Effects of MEH-PPV Molecular Ordering in the Emitting Layer on the Luminescence Efficiency of Organic Light-Emitting Diodes
Abstract
1. Introduction
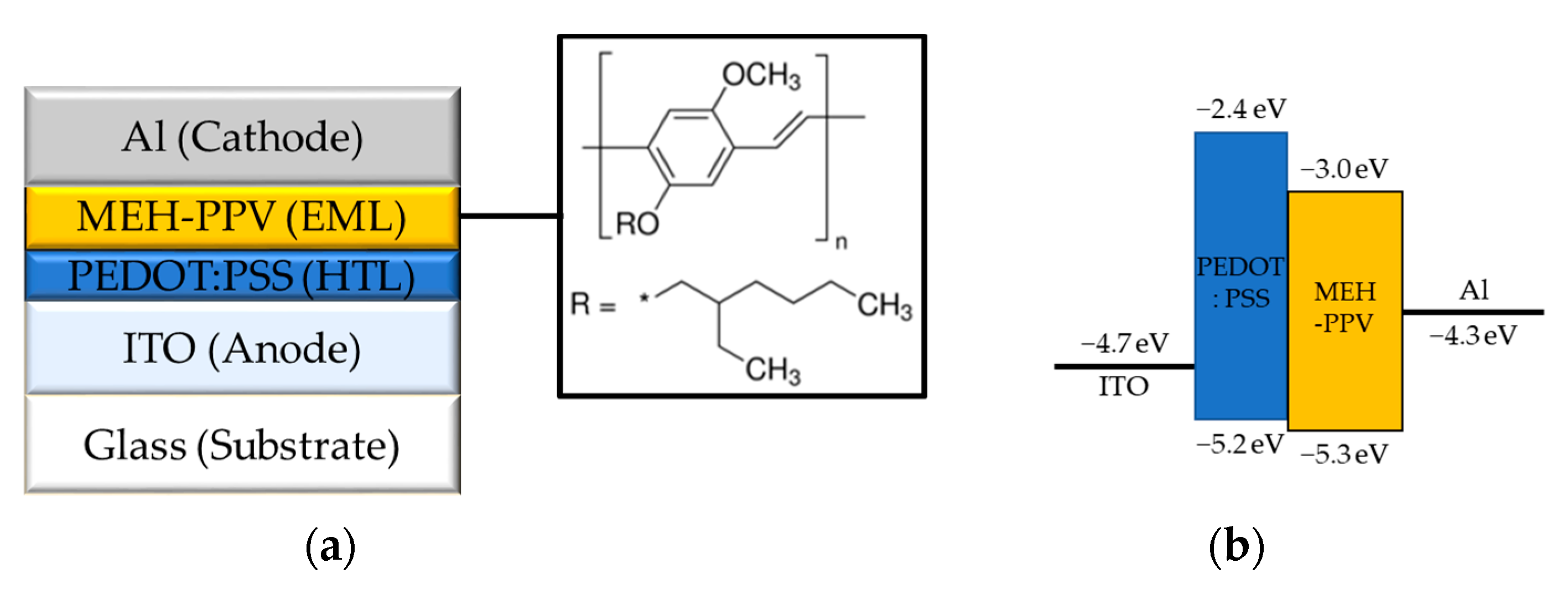
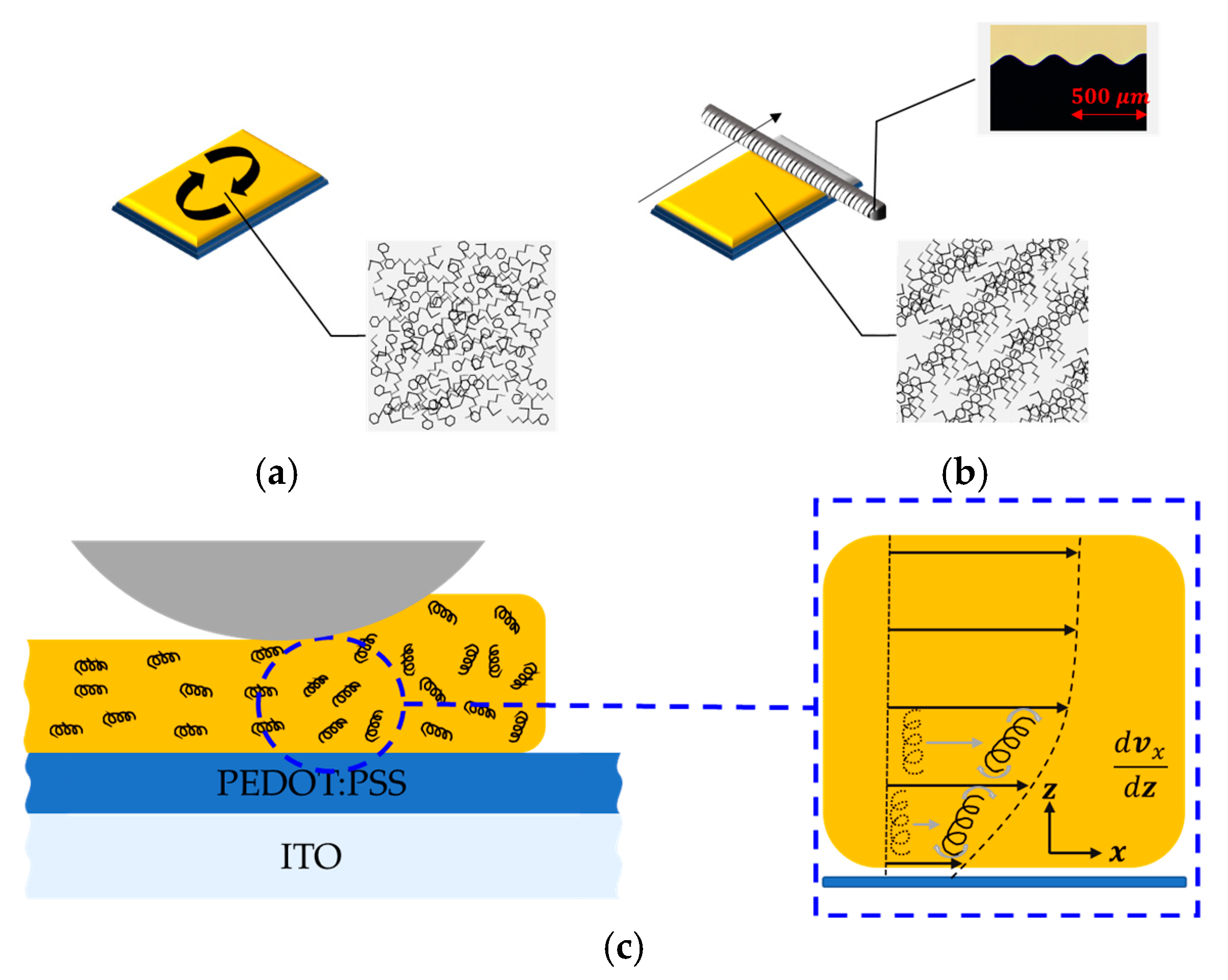
2. Materials and Fabrication Methods
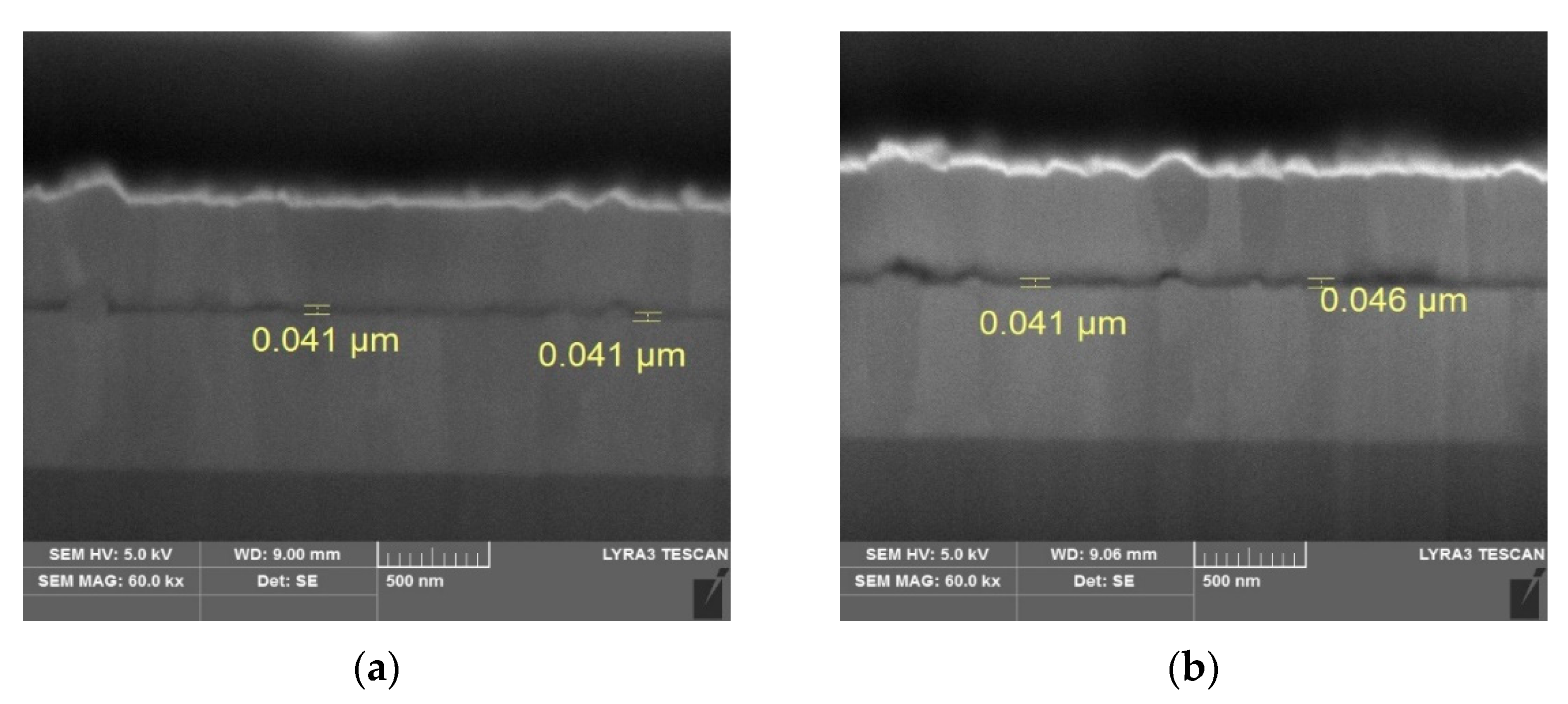
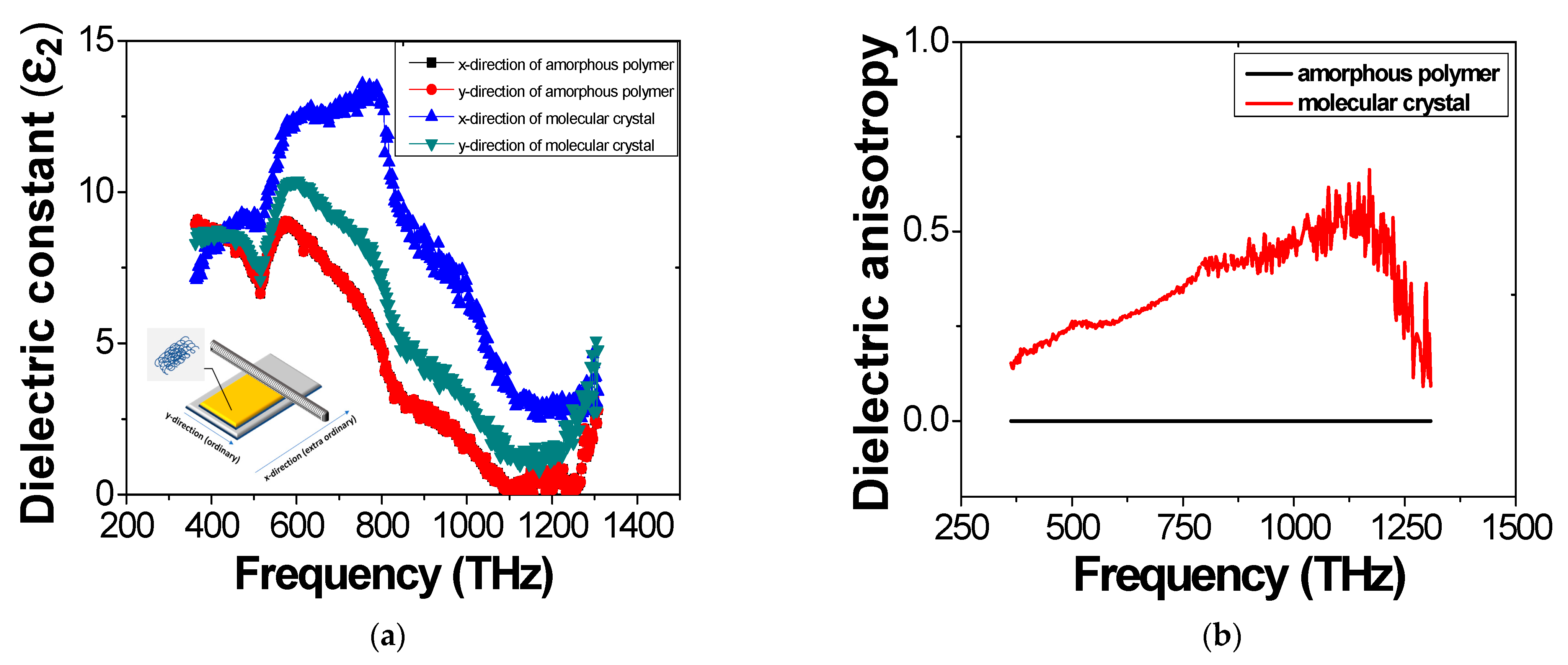
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liu, B.; Nie, H.; Zhou, X.; Hu, S.; Luo, D.; Gao, D.; Zou, J.; Xu, M.; Wang, L.; Zhao, Z.; et al. Manipulation of Charge and Exciton Distribution Based on Blue Aggregation-Induced Emission Fluorophors: A Novel Concept to Achieve High-Performance Hybrid White Organic Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 776–783. [Google Scholar] [CrossRef]
- Luo, D.; Chen, Q.; Gao, Y.; Zhang, M.; Liu, B. Extremely Simplified, High-Performance, and Doping-Free White Organic Light-Emitting Diodes Based on a Single Thermally Activated Delayed Fluorescent Emitter. ACS Energy Lett. 2018, 3, 1531–1538. [Google Scholar] [CrossRef]
- Liu, B.; Hu, S.; Zhang, L.; Xiao, P.; Huang, L.; Liu, C. Blue Molecular Emitter-Free and Doping-Free White Organic Light-Emitting Diodes with High Color Rendering. IEEE Electron. Device Lett. 2021, 42, 387. [Google Scholar] [CrossRef]
- Helfrich, W.; Schneider, W.G. Recombination radiation in anthracene crystals. Phys. Rev. Lett. 1965, 14, 229. [Google Scholar] [CrossRef]
- Tang, C.W.; VanSlyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913. [Google Scholar] [CrossRef]
- Tang, C.W.; VanSlyke, S.A.; Chen, C.H. Electroluminescence of doped organic thin films. J. Appl. Phys. 1989, 65, 3610. [Google Scholar] [CrossRef]
- Adachi, C.; Baldo, M.A.; Thompson, M.E.; Forrest, S.R. Nearly 100% internal phosphorescence efficiency in an organic light-emitting device. J. Appl. Phys. 2001, 90, 5048. [Google Scholar] [CrossRef]
