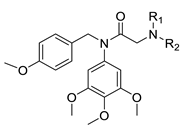
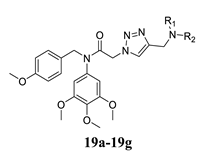
Abstract
Tubulin has been regarded as an attractive and successful molecular target in cancer therapy and drug discovery. Vicinal diaryl is a simple scaffold found in many colchicine site tubulin inhibitors, which is also an important pharmacophoric point of tubulin binding and anti-cancer activity. As the continuation of our research work on colchicine binding site tubulin inhibitors, we designed and synthesized a series of diarylamide N-containing heterocyclic derivatives by the combination of vicinal diaryl core and N-containing heterocyclic skeletons into one hybrid though proper linkers. Among of these compounds, compound 15b containing a 5-methoxyindole group exhibited the most potent inhibitory activity against the tested three human cancer cell lines (MGC-803, PC-3 and EC-109) with IC50 values of 1.56 μM, 3.56 μM and 14.5 μM, respectively. Besides, the SARs of these compounds were preliminarily studied and summarized. The most active compound 15b produced the inhibition of tubulin polymerization in a dose-dependent manner and caused microtubule network disruption in MGC-803 cells. Therefore, compound 15b was identified as a novel tubulin polymerization inhibitor targeting the colchicine binding site. In addition, the results of molecular docking also suggested compound 15b could tightly bind into the colchicine binding site of β-tubulin.
1. Introduction
Tubulin is an important component of the cytoskeleton and plays a vital role in the process of maintaining normal cell formation, cell mitosis, signal transduction and material transportation [1,2]. Tubulin has been regarded as an attractive and successful molecular target in cancer therapy and drug discovery [3]. Tubulin-targeting agents with excellent anti-cancer activity such as paclitaxel/Taxol, vincristine and vinblastine have been successfully used in clinical treatment [4]. However, owing to the poor water solubility, drug resistance or side effects of clinical used tubulin inhibitors [5,6,7], it is necessary to develop novel tubulin inhibitors [6]. However, colchicine site tubulin inhibitors have received extraordinary attention in the recent years, which could largely overcome the above drawbacks and have more therapeutic advantages over other binding sites tubulin inhibitors [8,9]. In addition, no colchicine site tubulin inhibitors have not been approved for clinical use; therefore, it is necessary to develop novel colchicine site tubulin inhibitors.
Vicinal diaryl is a simple scaffold found in many colchicine site tubulin inhibitors, which is also an important pharmacophoric point of tubulin binding and anti-cancer activity [10,11,12,13]. Natural colchicine site tubulin inhibitor combretastatin A-4 (CA-4) is a 1,2-diarylethylene analog, which exhibits potent inhibitory potency against many cancer cells including multidrug resistant cancer cells and could effectively inhibit the polymerization of tubulin [14]. However, due to the poor water solubility, low bioavailability and unstable cis double bond of CA-4 [12,15], its clinical application is limited; therefore, clinical work with CA-4 was carried out with the corresponding water-soluble phosphate prodrug salt (CA-4P) [16]. Therefore, many groups have reported novel colchicine site tubulin inhibitors with vicinal diaryl cores base on the structural optimizations of CA-4 [17,18,19,20,21,22,23,24,25]. Cushman et al. reported a novel tubulin inhibitor benzyl aniline 1 in which the olefinic bridge of the CA-4 was replaced by an aminomethylene hydrochloride moiety. Compound 1 retained significant anti-proliferative activity and inhibitory potency of tubulin polymerization [23]. Romagnoli et al. replaced the usual ethylene bridge of the led CA-4 with a five membered heterocyclic ring to obtain 1,5-disubstituted 1,2,4-triazole 2. Compound 2 exhibited potent inhibitory effect on tubulin polymerization (IC50 = 2.3 μM), and significantly suppressed the growth of human cancer cells at nano-molar levels [24]. Meegan′s group reported a class of diaryl-β-lactam derivatives which contained the β-lactam ring system in place of the ethylene bridge of the led CA-4 [25]. Compound 3 displayed sub nano-molar inhibitory activity against breast cancer cells MCF-7 and MDA-MB-231 (IC50 = 34 and 78 nM, respectively) together with significant inhibition of tubulin polymerization (Figure 1). Therefore, tubulin inhibitors bearing the vicinal diaryl moiety may be favorable for the interactions with tubulin.
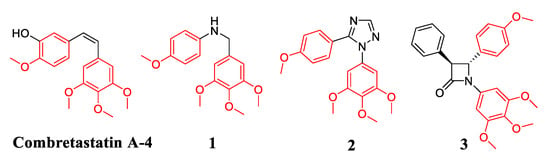
Figure 1.
Structures of CA-4 and CA-4 analogs as tubulin inhibitors.
N-containing heterocyclic skeleton is one of most attractive frameworks in bioactive compounds with strong pharmacological significance [26], especially the anti-cancer ability. For example, indole [27,28,29,30,31,32], indoline [33,34,35] and tetrahydroquinoline derivatives [36,37,38] have also been used to design novel colchicine binding site tubulin inhibitors with potent anti-cancer activity. Chalcone indole derivative 4 potently inhibited cancer cell growth with IC50 values ranging from 0.22 to 1.80 μM [30]. Compound 4 induced cell cycle arrest in G2/M phase and effectively inhibited the polymerization of tubulin. Quinoline-indole derivative 5 as an anti-tubulin agent targeting the colchicine binding site showed effective inhibition effect against tested cancer cell lines with IC50 values ranging from 2 to 11 nM together with significant in vitro inhibition of tubulin polymerization (IC50 = 2.09 μM) [31]. Benzimidazole-indole derivative 6 exhibited potent inhibitory effects on the growth of cancer cells with an average IC50 value of 50 nM together with significant in vitro inhibition of tubulin polymerization (IC50 = 2.52 μM) [32]. The 7-Aroyl-aminoindoline-1-sulfonamide 7 potently inhibited the tubulin polymerization (IC50 = 1.1 μM) by binding to colchicine binding site. Compound 7 effectively suppressed the growth of KB, MKN45, H460, HT29 and TSGH cells with IC50 values ranging from 8.6 nM to 10.8 nM [35]. Tetrahydroquinoline-pyrimidine [38] showed strong anti-proliferative activity (IC50 values ranging from 5.6 to 18.3 nM) via tubulin polymerization inhibition and potently inhibited tumor growth in an A375 melanoma xenograft model (Figure 2).
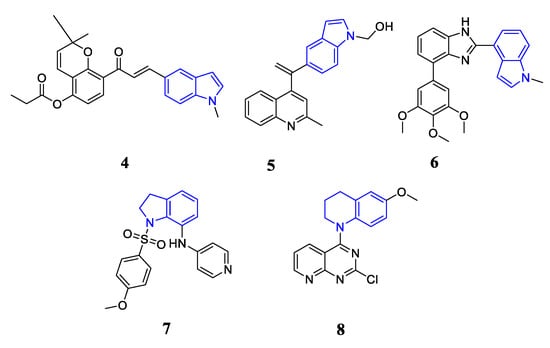
Figure 2.
Structures of N-containing heterocyclic derivatives as tubulin inhibitors.
