Influence of Prebiotic Fructans on Retronasal Aroma from Elderly Individuals
Abstract
1. Introduction
2. Results and Discussion
2.1. Effects of Oligofructose on the In Vivo Aroma Release from Elderly Individuals
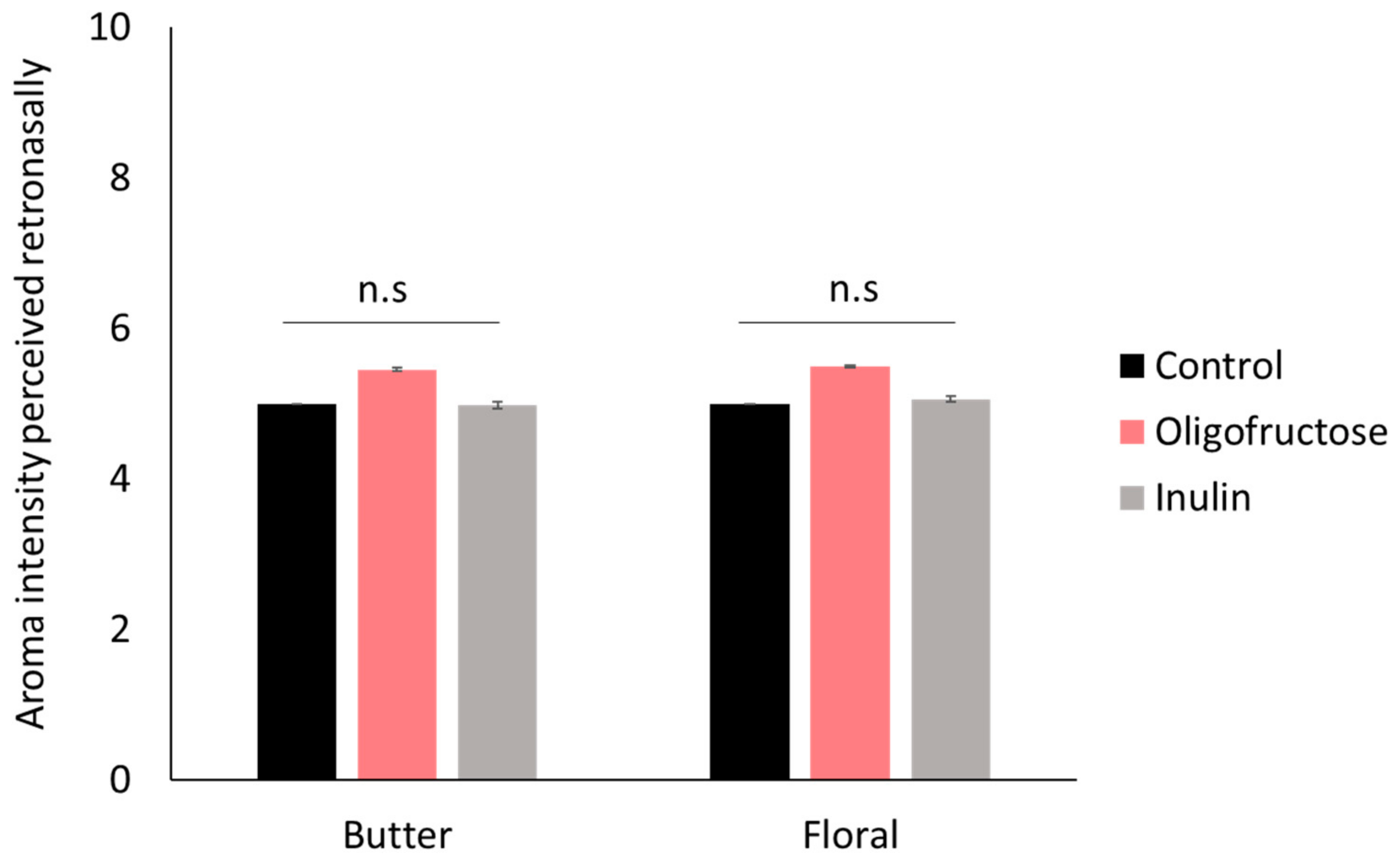
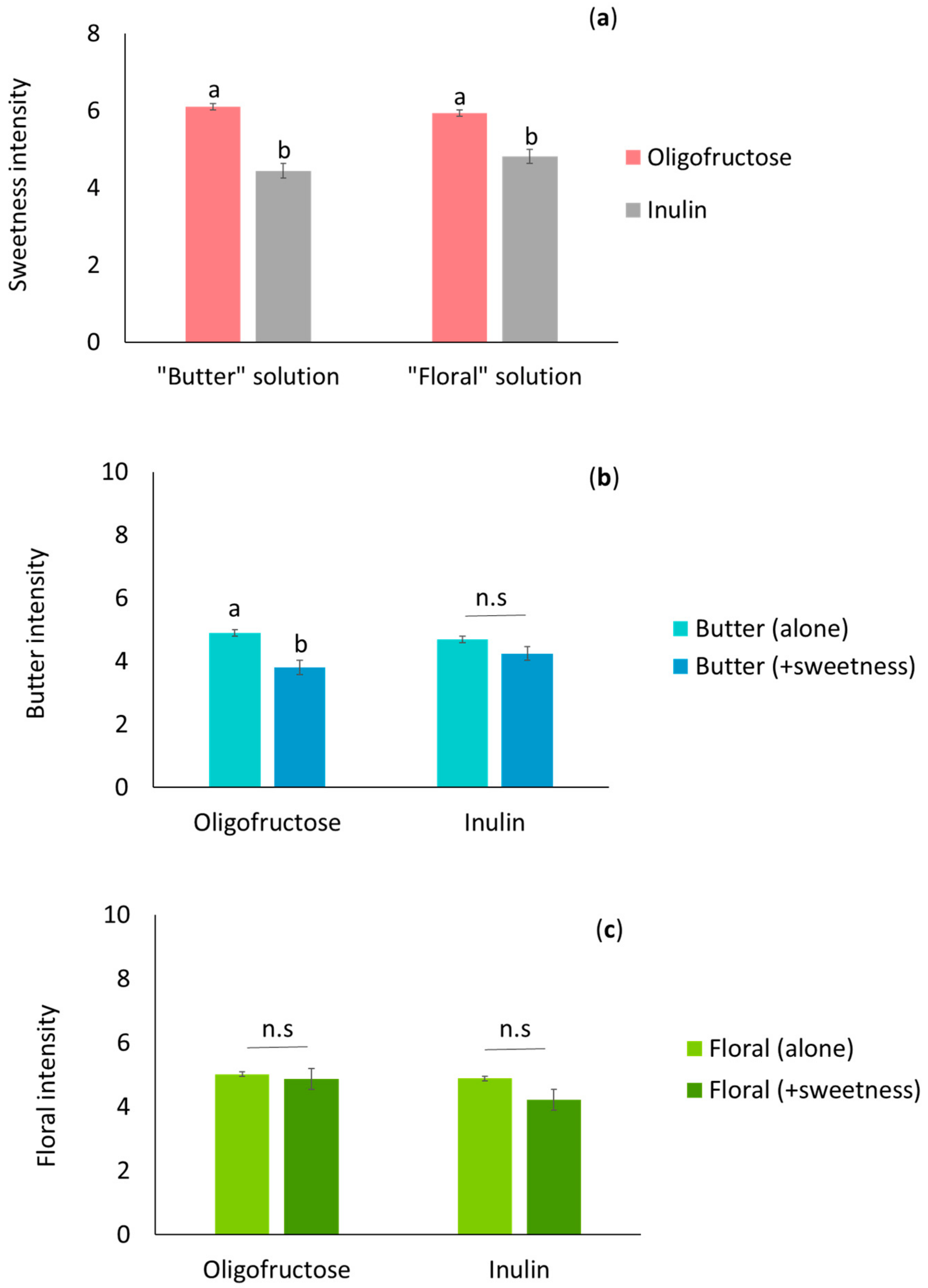
2.2. Effects of Prebiotic Fructans (Oligofructose and Inulin) on Aroma Perception in Elderly Individuals
3. Materials and Methods
3.1. Ingredients
3.2. In Vivo Retronasal Aroma Release by PTR-ToF-MS
3.3. Sensory Analyses
3.4. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tiihonen, K.; Ouwehand, A.C.; Rautonen, N. Human intestinal microbiota and healthy ageing. Ageing Res. Rev. 2010, 9, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Comparato, G.; Pilotto, A.; Franzè, A.; Franceschi, M.; Di Mario, F. Diverticular disease in the elderly. Dig. Dis. 2007, 25, 151–159. [Google Scholar] [CrossRef]
- Laurin, D.; Brodeur, J.M.; Bourdages, J.; Vallee, R.; Lachapelle, D. Fibre intake in elderly individuals with poor masticatory performance. J. Ca. Dent. Assoc. 1994, 60, 443–446, 449. [Google Scholar]
- Yoshida, M.; Kikutani, T.; Yoshikawa, M.; Tsuga, K.; Kimura, M.; Akagawa, Y. Correlation between dental and nutritional status in community-dwelling elderly Japanese. Geriatr. Gerontol. Int. 2011, 11, 315–319. [Google Scholar] [CrossRef]
- Affoo, R.H.; Foley, N.; Garrick, R.; Siqueira, W.L.; Martin, R.E. Meta-analysis of salivary flow rates in young and older adults. J. Am. Geriatr. Soc. 2015, 63, 2142–2151. [Google Scholar] [CrossRef]