- Baldo, M.A.; Thompson, M.E.; Forrest, S.R. High-efficiency fluorescent organic light-emitting devices using a phosphorescent sensitizer. Nature 2000, 403, 750. [Google Scholar] [CrossRef]
- Baldo, M.A.; Forrest, S.R. Transient analysis of organic electrophosphorescence: I. Transient analysis of triplet energy transfer. Phys. Rev. B 2000, 62, 10958. [Google Scholar] [CrossRef]
- Baldo, M.A.; Adachi, C.; Forrest, S.R. Transient analysis of organic electrophosphorescence. II. Transient analysis of triplet-triplet annihilation. Phys. Rev. B 2000, 62, 10967. [Google Scholar] [CrossRef]
- Hung, L.S.; Tang, C.W.; Mason, M.G. Enhanced electron injection in organic electroluminescence devices using an Al/LiF electrode. Appl. Phys. Lett. 1997, 70, 152. [Google Scholar] [CrossRef]
- Tessler, N.; Medvedev, V.; Kazes, M.; Kan, S.H.; Banin, U. Efficient near-infrared polymer nanocrystal light-emitting diodes. Science 2002, 295, 1506. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.S.; Klubek, K.P.; Tang, C.W. High-efficiency tandem organic light-emitting diodes. Appl. Phys. Lett. 2004, 84, 167. [Google Scholar] [CrossRef]
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nat. Lett. 2012, 492, 234. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.; Shaw, L.; Bao, Z.; Mannsfeld, S.C.B. Morphology Control Strategies for Solution-Processed Organic Semiconductor Thin films. Energy Environ. Sci. 2014, 7, 2145–2159. [Google Scholar] [CrossRef]
