Synthesis and Chemical and Biological Evaluation of a Glycine Tripeptide Chelate of Magnesium
Abstract
1. Introduction
2. Results
2.1. Characterization of MgG3
2.1.1. Electrospray Ionization Mass Spectroscopy
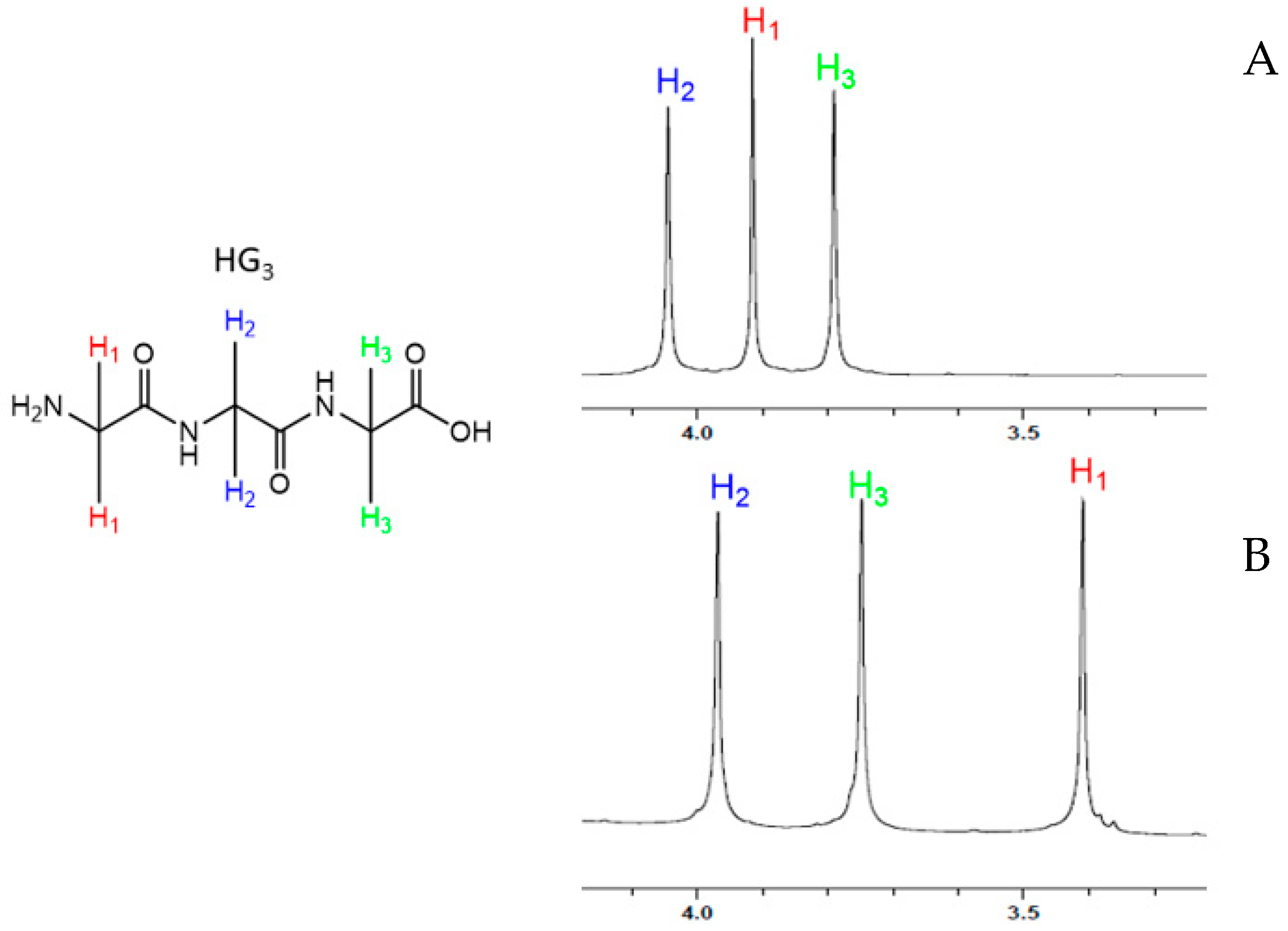
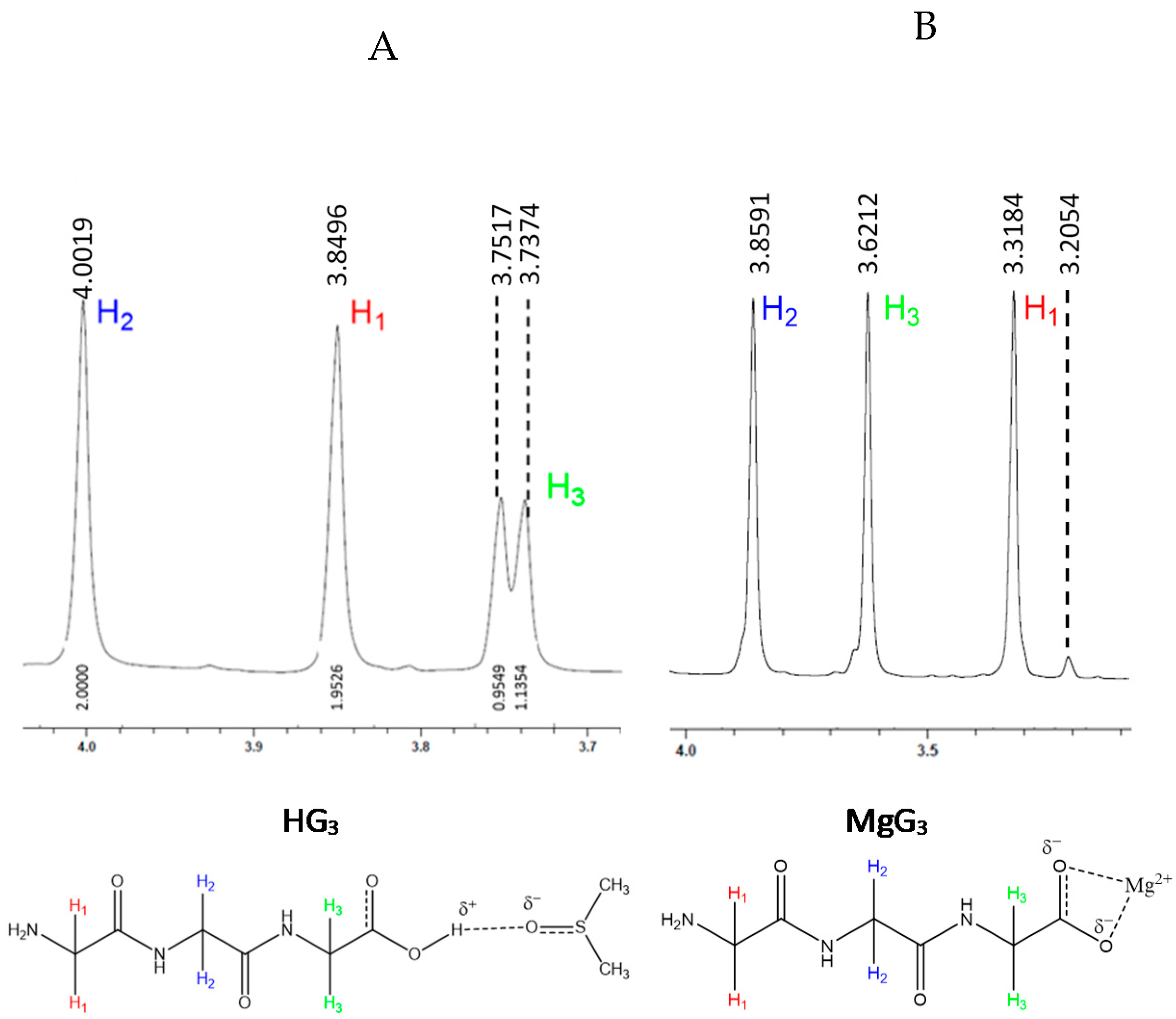
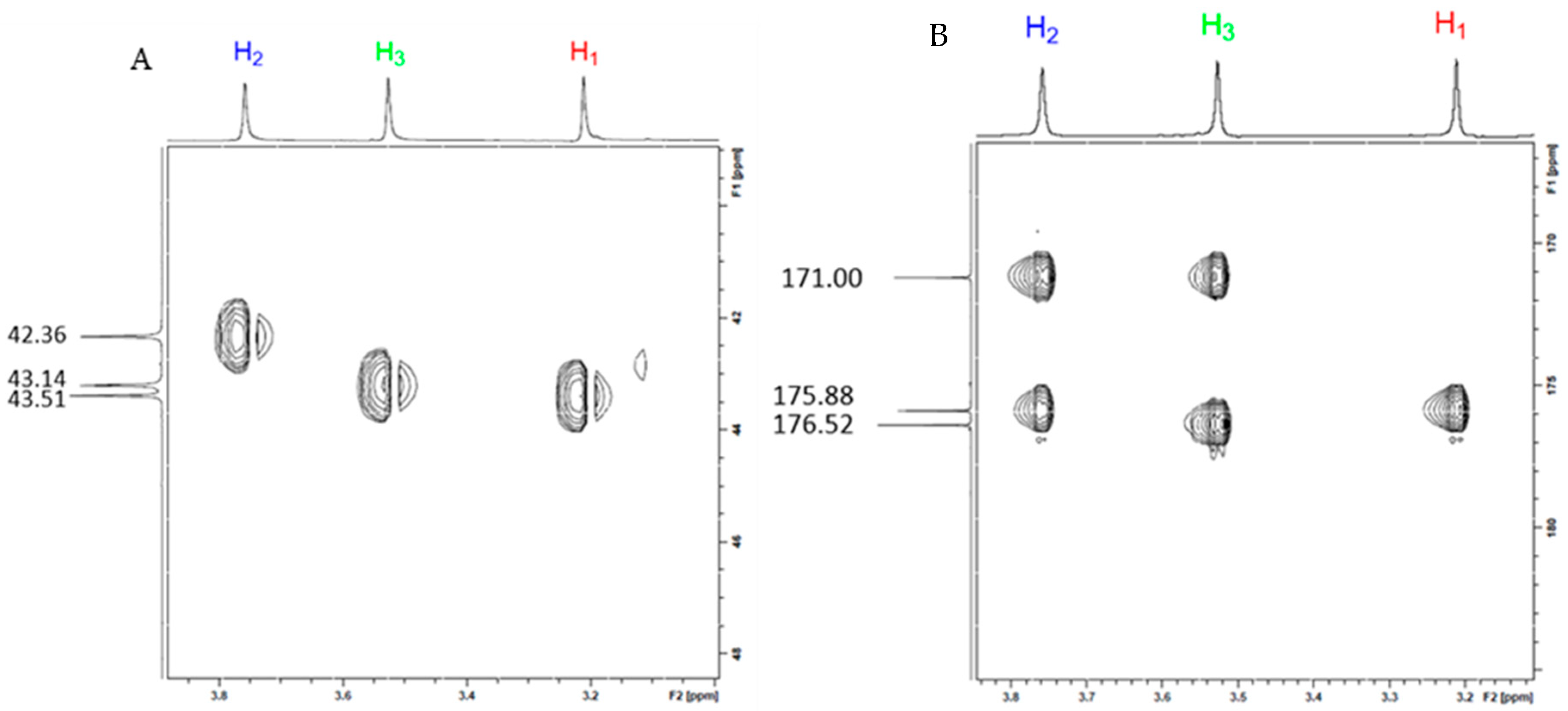
2.1.2. Structural Characterization of MgG3 via 1H-NMR and 2D 1H/13C HSQC/HMBC NMR
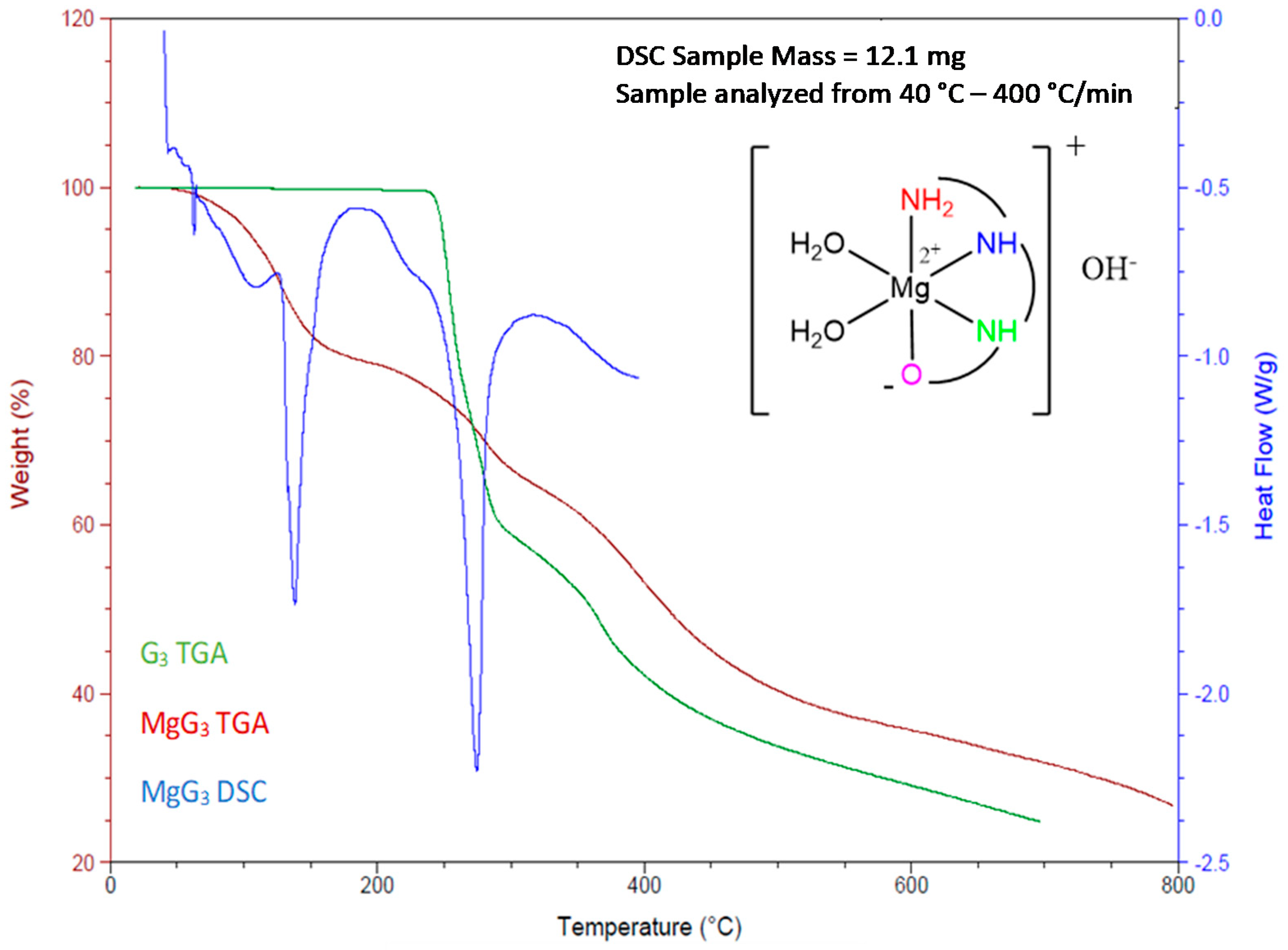
2.1.3. Determination of the Chemical Composition of MgG3 via TGA/DSC and EA
2.1.4. Evaluating the Solubility of MgG3
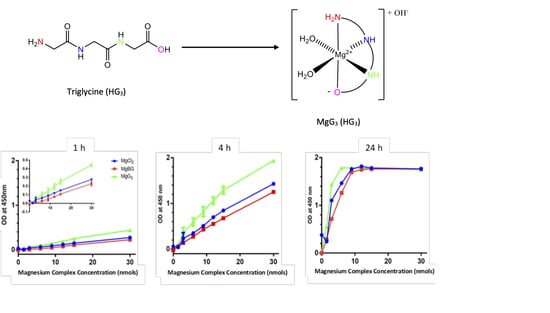
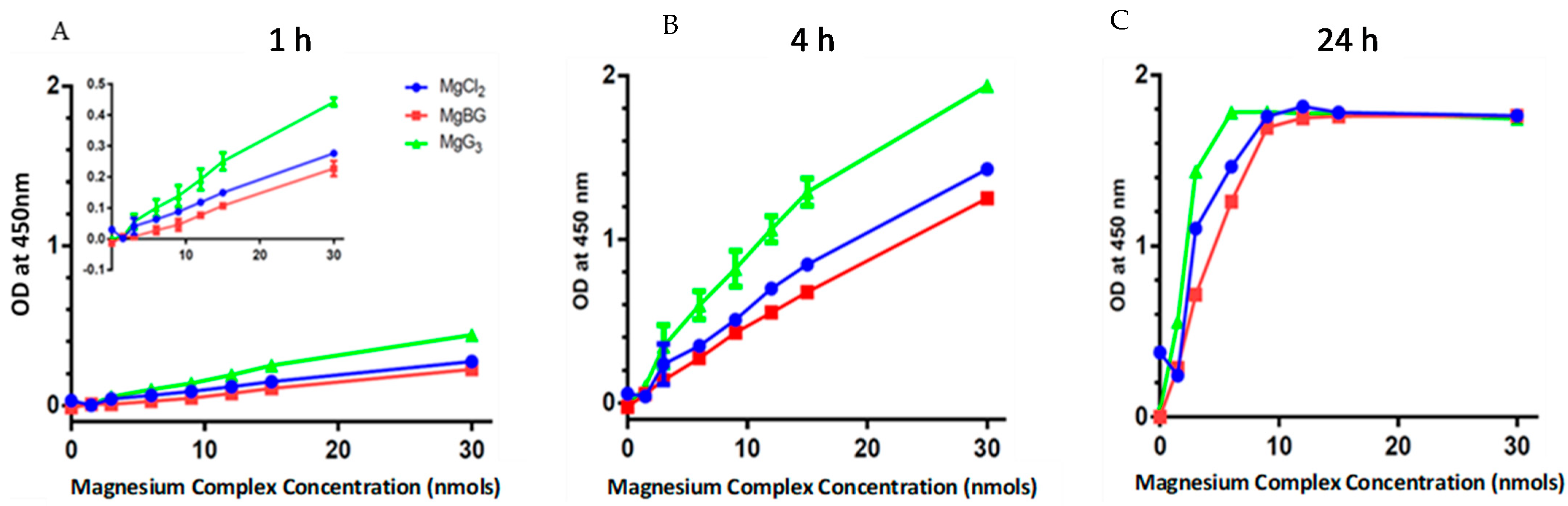
2.2. Cellular Uptake of MgG3
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Characterization of MgG3
4.2.2. Culturing of CaCo-2 and HEK293 Cells
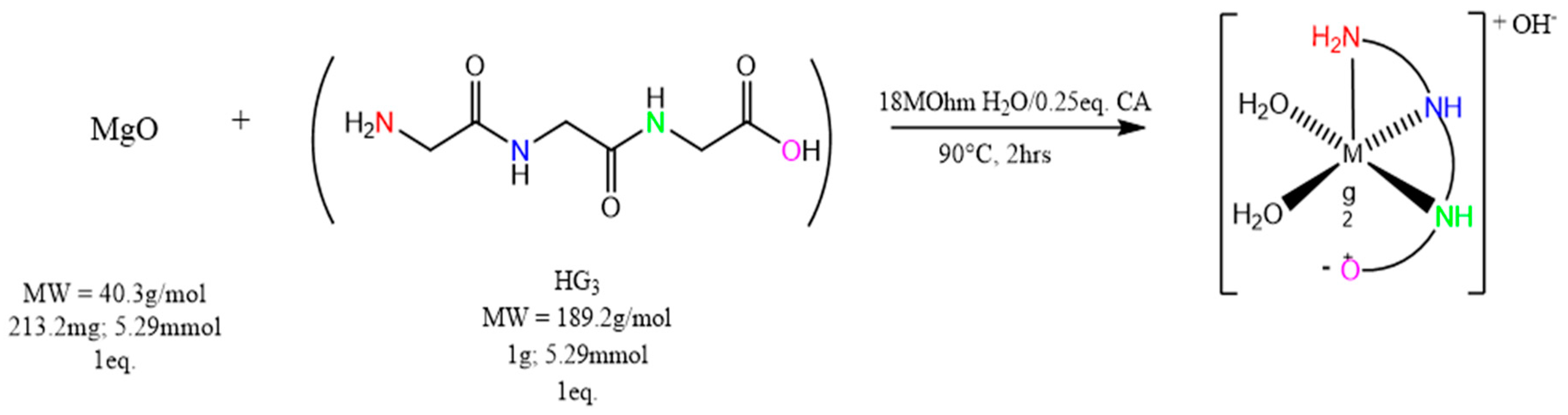
4.3. Synthesis of Magnesium Triglycine ([Mg(G3)(H2O)2]OH—(MgG3)
4.4. Determining Magnesium Uptake in CaCo-2 Human Cells
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. CKJ Clin. Kidney J. 2012, 5 (Suppl. 1). [Google Scholar] [CrossRef]
- Geiger, H.; Wanner, C. Magnesium in disease. CKJ Clin. Kidney J. 2012, 5 (Suppl. 1). [Google Scholar] [CrossRef] [PubMed]
- De Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Vormann, J. Magnesium: Nutrition and metabolism. Mol. Aspects Med. 2003, 24, 27–37. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H.; Wilson, W. Subclinical magnesium deficiency: A principal driver of cardiovascular disease and a public health crisis. Open Hear. 2018, 5, e000668. [Google Scholar] [CrossRef]
- Rude, R.K.; Gruber, H.E. Magnesium deficiency and osteoporosis: Animal and human observations. J. Nutr. Biochem. 2004, 15, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Workinger, J.L.; Doyle, R.P.; Bortz, J. Challenges in the diagnosis of magnesium status. Nutrients 2018, 10, 1202. [Google Scholar] [CrossRef] [PubMed]
