Self-Crosslinked Ellipsoidal Poly(Tannic Acid) Particles for Bio-Medical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
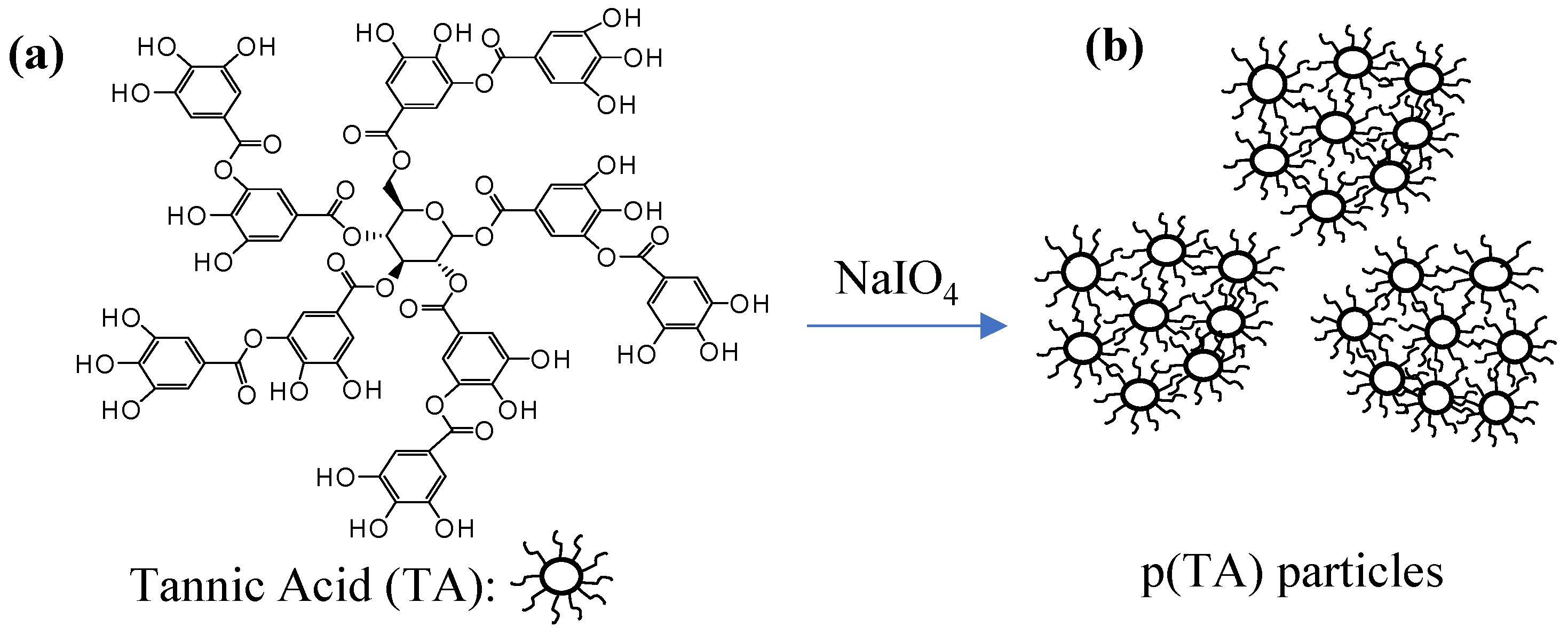
2.2. Preparation of p(TA) Particles
2.3. Characterization of p(TA) Particles
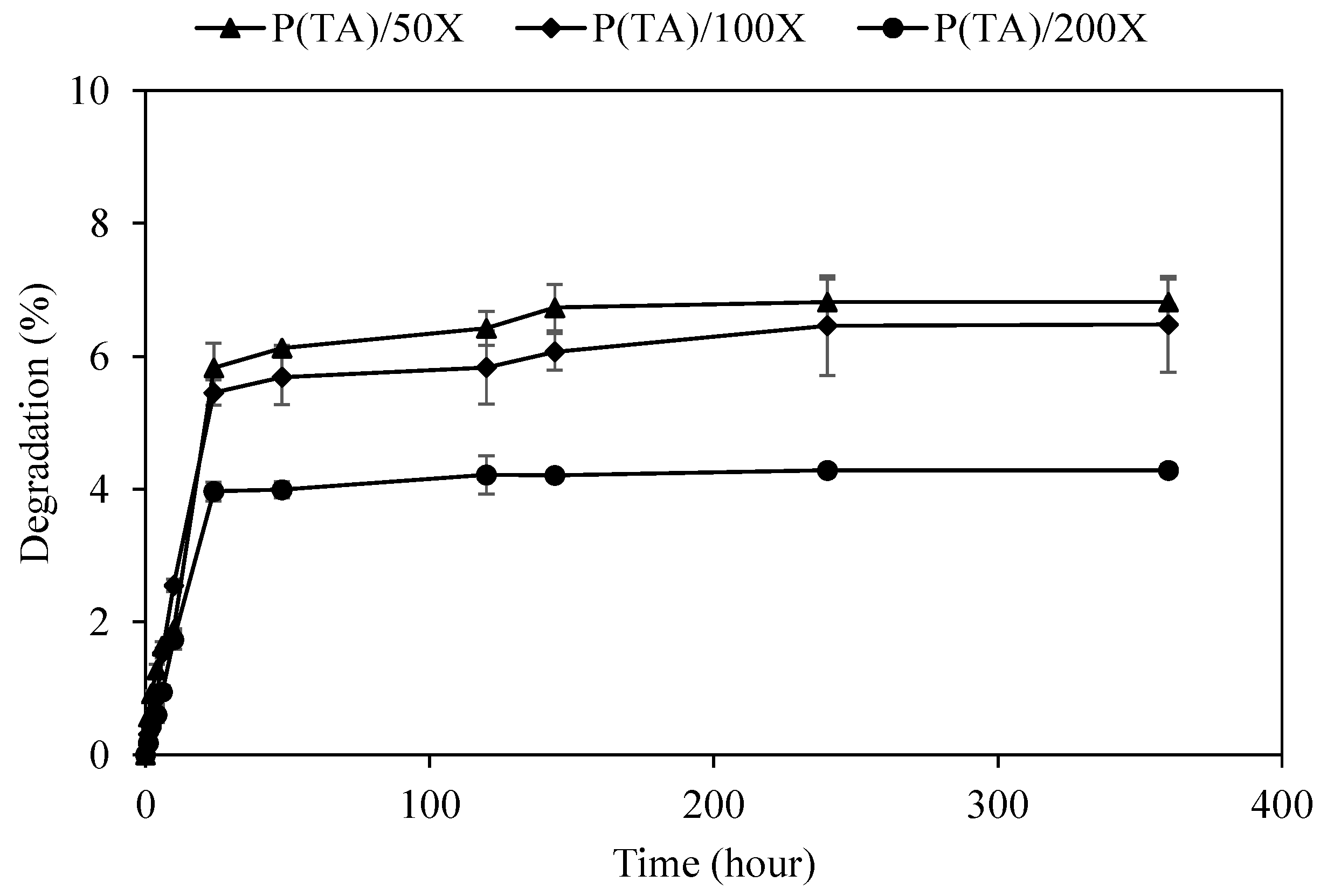
2.4. Hydrolytic Degradation Studies of p(TA) Particles
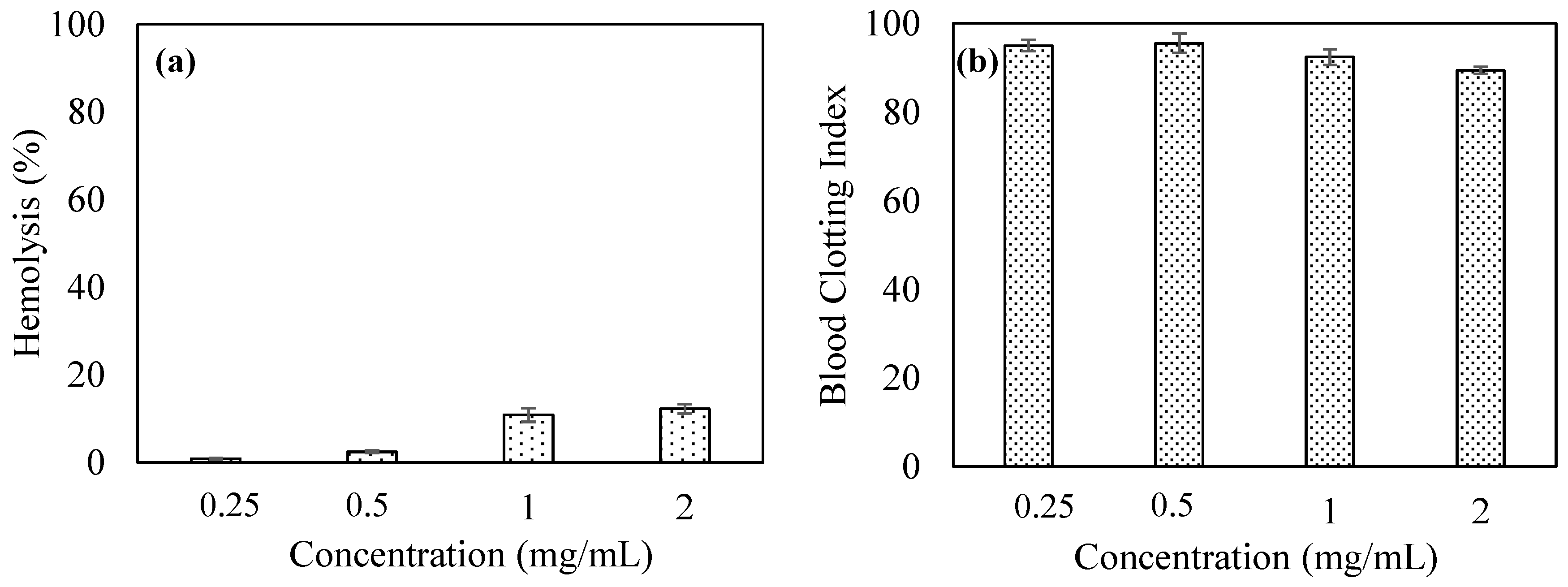
2.5. Blood Compatibility Studies of p(TA) Particles
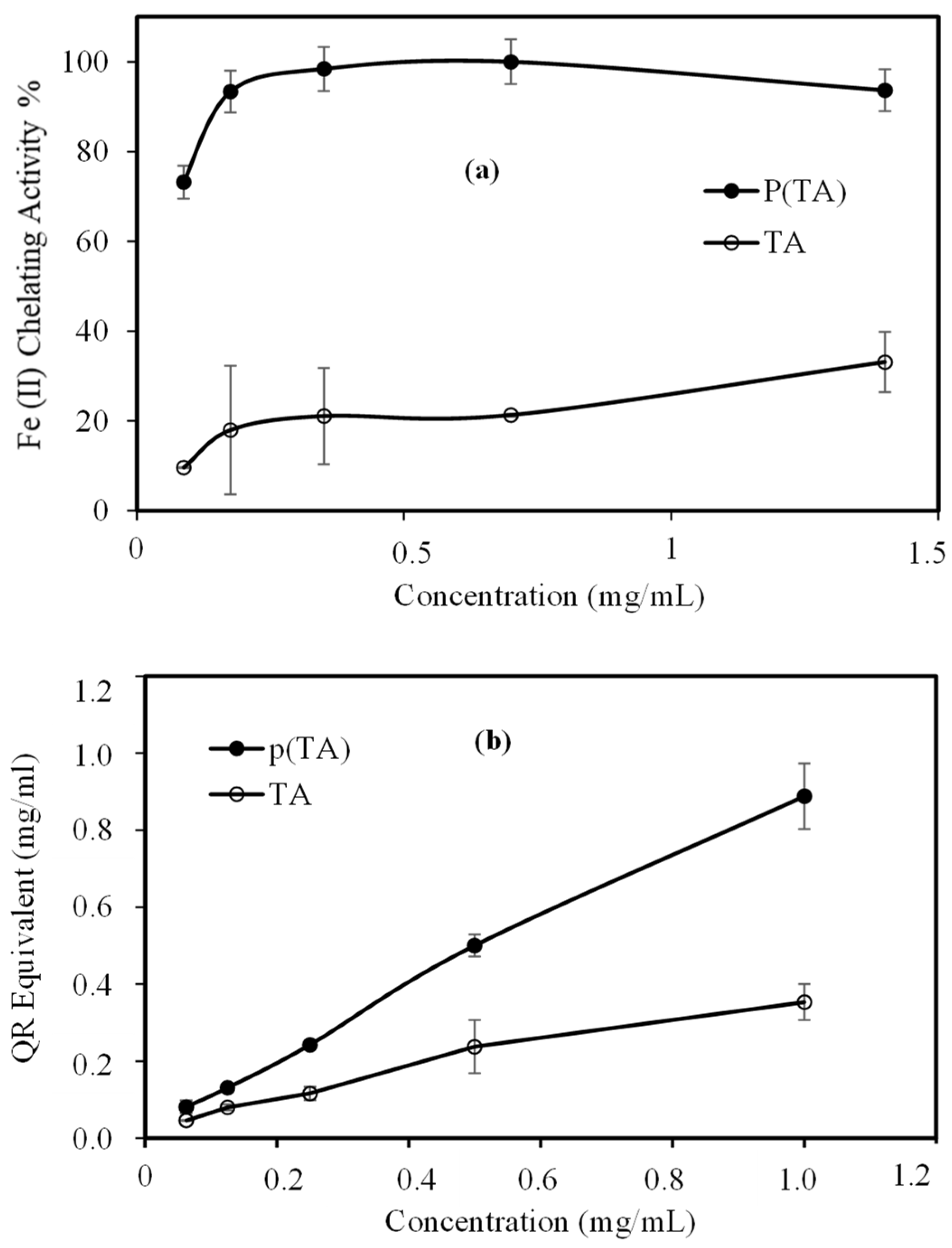
2.6. Antioxidant Capabilities of p(TA) Particles
2.7. Antibacterial Study of p(TA) Particles
3. Results and Discussions
3.1. Characterization of p(TA) Particles
3.2. Degradation of p(TA) Particles
3.3. Blood Compatibility Study of p(TA) Particles
3.4. Antioxidant Capabilities of p(TA) Particles
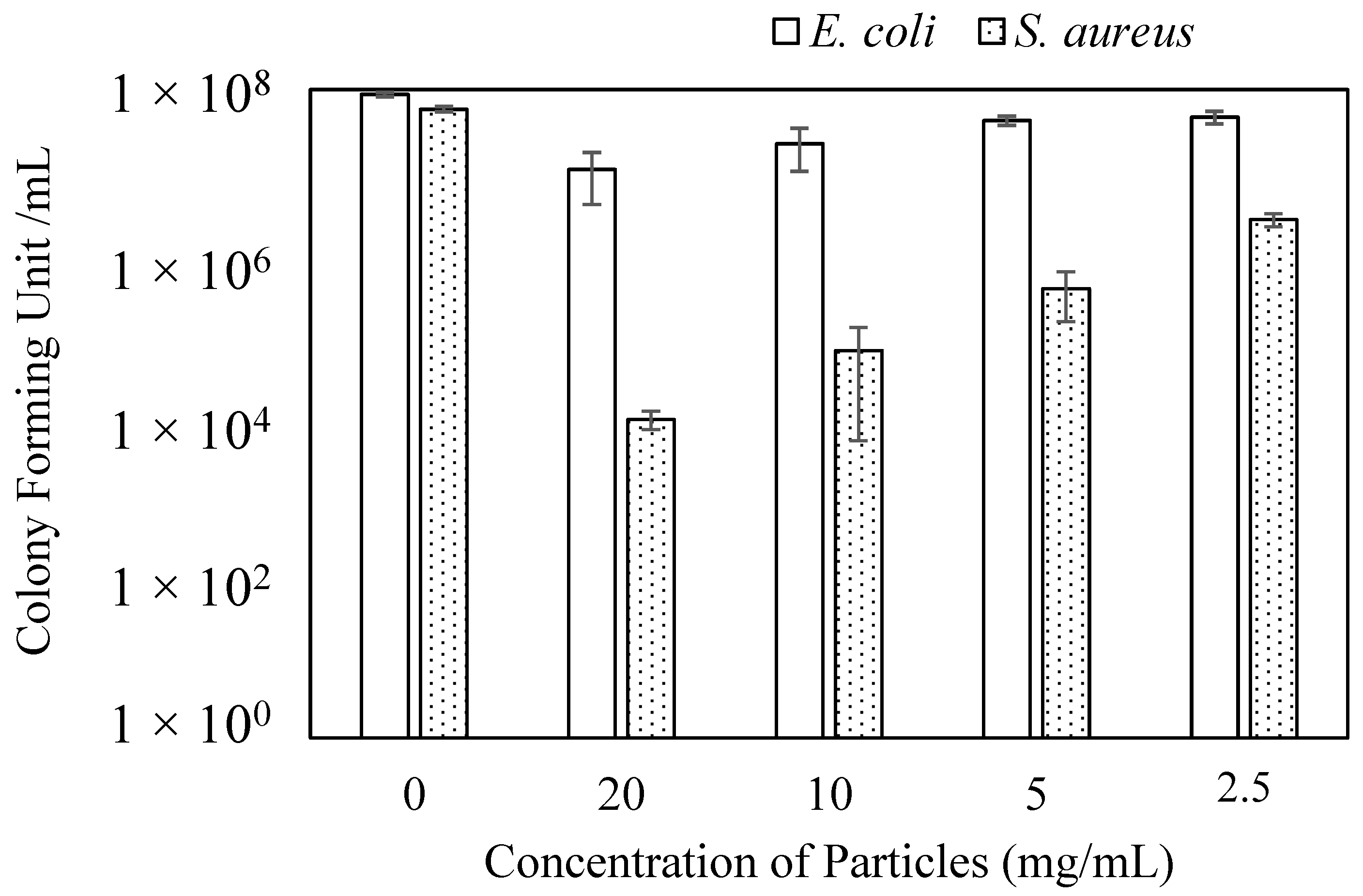
3.5. Antibacterial Study of p(TA) Particles
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Li, R.; Dai, T.; Zhou, W.; Fu, G.; Wan, Y.; McClements, D.J.; Li, J. Impact of pH, ferrous ions, and tannic acid on lipid oxidation in plant-based emulsions containing saponin-coated flaxseed oil droplets. Food Res. Int. 2020, 136, 109618. [Google Scholar] [CrossRef]
- Ahmed, G.H.G.; Laíño, R.B.; Calzón, J.A.G.; García, M.E.D. Fluorescent carbon nanodots for sensitive and selective detection of tannic acid in wines. Talanta 2015, 132, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, H.; Lv, X.; Chu, L.; Liu, Z.; Wei, X.; Chen, Q.; Zhu, L.; Cui, W. Cardioprotective Effects of Tannic Acid on Isoproterenol-Induced Myocardial Injury in Rats: Further Insight into ‘French Paradox’. Phytother. Res. 2015, 29, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Chu, X.; Wang, H.; Zhang, X.; Zhang, Y.; Liu, Z.; Guo, H.; Liu, H.; Liu, Y.; Chu, L.; et al. New Findings on the Effects of