- Kang, B.; Lee, W.H.; Cho, K. Recent Advances in Organic Transistor Printing Processes. ACS Appl. Mater. Interfaces 2013, 5, 2302–2315. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Lim, G.T.; Park, B.; Reichmanis, E. Control of Molecular Ordering, Alignment, and Charge Transport in Solution-Processed Conjugated Polymer Thin Films. Polymers 2017, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Burroughes, J.H.; Bradley, D.D.C.; Brown, A.R.; Marks, R.N.; Mackay, K.; Friend, R.H.; Burns, P.L.; Holmes, A.B. Light-emitting diodes based on conjugated polymers. Nat. Lett. 1990, 347, 539. [Google Scholar] [CrossRef]
- Choi, D.; Chang, M.; Reichmanis, E. Controlled Assembly of Poly(3-hexylthiophene): Managing the Disorder to Order Transition on the Nano- through Meso-Scales. Adv. Funct. Mater. 2015, 25, 920–927. [Google Scholar] [CrossRef]
- Chang, M.; Lee, J.; Kleinhenz, N.; Fu, B.; Reichmanis, E. Photoinduced Anisotropic Supramolecular Assembly and Enhanced Charge Transport of Poly(3-hexylthiophene) Thin Films. Adv. Funct. Mater. 2014, 24, 4457–4465. [Google Scholar] [CrossRef]
- Chang, M.; Choi, D.; Wang, G.; Kleinhenz, N.; Persson, N.; Park, B.; Reichmanis, E. Photoinduced Anisotropic Assembly of Conjugated Polymers in Insulating Polymer Blends. ACS Appl. Mater. Interfaces 2015, 7, 14095–14103. [Google Scholar] [CrossRef]
- Chang, M.; Lee, J.; Chu, P.-H.; Choi, D.; Park, B.; Reichmanis, E. Anisotropic Assembly of Conjugated Polymer Nanocrystallites for Enhanced Charge Transport. ACS Appl. Mater. Interfaces 2014, 6, 21541–21549. [Google Scholar] [CrossRef]
- Diao, Y.; Tee, B.C.-K.; Giri, G.; Xu, J.; Kim, D.H.; Becerril, H.A.; Stoltenberg, R.M.; Lee, T.H.; Xue, G.; Mannsfeld, S.C.B.; et al. Solution Coating of Large-Area Organic Semiconductor Thin Films with Aligned Single-Crystalline Domains. Nat. Mater. 2013, 12, 665–671. [Google Scholar] [CrossRef]
- Khim, D.; Han, H.; Baeg, K.-J.; Kim, J.; Kwak, S.-W.; Kim, D.-Y.; Noh, Y.-Y. Simple Bar-Coating Process for Large-Area, High-Performance Organic Field-Effect Transistors and Ambipolar Complementary Integrated Circuits. Adv. Mater. 2013, 25, 4302–4308. [Google Scholar] [CrossRef] [PubMed]