- Costello, R.B.; Elin, R.J.; Rosanoff, A.; Wallace, T.C.; Guerrero-Romero, F.; Hruby, A.; Lutsey, P.L.; Nielsen, F.H.; Rodriguez-Moran, M.; Song, Y.; et al. Perspective: The Case for an Evidence-Based Reference Interval for Serum Magnesium: The Time Has Come. Adv. Nutr. Int. Rev. J. 2016, 7, 977–993. [Google Scholar] [CrossRef]
- Al Alawi, A.M.; Majoni, S.W.; Falhammar, H. Review Article Magnesium and Human Health: Perspectives and Research Directions. Int. J. Endocrinol. 2018, 2018, 9041694. [Google Scholar] [CrossRef] [PubMed]
- Piuri, G.; Zocchi, M.; Porta, M.D.; Ficara, V.; Manoni, M.; Zuccotti, G.V.; Pinotti, L.; Maier, J.A.; Cazzola, R. Magnesium in Obesity, Metabolic Syndrome, and Type 2 Diabetes. Nutrients 2021, 2, 1–16. [Google Scholar]
- Razzaque, M.S.; Al Alawi, A.M.; Majoni, S.W.; Falhammar, H.; Piuri, G.; Zocchi, M.; Porta, M.D.; Ficara, V.; Manoni, M.; Zuccotti, G.V.; et al. Magnesium: Are we consuming enough? Nutrients 2018, 10, 1863. [Google Scholar] [CrossRef] [PubMed]
- Olza, J.; Aranceta-bartrina, J.; Gonz, M.; Ortega, R.M. Reported Dietary Intake, Disparity between the Adequacy and Food Sources of Calcium, Phosphorus, Magnesium and Vitamin D in the Spanish Population: Findings from the ANIBES Study. Nutrients 2017, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Elin, R.J. Magnesium Metabolism in Health and Disease. Disease-A-Month 1988, 34, 166–218. [Google Scholar] [CrossRef]
- Ford, E.S.; Mokdad, A.H. Nutritional Epidemiology—Research Communication Dietary Magnesium Intake in a National Sample of U.S. Adults. J. Nutr. 2003, 133, 2879–2882. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, S.; Cappuccio, F.P.; Nichols, R.; Elliott, P. Dietary magnesium intake and blood pressure: A qualitative overview of the observational studies. J. Hum. Hypertens. 1998, 12, 447–453. [Google Scholar] [CrossRef]
