How Different Snacks Produce a Distinct Effect in Salivary Protein Composition
Abstract
1. Introduction
2. Results
2.1. Flow Rate, Total Protein Concentration and Alpha-Amylase Enzymatic Activity
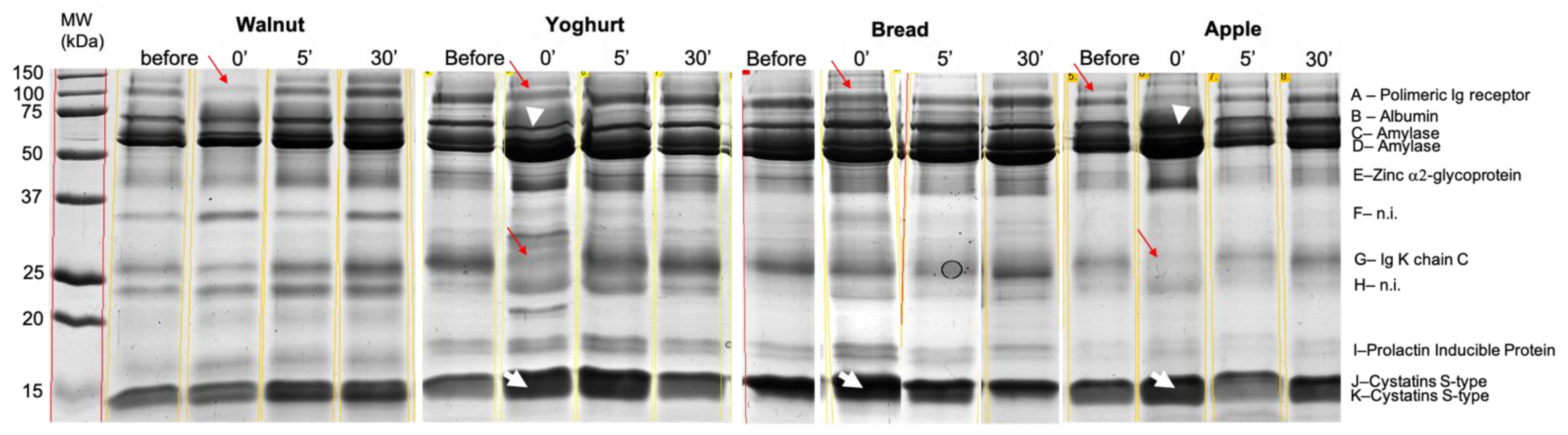
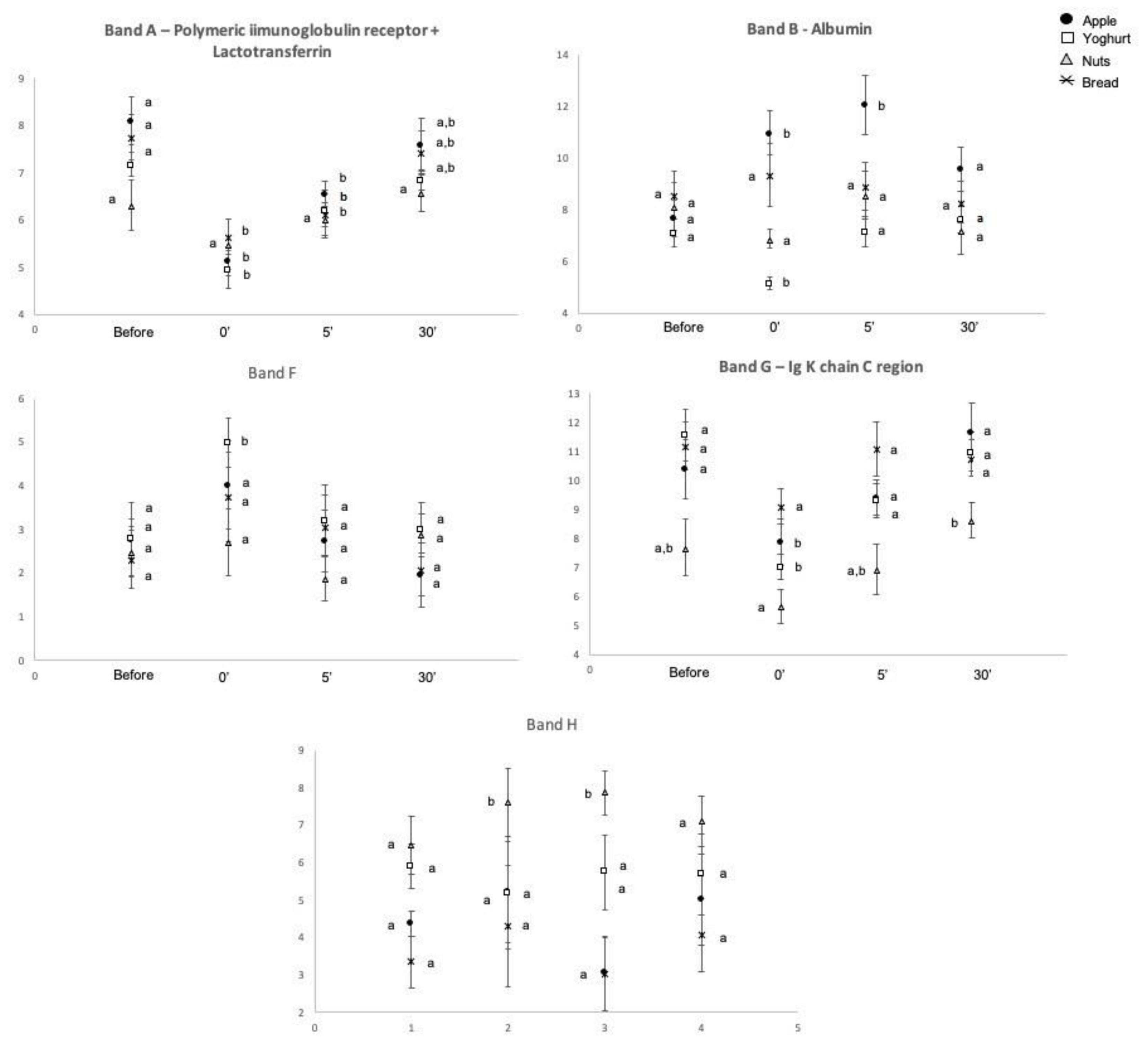
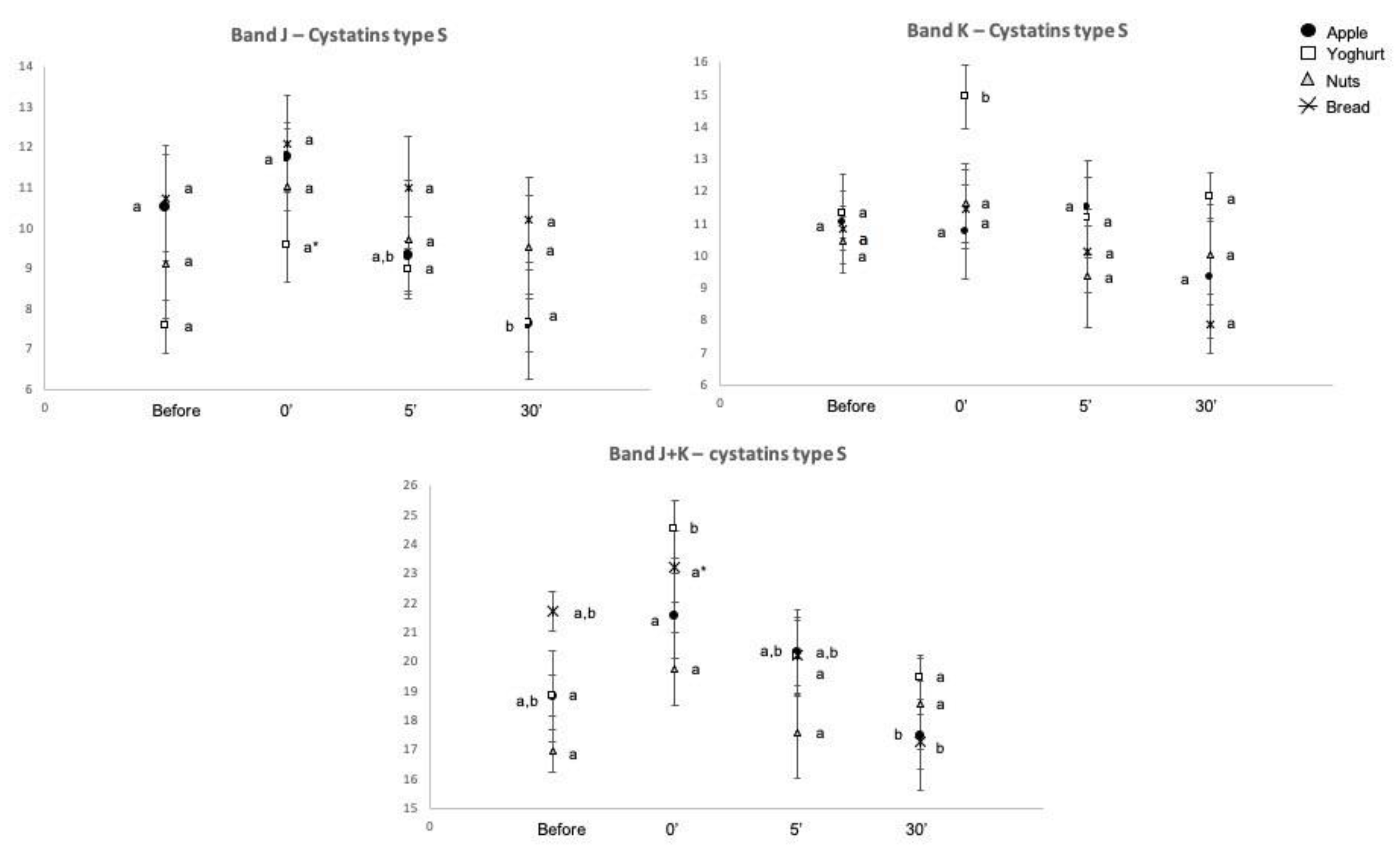
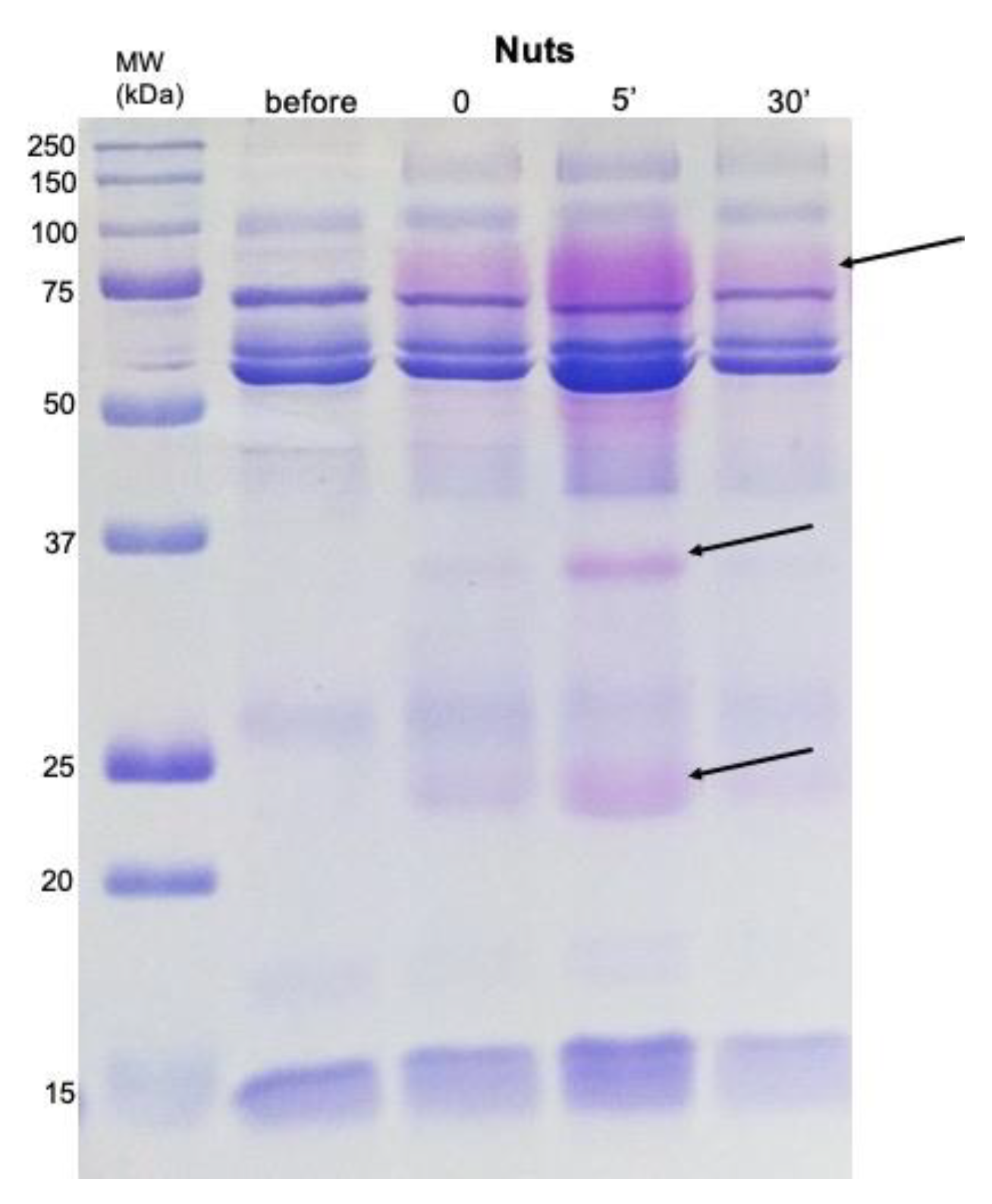
2.2. Salivary Protein Profile
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Experimental Design and Food Stimulus
4.3. Saliva Collection and Laboratory Analysis
4.3.1. Salivary Flow Rate, Total Protein Concentration and Alpha Amylase Activity
4.3.2. SDS-PAGE Salivary Protein Separation
4.3.3. Protein Identification by Mass Spectrometry
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tvarijonaviciute, A.; Martinez-Subiela, S.; Lopez-Jornet, P.; Lamy, E. (Eds.) Saliva in Health and Disease: The Present and Future of a Unique Sample for Diagnosis, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Lamy, E.; Torregrossa, A.-M.; Castelo, P.M.; e Silva, F.C. Saliva in Ingestive Behavior Research: Association with Oral Sensory Perception and Food Intake. In Saliva in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Proctor, G.B. The physiology of salivary secretion. Periodontology 2000 2016, 70, 11–25. [Google Scholar] [CrossRef]
- Dawes, C. Factors influencing salivary flow rate and composition. In Saliva and Oral Health; Edgar, M., Dawes, C., O’Mullane, D., Eds.; Wrigley: Cork, Ireland, 1996; pp. 37–56. [Google Scholar]