Based on the above findings and as the continuation of our group work on colchicine binding site tubulin inhibitors, we designed and synthesized a series of diarylamide N-containing heterocyclic derivatives by the combination of vicinal diaryl core and N-containing heterocyclic skeleton into one hybrid though proper linkers (Figure 3). Among of these compounds, compound 15b was identified as a tubulin inhibitor with potent anti-cancer activity.
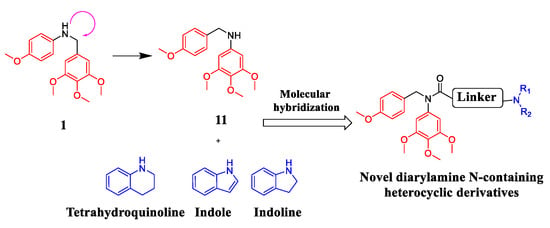
Figure 3.
Design of diarylamide N-containing heterocyclic derivatives in this work.
2. Results and Discussion
2.1. Chemistry
As shown in Scheme 1, all the target compounds 15a–15h and 19a–19g were synthesized by starting from commercially available 4-methoxybenzylchloride 9 and 3,4,5-trimethoxyaniline 10. The 4-Methoxybenzylchloride 9 reacted with the 3,4,5-trimethoxyaniline 10 in the presence of K2CO3 in DMF to give compound 11. Then compound 11 reacted with chloroacetyl chloride to afford compound 13 in DMF. Substitution reactions between compound 13 with indoles, indolines or tetrahydroquinolines 14 in the presence of K2CO3 in acetonitrile gave compounds 15a–h. In the synthesis of another series of compounds, compounds 14 reacted with 3-bromopropyne 16 in the presence of K2CO3 in acetonitrile to obtain compound 17. Substitution reaction between compound 15 with sodium azide in the presence of K2CO3 in acetonitrile gave compound 18. Then click reactions between compound 17 with 18 in the presence of CuSO4 and sodium ascorbate in THF/H2O (1:1) gave compounds 19a–e. Finally, all the target compounds were fully characterized by NMR and HMRS which was showed in the Supplementary Materials.
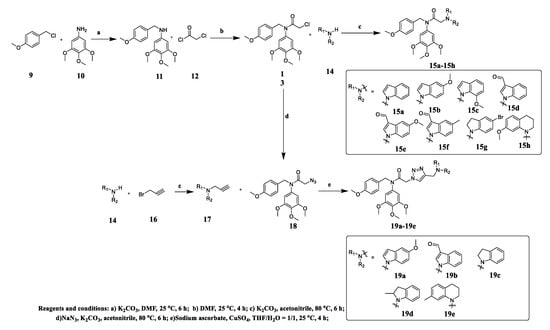
Scheme 1.
Synthetic routes of diarylamide N-containing heterocyclic derivatives.
2.2. Anti-proliferative Activity and Structure Activity Relationships
According to the latest cancer data in China [39] and the actual situation of our laboratory, the in vitro anti-proliferative activity of new target compounds 15a–h and 19a–e were evaluated against MGC-803 cell line (human gastric cancer), HCT-116 cell line (human colon cancer) and PC-3 cell line (human prostate cancer) using MTT assays with the well characterized tubulin inhibitor colchicine was used as a positive control. The following Table 1 and Table 2 depicted the results of in vitro anti-proliferative activity.

Table 1.
In vitro anti-proliferative activity of compounds 15a–h against MGC-803, PC-3 and EC-109 cells.

Table 2.
In vitro anti-proliferative activity of compounds 19a–e against MGC-803, PC-3 and EC-109 cells.
To explore the relationships between chemical groups and anti-proliferative activity, compounds 15a–h were designed and synthesized. Representative chemical features of compounds 15a–h are that different moiety such as indoles, indolines and tetrahydroquinoline, which are linked to diarylamide core through an alkyl linker. Most of these compounds exhibited certain anti-proliferative potency against three human cancer cell lines. Particularly, compound 15b exhibited the most potent inhibitory activity against the tested three human cancer cell lines (MGC-803, PC-3 and EC-109) with IC50 values of 1.56 µM, 3.56 μM and 14.5 μM, respectively. The anti-proliferative activity of the compounds varies with its substituent moieties. Compared compounds 15a with 15g and 15h, compounds with an indole group exhibited better anti-proliferative potency than compounds 15g and 15h with indoline and tetrahydroquinoline. Similarly, the substituent groups of indole group were also important for anti-proliferative activity. When indole ring is substituted by methoxy group at 5-position, the activity of the compound is the best. However, the anti-proliferative activity of compound was less potent when there was an aldehyde group at the 3-position of indole ring (compounds 15d, 15e and 15f) than that of compounds 15a and 15b. In addition, most of compound were more sensitive to MGC-803 cells than to PC-3 and EC-109 cells. These inhibitory results suggested that substituent moieties of compounds exhibited significant effects on anti-proliferative efficacy.
The 1,2,3-Triazole was usually used as a potentially pharmacological linker and fragment to design novel anti-cancer hybrids in medicinal chemistry [40]. Therefore, compounds 19a–e were designed and synthesized by replacing the alkyl linker with a 1,2,3-triazole linker to explore the effects of linkers on activity further. As shown in Table 2, the inhibitory potency of compounds 19a–e was decreased when the alkyl linker was replaced by a 1,2,3-triazole linker (compounds 19a vs. 15b and 19b vs. 15d), indicating that the 1,2,3-triazole linker could not improve the inhibitory potency for the diarylamide indole derivatives. Besides, compound 19b with a 5-methoxyindole group also exhibited the strongest inhibitory activity in this series compounds, which was consist with that of compound 15b. Most of this series compounds were also more sensitive to MGC-803 cells than to PC-3 and EC-109 cells.
Based on the above inhibitory activity results of compounds 15a–h and 19a–e, the structure–activity relationships were summarized. We concluded that nitrogen heterocycle played a significant role in anti-proliferative activity (indole > indoline > tetrahydroquinoline). Proper liner is beneficial to maintain anti-proliferative activity (alkyl linker > 1,2,3-triazole linker).
2.3. Compound 15b Inhibited Tubulin Polymerization
As its target protein, inhibitory effects on tubulin polymerization of compound 15b was first to be detected. Cell free tubulin polymerization assay was performed to evaluate anti-tubulin polymerization activity of compound 15b at different concentrations with the famous tubulin inhibitors colchicine and paclitaxel. As shown in Figure 4A, when tubulin was incubated with 15b (10 µM and 20 µM), the increased tendency of the fluorescence intensity was obviously slowed down with a similar action to that of colchicine, which indicated that compound 15b inhibited tubulin polymerization in a dose-dependent manner. However, the inhibitory activity of compound 15b on tubulin polymerization is less potent than that of colchicine, which is also consistent with results the anti-proliferative activity. Next, to investigate the effects to microtubules, compound 15b was selected to do immunofluorescence assay by staining tubulin. As shown in Figure 4B, cells’ morphologies were captured with immunofluorescence (IF) assay. MGC-803 cells treated with 15b at various concentrations (0.5 µM, 1 µM, and 2 µM) for 24 h resulted in disruption of microtubule networks, while the tubulins were polymerized to micro-tubes in control group. These results indicated that compound 15b produced the inhibition of tubulin polymerization a dose-dependent manner and caused microtubule network disruption in MGC-803 cells.