- Altura, B.M.; Altura, B.T. Tension headaches and muscle tension: Is there a role for magnesium? Med. Hypotheses 2001, 57, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Sales, C.H.; Pedrosa Lde, F. Magnesium and diabetes mellitus: Their relation. Clin. Nutr. 2006, 25, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, F.P. Sodium, potassium, calcium and magnesium and cardiovascular risk. Eur. J. Prev. Cardiol. 2000, 7, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Hayhoe, R.P.G.; Lentjes, M.A.H.; Luben, R.N.; Khaw, K.T.; Welch, A.A. Dietary magnesium and potassium intakes and circulating magnesium are associated with heel bone ultrasound attenuation and osteoporotic fracture risk in the EPIC-Norfolk cohort study. Am. J. Clin. Nutr. 2015, 102, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Brauman, J.; Schoutens, A. Bone Mineral Content of the Radius: Good Correlations With Physicochemical Determinations in Iliac Crest Trabecular Bone of Normal and Osteoporotic Subjects. Metabolism 1981, 30, 57–62. [Google Scholar]
- Classen, H.-G.; Kisters, K. Magnesium and osteoporosis. Trace Elem. Electrolytes 2017, 34, 100–103. [Google Scholar] [CrossRef]
- Kass, L.; Weekes, J.; Carpenter, L. Effect of magnesium supplementation on blood pressure: A meta-analysis. Eur. J. Clin. Nutr. 2012, 66, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Crook, M.; Couchman, S.; Tutt, P.; Amiel, S.; Swaminathan, R. Erythrocyte, plasma total, ultrafiltrable and platelet magnesium in type 2 (non-insulin dependent) diabetes mellitus. Diabetes Res. 1994, 27, 73–79. [Google Scholar] [PubMed]
- Guerrera, M.; Volpe, S.; Mao, J. Therapeutic Uses of Magnesium. Am. Fam. Physician 2009, 80, 157–162. [Google Scholar] [PubMed]
- Ropp, R.C. Group 16 (O, S, Se, Te) Alkaline Earth Compounds; Newnes: Londong, UK, 2013; Volume 16, ISBN 9780444595508. [Google Scholar]
- Perry, D.L. Handbook of Inorganic Compounds, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011; Volume 33, ISBN 9781439814628. [Google Scholar]
- Clynne, M.A.; Potter, R.W. Solubility of Some Alkali and Alkaline Earth Chlorides in Water at Moderate Temperatures. J. Chem. Eng. Data 1979, 24, 338–340. [Google Scholar] [CrossRef]