- Peyron, M.A.; Santé-Lhoutellier, V.; François, O.; Hennequin, M. Oral declines and mastication deficiencies cause alteration of food bolus properties. Food Funct. 2018, 9, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-González, C.; Brulé, M.; Feron, G.; Canon, F. Does interindividual variability of saliva affect the release and metabolization of aroma compounds ex vivo? The particular case of elderly suffering or not from hyposalivation. J. Texture Stud. 2019, 50, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-González, C.; Vandenberghe-Descamps, M.; Feron, G.; Canon, F.; Labouré, H.; Sulmont-Rossé, C. Association between Salivary Hypofunction and Food Consumption in the Elderlies. A Systematic Literature Review. J. Nutr. Health Aging 2018, 22, 407–419. [Google Scholar] [CrossRef]
- Kossioni, A.E. The association of poor oral health parameters with malnutrition in older adults: A review considering the potential implications for cognitive impairment. Nutrients 2018, 10, 1709. [Google Scholar] [CrossRef]
- Aliani, M.; Udenigwe, C.C.; Girgih, A.T.; Pownall, T.L.; Bugera, J.L.; Eskin, M.N.A. Aroma and Taste Perceptions With Alzheimer Disease and Stroke. Crit. Rev. Food Sci. Nutr. 2013, 53, 760–769. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Al-Sheraji, S.H.; Ismail, A.; Manap, M.Y.; Mustafa, S.; Yusof, R.M.; Hassan, F.A. Prebiotics as functional foods: A review. J. Funct. Foods 2013, 5, 1542–1553. [Google Scholar] [CrossRef]
- Tayyebymoghadam S, E.M. Comparison of the effect of extracted inulin from native chicory root with commercial inulin on the viability of probiotics and physicochemical, rheological and sensory properties of synbiotic yogurt. JFST 2020, 17, 91–110. [Google Scholar]