- Giri, G.; DeLongchamp, D.M.; Reinspach, J.; Fischer, D.A.; Richter, L.J.; Xu, J.; Benight, S.; Ayzner, A.; He, M.; Fang, L.; et al. Effect of Solution Shearing Method on Packing and Disorder of Organic Semiconductor Polymers. Chem. Mater. 2015, 27, 2350–2359. [Google Scholar] [CrossRef]
- Shin, J.; Hong, T.R.; Lee, T.W.; Kim, A.; Kim, Y.H.; Cho, M.J.; Choi, D.H. Template-Guided Solution-Shearing Method for Enhanced Charge Carrier Mobility in Diketopyrrolpyrrole-Based Polymer Field-Effect Transistors. Adv. Mater. 2014, 26, 6031–6035. [Google Scholar] [CrossRef]
- Chang, M.; Choi, D.; Egap, E. Macroscopic Alignment of One-Dimensional Conjugated Polymer Nanocrystallites for High-Mobility Organic Field-Effect Transistors. ACS Appl. Mater. Interfaces 2016, 8, 13484–13491. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Su, Z.; Egap, E. Alignment and Charge Transport of One-Dimensional Conjugated Polymer Nanowires in Insulating Polymer Blends. Macromolecules 2016, 49, 9449–9456. [Google Scholar] [CrossRef]
- Heil, H.; Finnberg, T.; von Malm, N.; Schmechel, R.; von Seggern, H. The Influence of Mechanical Rubbing on the Field-Effect Mobility in Polyhexylthiophene. J. Appl. Phys. 2003, 93, 1636. [Google Scholar] [CrossRef]
- Choi, G.J.; Le, Q.V.; Choi, K.S.; Kwon, K.C.; Jang, H.W.; Gwag, J.S.; Kim, S.Y. Polarized Light-Emitting Diodes Based on Patterned MoS2 Nanosheet Hole Transport Layer. Adv. Mater. 2017, 29, 1700907. [Google Scholar] [CrossRef]
- Geffroy, B.; Roy, P.L.; Prat, C. Organic light-emitting diode (OLED) technology: Materials, devices and display technologies. Polym. Int. 2006, 55, 572. [Google Scholar] [CrossRef]
