[CrIII8NiII6]n+ Heterometallic Coordination Cubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Crystallographic Details
2.3. Magnetic and Spectroscopic Measurements
3. Results and Discussion
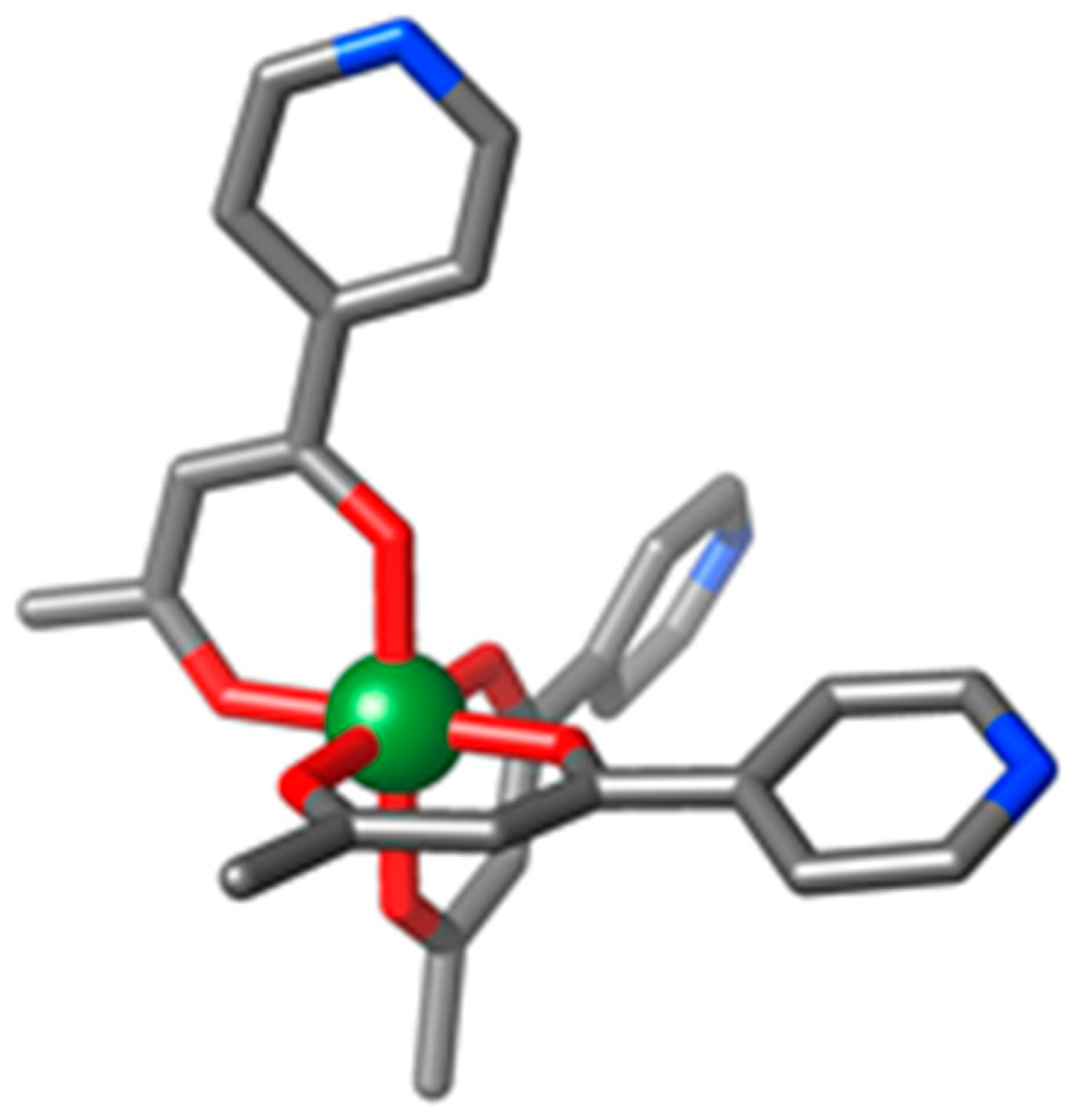
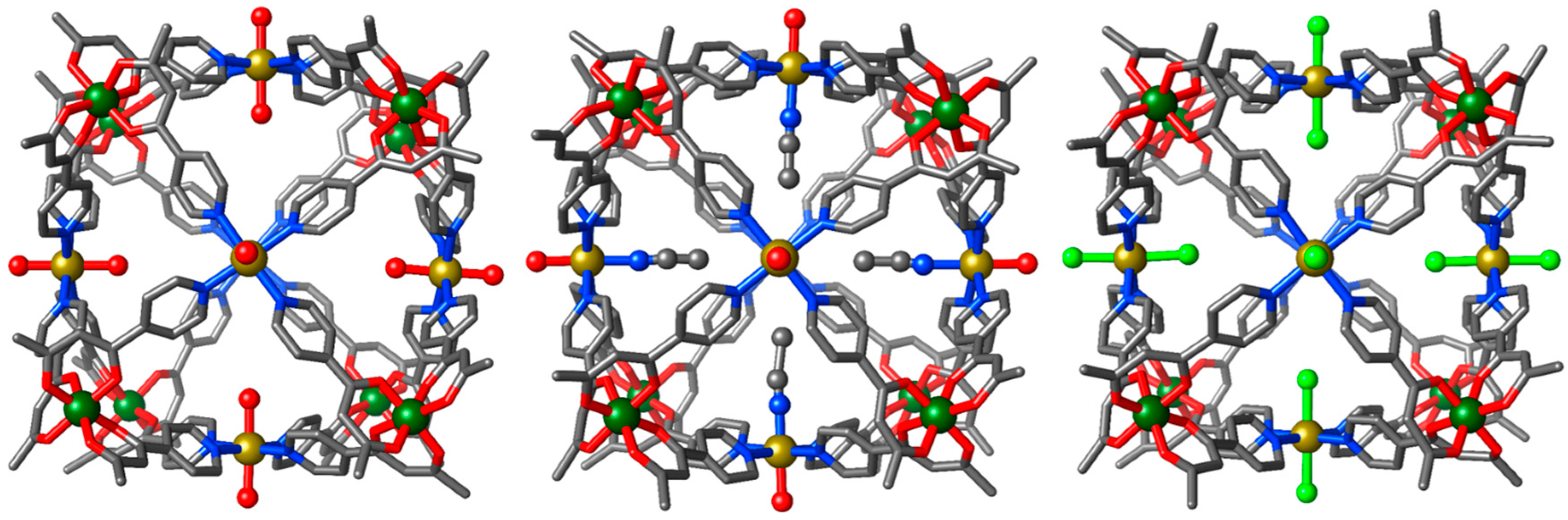
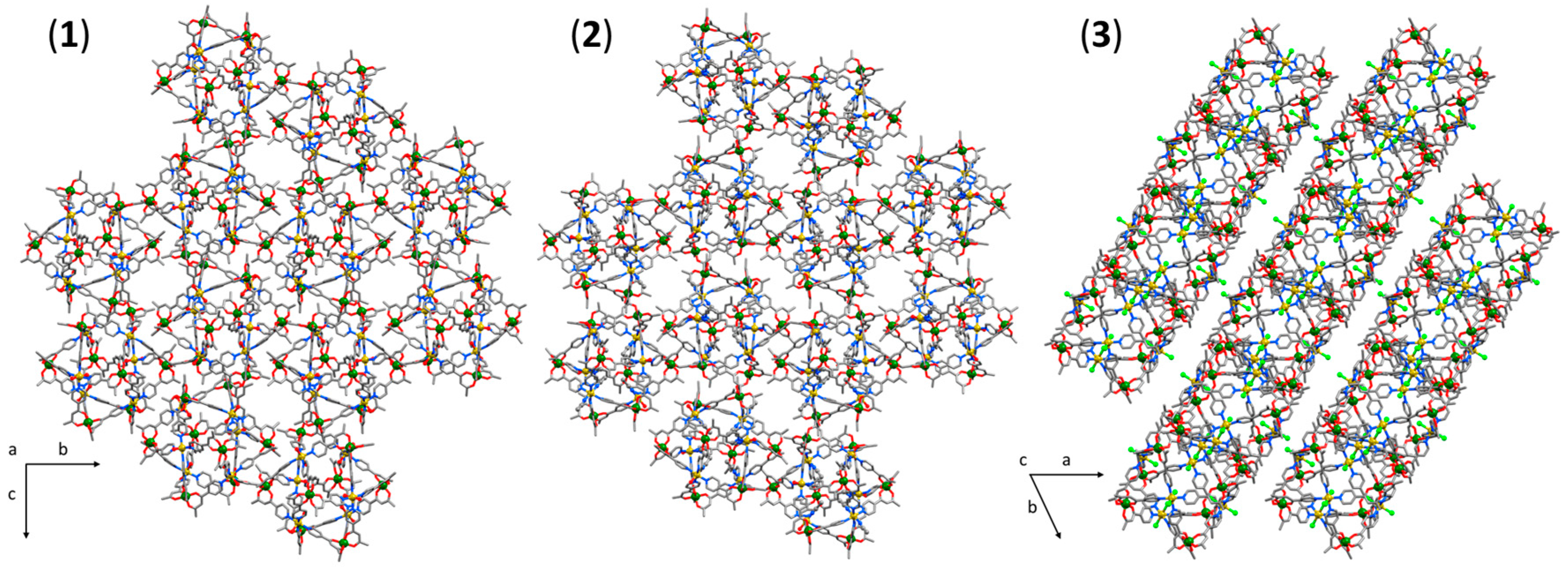
3.1. Structural Description
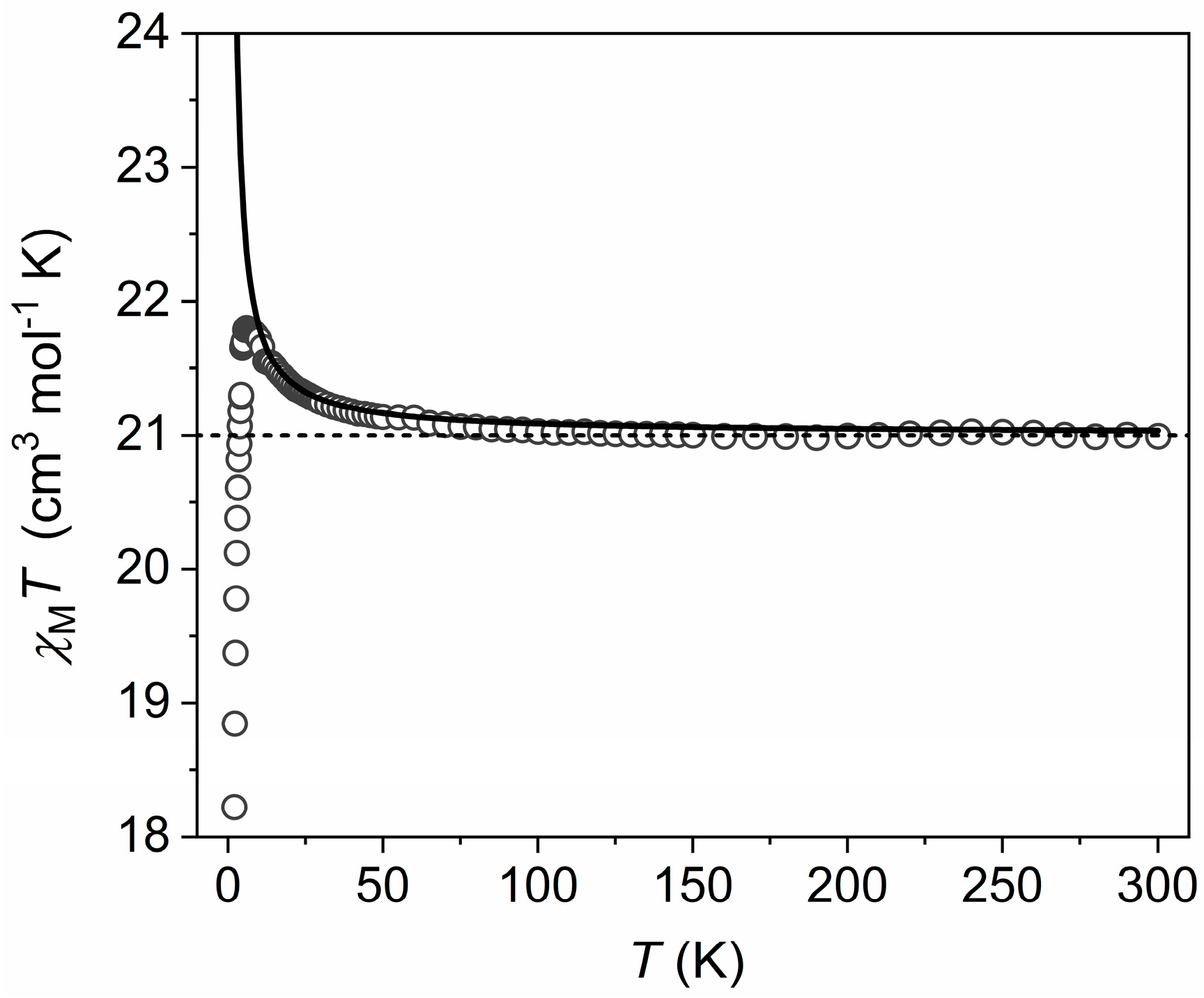
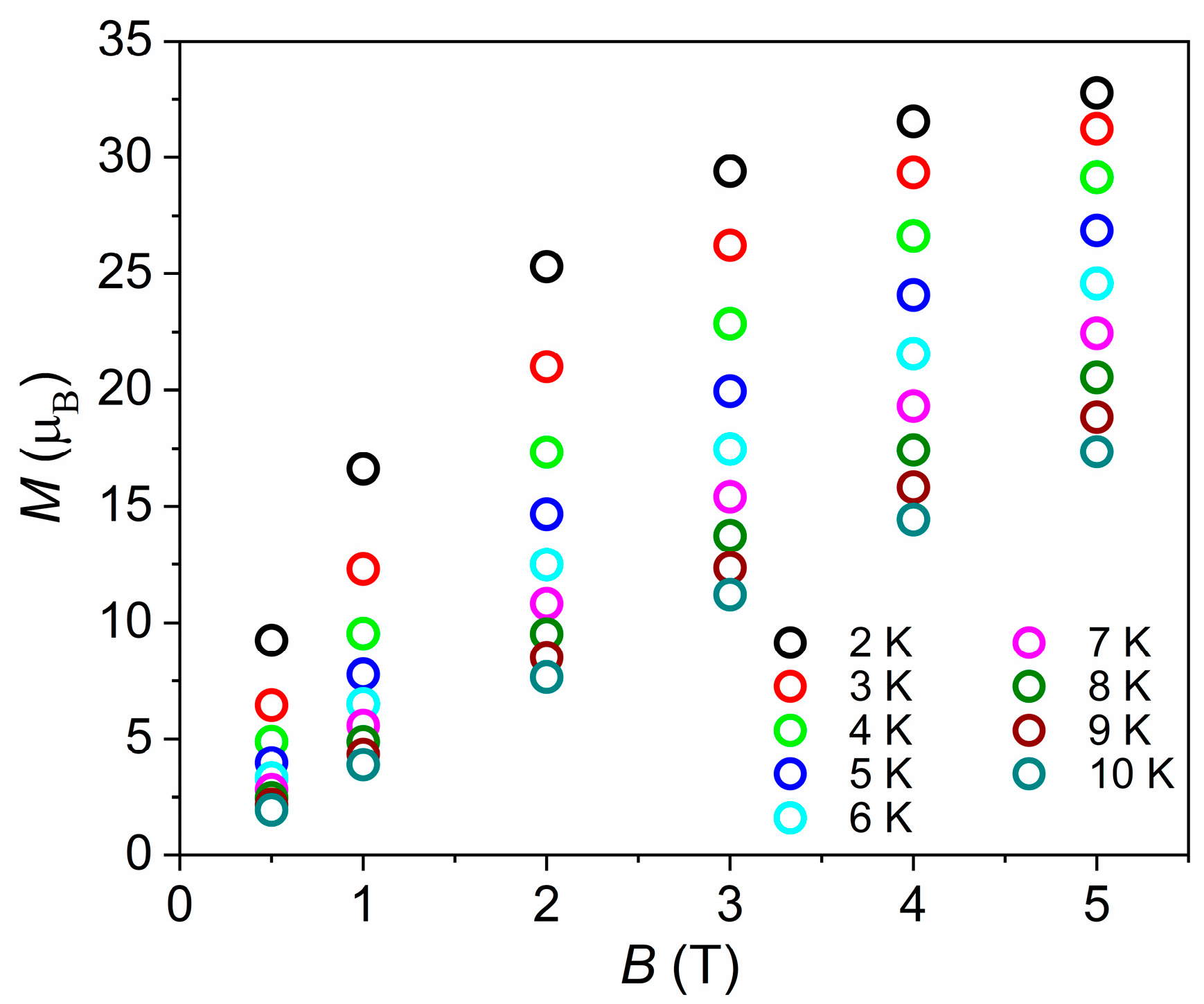