- Majuwana, K.; Menaka, G.; Kariyawasam, M.; Lee, N.; Paik, H. Food Bioscience Synbiotic yoghurt supplemented with novel probiotic Lactobacillus brevis KU200019 and fructooligosaccharides. Food Biosci. 2021, 39, 100835. [Google Scholar] [CrossRef]
- Dolores, M.; Iriondo-dehond, A.; Iriondo-dehond, M.; Gonzalez, I.; Medrano, A.; Filip, R.; Uribarri, J. Healthy eating recommendations: Good for reducing dietary contribution to the body’s advanced glycation/lipoxidation end products pool? Nutr. Res. Rev. 2020, 1–6. [Google Scholar] [CrossRef]
- Chand, P.; Kumar, M.D.; Singh, A.K.; Deshwal, G.K.; Rao, P.S.; Tomar, S.K.; Sharma, H. Low-calorie synbiotic yoghurt from indigenous probiotic culture and combination of inulin and oligofructose: Improved sensory, rheological, and textural attributes. J. Food Process. Preserv. 2021, 45, e15322. [Google Scholar] [CrossRef]
- Al-shawi, S.G.; Dang, D.S.; Yousif, A.Y.; Al-younis, Z.K.; Najm, T.A.; Matarneh, S.K. The Potential Use of Probiotics to Improve Animal Health, Efficiency, and Meat Quality: A Review. Agriculture 2020, 10, 452. [Google Scholar] [CrossRef]
- Yousefvand, M.G.A.; Zarei, M.; Farhangnia, P. Developing novel synbiotic low-fat yogurt with fucoxylogalacturonan from tragacanth gum: Investigation of quality parameters and Lactobacillus casei survival. Food Sci. Nutr. 2020, 8, 4491–4504. [Google Scholar] [CrossRef]
- Ruiz-Aceituno, L.; Hernandez-Hernandez, O.; Moreno, F.J.; Methven, L. Sweetness and sensory properties of commercial and novel oligosaccharides of prebiotic potential. LWT Food Sci. Technol. 2018, 97, 476–482. [Google Scholar] [CrossRef]