- Ho, S.; Liu, S.; Chen, Y.; So, F. Review of recent progress in multilayer solution-processed organic light-emitting diodes. J. Photonic Energy 2015, 5, 057611. [Google Scholar] [CrossRef]
- Zheng, H.; Zheng, Y.; Liu, N.; Ai, N.; Wang, Q.; Wu, S.; Zhou, J.; Hu, D.; Yu, S.; Han, S.; et al. All-solution processed polymer light-emitting diode displays. Nat. Commun. 2013, 4, 1971. [Google Scholar] [CrossRef]
- You, J.D.; Tseng, S.R.; Meng, H.F.; Yen, F.W.; Lin, I.F.; Horng, S.F. All-solution-processed blue small molecular organic light-emitting diodes with multilayer device structure. Org. Electron. 2009, 10, 1610–1614. [Google Scholar] [CrossRef]
- Zhang, M.; Höfle, S.; Czolk, J.; Mertens, A.; Colsmann, A. All-solution processed transparent organic light emitting diodes. Nanoscale 2015, 7, 20009–20014. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Xiao, T.; Hellerich, E.; Chen, Y.; Shinar, R.; Shinar, J. High-efficiency solution-processed small molecule electrophosphorescent organic light-emitting diodes. Adv. Mater. 2011, 23, 3590. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Hou, L.; Lee, T.W.; Qiao, J.; Zhang, D.; Dong, G.; Wang, L.; Qiu, Y. Solution processable small molecules for organic light-emitting diodes. J. Mater. Chem. 2010, 20, 6392–6407. [Google Scholar] [CrossRef]
- Hebner, T.R.; Wu, C.C.; Marcy, D.; Lu, M.H.; Sturm, J.C. Ink-jet printing of doped polymers for organic light emitting devices. Appl. Phys. Lett. 1998, 72, 519. [Google Scholar] [CrossRef]
- Tseng, S.R.; Meng, H.F.; Lee, K.C.; Horng, S.F. Multilayer polymer light-emitting diodes by blade coating method. Appl. Phys. Lett. 2008, 93, 153308. [Google Scholar] [CrossRef]
- Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/catalog/product/aldrich/541435 (accessed on 5 March 2021).
