implementing methods for predicting optimal cancer therapy and assessing early response to treatment (precision medicine). His particular interests have long related to prostate and breast cancer and most recently lung and kidney. He serves as advisor to graduate students in biomedical engineering and cancer biology and as mentor to postdocs and young faculty. He has been the recipient of multiple awards from the NCI and CPRIT. For 10 years, he has collaborated closely with the team at Baylor University to evaluate novel small molecule vascular disrupting agents for selective cancer treatment. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; O’Kelly, D.; Schuetze, R.; Carlson, G.; Zhou, H.; Trawick, M.L.; Pinney, K.G.; Mason, R.P. Non-Invasive Evaluation of Acute Effects of Tubulin Binding Agents: A Review of Imaging Vascular Disruption in Tumors. Molecules 2021, 26, 2551. https://doi.org/10.3390/molecules26092551
Liu L, O’Kelly D, Schuetze R, Carlson G, Zhou H, Trawick ML, Pinney KG, Mason RP. Non-Invasive Evaluation of Acute Effects of Tubulin Binding Agents: A Review of Imaging Vascular Disruption in Tumors. Molecules. 2021; 26(9):2551. https://doi.org/10.3390/molecules26092551
Chicago/Turabian StyleLiu, Li, Devin O’Kelly, Regan Schuetze, Graham Carlson, Heling Zhou, Mary Lynn Trawick, Kevin G. Pinney, and Ralph P. Mason. 2021. "Non-Invasive Evaluation of Acute Effects of Tubulin Binding Agents: A Review of Imaging Vascular Disruption in Tumors" Molecules 26, no. 9: 2551. https://doi.org/10.3390/molecules26092551
APA StyleLiu, L., O’Kelly, D., Schuetze, R., Carlson, G., Zhou, H., Trawick, M. L., Pinney, K. G., & Mason, R. P. (2021). Non-Invasive Evaluation of Acute Effects of Tubulin Binding Agents: A Review of Imaging Vascular Disruption in Tumors. Molecules, 26(9), 2551. https://doi.org/10.3390/molecules26092551