- Sales Baptista, E.; Lamy, E.; Mau, M.; e Silva, F.C.; Coelho, A.V. Variation in Salivary Protein Composition Related to Feeding Behavior and Its Ecological Implications; Nova Science Publishers Inc.: New York, NY, USA, 2013. [Google Scholar]
- Pajic, P.; Pavlidis, P.; Dean, K.; Neznanova, L.; Romano, R.A.; Garneau, D.; Daugherity, E.; Globig, A.; Ruhl, S.; Gokcumen, O. Independent amylase gene copy number bursts correlate with dietary preferences in mammals. Elife 2019, 8. [Google Scholar] [CrossRef]
- De Wijk, R.A.; Prinz, J.F. The role of friction in perceived oral texture. Food Qual. Prefer. 2005, 16. [Google Scholar] [CrossRef]
- Dinnella, C.; Recchia, A.; Vincenzi, S.; Tuorila, H.; Monteleone, E. Temporary modification of salivary protein profile and individual responses to repeated phenolic astringent stimuli. Chem. Senses 2010, 35, 75–85. [Google Scholar] [CrossRef]
- Dsamou, M.; Palicki, O.; Septier, C.; Chabanet, C.; Lucchi, G.; Ducoroy, P.; Chagnon, M.-C.; Morzel, M. Salivary protein profiles and sensitivity to the bitter taste of caffeine. Chem. Senses 2012, 37, 87–95. [Google Scholar] [CrossRef]
- Cabras, T.; Melis, M.; Castagnola, M.; Padiglia, A.; Tepper, B.J.; Messana, I.; Barbarossa, I.T. Responsiveness to 6-n-propylthiouracil (PROP) is associated with salivary levels of two specific basic proline-rich proteins in humans. PLoS ONE 2012, 7, e30962. [Google Scholar] [CrossRef]
- Rodrigues, L.; Espanca, R.; Costa, A.R.; Antunes, C.M.; Pomar, C.; Capela-Silva, F.; Pinheiro, C.C.; Domingues, P.; Amado, F.; Lamy, E. Comparison of salivary proteome of children with different sensitivities for bitter and sweet tastes: Association with body mass index. Int. J. Obes. 2019, 43. [Google Scholar] [CrossRef]