Tannic Acid: Inhibition of L-Type Calcium Channels, Calcium Transient and Contractility in Rat Ventricular Myocytes. Phytother. Res. 2016, 30, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, S.P.; Perumal, S.S.; Balakrishnan, A. Scope of Hydrolysable Tannins as Possible Antimicrobial Agent. Phytother. Res. 2016, 30, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Hazer, B.; Ashby, R.D. Synthesis of a novel tannic acid-functionalized polypropylene as antioxidant active-packaging materials. Food Chem. 2021, 344, 128644. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, P.; Nagesh, P.K.; Hatami, E.; Wagh, S.; Dan, N.; Tripathi, M.K.; Khan, S.; Hafeez, B.B.; Meibohm, B.; Chauhan, S.C.; et al. Tannic acid-inspired paclitaxel nanoparticles for enhanced anticancer effects in breast cancer cells. J. Colloid Interface Sci. 2019, 535, 133–148. [Google Scholar] [CrossRef]
- Rahimi, J.; Bahrami, N.; Niksefat, M.; Kamalzare, M.; Maleki, A. A novel biodegradable magnetic bionanocomposite based on tannic acid as a biological molecule for selective oxidation of alcohols. Solid State Sci. 2020, 105, 106284. [Google Scholar] [CrossRef]
- Serrano, J.; Puupponen-Pimiä, R.; Dauer, A.; Aura, A.-M.; Calixto, F.D.S. Tannins: Current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 2009, 53 (Suppl. 2), S310–S329. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.F.; Nascimento, J.M.D.; Sobral, R.V.D.S.; De Amorim, E.L.C.; Silva, R.O.; Leite, A.C.L. Tannic acid as a precipitating agent of human plasma proteins. Eur. J. Pharm. Sci. 2019, 138, 105018. [Google Scholar] [CrossRef]
- Adamczyk, B.; Simon, J.; Kitunen, V.; Adamczyk, S.; Smolander, A. Tannins and Their Complex Interaction with Different Organic Nitrogen Compounds and Enzymes: Old Paradigms versus Recent Advances. ChemistryOpen 2017, 6, 610–614. [Google Scholar] [CrossRef]