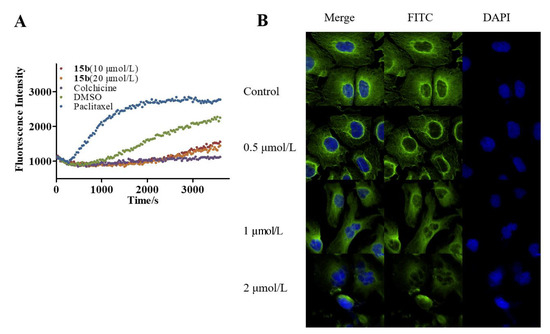
Figure 4.
Compound 15b inhibited tubulin polymerization. (A). Cell Free Tubulin Polymerization Assay, concentrations of Paclitaxel and Colchicine were 3.0 μmol/L; (B). β-tubulin (green) nucleus (blue) in MGC-803 cells. Cells were incubated with 0.5, 1 and 2 μM compound 15b for 24 h.
2.4. Compound 15b Bound to the Colchicine Site of β-tubulin and Molecular Docking Study
The N,N′-ethylenebis (iodoacetamide) (EBI) assay is usually used to test the binding ability of small molecules to β-tubulin at colchicine binding sites. Therefore, whether compound 15b acts on the colchicine binding site of tubulin was next to detected. As shown in Figure 5A, the results showed that with the increase of the concentration of compound 15b, β-tubulin adducts decreased gradually, which indicated that compound 15b directly bound to the colchicine site of β-tubulin. The Cellular Thermo Shift Assay (CTSA) can detect the direct interaction between compound and proteins. As shown in Figure 5C,D, compound 15b (100 μM) obviously accelerated the decrease of β-tubulin. These results suggested that compound 15b directly interacted with β-tubulin and targeted the colchicine binding site.
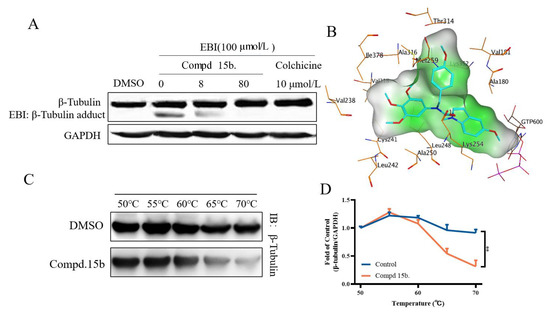
Figure 5.
Compound 15b bind to the colchicine site. (A) EBI competition assay, cells were incubated with compound/colchicine and EBI; (B) The docking of the colchicine binding site and compound 15b; (C,D) The Cellular Thermo Shift Assay. Cell Lysates were incubated with or without compound 15b. Then were incubated in different temperatures.
Compound 15b displayed inhibitory effects on tubulin polymerization in the screening above, and we then selected it as the optimized compound for the molecular docking studies by Autodock software. To investigate the binding site of compound 15b with the tubulin-microtubule system, PDB code 1SA0 was selected. The docking results were listed in Figure 5B, trimethoxyphenyl is located in a hydrophobic pocket consist of Val238, Cys241, Leu242, Leu248, Leu259, Ala316, Val319, Ile378 and other residues, and forms a strong hydrophobic interaction with this pocket. 4-methoxyphenyl occupies another hydrophobic pocket and forms extensive hydrophobic interactions with Val191, Met259, Thr314, Ala316, Lys352 and other residues. In addition, the substituted methoxy group on the indole ring faces the vicinity of the electronegative phosphate of the GTP molecule, forming a favorable electrostatic match with this region. At the same time, it also forms a certain hydrophobic effect with residues such as Ala190, Leu248 and Lys254. The above results indicated that compound 15b bound tightly to the colchicine binding site of β-tubulin and showed polymerization inhibitory activity on β-tubulin.
3. Materials and Methods
All the chemical reagents were purchased from commercial suppliers (Energy chemical Company, Shanghai, China and Zhengzhou HeQi Company, Zhengzhou, China). Melting points were determined on an X-5 micromelting apparatus. NMR spectra data was recorded with a Bruker spectrometer. HRMS spectra data was obtained using a Waters Micromass spectrometer. HPLC conditions: injection volume: 10 μL, flow rate, 1 mL/min with a mobile phase of H2O/MeOH; H2O/MeOH = 55/45 was initially held for 3 min, followed by a linear gradient from 55/45 to 5/95 = H2O/MeOH over 15 min, which was then held for 12 min.
3.1. Synthesis of Compound 11
A solution of commercially available 4-methoxybenzylchloride 9 (1.0 mmol, 1.0 eq), 3,4,5-trimethoxyaniline 10 (1.0 mmol, 1.0 eq), K2CO3 (2.0 mmol, 2.0 eq) were added into 20 mL DMF, and the reaction was stirred for 6 h at 25 °C. Upon completion, add 15 mL water and extract aqueous layer three times using ethyl acetate (20 mL). The collected organic layer was washed with saturated salt water, dried with magnesium sulfate anhydrous and evaporated to get crude product. The crude product was purified by column chromatography to obtain compound 11.
3.2. Synthesis of Compound 13
A solution of compound 11 (1.0 mmol, 1.0 eq) and chloroacetyl chloride 12 (1.5 mmol, 1.5 eq) was added into 20 mL dichloromethane, and the reaction was stirred for 4 h at 25 °C. Upon completion, organic phase was collected to obtain crude products and then were purified with column chromatography to give compound 13.
3.3. Synthesis of Compounds 15a–h
A solution of compound 13 (1.0 mmol, 1.0 eq), substituted indoles, indolines or tetrahydroquinoline 14 (1.5 mmol, 1.5 eq) and K2CO3 (2.0 mmol, 2.0 eq) were added were 20 mL acetonitrile, and the reactions were stirred for 8 h at 80 °C. Upon completion, organic phase was collected to obtain crude products and then were purified with column chromatography to give compound 15a–h.
2-(1H-Indol-1-yl)-N-(4-methoxybenzyl)-N-(3,4,5-trimethoxyphenyl) acetamide (15a). Yield, 47%, m.p. 162–163 °C, White solid. 1H NMR (400 MHz, DMSO-d6) δ 7.52 (d, J = 7.6 Hz, 1H), 7.25 (d, J = 8.2 Hz, 1H), 7.08 (ddd, J = 35.7, 15.9, 8.0 Hz, 5H), 6.86 (d, J = 8.1 Hz, 2H), 6.54 (s, 2H), 6.39 (s, 1H), 4.81 (d, J = 38.6 Hz, 4H), 3.69 (t, J = 12.8 Hz, 12H). 15C NMR (101 MHz, DMSO-d6) δ 167.03, 158.48, 153.04, 157.02, 156.27, 156.08, 129.78, 129.62, 129.25, 127.98, 120.88, 120.16, 118.94, 115.60, 109.70, 105.99, 100.65, 60.00, 55.96, 55.01, 51.86, 47.88, 40.11, 39.90, 39.70, 39.49, 39.28, 39.07, 38.86. HR-MS (ESI): Calcd. C27H28N2O5, [M + H]+ m/z: 461.2071, found: 461.2074. HPLC: tR 7.06 min, purity 93.97%.