- Rodrigues, L.; Costa, G.; Cordeiro, C.; Pinheiro, C.; Amado, F.; Lamy, E. Salivary proteome and glucose levels are related with sweet taste sensitivity in young adults. Food Nutr. Res. 2017, 61, 1389208. [Google Scholar] [CrossRef]
- Rodrigues, L.; da Costa, G.; Cordeiro, C.; Pinheiro, C.C.; Amado, F.; Lamy, E. Relationship between saliva protein composition and 6-n-Propylthiouracil bitter taste responsiveness in young adults. J. Sens. Stud. 2017, 32, e12275. [Google Scholar] [CrossRef]
- Lee, C.A.; Ismail, B.; Vickers, Z.M. The role of salivary proteins in the mechanism of astringency. J. Food Sci. 2012, 77, C381–C387. [Google Scholar] [CrossRef]
- Lamy, E.; Graça, G.; da Costa, G.; Franco, C.; e Silva, F.C.; Baptista, E.S.; Coelho, A.V. Changes in mouse whole saliva soluble proteome induced by tannin-enriched diet. Proteome Sci. 2010, 8, 65. [Google Scholar] [CrossRef]
- Da Costa, G.; Lamy, E.; e Silva, F.C.; Andersen, J.; Baptista, E.S.; Coelho, A.V. Salivary amylase induction by tannin-enriched diets as a possible countermeasure against tannins. J. Chem. Ecol. 2008, 34, 376–387. [Google Scholar] [CrossRef]
- Yousaf, N.Y.; Melis, M.; Mastinu, M.; Contini, C.; Cabras, T.; Barbarossa, I.T.; Tepper, B.J. Time course of salivary protein responses to cranberry-derived polyphenol exposure as a function of prop taster status. Nutrients 2020, 12, 2878. [Google Scholar] [CrossRef]
- Crawford, C.R.; Running, C.A. Addition of chocolate milk to diet corresponds to protein concentration changes in human saliva. Physiol. Behav. 2020, 225. [Google Scholar] [CrossRef]