- Xu, F.; Weng, B.; Gilkerson, R.; Materon, L.A.; Lozano, K. Development of tannic acid/chitosan/pullulan composite nanofibers from aqueous solution for potential applications as wound dressing. Carbohydr. Polym. 2015, 115, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Taheri, P.; Jahanmardi, R.; Koosha, M.; Abdi, S. Physical, mechanical and wound healing properties of chitosan/gelatin blend films containing tannic acid and/or bacterial nanocellulose. Int. J. Biol. Macromol. 2020, 154, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Wang, J.; Zhang, Q.; Jin, Z. Tannic Acid-Based Multifunctional Hydrogels with Facile Adjustable Adhesion and Cohesion Contributed by Polyphenol Supramolecular Chemistry. ACS Omega 2017, 2, 6668–6676. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Wang, L.; Feng, X.; Bu, Y.; Wu, D.; Jin, Z. Supramolecular Hydrogel Formation Based on Tannic Acid. Macromolecules 2017, 50, 666–676. [Google Scholar] [CrossRef]
- Farha, A.K.; Yang, Q.-Q.; Kim, G.; Li, H.-B.; Zhu, F.; Liu, H.-Y.; Gan, R.-Y.; Corke, H. Tannins as an alternative to antibiotics. Food Biosci. 2020, 38, 100751. [Google Scholar] [CrossRef]
- Antonia, D.S.L.; Laynne, H.D.C.L.; Davi, D.S.; Livio, C.C.N.; Jose, A.D.L. Incorporation of tannic acid in formulations for topical use in wound healing: A technological prospecting. Afr. J. Pharm. Pharmacol. 2015, 9, 662–674. [Google Scholar] [CrossRef]
- Baer-Dubowska, W.; Szaefer, H.; Majchrzak-Celińska, A.; Krajka-Kuźniak, V. Tannic Acid: Specific Form of Tannins in Cancer Chemoprevention and Therapy-Old and New Applications. Curr. Pharmacol. Rep. 2020, 6, 28–37. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Demirci, S. Degradable tannic acid/polyethyleneimine polyplex particles with highly antioxidant and antimicrobial effects. Polym. Degrad. Stab. 2016, 133, 152–161. [Google Scholar] [CrossRef]