3.2. Magnetic Properties
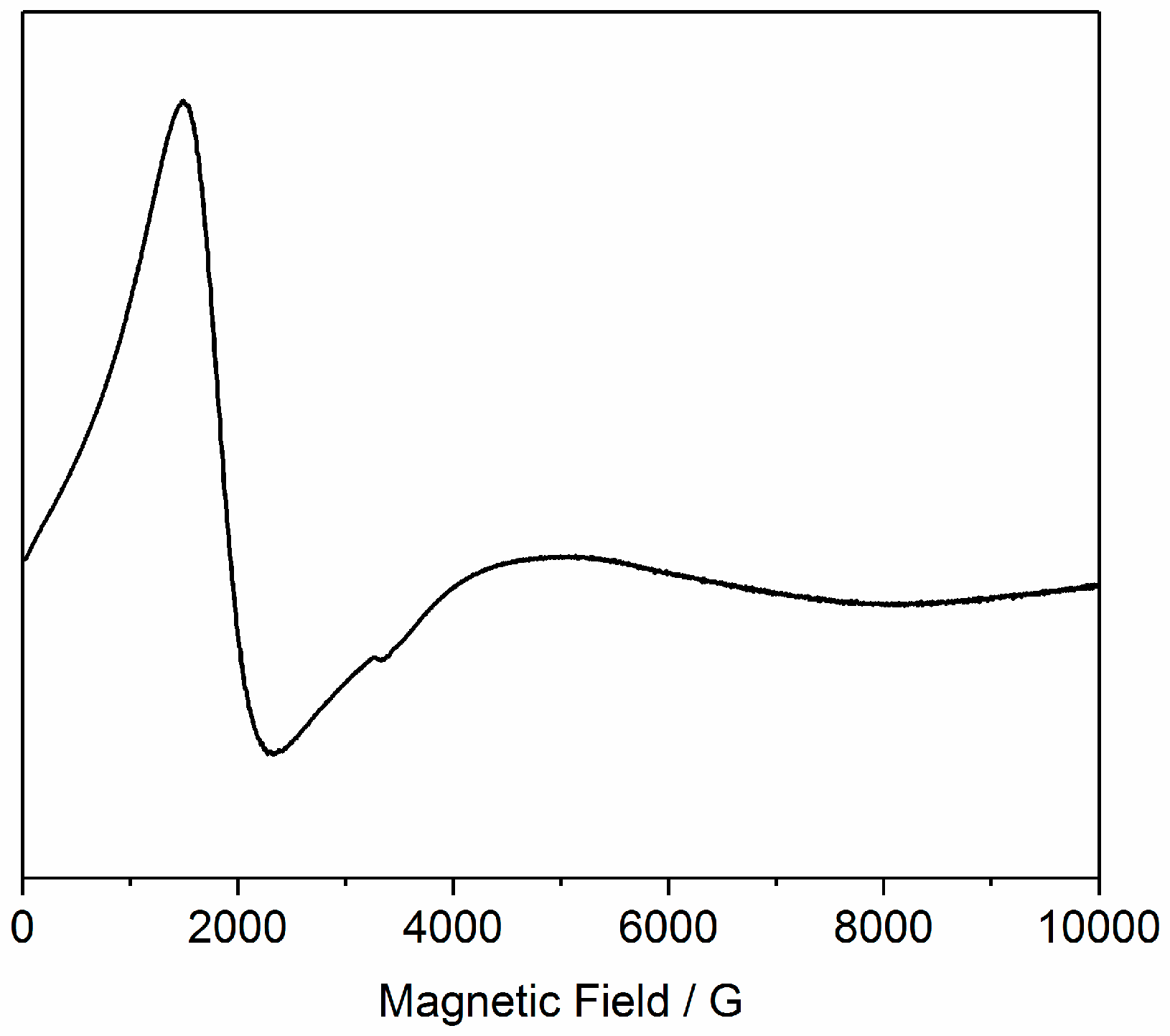
3.3. EPR Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barton, B.E.; Whaley, C.M.; Rauchfuss, T.B.; Gray, D.L. Nickel−Iron Dithiolato Hydrides Relevant to the [NiFe]-Hydrogenase Active Site. J. Am. Chem. Soc. 2009, 131, 6942–6943. [Google Scholar] [CrossRef]
- Canaguier, S.; Field, M.; Oudart, Y.; Pécaut, J.; Fontecave, M.; Artero, V. A structural and functional mimic of the active site of NiFe hydrogenases. Chem. Commun. 