- Siefarth, C.; Tyapkova, O.; Beauchamp, J.; Schweiggert, U.; Buettner, A.; Bader, S. Influence of polyols and bulking agents on flavour release from low-viscosity solutions. Food Chem. 2011, 129, 1462–1468. [Google Scholar] [CrossRef]
- Carr, J.; Baloga, D.; Guinard, J.; Lawter, L.; Marty, C.; Squire, C. The Effect of Gelling Agent Type and Concentration on Flavor Release in Model Systems. In Flavor-Food Interactions; American Chemical Society: Washington, DC, USA, 1996; pp. 98–108. [Google Scholar]
- Hansson, A.; Andersson, J.; Leufve, A. The efect of sugars and pectin on flavour release from a soft drink-related model system. Food Chem. 2001, 72, 363–368. [Google Scholar] [CrossRef]
- Hilippe, E.L.P.; Euvre, A.N.; Olas, B.E.C.; Angendorff, V.I.L.; Chippa, C.H.S.; Ndre, A.; Oilley, Ä.E.V. Behavior of Flavor Compounds in Model Food Systems: A Thermodynamic Study. J. Agric. Food Chem. 2003, 51, 1393–1398. [Google Scholar] [CrossRef]
- Malone, M.E.; Appelqvist, I.A.M.; Norton, I.T. Oral behaviour of food hydrocolloids and emulsions. Part 2. Taste and aroma release. Food Hydrocoll. 2003, 17, 775–784. [Google Scholar] [CrossRef]
- Ployon, S.; Brulé, M.; Andriot, I.; Morzel, M.; Canon, F. Understanding retention and metabolization of aroma compounds using an in vitro model of oral mucosa. Food Chem. 2020, 318, 126468. [Google Scholar] [CrossRef] [PubMed]
- Pangborn, R.M.; Szczesniak, A. Effect of hydrocolloids and viscosity on flavor and odor intensities of aromatic flavor compounds. J. Texture Stud. 1974, 4, 467–482. [Google Scholar] [CrossRef]