- Allais, M.; Mailley, D.; Hébraud, P.; Ihiawakrim, D.; Ball, V.; Meyer, F.; Hébraud, A.; Schlatter, G. Polymer-free electrospinning of tannic acid and cross-linking in water for hybrid supramolecular nanofibres. Nanoscale 2018, 10, 9164–9173. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xiao, Y.; Guo, D.; Shen, L.; Li, R.; Jiao, Y.; Xu, Y.; Lin, H. In-situ coating TiO2 surface by plant-inspired tannic acid for fabrication of thin film nanocomposite nanofiltration membranes toward enhanced separation and antibacterial performance. J. Colloid Interface Sci. 2020, 572, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-Y.; Yu, P.; Tang, L.-S.; Wang, Q.-Y.; Bao, R.-Y.; Liu, Z.-Y.; Yang, M.-B.; Yang, W. Tannic acid functionalized graphene hydrogel for organic dye adsorption. Ecotoxicol. Environ. Saf. 2018, 165, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Koopmann, A.-K.; Schuster, C.; Torres-Rodríguez, J.; Kain, S.; Pertl-Obermeyer, H.; Petutschnigg, A.; Hüsing, N. Tannin-Based Hybrid Materials and Their Applications: A Review. Molecules 2020, 25, 4910. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, L.; Dong, S.; Cui, J.; Hao, J. Microgels in biomaterials and nanomedicines. Adv. Colloid Interface Sci. 2019, 266, 1–20. [Google Scholar] [CrossRef]
- Agili, F.A.; Aly, S.F. Physicochemical characterization and release properties of oral drug delivery: A pH-sensitive nanocomposite based on sodium alginate–pectin–tannic acid–silver. Polym. Polym. Compos. 2020, 28, 598–608. [Google Scholar] [CrossRef]