- Rodrigues, L.; Mouta, R.; Costa, A.R.; Pereira, A.; e Silva, F.C.; Amado, F.; Antunes, C.M.; Lamy, E. Effects of high fat diet on salivary α-amylase, serum parameters and food consumption in rats. Arch. Oral Biol. 2015, 60. [Google Scholar] [CrossRef] [PubMed]
- Carreira, L.; Midori Castelo, P.; Simões, C.; e Silva, F.C.; Viegas, C.; Lamy, E. Changes in Salivary Proteome in Response to Bread Odour. Nutrients 2020, 12, 1002. [Google Scholar] [CrossRef]
- Lamy, E.; Santos, V.; Barrambana, S.; Simões, C.; Carreira, L.; Infante, P.; e Silva, F.C. Saliva Protein Composition Relates with Interindividual Variations in Bread Sensory Ratings. Starch/Staerke 2020, 73. [Google Scholar] [CrossRef]
- Guota, N.; Mutreja, S.; Kamate, S.; Gupta, B. Evaluation of Change in Salivary Ph, Following Consumption of Different Snacks and Beverages and Estimation of Their Oral Clearance Time. Int. J. Prev. Clin. Dent. Res. 2015, 3, 25–31. [Google Scholar]
- Piombino, P.; Genovese, A.; Esposito, S.; Moio, L.; Cutolo, P.P.; Chambery, A.; Severino, V.; Moneta, E.; Smith, D.P.; Owens, S.M.; et al. Saliva from obese individuals suppresses the release of aroma compounds from wine. PLoS ONE 2014, 9, e85611. [Google Scholar] [CrossRef]
- Martin, L.E.; Nikonova, L.V.; Kay, K.E.; Torregrossa, A.M. Altering salivary protein profile can increase acceptance of a novel bitter diet. Appetite 2019, 136. [Google Scholar] [CrossRef]
