Synthesis and Single Crystal Structures of N-Substituted Benzamides and Their Chemoselective Selenation/Reduction Derivatives
Abstract
1. Introduction
2. Results and Discussion
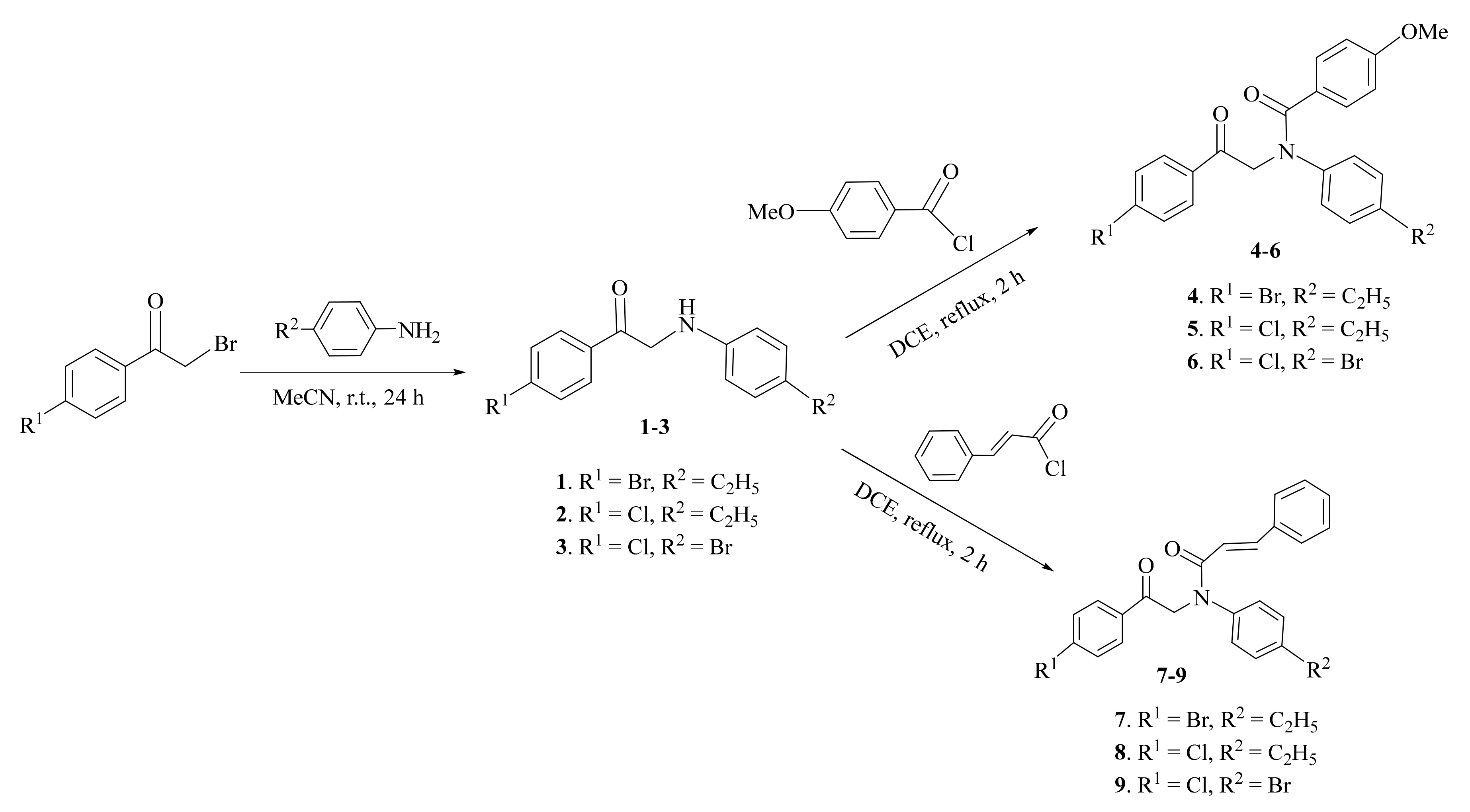
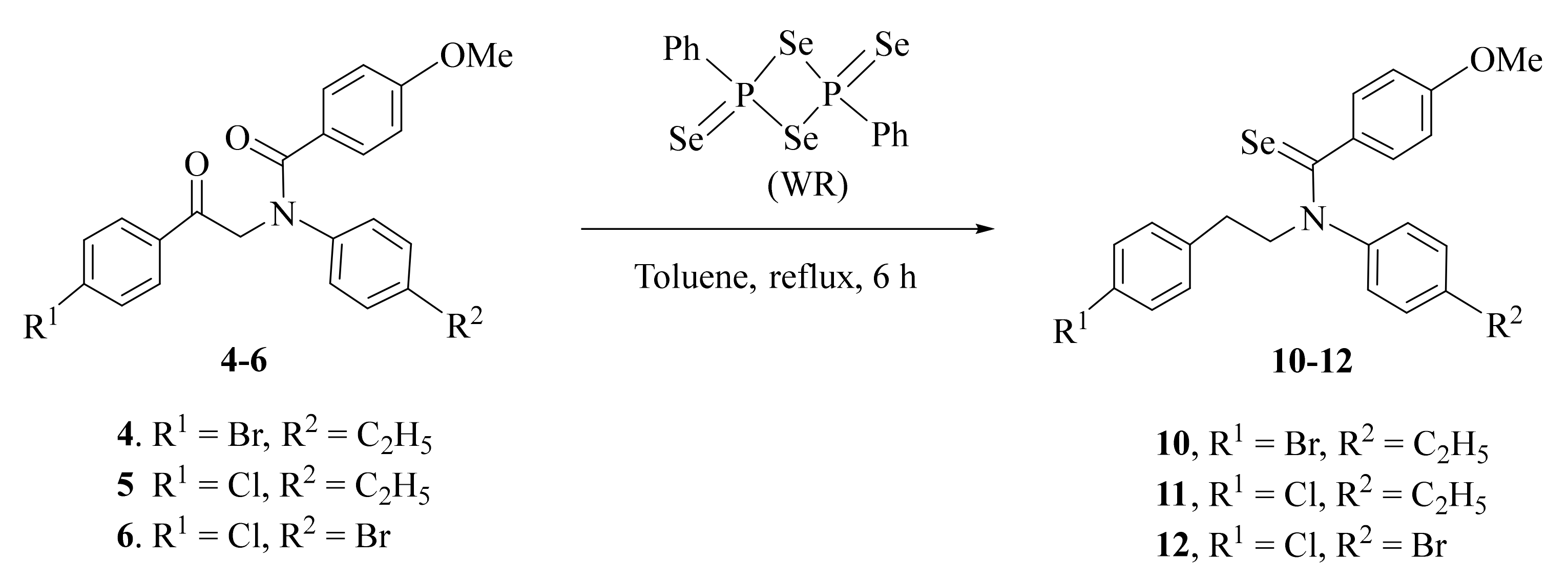
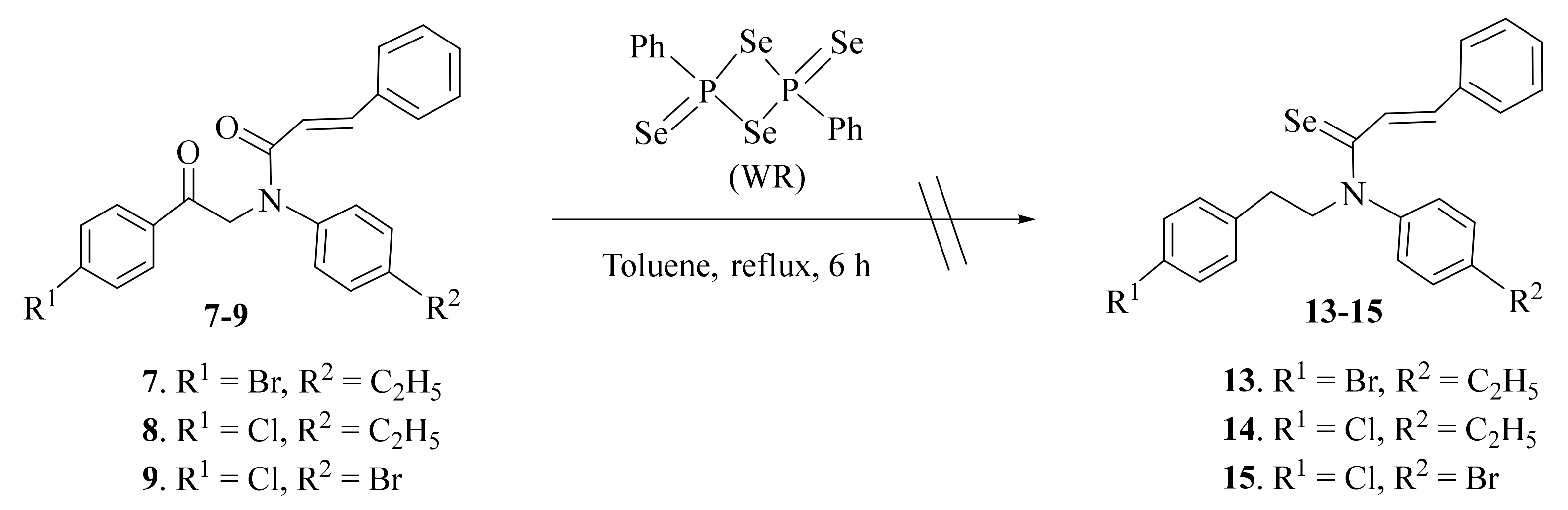
2.1. Synthesis and Characterization
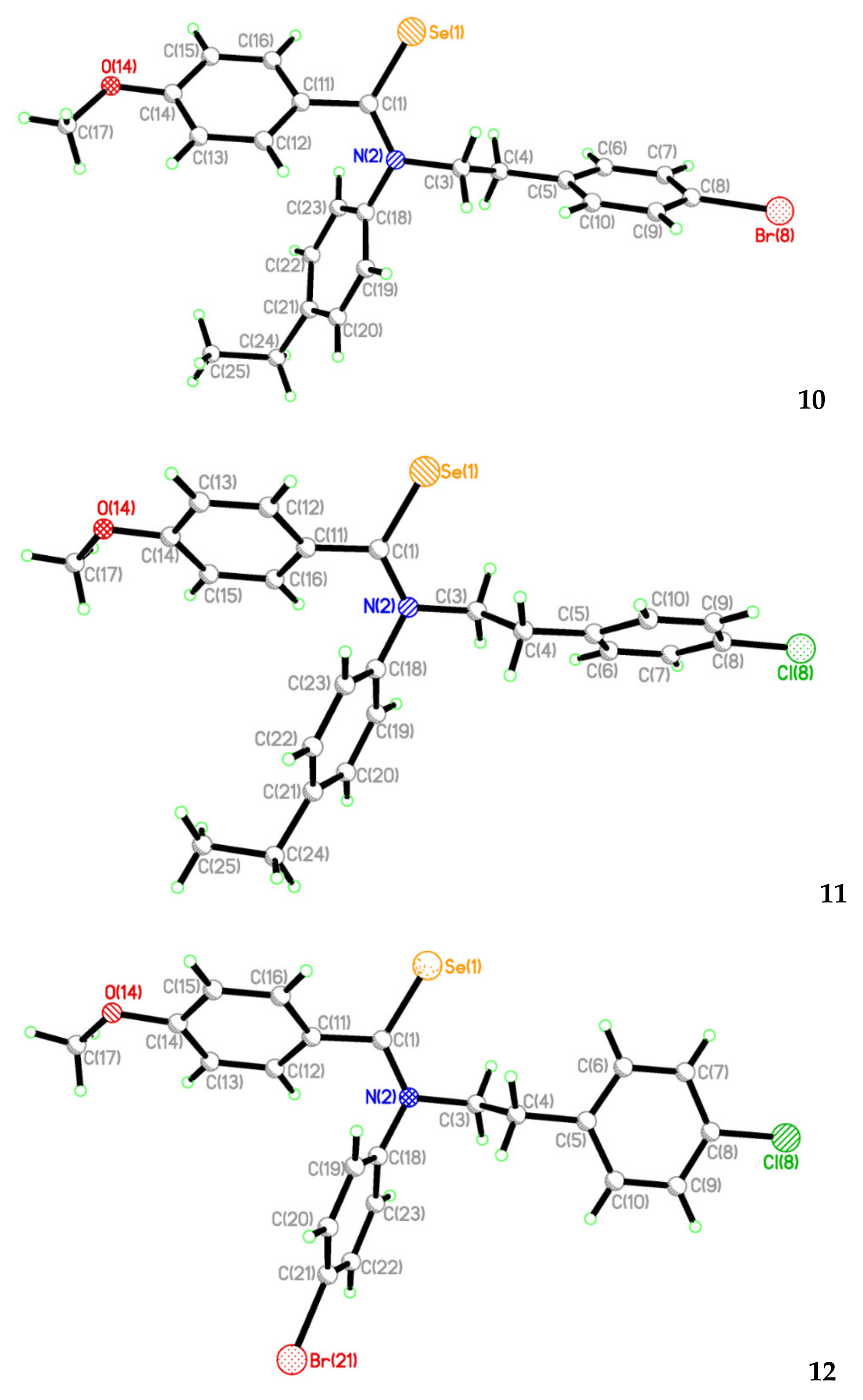
2.2. Single Crystal Structure Analysis
3. Materials and Methods
3.1. General
Crystallography
3.2. Synthesis
3.2.1. General Procedure for Synthesis of Compounds 1–3
3.2.2. General Procedure for Synthesis of Compounds 4–9
3.2.3. General Procedure for Synthesis of Compounds 10–12
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hua, G.; Woollins, J.D. Formation and reactivity of phosphorus-selenium rings. Angew. Chem. Int. Ed. 2009, 48, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Zhang, Q.; Li, Y.; Slawin, A.M.Z.; Woollins, J.D. Novel heterocyclic selenazadiphospholaminediselenides, zwitterionic carbamimidoyl(phenyl)phosphinodiselenoic acids and selenoureas derived from cyanamides. Tetrahedron 2009, 65, 6074–6082. [Google Scholar] [CrossRef]
- Hua, G.; Li, Y.; Fuller, A.L.; Slawin, A.M.Z.; Woollins, J.D. Facile synthesis and structure of novel 2,5-disubstituted 1,3,4-selenadiazoles. Eur. J. Org. Chem. 2009, 2009, 1612–1618. [Google Scholar] [CrossRef]