- Abouelmagd, S.A.; Ellah, N.H.A.; Amen, O.; Abdelmoez, A.; Mohamed, N.G. Self-assembled tannic acid complexes for pH-responsive delivery of antibiotics: Role of drug-carrier interactions. Int. J. Pharm. 2019, 562, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Javanbakht, S.; Shadi, M.; Mohammadian, R.; Shaabani, A.; Ghorbani, M.; Rabiee, G.; Amini, M.M. Preparation of Fe3O4@SiO2@Tannic acid double core-shell magnetic nanoparticles via the Ugi multicomponent reaction strategy as a pH-responsive co-delivery of doxorubicin and methotrexate. Mater. Chem. Phys. 2020, 247, 122857. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Wekwejt, M.; Nadolna, K.; Owczarek, A.; Mazur, O.; Pałubicka, A. The mechanical properties and bactericidal degradation effectiveness of tannic acid-based thin films for wound care. J. Mech. Behav. Biomed. Mater. 2020, 110, 103916. [Google Scholar] [CrossRef] [PubMed]
- Picchio, M.L.; Linck, Y.G.; Monti, G.A.; Gugliotta, L.M.; Minari, R.J.; Igarzabal, C.I.A. Casein films crosslinked by tannic acid for food packaging applications. Food Hydrocoll. 2018, 84, 424–434. [Google Scholar] [CrossRef]
- Halim, A.L.A.; Kamari, A.; Phillip, E. Chitosan, gelatin and methylcellulose films incorporated with tannic acid for food packaging. Int. J. Biol. Macromol. 2018, 120, 1119–1126. [Google Scholar] [CrossRef]
- Dai, Z.; Ngai, T. Microgel particles: The structure-property relationships and their biomedical applications. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 2995–3003. [Google Scholar] [CrossRef]
- Fernandez-Rodriguez, M.A.; Martín-Molina, A.; Maldonado-Valderrama, J. Microgels at interfaces, from mickering emulsions to flat interfaces and back. Adv. Colloid Interface Sci. 2021, 288, 102350. [Google Scholar] [CrossRef]
- Taokaew, S.; Ofuchi, M.; Kobayashi, T. Size Distribution and Characteristics of Chitin Microgels Prepared via Emulsified Reverse-Micelles. Materials 2019, 12, 1160. [Google Scholar] [CrossRef]