- Kanunnikova, O.M.; Aksenova, V.V.; Karban, O.V.; Muhgalin, V.V.; Senkovski, B.V.; Ladjanov, V.I. Mechanical activation effect on structure, physicochemical, and biological properties of potassium/magnesium orotates. IOP Conf. Ser. Mater. Sci. Eng. 2018, 283. [Google Scholar] [CrossRef]
- McCarty, M.F. Magnesium Taurate and Other Mineral Taurates. U.S. Patent 5,582,839, 10 December 1996. [Google Scholar]
- Apelblat, A.; Manzurola, E. Solubilities of o-acetylsalicylic, 4-aminosalicylic, 3,5-dinitrosalicylic, and p-toluic acid, and magnesium-DL-aspartate in water from T = (278 to 348) K. J. Chem. Thermodyn. 1999, 31, 85–91. [Google Scholar] [CrossRef]
- Liu, G.; Mao, F. Slow Release Magnesium Composition and Uses Thereof. U.S. Patent 8,377,473 B2, 19 February 2013. [Google Scholar]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gundert-Remy, U.; Kuhnle, G.G.; et al. Evaluation of di-magnesium malate, used as a novel food ingredient and as a source of magnesium in foods for the general population, food supplements, total diet replacement for weight control and food for special medical purposes. EFSA J. 2018, 16. [Google Scholar] [CrossRef]
- Hartle, J.; Ashmead, S.D.; Kreitlow, R. Dimetal Hydroxy Malates. U.S. Patent 6,706,904, 16 March 2004. [Google Scholar]
- Mühlbauer, B.; Schwenk, M.; Coram, W.M.; Antonin, K.H.; Etienne, P.; Bieck, P.R.; Douglas, F.L. Magnesium-L-aspartate-HCl and magnesium-oxide: Bioavailability in healthy volunteers. Eur. J. Clin. Pharmacol. 1991, 40, 437–438. [Google Scholar] [CrossRef] [PubMed]
- Firoz, M.; Graber, M. Bioavallability of US commercial magnesium preparations. Magnes. Res. 2001, 14, 257–262. [Google Scholar] [PubMed]
- Kappeler, D.; Heimbeck, I.; Herpich, C.; Naue, N.; Höfler, J.; Timmer, W.; Michalke, B. Higher bioavailability of magnesium citrate as compared to magnesium oxide shown by evaluation of urinary excretion and serum levels after single-dose administration in a randomized cross-over study. BMC Nutr. 2017, 3, 1–12. [Google Scholar] [CrossRef]