- Gómez Castaño, J.A.; Romano, R.M.; Beckers, H.; Willner, H.; Della Védova, C.O. Trifluoroselenoacetic acid, CF3C(O)SeH: Preparation and properties. Inorg. Chem. 2010, 49, 9972–9977. [Google Scholar] [CrossRef]
- Hua, G.; Li, Y.; Fuller, A.L.; Slawin, A.M.Z.; Woollins, J.D. Synthesis and X-ray structures of new phosphorus-selenium heterocycles with an E-P(Se)-E’ (E, E’ = N, S, Se) linkage. New J. Chem. 2010, 34, 1565–1571. [Google Scholar] [CrossRef]
- Huang, Y.; Jahreis, G.; Lücke, C.; Wildemann, D.; Fischer, G. Modulation of the Peptide Backbone Conformation by the Selenoxo Photoswitch. J. Am. Chem. Soc. 2010, 132, 7578–7579. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Fuller, A.; Slawin, A.M.; Woollins, J.D. Novel five- to ten-membered organoselenium heterocycles from the selenation of aryl-diols. Eur. J. Org. Chem. 2010, 2010, 2607–2615. [Google Scholar] [CrossRef]
- Hua, G.; Henry, J.B.; Li, Y.; Mount, A.R.; Slawin, A.M.Z.; Woollins, J.D. Synthesis of novel 2,5-diarylselenophenes from selenation of 1,4-diarylbutane-1,4-diones or methanol/arylacetylenes. Org. Biomol. Chem. 2010, 8, 1655–1660. [Google Scholar] [CrossRef]
- Hua, G.; Fuller, A.L.; Slawin, A.M.; Woollins, J.D. Formation of new organoselenium heterocycles and ring reduction of ten-membered heterocycles into seven-memered heterocycle. Polyhedron 2011, 30, 805–808. [Google Scholar] [CrossRef]