- Beeley, J.A.; Sweeney, D.; Lindsay, J.C.; Buchanan, M.L.; Sarna, L.; Khoo, K.S. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis of human parotid salivary proteins. Electrophoresis 1991, 12, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Fearing, F.; Pavlov, I.P.; Anrep, G.V. Conditioned Reflexes. An Investigation of the Physiological Activity of the Cerebral Cortex. J. Am. Inst. Crim. Law Criminol. 1929, 20. [Google Scholar] [CrossRef]
- Batista, T.B.D.; Chaiben, C.L.; Penteado, C.A.S.; Nascimento, J.M.C.; Ventura, T.M.O.; Dionizio, A.; Rosa, E.A.R.; Buzalaf, M.A.R.; Azevedo-Alanis, L.R. Salivary proteome characterization of alcohol and tobacco dependents. Drug Alcohol Depend. 2019, 204. [Google Scholar] [CrossRef]
- Van Langeveld, A.W.B.; Teo, P.S.; De Vries, J.H.M.; Feskens, E.J.M.; De Graaf, C.; Mars, M. Dietary taste patterns by sex and weight status in the Netherlands. Br. J. Nutr. 2018, 119. [Google Scholar] [CrossRef]
- Spielman, A.I. Interaction of Saliva and Taste. J. Dent. Res. 1990, 69. [Google Scholar] [CrossRef]
- Al-Manei, K.; Almotairy, N.; Bostanci, N.; Kumar, A.; Grigoriadis, A. Effect of Chewing on the Expression of Salivary Protein Composition: A Systematic Review. Proteom. Clin. Appl. 2019, 14, e1900039. [Google Scholar] [CrossRef]
- Neyraud, E.; Bult, J.H.F.; Dransfield, E. Continuous analysis of parotid saliva during resting and short-duration simulated chewing. Arch. Oral Biol. 2009, 54. [Google Scholar] [CrossRef]
- Joubert, M.; Septier, C.; Brignot, H.; Salles, C.; Panouillé, M.; Feron, G.; Tournier, C. Chewing bread: Impact on alpha-amylase secretion and oral digestion. Food Funct. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.; Fichtel, S.L.; Taylor, A.J. Impact of calcium on salivary α-amylase activity, starch paste apparent viscosity and thickness perception. Chemosens. Percept. 2011, 4, 116–122. [Google Scholar] [CrossRef]