- Zhou, Y.; Mei, S.; Sun, D.; Liu, N.; Shi, W.; Feng, J.; Mei, F.; Xu, J.; Jiang, Y.; Cao, X. Improved Efficiency of Perovskite Light-Emitting Diodes Using a Three-Step Spin-Coated CH3NH3PbBr3 Emitter and a PEDOT:PSS/MoO3-Ammonia Composite Hole Transport Layer. Micromachines 2019, 10, 459. [Google Scholar] [CrossRef]
- Alam, M.M.; Jenekhe, S.A. Polybenzobisazoles are efficient electron transport materials for improving the performance and stability of polymer light-emitting diodes. Chem. Mater. 2002, 14, 4775. [Google Scholar] [CrossRef]
- Tonzola, C.J.; Alam, M.M.; Kaminsky, W.; Jenekhe, S.A. New n-Type Organic Semiconductors: Synthesis, Single Crystal Structures, Cyclic Voltammetry, Photophysics, Electron Transport, and Electroluminescence of a Series of Diphenylanthrazolines. J. Am. Chem. Soc. 2003, 125, 13548. [Google Scholar] [CrossRef]
- Hoven, C.V.; Garcia, A.; Bazan, G.C.; Nguyen, T.Q. Recent applications of conjugated polyelectrolytes in optoelectronic devices. Adv. Mater. 2008, 20, 3793. [Google Scholar] [CrossRef]
- Garcia, A.; Yang, R.; Jin, Y.; Walker, B.; Nguyen, T.Q. Structure-function relationships of conjugated polyelectrolyte electron injection layers in polymer light emitting diodes. Appl. Phys. Lett. 2007, 91, 153502. [Google Scholar] [CrossRef]
- Kwon, T.W.; Alam, M.M.; Jenekhe, S.A. n-type conjugated dendrimers: Convergent synthesis, photophysics, electroluminescence, and use as electron-transport materials for light-emitting diodes. Chem. Mater. 2004, 16, 4657. [Google Scholar] [CrossRef]
- Volkov, V.S.; Kulichikhin, V.G. Rheology of Complex Anisotropic Fluids. Colloid J. 2011, 73, 614–620. [Google Scholar] [CrossRef]
- Jhun, C.G.; Choi, J.H.; Yoon, T.H.; Kim, J.C. Optical Bouncing in Bistable Chiral Splay Nematic Liquid Crystal Device. Jpn. J. Appl. Phys. 2006, 45, 128–132. [Google Scholar] [CrossRef]
- van Doorn, C.Z. Dynamic Behavior of Twisted Nematic Liquid–Crystal Layers in Switched Fields. J. Appl. Phys. 1975, 46, 3738–3745. [Google Scholar] [CrossRef]