- Hua, G.; Fuller, A.L.; Buehl, M.; Slawin, A.M.; Woollins, J.D. Selenation/Thionation of α-Amino Acids: Formation and X-ray Structures of Diselenopiperazine and Dithiopiperazine and Related Compounds. Eur. J. Org. Chem. 2011, 2011, 3067–3073. [Google Scholar] [CrossRef]
- Hua, G.; Cordes, D.B.; Li, Y.; Slawin, A.M.; Woollins, J.D. Symmetrical organophosphorus spiroheterocycles from selenation of carbohydrazides. Tetrahedron Lett. 2011, 52, 3311–3314. [Google Scholar] [CrossRef]
- Wong, R.C.S.; Ooi, M.L. A new approach to coordination chemistry involving phosphorus-selenium based ligands: Ring opening, deselenation and phosphorus-–phosphorus coupling of Woollins’ Reagent. Inorg. Anica Chim. Acta 2011, 366, 350–356. [Google Scholar] [CrossRef]
- Gray, I.P.; Bhattacharyya, P.; Slawin, A.M.; Woollins, J.D. A new synthesis of (PhPSe2)2 (Woollins’ Reagnet) and its use in the synthesis of novel P-Se heterocycles. Chem. Eur. J. 2005, 11, 6221–6227. [Google Scholar] [CrossRef]
- Hua, G.; Li, Y.; Slawin, A.M.; Woollins, J.D. Synthesis and structure of eight-, nine- and ten-membered rings with P-Se-Se-P linkages. Angew. Chem. 2008, 120, 2899–2901. [Google Scholar]
- Hua, G.; Griffin, J.M.; Ashbrook, S.E.; Slawin, A.M.; Woollins, J.D. Octaselenocycododecane. Angew. Chem. Int. Ed. 2011, 123, 4209–4212. [Google Scholar] [CrossRef]
- Mandal, M.; Chatterjee, S.; Jaisankar, O. Woollins’ reagent: A chemoselective reducing agent for 1,4-enediones and 1,4-ynediones to saturated 1,4-diones. Synlett 2012, 23, 2615. [Google Scholar] [CrossRef]