2-(5-Methoxy-1H-indol-1-yl)-N-(4-methoxybenzyl)-N-(3,4,5-trimethoxyphenyl) acetamide (15b). Yield, 50%, m.p.: 173–174 °C. 1H NMR (400 MHz, DMSO-d6) δ 7.19–7.09 (m, 4H), 7.03 (d, J = 2.3 Hz, 1H), 6.86 (d, J = 8.5 Hz, 2H), 6.74 (dt, J = 11.9, 6.0 Hz, 1H), 6.52 (s, 2H), 6.30 (d, J = 3.0 Hz, 1H), 4.80 (d, J = 15.3 Hz, 2H), 4.76 (s, 2H), 3.74 (s, 3H), 3.71 (d, J = 8.6 Hz, 9H), 3.65 (s, 3H). 15C NMR (100 MHz, DMSO-d6) δ 198.96, 168.05, 162.86, 156.28, 152.78, 149.30, 159.04, 156.65, 153.86, 129.86, 129.24, 128.61, 125.30, 124.70, 124.15, 120.40, 34.84, 30.79. HR-MS (ESI): Calcd. C28H30N2O6, [M + H]+ m/z: 491.2197, found: 491.2180. HPLC: tR 7.76 min, purity 92.32%.
2-(6-Methoxy-1H-indol-1-yl)-N-(4-methoxybenzyl)-N-(3,4,5-trimethoxyphenyl) acetamide (15c). Yield, 38%, m.p. 146–147 °C, White solid. 1H NMR (400 MHz, DMSO-d6) δ 7.38 (d, J = 8.4 Hz, 1H), 7.14 (d, J = 8.4 Hz, 2H), 7.02 (d, J = 3.0 Hz, 1H), 6.86 (d, J = 8.5 Hz, 2H), 6.71–6.63 (m, 2H), 6.49 (s, 2H), 6.31 (d, J = 3.0 Hz, 1H), 4.79 (d, J = 18.2 Hz, 4H), 3.75 (s, 3H), 3.72 (s, 3H), 3.68 (s, 6H), 3.64 (s, 3H).15C NMR (101 MHz, DMSO-d6) δ 167.11, 158.49, 155.43, 152.99, 156.97, 156.12, 129.78, 129.33, 128.23, 122.11, 120.68, 115.59, 108.78, 105.82, 100.73, 93.28, 59.94, 55.88, 55.15, 55.02, 51.86, 47.89. HR-MS (ESI): Calcd. C28H30N2O6, [M + H]+ m/z: 491.2197, found: 491.2180. HPLC: tR 6.73 min, purity 93.22%.
2-(3-Formyl-1H-indol-1-yl)-N-(4-methoxybenzyl)-N-(3,4,5-trimethoxyphenyl) acetamide (15d). Yield, 51%, m.p. 151–152 °C, 1H NMR (400 MHz, DMSO-d6) δ 9.95 (s, 1H), 8.15 (d, J = 7.3 Hz, 2H), 7.49 (d, J = 7.9 Hz, 1H), 7.38–7.28 (m, 2H), 7.19 (d, J = 8.5 Hz, 2H), 6.91 (d, J = 8.5 Hz, 2H), 6.63 (s, 2H), 5.07 (s, 2H), 4.83 (s, 2H), 3.77 (s, 9H), 3.72 (s, 3H). White solid. 15C NMR (101 MHz, DMSO-d6) δ 184.63, 166.11, 158.53, 153.10, 142.01, 157.66, 157.12, 155.71, 129.83, 129.06, 124.38, 123.38, 122.35, 120.81, 119.32, 115.61, 111.06, 106.04, 60.00, 55.99, 55.02, 51.99, 48.60. HR-MS (ESI): Calcd. C28H28N2O6, [M + H]+ m/z: 489.2020, found: 489.2026. HPLC: tR 5.01 min, purity 98.91%.
2-(6-Methoxy-1H-indol-1-yl)-N-(4-methoxybenzyl)-N-(3,4,5-trimethoxyphenyl) acetamide (15e). Yield, 38%, m.p. 162–163 °C, White solid. 1H NMR (400 MHz, DMSO-d6) δ 9.86 (s, 1H), 8.03 (s, 1H), 7.59 (d, J = 2.3 Hz, 1H), 7.34 (d, J = 8.9 Hz, 1H), 7.14 (d, J = 8.4 Hz, 2H), 6.96–6.82 (m, 3H), 6.57 (s, 2H), 4.99 (s, 2H), 4.78 (s, 2H), 3.80 (s, 3H), 3.72 (d, J = 2.1 Hz, 9H), 3.67 (s, 3H), 3.35 (s, 4H). 15C NMR (101 MHz, DMSO-d6) δ 184.48, 166.12, 158.52, 155.87, 153.08, 141.94, 157.08, 155.70, 152.54, 129.82, 129.06, 125.15, 119.09, 115.60, 115.02, 111.95, 106.00, 102.61, 59.98, 55.97, 55.35, 55.01, 51.99, 48.78. HR-MS (ESI): Calcd. C29H30N2O7, [M + H]+ m/z: 519.2126, found: 519.2151. HPLC: tR 4.90 min, purity 92.22%.
2-(3-Formyl-5-methyl-1H-indol-1-yl)-N-(4-methoxybenzyl)-N-(3,4,5-trimethoxyphenyl) acetamide (15f). Yield, 33%, m.p. 170–171 °C, White solid. 1H NMR (400 MHz, DMSO-d6) δ 9.86 (s, 1H), 8.03 (s, 1H), 7.90 (s, 1H), 7.31 (d, J = 8.3 Hz, 1H), 7.15 (t, J = 7.1 Hz, 3H), 6.86 (d, J = 8.3 Hz, 2H), 6.57 (s, 2H), 4.98 (s, 2H), 4.77 (s, 2H), 3.69 (d, J = 22.0 Hz, 12H), 2.42 (s, 3H).15C NMR (101 MHz, DMSO-d6) δ 184.49, 166.14, 158.52, 153.09, 141.96, 157.10, 156.06, 155.72, 151.39, 129.82, 129.07, 124.77, 124.63, 120.58, 119.01, 115.60, 110.68, 106.01, 59.99, 55.97, 55.01, 51.98, 48.62, 21.07. HR-MS (ESI): Calcd. C29H30N2O6, [M + Na]+ m/z: 525.1996, found: 525.2001. HPLC: tR 5.74 min, purity 90.88%.
2-(5-Bromoindolin-1-yl)-N-(4-methoxybenzyl)-N-(3,4,5-trimethoxyphenyl) acetamide (15g). Yield, 54%, m.p. 149–150 °C, White solid. 1H NMR (400 MHz, DMSO-d6) δ 7.15–7.01 (m, 4H), 6.86 (d, J = 8.5 Hz, 2H), 6.48 (s, 2H), 6.22 (d, J = 8.3 Hz, 1H), 4.75 (s, 2H), 3.80 (s, 2H), 3.72 (s, 3H), 3.67 (s, 6H), 3.63 (s, 3H), 3.43 (t, J = 8.5 Hz, 2H), 2.88 (t, J = 8.4 Hz, 2H).15C NMR (101 MHz, DMSO-d6) δ 167.83, 158.44, 152.97, 151.05, 156.91, 156.58, 151.87, 129.68, 129.49, 129.15, 126.63, 115.61, 107.37, 107.07, 105.84, 60.01, 55.99, 55.02, 52.45, 51.58, 49.22, 27.66. HR-MS (ESI): Calcd. C27H29BrN2O5, [M + H]+ m/z: 541.1533, found: 541.1537. HPLC: tR 10.97 min, purity 95.64%.