- Nahon, D.F.; Roozen, J.P.; Posthumus, M.A. Flavor Release from Mixtures of Sodium Cyclamate, Sucrose, and an Orange Aroma. J. Agric. Food Chem. 1998, 46, 4963–4968. [Google Scholar] [CrossRef]
- Goubet, I.; Voilley, A.J. Retention of Aroma Compounds by Carbohydrates: Influence of Their Physicochemical Characteristics and of Their Physical State. J. Agric. Food Chem. 1998, 46, 1981–1990. [Google Scholar] [CrossRef]
- Muñoz-gonzález, C.; Feron, G.; Brulé, M.; Canon, F. Understanding the release and metabolism of aroma compounds using micro-volume saliva samples by ex vivo approaches. Food Chem. 2018, 240, 275–285. [Google Scholar] [CrossRef]
- Roberts, D.D.; Acree, T.E. Effects of Heating and Cream Addition on Fresh Raspberry Aroma Using a Retronasal Aroma Simulator and Gas Chromatography Olfactometry. J. Agric. Food Chem. 1996, 44, 3919–3925. [Google Scholar] [CrossRef]
- Saint-Eve, A.; Déléris, I.; Aubin, E.; Semon, E.; Feron, G.; Rabillier, J.M.; Ibarra, D.; Guichard, E.; Souchon, I. Influence of composition (CO2 and sugar) on aroma release and perception of mint-flavored carbonated beverages. J. Agric. Food Chem. 2009, 57, 5891–5898. [Google Scholar] [CrossRef]
- Clark, C.C.; Lawless, H.T. Limiting response alternatives in time-intensity scaling: An examination of the halo-dumping effect. Chem. Senses 1994, 19, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-González, C.; Feron, G.; Canon, F. Physiological and oral parameters contribute prediction of retronasal aroma release in an elderly cohort. Food Chem. 2021, 342, 128355. [Google Scholar] [CrossRef]