- Berreman, D.W. Liquid-crystal Twist Cell Dynamics with Backflow. J. Appl. Phys. 1975, 46, 3746–3751. [Google Scholar] [CrossRef]
- Kenneth, K.L. Electrostatic Discharge; CRC Press: Boca Raton, FL, USA, 2005; p. 128. [Google Scholar]
- Seybold, J.S. Introduction to RF Propagation; John Wiley & Sons: Hoboken, NJ, USA, 2005; p. 22. [Google Scholar]
- Yokoyama, D. Molecular orientation in small-molecule organic light-emitting diodes. J. Mater. Chem. 2011, 21, 19187. [Google Scholar] [CrossRef]
- Ou-Yang, W.C.; Wu, T.Y.; Lin, Y.C. Supramolecular Structure of Poly[2-methoxy-5-(2′-ethylhexyloxy) -1,4-phenylenevinylene] (MEH-PPV) Probed Using Wide-angle X-ray Diffraction and Photoluminescence. Iran. Polym. J. 2009, 18, 453. [Google Scholar]
- Kohler, A.; Bassler, H. Electronic Processes in Organic Semiconductors; VCH: Weinheim, Germany, 2015. [Google Scholar]
- May, F.; Baumeier, B.; Lennartz, C.; Andrienko, D. Can lattice models predict the density of states of amorphous organic semiconductors? Phys. Rev. Lett. 2012, 109, 136401. [Google Scholar] [CrossRef]
- Baranovskii, S.D. Theoretical description of charge transport in disordered organic semiconductors. Phys. Status Solidi B Basic Solid State Phys. 2014, 251, 487. [Google Scholar] [CrossRef]
- Novikov, S.V.; Dunlap, D.H.; Kenkre, V.M.; Parris, P.E.; Vannikov, A.V. Essential role of correlations in governing charge transport in disordered organic materials. Phys. Rev. Lett. 1998, 81, 4472. [Google Scholar] [CrossRef]
- Yokoyama, D.; Sakaguchi, A.; Suzuki, M.; Adachi, C. Enhancement of electron transport by horizontal molecular orientation of oxadiazole planar molecules in organic amorphous films. Appl. Phys. Lett. 2009, 95, 243303. [Google Scholar] [CrossRef]
- Yokoyama, D.; Setoguchi, Y.; Sakaguchi, A.; Suzuki, M.; Adachi, C. Orientation control of linear-shaped molecules in vacuum-deposited organic amorphous films and its effect on carrier mobilities. Adv. Funct. Mater. 2010, 20, 386. [Google Scholar] [CrossRef]