- Bonham, J.A.; Faers, M.A.; Van Duijneveldt, J.S. Non-aqueous microgel particles: Synthesis, properties and applications. Soft Matter 2014, 10, 9384–9398. [Google Scholar] [CrossRef]
- Bin Hamzah, Y.; Hashim, S.; Rahman, W.A.W.A. Synthesis of polymeric nano/microgels: A review. J. Polym. Res. 2017, 24, 134. [Google Scholar] [CrossRef]
- Thorne, J.B.; Vine, G.J.; Snowden, M.J. Microgel applications and commercial considerations. Colloid Polym. Sci. 2011, 289, 625–646. [Google Scholar] [CrossRef]
- Hua, M.; Du, Y.; Song, J.; Sun, M.; He, X. Surfactant-free fabrication of pNIPAAm microgels in microfluidic devices. J. Mater. Res. 2019, 34, 206–213. [Google Scholar] [CrossRef]
- Anakhov, M.V.; Gumerov, R.A.; Potemkin, I.I. Stimuli-responsive aqueous microgels: Properties and applications. Mendeleev Commun. 2020, 30, 555–562. [Google Scholar] [CrossRef]
- Kozhunova, E.Y.; Gvozdik, N.A.; Motyakin, M.V.; Vyshivannaya, O.V.; Stevenson, K.J.; Itkis, D.M.; Chertovich, A.V. Redox-Active Aqueous Microgels for Energy Storage Applications. J. Phys. Chem. Lett. 2020, 11, 10561–10565. [Google Scholar] [CrossRef]
- Shewan, H.M.; Stokes, J.R. Review of techniques to manufacture micro-hydrogel particles for the food industry and their applications. J. Food Eng. 2013, 119, 781–792. [Google Scholar] [CrossRef]
- De Lima, C.S.A.; Balogh, T.S.; Varca, J.P.R.O.; Varca, G.H.C.; Lugão, A.B.; Camacho-Cruz, L.A.; Bucio, E.; Kadlubowski, S.S. An Updated Review of Macro, Micro, and Nanostructured Hydrogels for Biomedical and Pharmaceutical Applications. Pharmaceutics 2020, 12, 970. [Google Scholar] [CrossRef]