- Thongon, N.; Krishnamra, N. Omeprazole decreases magnesium transport across Caco-2 monolayers. World J. Gastroenterol. 2011, 17, 1574–1583. [Google Scholar] [CrossRef]
- Thongon, N.; Krishnamra, N. Apical acidity decreases inhibitory effect of omeprazole on Mg2+ absorption and claudin-7 and -12 expression in Caco-2 monolayers. Exp. Mol. Med. 2012, 44, 684–693. [Google Scholar] [CrossRef]
- Coudray, C.; Feillet-Coudray, C.; Rambeau, M.; Tressol, J.C.; Gueux, E.; Mazur, A.; Rayssiguier, Y. The effect of aging on intestinal absorption and status of calcium, magnesium, zinc, and copper in rats: A stable isotope study. J. Trace Elem. Med. Biol. 2006, 20, 73–81. [Google Scholar] [CrossRef]
- Blaquiere, C.; Berthon, G. Speciation studies in relation to magnesium bioavailability. Formation of Mg(II) complexes with glutamate, aspartate, glycinate, lactate, pyroglutamate, pyridoxine and citrate, and appraisal of their potential significance towards magnesium gastrointestin. Inorg. Chim. Acta 1987, 135, 179–189. [Google Scholar] [CrossRef]
- Hartshorn, R.M.; Hellwich, K.H.; Yerin, A.; Damhus, T.; Hutton, A.T. Brief guide to the nomenclature of inorganic chemistry. Pure Appl. Chem. 2015, 87, 1039–1049. [Google Scholar] [CrossRef]
- Fowden, L.; Smith, A. Peptides From Blighia Sapida Seed. Phytochemistry 1969, 8, 1043–1045. [Google Scholar] [CrossRef]
- Yin, L.H.; Liu, X.P.; Yi, L.Y.; Wang, J.; Zhang, Y.J.; Feng, Y.F. Structural characterization of calcium glycinate, magnesium glycinate and zinc glycinate. J. Innov. Opt. Health Sci. 2017, 10, 1–10. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Classen, H.G.; Helbig, J. Aspartic and Glutamic Acid as Ligands to Alkali and Alkaline-Earth Metals: Structural Chemistry as Related to Magnesium Therapy. Angew. Chem. Int. Ed. Engl. 1990, 29, 1090–1103. [Google Scholar] [CrossRef]