- Thomas-Danguin, T.; Rouby, C.; Sicard, G.; Vigouroux, M.; Farget, V.; Johanson, A.; Bengtzon, A.; Hall, G. Development of the ETOC: A European Test of Olfactory Capabilities. Rhinology 2003, 41, 142–151. [Google Scholar] [PubMed]
- Abdi, H. What can cognitive psychology and sensory evaluation learn from each other? Food Qual. Prefer. 2002, 13, 445–451. [Google Scholar] [CrossRef]
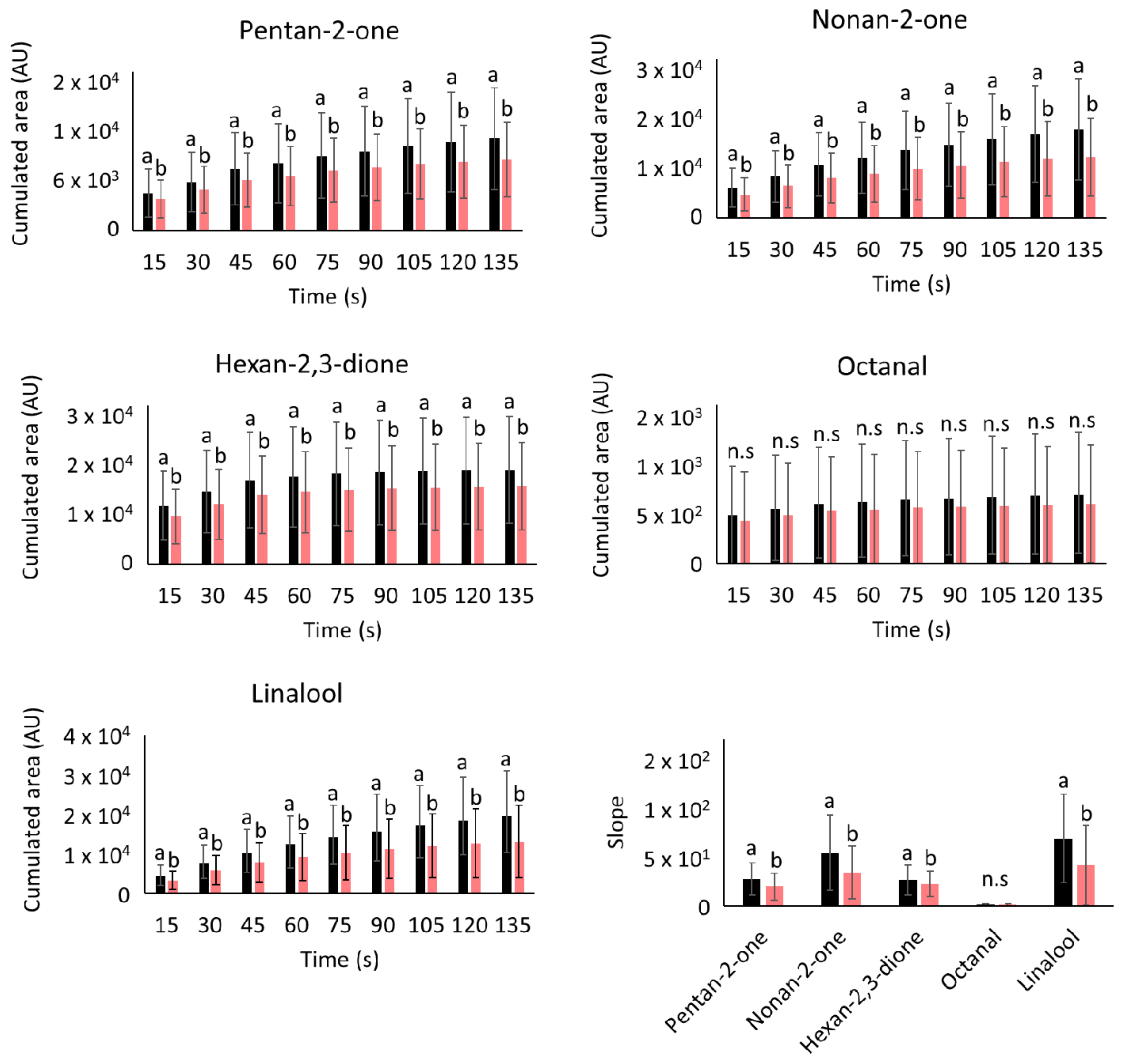
| Aroma Compounds | MW a (g/mol) | Log P b | BP c (°C) | Reduction in Aroma Released (%) |
|---|---|---|---|---|
| Hexan-2,3-dione | 114 | −0.35 | 162 | 17.29 ab |
| Pentan-2-one | 86 | 0.75 | 95 | 23.02 ab |
| Nonan-2-one | 142 | 2.70 | 185 | 31.22 ab |
| Octanal | 128 | 2.80 | 176 | 13.56 b |
| Linalool | 154 | 2.97 | 204 | 32.55 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-González, C.; Brule, M.; Martin, C.; Feron, G.; Canon, F. Influence of Prebiotic Fructans on Retronasal Aroma from Elderly Individuals. Molecules 2021, 26, 2906. https://doi.org/10.3390/molecules26102906
Muñoz-González C, Brule M, Martin C, Feron G, Canon F. Influence of Prebiotic Fructans on Retronasal Aroma from Elderly Individuals. Molecules. 2021; 26(10):2906. https://doi.org/10.3390/molecules26102906
Chicago/Turabian StyleMuñoz-González, Carolina, Marine Brule, Christophe Martin, Gilles Feron, and Francis Canon. 2021. "Influence of Prebiotic Fructans on Retronasal Aroma from Elderly Individuals" Molecules 26, no. 10: 2906. https://doi.org/10.3390/molecules26102906
APA StyleMuñoz-González, C., Brule, M., Martin, C., Feron, G., & Canon, F. (2021). Influence of Prebiotic Fructans on Retronasal Aroma from Elderly Individuals. Molecules, 26(10), 2906. https://doi.org/10.3390/molecules26102906