- Pizzo, C.; Graciela, M. Woollins’ reagent promotes selective reduction of α, β-unsaturated thiazo and selenazolidinones. Tetrahedron Lett. 2017, 58, 1445–1447. [Google Scholar] [CrossRef]
- Yu, Y.; Furuyama, T.; Tang, J.; Wu, Z.Y.; Chen, J.Z.; Kobayashi, N.; Zhang, J.L. Stable iso-bacteriochlorin mimics from porpholactone: Effect of α,β-oxazolone moiety on the frontier π-molecular orbitals. Inorg. Chem. Front. 2015, 2, 671–677. [Google Scholar] [CrossRef]
- Wirth, T. Organoselenium chemistry in stereoselective reactions. Angew. Chem. Int. Ed. 2000, 39, 3740–3749. [Google Scholar] [CrossRef]
- Rhoden, C.R.; Zeni, G. New development of synthesis and reactivity of seleno- and tellurophenes. Org. Biomol. Chem. 2011, 9, 1301–1303. [Google Scholar] [CrossRef] [PubMed]
- Uemoto, T.; Emmanuel, M. S- Se-, Te-(perfluoroalkyl)dibenzothiophenium, -selenophenium, and -tellurophenium salts. Adv. Heterocycl. Chem. 1995, 64, 323–339. [Google Scholar] [CrossRef]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B.T. Organoselenium and Organotellurium Compounds: Toxicology and Pharmacology. Chemin Rev. 2005, 36, 6255–6286. [Google Scholar] [CrossRef]
- Lakner, F.J.; Parker, M.A.; Rogovoy, B.; Khvat, A.; Ivachtchenko, A. Synthesis of novel trisubstituted imidazolines. Synthesis 2009, 12, 1987–1990. [Google Scholar]