- Murphy, C.B.; Martell, A.M. Metal Chelates of Glycine and Glycine Peptides. J. Biol. Chem. 1957, 226, 37–50. [Google Scholar] [CrossRef]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, B.M.; Cabarga, M.M.; Navarro, A.S.; Hurlé, A.D.G. A physico-chemical study of the interaction of ciprofloxacin and ofloxacin with polivaient cations. Int. J. Pharm. 1994, 106, 229–235. [Google Scholar] [CrossRef]
- Drevenšek, P.; Košmrlj, J.; Giester, G.; Skauge, T.; Sletten, E.; Sepčić, K.; Turel, I. X-Ray crystallographic, NMR and antimicrobial activity studies of magnesium complexes of fluoroquinolones-racemic ofloxacin and its S-form, levofloxacin. J. Inorg. Biochem. 2006, 100, 1755–1763. [Google Scholar] [CrossRef] [PubMed]
- Storm, C.B.; Corwin, A.H. Proton Magnetic Resonance Evidence for Ligand-Porphyrin Interaction in Magnesium Porphyrins. J. Org. Chem. 1964, 29, 3700–3702. [Google Scholar] [CrossRef]
- Leifer, A.; Lippincott, E.R. The Infrared Spectra of Some Amino Acids. J. Am. Chem. Soc. 1957, 79, 5098–5101. [Google Scholar] [CrossRef]
- Maguire, M.E.; Cowan, J.A. Magnesium chemistry and biochemistry. BioMetals 2002, 15, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Black, C.B.; Huang, H.W.; Cowan, J.A. Biological coordination chemistry of magnesium, sodium, and potassium ions. Protein and nucleotide binding sites. Coord. Chem. Rev. 1994, 135–136, 165–202. [Google Scholar] [CrossRef]
- Dudev, T.; Cowan, J.A.; Lim, J.A. Competition between Protein Ligands and Cytoplasmic Inorganic Anions for the Metal Cation: A DFT/CDM Study. J. Am. Chem. Soc. 1999, 121, 7665–7673. [Google Scholar] [CrossRef]
- Burgess, M.A. Metal Ions in Solution; Horwood, E., Ed.; Prentice Hall: London, UK, 1978. [Google Scholar]