2-(6-Methoxy-3,4-dihydroquinolin-1(2H)-yl)-N-(4-methoxybenzyl)-N-(3,4,5-trimethoxyphenyl) acetamide (15h). Yield, 32%, m.p. 139–140 °C, White solid. 1H NMR (400 MHz, DMSO-d6) δ 7.12 (d, J = 8.4 Hz, 2H), 6.85 (d, J = 8.5 Hz, 2H), 6.59–6.45 (m, 4H), 6.23 (d, J = 8.7 Hz, 1H), 4.74 (s, 2H), 3.83 (s, 2H), 3.70 (d, J = 15.4 Hz, 9H), 3.64 (d, J = 6.2 Hz, 6H), 3.25–3.18 (m, 2H), 2.64 (t, J = 6.1 Hz, 2H), 1.83–1.70 (m, 2H). 13C NMR (100 MHz, DMSO-d6) δ 169.06, 158.40, 153.00, 150.35, 139.44, 136.87, 136.75, 129.65, 129.62, 123.12, 114.73, 113.56, 111.98, 110.86, 105.61, 60.02, 55.99, 55.23, 55.00, 53.19, 51.49, 49.66, 27.58, 21.92. HR-MS (ESI): Calcd. C29H34N2O6, [M + H]+ m/z: 507.2490, found: 507.2496. HPLC: tR 10.97 min, purity 95.64%.
3.4. Synthesis of Compounds 17
A solution of commercially available 3-bromopropyne 16 (1.5 mmol, 1.0 eq), indolines or tetrahydroquinoline 14 (1.5 mmol, 1.0 eq) and K2CO3 (2.0 mmol, 1.0 eq) was added into 20 mL acetonitrile, and the reactions were stirred for 6 h at 80 °C. Upon completion, the organic phase was collected to obtain crude products and then were purified with column chromatography to give compounds 17.
3.5. Synthesis of Compounds 18
A solution of compound 13 (1.0 mmol, 1.0 eq), sodium azide (2.0 mmol, 2.0 eq) and K2CO3 (2.0 mmol, 2.0 eq) was added to 20 mL acetonitrile, and the reactions were stirred for 2 h at 80 °C. Upon completion, the organic phase was collected to obtain crude products and then were purified with column chromatography to give compound 18.
3.6. Synthesis of Compounds 19a–e
A solution of compound 17 (1.0 mmol, 1.0 eq), compounds 18 (1.0 mmol, 1.0 eq), CuSO4 (0.1 mmol, 0.1 eq) and sodium ascorbate (0.1 mmol, 0.1 eq) was added to THF/H2O (5 mL/5 mL), and the reactions were stirred for 4 h at 25 °C. Upon completion, the organic phase was collected to obtain crude products and then were purified with column chromatography to give compounds 19a–e.
2-(4-((5-Methoxy-1H-indol-1-yl)methyl)-1H-1,2,3-triazol-1-yl)-N-(4-methoxybenzyl)-N-(3,4,5-trimethoxyphenyl)acetamide (19a). Yield, 47%, m.p. 158–159 °C, White solid. 1H NMR (400 MHz, DMSO-d6) δ 7.85 (s, 1H), 7.46 (d, J = 8.8 Hz, 1H), 7.38 (s, 1H), 7.12 (d, J = 7.5 Hz, 2H), 7.04 (s, 1H), 6.85 (d, J = 7.6 Hz, 2H), 6.77 (d, J = 8.9 Hz, 1H), 6.55 (s, 2H), 6.35 (s, 1H), 5.43 (s, 2H), 5.08 (s, 2H), 4.76 (s, 2H), 3.74 (s, 3H), 3.70 (d, J = 8.4 Hz, 9H), 3.65 (s, 3H).15C NMR (101 MHz, DMSO-d6) δ 165.65, 159.02, 154.03, 153.55, 143.89, 157.60, 155.94, 151.32, 150.30, 129.52, 129.41, 129.14, 125.15, 114.10, 111.68, 111.24, 106.56, 102.65, 101.09, 60.49, 56.45, 55.79, 55.51, 52.33, 51.50, 41.46. HR-MS (ESI): Calcd. C31H33N5O6, [M + H]+ m/z: 572.2504, found: 572.2509. HPLC: tR 5.29 min, purity 92.17%.
2-(4-((2-Formyl-1H-indol-1-yl)methyl)-1H-1,2,3-triazol-1-yl)-N-(4-methoxybenzyl)-N-(3,4,5-trimethoxyphenyl)acetamide (19b). Yield, 47%, m.p. 168–169 °C, White solid. 1H NMR (400 MHz, DMSO-d6) δ 9.94 (s, 1H), 8.41 (s, 1H), 8.15–8.02 (m, 2H), 7.73 (d, J = 8.0 Hz, 1H), 7.29 (dd, J = 15.6, 7.5 Hz, 2H), 7.12 (d, J = 8.1 Hz, 2H), 6.85 (d, J = 8.2 Hz, 2H), 6.56 (s, 2H), 5.64 (s, 2H), 5.11 (s, 2H), 4.77 (s, 2H), 3.69 (t, J = 12.0 Hz, 12H).15C NMR (101 MHz, DMSO-d6) δ 185.20, 165.63, 159.03, 153.58, 142.49, 141.11, 157.64, 157.30, 155.93, 150.32, 129.40, 125.78, 125.21, 124.08, 123.07, 121.52, 119.90, 114.10, 111.84, 106.57, 60.49, 56.45, 55.50, 52.37, 51.62, 42.04. HR-MS (ESI): Calcd. C31H31N5O6, [M + H]+ m/z: 592.2167, found: 592.2192. HPLC: tR 4.41 min, purity 95.57%.
N-(4-Methoxybenzyl)-2-(4-((2-methylindolin-1-yl)methyl)-1H-1,2,3-triazol-1-yl)-N-(3,4,5-trimethoxyphenyl)acetamide (19c). Yield, 39%, m.p. 171–172 °C, 1H NMR (400 MHz, DMSO-d6) δ 7.78 (s, 1H), 7.12 (d, J = 8.5 Hz, 2H), 6.95 (dt, J = 7.2, 3.7 Hz, 2H), 6.85 (d, J = 8.6 Hz, 2H), 6.56 (dd, J = 15.9, 7.6 Hz, 4H), 5.07 (s, 2H), 4.76 (d, J = 2.5 Hz, 2H), 4.52 (d, J = 16.0 Hz, 1H), 4.25 (d, J = 15.9 Hz, 1H), 3.70 (d, J = 7.1 Hz, 9H), 3.65 (s, 3H), 3.02 (dd, J = 15.5, 8.4 Hz, 1H), 1.31 (d, J = 6.1 Hz, 3H). 15C NMR (101 MHz, DMSO-d6) δ 165.73, 159.02, 153.57, 152.02, 143.18, 157.61, 156.00, 150.29, 129.44, 129.02, 127.53, 125.22, 124.36, 119.69, 114.10, 107.60, 106.56, 60.49, 59.08, 56.45, 55.50, 52.32, 51.45, 37.08, 19.26. HR-MS (ESI): Calcd. C31H35N5O5, [M + H]+ m/z: 558.2711, found: 558.2715. HPLC: tR 5.19 min, purity 96.55%.