- Alzanbaki, H.; Moretti, M.; Hauser, C.A.E. Engineered Microgels—Their Manufacturing and Biomedical Applications. Micromachines 2021, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Nalawade, P.; Mukherjee, P.; Kapoor, S. Triethylamine induced synthesis of silver and bimetallic (Ag/Au) nanoparticles in glycerol and their antibacterial study. J. Nanostruct. Chem. 2014, 4, 113. [Google Scholar] [CrossRef]
- Gui, Y.; Tian, K.; Liu, J.; Yang, L.; Zhang, H.; Wang, Y. Superior triethylamine detection at room temperature by {-112} faceted WO3 gas sensor. J. Hazard. Mater. 2019, 380, 120876. [Google Scholar] [CrossRef] [PubMed]
- Fiol, M.; Weckmüller, A.; Neugart, S.; Schreiner, M.; Rohn, S.; Krumbein, A.; Kroh, L.W. Thermal-induced changes of kale’s antioxidant activity analyzed by HPLC–UV/Vis-online-TEAC detection. Food Chem. 2013, 138, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, D.; Sun, D.; Wang, C.; Zhang, J.; Xie, Y.; Guo, T. Total phenolic, flavonoid content, and antioxidant activity of flour, noodles, and steamed bread made from different colored wheat grains by three milling methods. Crop J. 2015, 3, 328–334. [Google Scholar] [CrossRef]
- Sudan, R.; Bhagat, M.; Gupta, S.; Singh, J.; Koul, A. Iron (FeII) Chelation, Ferric Reducing Antioxidant Power, and Immune Modulating Potential of Arisaema jacquemontii (Himalayan Cobra Lily). BioMed Res. Int. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Lee, B.P.; Dalsin, J.L.; Messersmith, P.B. Synthesis and Gelation of DOPA-Modified Poly(ethylene glycol) Hydrogels. Biomacromolecules 2002, 3, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Phillips, I.R.; Sheehan, K.J. Importance of surface charge characteristics when selecting soils for wastewater re-use. Aust. J. Soil Res. 2005, 43, 915–927. [Google Scholar] [CrossRef]
- Florence, T.M. The role of free radicals in disease. Aust. N. Z. J. Ophthalmol. 1995, 23, 3–7. [Google Scholar] [CrossRef]