- Ivanova, B.; Kolev, T.; Zareva, S. Solid-State IR-LD Spectroscopic and Theoretical Analysis of Glycine-Containing Peptides and Their Hydrochlorides. Biopolymers 2006, 82, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, M.; Gharib, F. Equilibrium studies of triphenyltin(IV) complexes with glycine, glycyl-glycine, and glycyl-glycyl-glycine in different aqueous solutions of ethanol. Can. J. Chem. 2010, 88, 877–885. [Google Scholar] [CrossRef]
- Manyak, A.R.; Murphy, C.B.; Martell, A.E. Metal chelate compounds of glycylglycine and glycylglycylglycine. Arch. Biochem. Biophys. 1955, 59, 373–382. [Google Scholar] [CrossRef]
- Van der Helm, D.; Willoughby, T.V. The crystal structure of CaCl2•glycylglycylglycine•3H2O. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1969, 25, 2317–2326. [Google Scholar] [CrossRef]
- Srikrishnan, T.; Winiewicz, N.; Parthasarathy, R. New patterns of hydrogen bonded interactions between polypeptide chains: Crystal and molecular structure of glycylglycylglycine. Int. J. Pept. Protein Res. 1982, 19, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Fine, K.D.; Santa Ana, C.A.; Porter, J.L.; Fordtran, J.S. Intestinal absorption of magnesium from food and supplements. J. Clin. Investig. 1991, 88, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Schuette, S.A.; Ziegler, E.E.; Nelson, S.E.; Janghorbani, M. Feasibility of using the stable isotope 25Mg to study mg metabolism in infants. Pediatr. Res. 1990, 27, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, M.; Grandvuillemin, A.; Kastenmayer, P.; Aeschliman, J.M.; Bouisset, F.; Arnaud, M.J.; Dumoulin, G.; Berthelot, A. Influence of the consumption pattern of magnesium from magnesium-rich mineral water on magnesium bioavailability. Br. J. Nutr. 2011, 106, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Younes, H.; Demigné, C.; Rémésy, C. Acidic fermentation in the caecum increases absorption of calcium and magnesium in the large intestine of the rat. Br. J. Nutr. 2005, 75, 301–314. [Google Scholar] [CrossRef]


| Form (Oxide/Salt/Chelate) | Acid/Base Chemistry | Solubility (g/100 mLs H2O) | MW g/mol (%Mg) | Reference |
|---|---|---|---|---|