2-(4-(Indolin-1-ylmethyl)-1H-1,2,3-triazol-1-yl)-N-(4-methoxybenzyl)-N-(3,4,5-trimethoxyphenyl) acetamide (19d). Yield, 51%, m.p. 165–166 °C, White solid. 1H NMR (400 MHz, DMSO-d6) δ 7.78 (s, 1H), 7.12 (d, J = 8.5 Hz, 2H), 6.95 (dt, J = 7.2, 3.7 Hz, 2H), 6.85 (d, J = 8.6 Hz, 2H), 6.56 (dd, J = 13.9, 7.6 Hz, 4H), 5.07 (s, 2H), 4.76 (d, J = 2.5 Hz, 2H), 4.52 (d, J = 16.0 Hz, 1H), 4.25 (d, J = 15.9 Hz, 1H), 3.70 (d, J = 7.1 Hz, 9H), 3.65 (s, 3H), 3.02 (dd, J = 15.5, 8.4 Hz, 1H), 1.31 (d, J = 6.1 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 165.23, 158.54, 153.08, 151.49, 143.29, 142.62, 157.18, 155.51, 129.80, 129.75, 128.94, 127.00, 124.88, 124.20, 119.38, 115.62, 107.39, 106.10, 60.01, 55.99, 55.02, 52.34, 51.85, 50.97, 43.19, 27.87. HR-MS (ESI): Calcd. C30H33N5O5, [M + H]+ m/z: 544.2554, found: 544.2559. HPLC: tR 7.1 min, purity 88.75%.
N-(4-Methoxybenzyl)-2-(4-((6-methyl-3,4-dihydroquinolin-1(2H)-yl)methyl)-1H-1,2,3-triazol-1-yl)-N-(3,4,5-trimethoxyphenyl)acetamide (19e). Yield, 44%, m.p. 162–163 °C, 1H NMR (400 MHz, DMSO-d6) δ 7.76 (s, 1H), 7.15 (d, J = 8.2 Hz, 2H), 6.85 (d, J = 8.2 Hz, 2H), 6.75–6.61 (m, 3H), 6.55 (s, 2H), 5.07 (s, 2H), 4.77 (s, 2H), 4.47 (s, 2H), 3.70 (d, J = 7.0 Hz, 9H), 3.65 (s, 3H), 3.31–3.26 (m, 2H), 2.62 (t, J = 6.0 Hz, 2H), 2.10 (s, 3H), 1.90–1.82 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 165.75, 159.02, 153.56, 144.07, 142.98, 157.60, 156.00, 150.29, 150.00, 129.44, 127.64, 124.74, 124.57, 122.68, 114.10, 111.87, 106.55, 60.49, 56.45, 55.50, 52.32, 51.44, 49.37, 46.44, 27.89, 22.42, 20.37. HR-MS (ESI): Calcd. C32H35N5O5, [M + Na]+ m/z: 594.2687, found: 594.2691. HPLC: tR 9.50 min, purity 86.08%.
3.7. Cell Culture
Cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin and 0.1 mg/mL streptomycin. All the cells were incubated at 37 °C and 5% CO2.
3.8. MTT Assay
A total of 5000 cells were seeded into 96-well cell culture plates. After 24 h, cells were treated with synthesized compounds. Then, MTT reagent was added 20 μL per well after 48 h treatment with synthesized compounds. Cells were then incubated for 4 h at 37 °C. Formazan was then dissolved with DMSO. Absorbencies of formazan solution at 490 nm were determined. SPSS version 10.0 was used for 50% inhibitory concentration (IC50) calculation [41,42].
3.9. Tubulin Polymerization Detection
Pig brain microtubule protein was isolated by three cycles of temperature-dependent assembly/disassembly in PIPES (pH 6.5, 100 mM), MgSO4 (1.0 mM), EGTA (2.0 mM), GTP (1.0 mM) and 2-mercaptoethanol (1.0 mM). In the first cycle of polymerization, glycerol and phenylmethylsulfonyl fluoride were added to 4 M and 0.2 mM, respectively. Homogeneous tubulin was prepared from microtubule protein by phosphocellulose (P11) chromatography. The purified proteins were stored in aliquots at −70 °C.
Re-suspend tubulin in proton exchange membrane buffer (containing 100 mmol/L PIPES, 1 mmol/L EGTA, 0.5 mmol/L Mgcl2, 1 mmol/L GTP, 10.2% glycerol), and the solution was incubated with different concentrations of compound 15b (10, 20 μmol/L), colchicine (3 μmol/L), paclitaxel (3 μmol/L) and the carrier DMSO on ice. Using a spectrophotometer to monitor the absorbance of the reaction at 420 nm (excitation wavelength is 340 nm) [43].
3.10. Cellular Thermal Shift Assay
Inoculate the cells in a petri dish, and collect the cells when the cells grow to 90%. The cells were re-suspended in PBS containing phosphatase inhibitor and protease inhibitor, and the cells were repeatedly frozen and thawed in liquid nitrogen. Compound 15b (100 μmol/L) and the same amount of DMSO were added to the protein respectively, and the mixture was heated at 50 °C, 55 °C, 60 °C, 65 °C and 70 °C for three times. The supernatant was centrifuged at 15,000× g rpm, and the sample was used for western blot analysis.
3.11. EBI Competition Experiment
In total, 3 × 105 MGC-903 cells ells were seeded into 6-well cell culture plates and cultured for 24 h, incubated with different concentrations of compound 15b, colchicine, paclitaxel, and DMSO for 2 h, and then treated with 100 μmol/L EBI for 1.5 h. The cells were collected, and the β-tubulin and β-tubulin adducts were determined with anti-β-tubulin antibody.
3.12. Immunofluorescence Experiment
MGC-803 cells were seeded in 96-well plates and treated with different concentrations of compound 15b for 24 h. Fix the cells with 4% paraformaldehyde for 10 min, and then infiltrate the cells with PBS containing 0.1% Triton X-100. After blocking with 5% BSA at room temperature for 1 h, incubate overnight with anti-β-tubulin antibody at 4 °C, stain with fluorescent antibody, and label cell nuclei with DAPI. Observe the cells using a fluorescence microscope.
3.13. Molecular Docking
The molecular docking study was performed using MOE 2015.10. The crystal structure of tubulin (PDB ID: 1SA0) was retrieved from RCSB Protein Data Bank (https://www.rcsb.org/structure/1SA0, accessed on 1 July 2021), and then was prepared by adding hydrogen atoms, removing water molecules and repairing the missing side chains. The protonation states of protein residues were calculated in the pKa at 7. The ligand compound 15b was built in Autodock software (Scripps Research Institute, La Jolla, CA, USA) and was prepared by energy minimization and conformational search. The ligand was docked into the colchicine binding site of tubulin and 20 poses were exported for the next analysis.
4. Conclusions
Tubulin has been regarded as an attractive and successful molecular target in cancer therapy and drug discovery. However, owing to the poor water solubility, drug resistance and side effects of clinical tubulin inhibitors, it is necessary to develop novel tubulin inhibitors. As the continuation of our group work on colchicine binding-site tubulin inhibitors, we designed and synthesized a series of diarylamide indole derivatives by the combination of vicinal diaryl core and indole skeleton into one hybrid though proper linkers. Among of these compounds, compound 15b exhibited the most potent inhibitory activity against the tested three human cancer cell lines (MGC-803, PC-3 and EC-109) with IC50 values of 1.56 µM, 3.56 μM and 14.5 μM, respectively. Besides, the SARs of these compounds were preliminarily studied and summarized. The most active compound 15b produced the inhibition of tubulin polymerization in a dose-dependent manner and caused microtubule network disruption in MGC-803 cells. Therefore, compound 15b was identified as a novel tubulin polymerization inhibitor targeting the colchicine binding site. In addition, the results of molecular docking also suggested compound 15b tightly bind into the colchicine binding site of β-tubulin.