- Schiffman, S.S.; Miletic, I.D. Effect of taste and smell on secretion rate of salivary iga in elderly and young persons. J. Nutr. Health Aging 1999, 3, 158–164. [Google Scholar]
- Allgrove, J.E.; Oliveira, M.; Gleeson, M. Stimulating whole saliva affects the response of antimicrobial proteins to exercise. Scand. J. Med. Sci. Sport. 2014, 24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gibbins, H.L.; Carpenter, G.H. Alternative Mechanisms of Astringency—What is the Role of Saliva? J. Texture Stud. 2013, 44, 364–375. [Google Scholar] [CrossRef]
- Lamy, E.; Mowe, M.; Pinheiro, C.; Rodrigues, L.; Lopes, O.; e Silva, F.C. Oral food perception and polyphenol-rich foods acceptance—The importance of knowing individuals saliva characteristics for promoting consumption. J. Int. Soc. Antioxid. 2016, 3. [Google Scholar] [CrossRef]
- Bayarri, S.; Carbonell, I.; Barrios, E.X.; Costell, E. Impact of sensory differences on consumer acceptability of yoghurt and yoghurt-like products. Int. Dairy J. 2011, 21. [Google Scholar] [CrossRef]
- Carter, B.G.; Foegeding, E.A.; Drake, M.A. Invited review: Astringency in whey protein beverages. J. Dairy Sci. 2020, 103, 5793–5804. [Google Scholar] [CrossRef]
- Sze-Tao, K.W.C.; Schrimpf, J.E.; Teuber, S.S.; Roux, K.H.; Sathe, S.K. Effects of processing and storage on walnut (Juglans regia L) tannins. J. Sci. Food Agric. 2001, 81. [Google Scholar] [CrossRef]