- Lou, W.; Chen, Y.; Ma, H.; Liang, G.; Liu, B. Antioxidant and α-amylase inhibitory activities of tannic acid. J. Food Sci. Technol. 2018, 55, 3640–3646. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, N.; Sagbas, S.; Aktas, N. Preparation of macro-, micro-, and nano-sized poly(Tannic acid) particles with controllable degradability and multiple biomedical uses. Polym. Degrad. Stab. 2016, 129, 96–105. [Google Scholar] [CrossRef]


| Particles/NaIO4% | Particle Sizes (nm) | Zeta Potential (mV) | Yield (%) |
|---|---|---|---|
| p(TA)/50% | 1582 ± 120 | −20 ± 3 | 18 ± 5 |
| p(TA)/100% | 1325 ± 116 | −24 ± 2 | 20 ± 6 |
| p(TA)/200% | 981 ± 76 | −22 ± 4 | 25 ± 5 |
| Sample | Total Phenol Content (TPC) in Terms of Gallic Acid Equivalency (µg/mL) | Ferric Reducing Antioxidant Potential (FRAP) (µmole Fe(III)) | Trolox Equivalent Antioxidant (TEAC) Value (mM Trolox Equivalent/g) |
|---|---|---|---|
| GA | 250.0 | 399.9 ± 35.91 | 9.1 ± 0.4 |
| TA | 168.0 ± 1.0 | 22.6 ± 1.2 | 5.1 ± 0.3 |
| p(TA) | 202.0 ± 2.0 | 10.6 ± 0.6 | 5.8 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahiner, N. Self-Crosslinked Ellipsoidal Poly(Tannic Acid) Particles for Bio-Medical Applications. Molecules 2021, 26, 2429. https://doi.org/10.3390/molecules26092429
Sahiner N. Self-Crosslinked Ellipsoidal Poly(Tannic Acid) Particles for Bio-Medical Applications. Molecules. 2021; 26(9):2429. https://doi.org/10.3390/molecules26092429
Chicago/Turabian StyleSahiner, Nurettin. 2021. "Self-Crosslinked Ellipsoidal Poly(Tannic Acid) Particles for Bio-Medical Applications" Molecules 26, no. 9: 2429. https://doi.org/10.3390/molecules26092429
APA StyleSahiner, N. (2021). Self-Crosslinked Ellipsoidal Poly(Tannic Acid) Particles for Bio-Medical Applications. Molecules, 26(9), 2429. https://doi.org/10.3390/molecules26092429