- Quan, S.; Teng, F.; Xu, Z.; Qian, L.; Hou, Y.; Wang, Y.; Xu, X. Solvent and concentration effects on fluorescence emission in MEH-PPV solution. Eur. Polym. J. 2006, 42, 228. [Google Scholar] [CrossRef]
- Kim, W.Y.; Kim, Y.H.; Jhun, C.G.; Wood, R.; Mascher, P.; Moon, C.B. Spectroscopic study of white organic light-emitting devices with various thicknesses of emissive layer. J. Appl. Phys. 2012, 111, 014507. [Google Scholar]

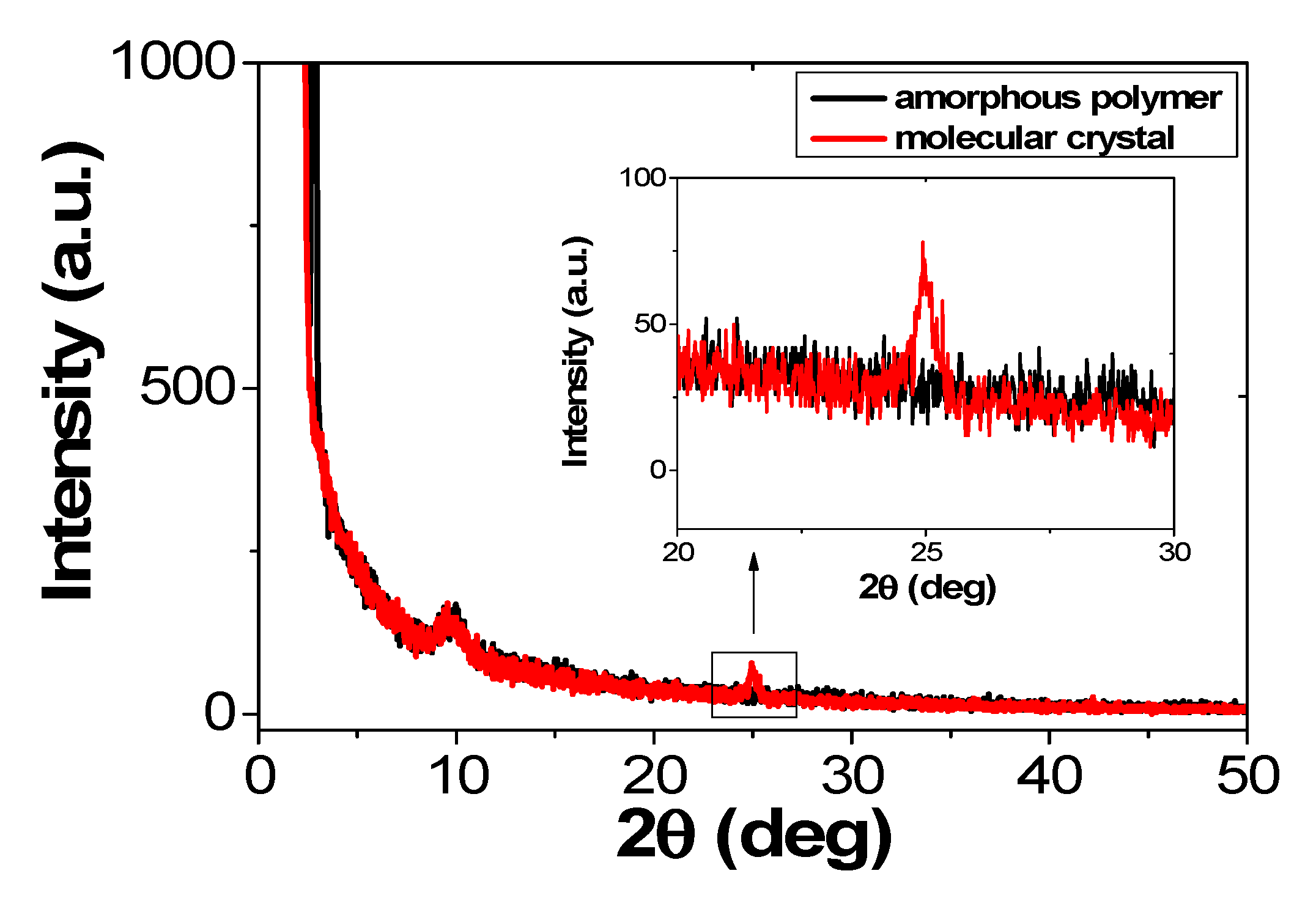
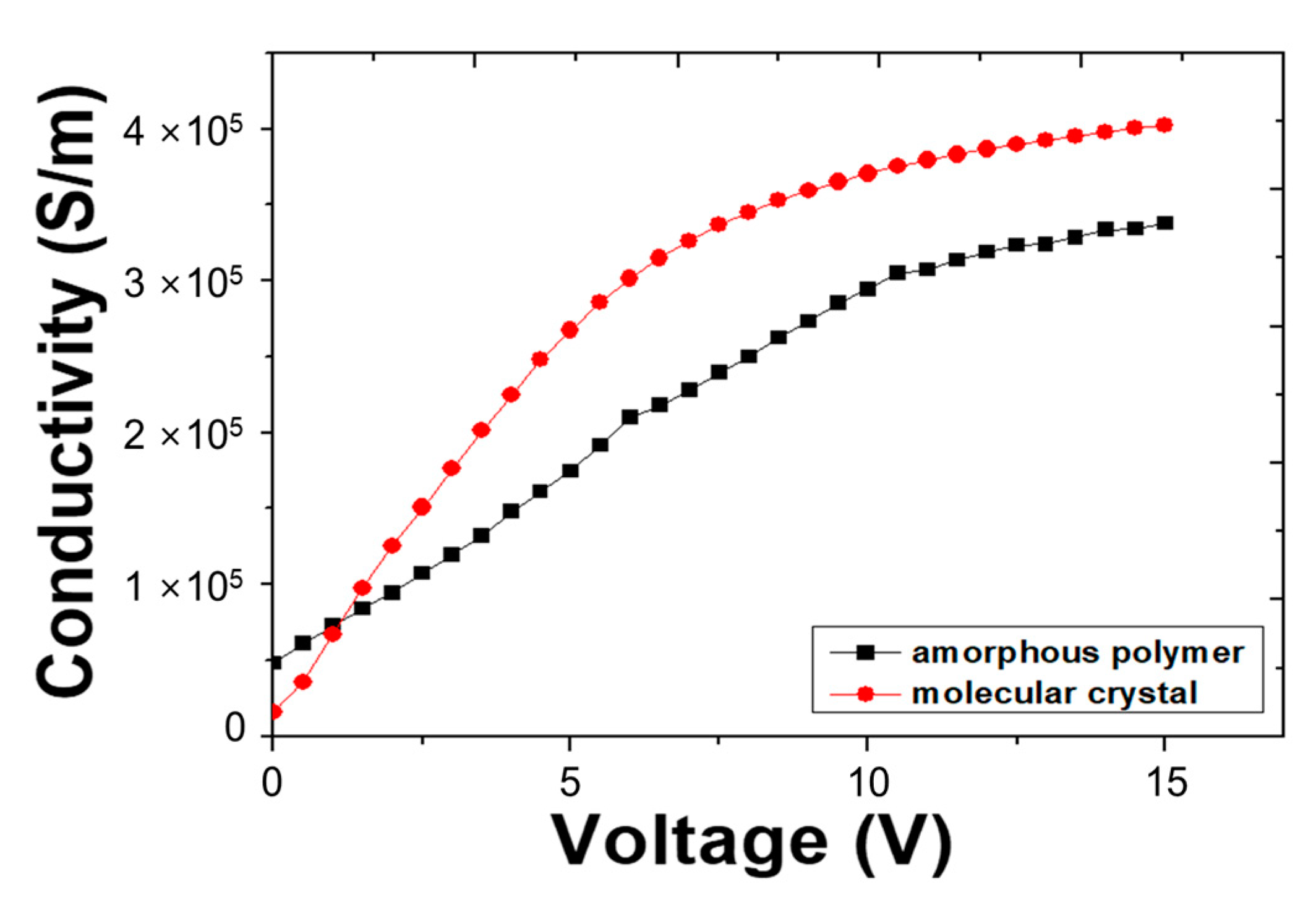

| Molecular State | Von | Lmax | LE (Max) | PE (Max) | EQE (Max) |
|---|---|---|---|---|---|
| amorphous polymer | 5.0 V | 35.75 cd/m2 | 0.024 cd/A | 0.013 lm/W | 0.012% |
| molecular crystal | 4.5 V | 120.3 cd/m2 | 0.123 cd/A | 0.068 lm/W | 0.066% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.J.; Li, J.; Lee, S.I.; Moon, C.-B.; Kim, W.Y.; Cao, J.; Jhun, C.G. Effects of MEH-PPV Molecular Ordering in the Emitting Layer on the Luminescence Efficiency of Organic Light-Emitting Diodes. Molecules 2021, 26, 2512. https://doi.org/10.3390/molecules26092512
Lee SJ, Li J, Lee SI, Moon C-B, Kim WY, Cao J, Jhun CG. Effects of MEH-PPV Molecular Ordering in the Emitting Layer on the Luminescence Efficiency of Organic Light-Emitting Diodes. Molecules. 2021; 26(9):2512. https://doi.org/10.3390/molecules26092512
Chicago/Turabian StyleLee, Seok Je, Jun Li, Seung Il Lee, Chang-Bum Moon, Woo Young Kim, Jin Cao, and Chul Gyu Jhun. 2021. "Effects of MEH-PPV Molecular Ordering in the Emitting Layer on the Luminescence Efficiency of Organic Light-Emitting Diodes" Molecules 26, no. 9: 2512. https://doi.org/10.3390/molecules26092512
APA StyleLee, S. J., Li, J., Lee, S. I., Moon, C.-B., Kim, W. Y., Cao, J., & Jhun, C. G. (2021). Effects of MEH-PPV Molecular Ordering in the Emitting Layer on the Luminescence Efficiency of Organic Light-Emitting Diodes. Molecules, 26(9), 2512. https://doi.org/10.3390/molecules26092512