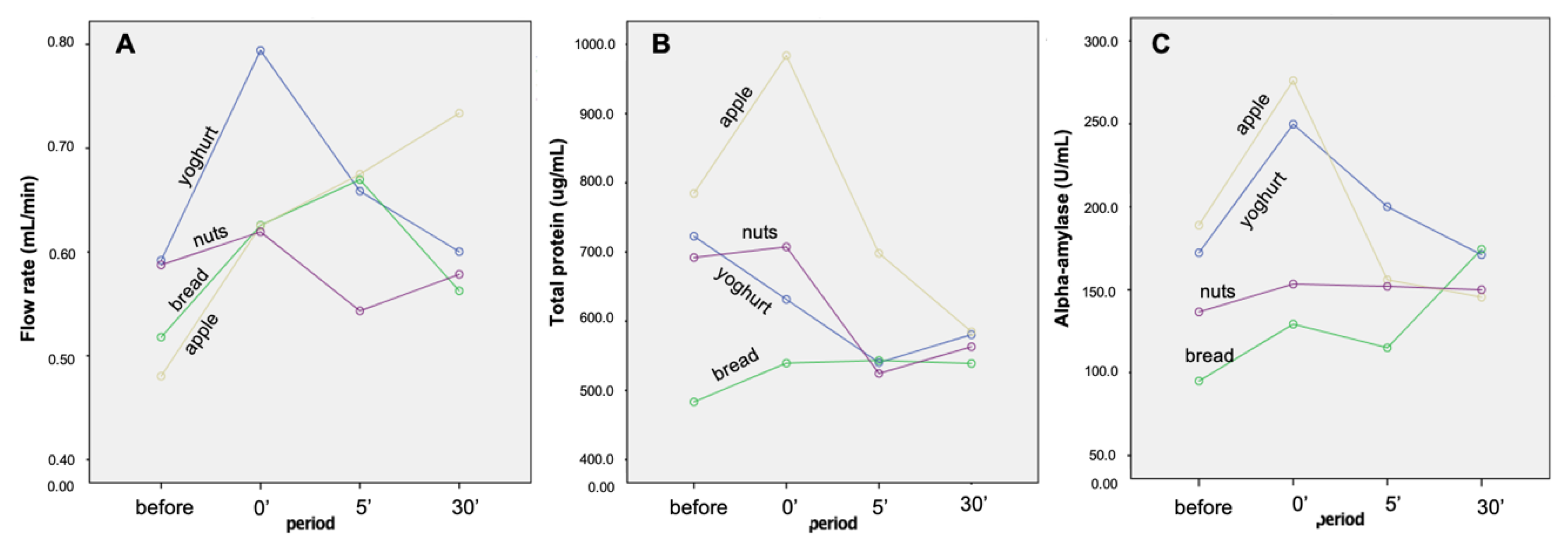
| Time | Band A | Band B | Band C | Band D | Band E | Band F | Band G | Band H | Band I | Band J | Band K | Band J + K | Flow Rate (mL/min) | Total Protein (µg/mL) | Amy_ Enzymatic Activity 1 (U/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | 7.3(0.2) A | 7.9(0.4) A | 9.5(0.5) | 14.0(0.9) | 10.2(0.5) | 2.5(0.3) A | 9.5(0.6) A | 4.9(0.4) | 6.5(0.4) | 9.3(0.6) A | 10.6(0.5) A | 18.9(0.8) A | 0.54(0.03) A | 670.7(38.9) A,B | 148.2(13.7) A |
| 0′ | 5.3(0.2) B | 8.1(0.4) A | 8.7(0.4) | 14.8(0.6) | 10.2(0.5) | 4.0(0.4) B | 7.4(0.3) B | 5.6(0.7) | 7.5(0.3) | 11.1(0.6) B | 12.1(0.6) B | 22.1(0.8) B | 0.67(0.04) B | 715.5(38.7) A | 202.2(14.3) B |
| 5′ after | 6.2(0.2) C | 9.2(0.5) B | 9.5(0.4) | 14.8(0.8) | 10.4(0.5) | 2.8(0.3) A | 9.0(0.4) A | 4.8(0.4) | 6.9(0.3) | 9.8(0.6) A,B | 10.6(0.7) A,B | 19.5(1.0) A | 0.64(0.03) A | 576.5(35.2) A,B | 155.8(16.3) A |
| 30′ after | 7.1(0.2) A | 8.2(0.4) A | 9.7(0.5) | 15.0(0.9) | 9.2(0.5) | 2.6(0.3) A | 10.1(0.5) A | 5.5(0.5) | 6.9(0.2) | 8.7(0.6) A | 9.8(0.6) A | 18.2(0.9) A | 0.62(0.04) A | 566.9(46.1) B | 160.3(15.6) A,B |
| p-value (effects) | |||||||||||||||
| Time | 0.0005 * | 0.012 * | 0.151 | 0.636 | 0.918 | 0.005 * | 0.0005 * | 0.437 | 0.176 | 0.025 * | 0.014 * | 0.0005 * | 0.007 * | 0.006 * | 0.007 * |
| Time * Snack | 0.273 | 0.0005 * | 0.909 | 0.243 | 0.747 | 0.298 | 0.684 | 0.498 | 0.833 | 0.539 | 0.114 | 0.224 | 0.031 * | 0.130 | 0.006 * |
| Protein Band | Protein ID | Accession Number (Uniprot) | MW (kDa) (Est/Theor.) # | MASCOT ID Score | N° Peptides Matched |
|---|---|---|---|---|---|