Supplementary Materials
The following are available.
Author Contributions
S.-Y.Z. and J.S. designed the research and contributed to revision of manuscript; X.-J.P., G.-X.Y., Y.-B.Z. performed the synthetic work. X.L., Y.L., W.-B.L., Y.-R.L. were responsible for the direction of the biological research; X.L. and X.-J.P. contributed to writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Sciences Foundations of China (No. 81903541, U2004123 for Sai-Yang Zhang and No. 81673322 for Yan-Bing Zhang) and China Postdoctoral Science Foundation (No. 2019M632812 for Sai-Yang Zhang) and the Henan Scientific Innovation Talent Team, Department for Education (No. 19ITSTHN001 for Wen Zhao, China). Henan Association of Science and Technology (No. 2020HYTP056 for Sai-Yang Zhang, China) and Science and Technology Department of Henan Province (No. 20202310144, for Sai-Yang Zhang, China). The open fund of state key laboratory of Pharmaceutical Biotechnology, Nan-jing University, China (Grant no. KF-GN-202101).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Howard, J.; Hyman, A. Dynamics and mechanics of the microtubule plus end. Nat. Cell Biol. 2003, 422, 753–758. [Google Scholar] [CrossRef]
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Binarová, P.; Tuszynski, J. Tubulin: Structure, Functions and Roles in Disease. Cells 2019, 8, 1294. [Google Scholar] [CrossRef] [PubMed]
- Borisy, G.; Heald, R.; Howard, J.; Janke, C.; Musacchio, A.; Nogales, E. Microtubules: 50 years on from the discovery of tubulin. Nat. Rev. Mol. Cell Biol. 2016, 17, 322–328. [Google Scholar] [CrossRef]
- Perez, E.A.; Shang, X.; Burlingame, S.M.; Okcu, M.F.; Ge, N.; Russell, H.V.; Egler, R.A.; David, R.D.; Vasudevan, S.A.; Yang, J.; et al. Microtubule inhibitors: Differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity, and resistance. Mol. Cancer Ther. 2009, 8, 2086–2095. [Google Scholar] [CrossRef]
- Kavallaris, M. Microtubules and resistance to tubulin-binding agents. Nat. Rev. Cancer 2010, 10, 194–204. [Google Scholar] [CrossRef]
- Bumbaca, B.; Li, W. Taxane resistance in castration-resistant prostate cancer: Mechanisms and therapeutic strategies. Acta Pharm. Sin. B 2018, 8, 518–529. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J.; Xiao, M.; Li, W.; Miller, D.D. An Overview of Tubulin Inhibitors That Interact with the Colchicine Binding Site. Pharm. Res. 2012, 29, 2943–2971. [Google Scholar] [CrossRef] [PubMed]
- Tangutur, A.D.; Kumar, D.; Krishna, K.V.; Kantevari, S. Microtubule Targeting Agents as Cancer Chemotherapeutics: An Overview of Molecular Hybrids as Stabilizing and Destabilizing Agents. Curr. Top. Med. Chem. 2017, 17, 2523–2537. [Google Scholar] [CrossRef]
- Ramajayam, R. Medicinal chemistry of vicinal diaryl scaffold: A mini review. Eur. J. Med. Chem. 2019, 162, 1–17. [Google Scholar] [CrossRef]
- Karatoprak, G.Ş.; Akkol, E.K.; Genç, Y.; Bardakci, H.; Yücel, Ç.; Sobarzo-Sánchez, E. Combretastatins: An Overview of Structure, Probable Mechanisms of Action and Potential Applications. Molecules 2020, 25, 2560. [Google Scholar] [CrossRef]
- Hamze, A.; Alami, M.; Provot, O. Developments of isoCombretastatin A-4 derivatives as highly cytotoxic agents. Eur. J. Med. Chem. 2020, 190, 112110. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.N.A.; Kumar, G.B.; Revankar, H.M.; Qin, H.-L. Development of combretastatins as potent tubulin polymerization inhibitors. Bioorg. Chem. 2017, 72, 130–147. [Google Scholar] [CrossRef]
- Pettit, G.R.; Singh, S.B.; Hamel, E.; Lin, C.M.; Alberts, D.S.; Garcia-Kendal, D. Isolation and structure of the strong cell growth and tubulin inhibitor combretastatin A-4. Cell. Mol. Life Sci. 1989, 45, 209–211. [Google Scholar] [CrossRef]
- Tron, G.C.; Pirali, T.; Sorba, G.; Pagliai, F.; Busacca, A.S.; Genazzani, A. Medicinal Chemistry of Combretastatin A4: Present and Future Directions. J. Med. Chem. 2006, 49, 3033–3044. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, I.G.; Loadman, P.M.; Swaine, D.J.; Anthoney, D.A.; Pettit, G.R.; Lippert, J.W.; Shnyder, S.; Cooper, P.A.; Bibby, M.C. Comparative Preclinical Pharmacokinetic and Metabolic Studies of the Combretastatin Prodrugs Combretastatin A4 Phosphate and A1 Phosphate. Clin. Cancer Res. 2004, 10, 1446–1453. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, L.; Jiang, S.; Li, X.; Liu, Y.; Su, J.; Chen, J. Recent advances in trimethoxyphenyl (TMP) based tubulin inhibitors targeting the colchicine binding site. Eur. J. Med. Chem. 2018, 151, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jian, X.-E.; Chen, Z.-R.; Chen, L.; Huo, X.-S.; Li, Z.-H.; You, W.-W.; Rao, J.-J.; Zhao, P.-L. Synthesis and biological evaluation of benzofuran-based 3,4,5-trimethoxybenzamide derivatives as novel tubulin polymerization inhibitors. Bioorganic Chem. 2020, 102, 104076. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Pollock, J.K.; Carr, M.; Knox, A.J.S.; Nathwani, S.M.; Wang, S.; Caboni, L.; Zisterer, D.M.; Meegan, M.J. β-Lactam Estrogen Receptor Antagonists and a Dual-Targeting Estrogen Receptor/Tubulin Ligand. J. Med. Chem. 2014, 57, 9370–9382. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.-J.; Li, P.; Wu, B.-W.; Cui, X.-X.; Zhao, C.-B.; Zhang, S.-Y. Molecular diversity of trimethoxyphenyl-1,2,3-triazole hybrids as novel colchicine site tubulin polymerization inhibitors. Eur. J. Med. Chem. 2019, 165, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.-J.; Yang, J.-J.; Li, P.; Hou, Y.-H.; Huang, S.-N.; Tippin, M.A.; Pham, V.; Song, L.; Zi, X.; Xue, W.-L.; et al. Bioactive heterocycles containing a 3,4,5-trimethoxyphenyl fragment exerting potent antiproliferative activity through microtubule destabilization. Eur. J. Med. Chem. 2018, 157, 50–61. [Google Scholar] [CrossRef]
- Song, J.; Gao, Q.-L.; Wu, B.-W.; Zhu, T.; Cui, X.-X.; Jin, C.-J.; Wang, S.-Y.; Wang, S.-H.; Fu, D.-J.; Liu, H.-M.; et al. Discovery of tertiary amide derivatives incorporating benzothiazole moiety as anti-gastric cancer agents in vitro via inhibiting tubulin polymerization and activating the Hippo signaling pathway. Eur. J. Med. Chem. 2020, 203, 112618. [Google Scholar] [CrossRef] [PubMed]