| Magnesium Oxide | Alkaline | 0.010 | 40.30 (60.3) | [25] |
| Magnesium Citrate | Acidic (pKa1 = 3.13) | 20 | 214.41 (11.3) | [26] |
| Magnesium Chloride | Neutral | 54.0 | 95.21 (25.5) | [27] |
| Magnesium Sulfate | Acidic (pKa1 = 3.0; pKa2 = 1.99) | 35.7 | 120.37 (20.1) | [25] |
| Magnesium Orotate | Acidic (pKa1 = 2.83) | Slightly Soluble | 334.48 (7.2) | [28] |
| Magnesium Taurate | Acidic (pKa = 1.50) | Slightly Soluble | 272.57 (8.9) | [29] |
| Magnesium Aspartate | Acidic (pKa1 = 1.88; pKa3 = 3.65) | 4.0 | 288.49 (8.5) | [30] |
| Magnesium Threonate | Acidic (pKa1 = 3.4) | Soluble | 294.50 (8.3) | [31] |
| Magnesium Malate | Acidic (pKa1 = 3.46; pKa2 = 5.10) | Slightly Soluble | 156.37 (15.5) | [32,33] |
| Magnesium Bisglycinate | Acidic (pKa = 2.34) | 1.36 | 172.42 (14.1) | In-house |
| Magnesium Hydroxide | Alkaline | 0.00069 | 58.32 (41.7) | [25] |
| Magnesium Carbonate | Weakly Alkaline | 0.18 | 84.31 (28.8) | [26] |
| Magnesium Triglycine | Acidic | 169 | 265.50 (9.1) | This Work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Case, D.R.; Zubieta, J.; Gonzalez, R.; Doyle, R.P. Synthesis and Chemical and Biological Evaluation of a Glycine Tripeptide Chelate of Magnesium. Molecules 2021, 26, 2419. https://doi.org/10.3390/molecules26092419
Case DR, Zubieta J, Gonzalez R, Doyle RP. Synthesis and Chemical and Biological Evaluation of a Glycine Tripeptide Chelate of Magnesium. Molecules. 2021; 26(9):2419. https://doi.org/10.3390/molecules26092419
Chicago/Turabian StyleCase, Derek R., Jon Zubieta, Ren Gonzalez, and Robert P. Doyle. 2021. "Synthesis and Chemical and Biological Evaluation of a Glycine Tripeptide Chelate of Magnesium" Molecules 26, no. 9: 2419. https://doi.org/10.3390/molecules26092419
APA StyleCase, D. R., Zubieta, J., Gonzalez, R., & Doyle, R. P. (2021). Synthesis and Chemical and Biological Evaluation of a Glycine Tripeptide Chelate of Magnesium. Molecules, 26(9), 2419. https://doi.org/10.3390/molecules26092419