- Porretta, G.C.; Biava, M.; Fioravanti, R.; Fischetti, M.; Melino, C.; Venza, F.; Bolle, P.; Tita, B. Research on antibacterial and antifungal agents. VIII. Synthesis and antimicrobial activity of 1,4-diarylpyrroles. Eur. J. Med. Chem. 1992, 27, 717–722. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Yoshida, M.; Ohkawa, S.; Hamanaka, S. New agents for the selective reduction of the carbon-carbon double bond of alpha/betal unsaturated carbonyl compounds. J. Org. Chem. 1991, 56, 6720–6722. [Google Scholar] [CrossRef]
- Mesquita, K.D.; Waskow, B.; Schumacher, R.F.; Perin, G.; Jacob, R.G.; Alves, D. Glycerol/hypophosphorus acid and PhSeSePh: An efficient and selective system for reactions in the carbon-carbon double bond of (E)-chalcones. J. Braz. Chem. Soc. 2014, 25, 1261–1269. [Google Scholar]
- Lalezari, I.; Ghanbarpour, F.; Niazi, M.; Jafari-Namin, R. Selenium heterocycles XIV. 2,6-Diaryltetrahydroselenopyran-4-ones. J. Heterocycl. Chem. 1974, 11, 469–470. [Google Scholar] [CrossRef]
- Miyashita, M.; Yoshikoehi, A. Facile and highly efficient conjugate addition of benzeneselenol to α,β-unsaturated carbonyl compounds. Synthesis 1980, 8, 664–666. [Google Scholar] [CrossRef]
- Li, Y.; Hua, G.-X.; Slawin, A.M.Z.; Woollins, J.D. The X-ray Crystal Structures of Primary Aryl Substituted Selenoamides. Molecules 2009, 14, 884–892. [Google Scholar] [CrossRef] [PubMed]
- CrystalClear-SM Expert v2.1; Rigaku Americas: The Woodlands, TX, USA; Rigaku Corporation: Tokyo, Japan, 2015.