| A | Mixture (Polymeric Immunoglobulin receptor + Lactotransferrin) | P01833 + P02788 | 102.0/84.4 and 80.0 | 164 | 24 |
| B | Albumin | P02768 | 71.0/71.3 | 150 | 15 |
| C | Alpha-amylase 1 | P04745 | 62.0/58.4 | 154 | 16 |
| D | Alpha-amylase 1 | P04745 | 58.0/58.4 | 135 | 15 |
| E | Mixture (Carbonic Anhydrase VI + Zinc-alpha-2-glycoprotein) | P25311 + P23280 | 42.0/34.5 and 35.4 | 196 | 20 |
| F | n.i. | ___ | ___ | ___ | ___ |
| G | Ig Kappa chain C region | P01834 | 71 | 4 | |
| H | n.i | ___ | ___ | ___ | ___ |
| I | Prolactin-inducible protein | P12273 | 17.0/16.8 | 92 | 7 |
| J + K * | Cystatin SN | P01037 | 14.0/16.6 | 99 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simões, C.; Caeiro, I.; Carreira, L.; Silva, F.C.e.; Lamy, E. How Different Snacks Produce a Distinct Effect in Salivary Protein Composition. Molecules 2021, 26, 2403. https://doi.org/10.3390/molecules26092403
Simões C, Caeiro I, Carreira L, Silva FCe, Lamy E. How Different Snacks Produce a Distinct Effect in Salivary Protein Composition. Molecules. 2021; 26(9):2403. https://doi.org/10.3390/molecules26092403
Chicago/Turabian StyleSimões, Carla, Inês Caeiro, Laura Carreira, Fernando Capela e Silva, and Elsa Lamy. 2021. "How Different Snacks Produce a Distinct Effect in Salivary Protein Composition" Molecules 26, no. 9: 2403. https://doi.org/10.3390/molecules26092403
APA StyleSimões, C., Caeiro, I., Carreira, L., Silva, F. C. e., & Lamy, E. (2021). How Different Snacks Produce a Distinct Effect in Salivary Protein Composition. Molecules, 26(9), 2403. https://doi.org/10.3390/molecules26092403