- Cushman, M.; He, H.M.; Lin, C.M.; Hamel, E. Synthesis and evaluation of a series of benzylaniline hydrochlorides as potential cytotoxic and antimitotic agents acting by inhibition of tubulin polymerization. J. Med. Chem. 1993, 36, 2817–2821. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, R.; Baraldi, P.G.; Cruz-Lopez, O.; Cara, C.L.; Carrion, M.D.; Brancale, A.; Hamel, E.; Chen, L.; Bortolozzi, R.; Basso, G.; et al. Synthesis and Antitumor Activity of 1,5-Disubstituted 1,2,4-Triazoles as Cis-Restricted Combretastatin Analogues. J. Med. Chem. 2010, 53, 4248–4258. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Carr, M.; Greene, L.M.; Bergin, O.; Nathwani, S.M.; McCabe, T.; Lloyd, D.G.; Zisterer, D.; Meegan, M.J. Synthesis and Evaluation of Azetidinone Analogues of Combretastatin A-4 as Tubulin Targeting Agents. J. Med. Chem. 2010, 53, 8569–8584. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, J.; Khan, A.A.; Ali, Z.; Haider, R.; Yar, M.S. Structure-activity relationship (SAR) study and design strategies of nitrogen-containing heterocyclic moieties for their anticancer activities. Eur. J. Med. Chem. 2017, 125, 143–189. [Google Scholar] [CrossRef] [PubMed]
- Dhuguru, J.; Skouta, R. Role of Indole Scaffolds as Pharmacophores in the Development of Anti-Lung Cancer Agents. Molecules 2020, 25, 1615. [Google Scholar] [CrossRef] [PubMed]
- Kode, J.; Kovvuri, J.; Nagaraju, B.; Jadhav, S.; Barkume, M.; Sen, S.; Kasinathan, N.K.; Chaudhari, P.; Mohanty, B.S.; Gour, J.; et al. Synthesis, biological evaluation, and molecular docking analysis of phenstatin based indole linked chalcones as anticancer agents and tubulin polymerization inhibitors. Bioorg. Chem. 2020, 105, 104447. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.-J.; Wang, J.; Li, W.; Miller, D.D. Structural Optimization of Indole Derivatives Acting at Colchicine Binding Site as Potential Anticancer Agents. ACS Med. Chem. Lett. 2015, 6, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, C.; He, L.; Lei, K.; Wang, F.; Pu, Y.; Yang, Z.; Cao, D.; Ma, L.; Chen, J.; et al. Design, synthesis and biological evaluation of a series of pyrano chalcone derivatives containing indole moiety as novel anti-tubulin agents. Bioorg. Med. Chem. 2014, 22, 2060–2079. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shuai, W.; Sun, H.; Xu, F.; Bi, Y.; Xu, J.; Ma, C.; Yao, H.; Zhu, Z.; Xu, S. Design, synthesis and biological evaluation of quinoline-indole derivatives as anti-tubulin agents targeting the colchicine binding site. Eur. J. Med. Chem. 2019, 163, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, Y.; Li, G.; Zhang, Z.; Ma, L.; Cheng, B.; Chen, J. Discovery of Novel Benzimidazole and Indazole Analogues as Tubulin Polymerization Inhibitors with Potent Anticancer Activities. J. Med. Chem. 2021, 64, 4498–4515. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.-J.; Ojha, R.; Lin, M.-H.; Liu, Y.-M.; Lee, H.-Y.; Lin, T.E.; Hsu, K.-C.; Chang, C.-Y.; Chen, M.-C.; Nepali, K.; et al. 1-Arylsulfonyl indoline-benzamides as a new antitubulin agents, with inhibition of histone deacetylase. Eur. J. Med. Chem. 2019, 162, 612–630. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, S.; Ji, L.; Zhang, C.; Yu, S.; Li, Z.; Meng, X. Synthesis and structure–activity relationship of 4-azaheterocycle benzenesulfonamide derivatives as new microtubule-targeting agents. Bioorg. Med. Chem. Lett. 2014, 24, 5055–5058. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-Y.; Hsieh, H.-P.; Chang, C.-Y.; Hsu, K.-S.; Chiang, Y.-F.; Chen, C.-M.; Kuo, A.C.-C.; Liou, J.-P. 7-Aroyl-aminoindoline-1-sulfonamides as a Novel Class of Potent Antitubulin Agents. J. Med. Chem. 2006, 49, 6656–6659. [Google Scholar] [CrossRef]
- Wang, X.-F.; Guan, F.; Ohkoshi, E.; Guo, W.; Wang, L.; Zhu, D.-Q.; Wang, S.-B.; Wang, L.-T.; Hamel, E.; Yang, D.; et al. Optimization of 4-(N-Cycloamino)phenylquinazolines as a Novel Class of Tubulin-Polymerization Inhibitors Targeting the Colchicine Site. J. Med. Chem. 2014, 57, 1390–1402. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-F.; Wang, S.-B.; Ohkoshi, E.; Wang, L.-T.; Hamel, E.; Qian, K.; Morris-Natschke, S.L.; Lee, K.-H.; Xie, L. N-Aryl-6-methoxy-1,2,3,4-tetrahydroquinolines: A novel class of antitumor agents targeting the colchicine site on tubulin. Eur. J. Med. Chem. 2013, 67, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Arnst, K.E.; Wang, Y.; Kumar, G.; Deng, S.; Yang, L.; Li, G.-B.; Yang, J.; White, S.W.; Li, W.; et al. Heterocyclic-Fused Pyrimidines as Novel Tubulin Polymerization Inhibitors Targeting the Colchicine Binding Site: Structural Basis and Antitumor Efficacy. J. Med. Chem. 2018, 61, 1704–1718. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, S.-J.; Liu, Y. 1,2,3-Triazole-containing hybrids as potential anticancer agents: Current developments, action mechanisms and structure-activity relationships. Eur. J. Med. Chem. 2019, 183, 111700. [Google Scholar] [CrossRef]
- Song, J.; Gao, Q.-L.; Wu, B.-W.; Li, D.; Shi, L.; Zhu, T.; Lou, J.-F.; Jin, C.-Y.; Zhang, Y.-B.; Zhang, S.-Y.; et al. Novel tertiary sulfonamide derivatives containing benzimidazole moiety as potent anti-gastric cancer agents: Design, synthesis and SAR studies. Eur. J. Med. Chem. 2019, 183, 111731. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Cui, X.-X.; Wu, B.-W.; Li, D.; Wang, S.-H.; Shi, L.; Zhu, T.; Zhang, Y.-B.; Zhang, S.-Y. Discovery of 1,2,4-triazine-based derivatives as novel neddylation inhibitors and anticancer activity studies against gastric cancer MGC-803 cells. Bioorg. Med. Chem. Lett. 2020, 30, 126791. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Wang, S.-H.; Li, D.; Wang, S.-Y.; Liu, X.; Song, J.; Wang, Y.-T.; Zhang, S.-Y. Progress of tubulin polymerization activity detection methods. Bioorg. Med. Chem. Lett. 2021, 37, 127698. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).