- CrysAlisPro v1.171.38.43.; Rigaku Oxford Diffraction; Rigaku Corporation: Oxford, UK, 2015.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Beurskens, P.T.; Beurskens, G.; de Gelder, R.; Garcia-Granda, S.; Gould, R.O.; Israel, R.; Smits, J.M.M. DIRDIF-99; Crystallography Laboratory, University of Nijmegen: Nijmegen, The Netherlands, 1999. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- CrystalStructure v4.3.0; Rigaku Americas: The Woodlands, TX, USA; Rigaku Corporation: Tokyo, Japan, 2018.

| Compound | 3 | 7 | 10 | 11 | 12 |
|---|---|---|---|---|---|
| Formula | C14H11BrClNO | C25H22ClNO2 | C24H24BrNOSe | C24H24ClNOSe | C22H19BrClNOSe |
| M | 324.60 | 403.91 | 501.32 | 456.87 | 507.72 |
| Temperature/K | 93 | 93 | 173 | 173 | 173 |
| Crystal system | triclinic | monoclinic | triclinic | triclinic | triclinic |
| Space group | P | P21/c | P | P | P |
| a/Å | 5.718 (3) | 6.00023 (18) | 9.2004 (17) | 9.1375 (12) | 9.98830 (10) |
| b/Å | 7.296 (4) | 22.8404 (6) | 10.541 (3) | 10.6864 (14) | 10.0160 (2) |
| c/Å | 15.461 (8) | 14.7270 (4) | 11.146 (3) | 11.1485 (15) | 12.3778 (18) |
| α | 95.472 (13) | 101.842 (4) | 103.062 (4) | 99.855 (14) | |
| β | 90.3200 (10) | 93.111 (3) | 94.351 (6) | 95.439 (3) | 110.822 (8) |
| γ | 906.126 (12) | 90.478 (6) | 91.412 (4) | 108.988 (12) | |
| U/A3 | 638.3 (6) | 2015.33 (10) | 1054.6 (5) | 1054.5 (2) | 1035.2 (2) |
| Z | 2 | 4 | 2 | 2 | 2 |
| µ/mm−1 | 3.424 | 0.211 | 3.695 | 1.922 | 3.890 |
| Reflections collected | 8491 | 34,715 | 14,106 | 21,665 | 21,452 |
| Independent reflections | 2296 | 4515 | 3785 | 3832 | 3776 |
| Rint | 0.0769 | 0.0669 | 0.0448 | 0.0283 | 0.0314 |
| R1 [I > 2σ (I)] | 0.0529 | 0.0341 | 0.0240 | 0.0304 | 0.0207 |
| wR2 | 0.1479 | 0.0883 | 0.0768 | 0.0973 | 0.0569 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, G.; Carpenter-Warren, C.L.; Cordes, D.B.; Slawin, A.M.Z.; Woollins, J.D. Synthesis and Single Crystal Structures of N-Substituted Benzamides and Their Chemoselective Selenation/Reduction Derivatives. Molecules 2021, 26, 2367. https://doi.org/10.3390/molecules26082367
Hua G, Carpenter-Warren CL, Cordes DB, Slawin AMZ, Woollins JD. Synthesis and Single Crystal Structures of N-Substituted Benzamides and Their Chemoselective Selenation/Reduction Derivatives. Molecules. 2021; 26(8):2367. https://doi.org/10.3390/molecules26082367
Chicago/Turabian StyleHua, Guoxiong, Cameron L. Carpenter-Warren, David B. Cordes, Alexandra M. Z. Slawin, and J. Derek Woollins. 2021. "Synthesis and Single Crystal Structures of N-Substituted Benzamides and Their Chemoselective Selenation/Reduction Derivatives" Molecules 26, no. 8: 2367. https://doi.org/10.3390/molecules26082367
APA StyleHua, G., Carpenter-Warren, C. L., Cordes, D. B., Slawin, A. M. Z., & Woollins, J. D. (2021). Synthesis and Single Crystal Structures of N-Substituted Benzamides and Their Chemoselective Selenation/Reduction Derivatives. Molecules, 26(8), 2367. https://doi.org/10.3390/molecules26082367