2010, 46, 5876–5878. [Google Scholar] [CrossRef]
- Buchwalter, P.; Rosé, J.; Braunstein, P. Multimetallic Catalysis Based on Heterometallic Complexes and Clusters. Chem. Rev. 2015, 115, 28–126. [Google Scholar] [CrossRef]
- Rice, A.M.; Leith, G.A.; Ejegbavwo, O.A.; Dolgopolova, E.A.; Shustova, N.B. Heterometallic Metal–Organic Frameworks (MOFs): The Advent of Improving the Energy Landscape. ACS Energy Lett. 2019, 4, 1938–1946. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Gao, W.X.; Lin, L.; Jin, G.X. Recent advances in the construction and applications of heterometallic macrocycles and cages. Coord. Chem. Rev. 2017, 344, 323–344. [Google Scholar] [CrossRef]
- Vigato, P.A.; Tamburini, S. Advances in acyclic compartmental ligands and related complexes. Coord. Chem. Rev. 2008, 252, 1871–1995. [Google Scholar] [CrossRef]
- Ferlay, S.; Mallah, T.; Ouahès, R.; Veillet, P.; Verdaguer, M. A room-temperature organometallic magnet based on Prussian blue. Nature 1995, 378, 701–703. [Google Scholar] [CrossRef]
- Alexandru, M.-G.; Visinescu, D.; Shova, S.; Lloret, F.; Julve, M.; Andruh, M. Two-Dimensional Coordination Polymers Constructed by [NiIILnIII] Nodes and [WIV(bpy)(CN)6]2− Spacers: A Network of [NiIIDyIII] Single Molecule Magnets. Inorg. Chem. 2013, 52, 11627–11637. [Google Scholar] [CrossRef]
- Yao, M.-X.; Wei, Z.-Y.; Gu, Z.-G.; Zheng, Q.; Xu, Y.; Zuo, J.-L. Syntheses, Structures, and Magnetic Properties of Low-Dimensional Heterometallic Complexes Based on the Versatile Building Block [(Tp)Cr(CN)3]−. Inorg. Chem. 2011, 50, 8636–8644. [Google Scholar] [CrossRef]
- Liu, W.; Wang, C.; Li, Y.; Zuo, J.; You, X. Structural and Magnetic Studies on Cyano-Bridged Rectangular Fe2M2 (M. = Cu, Ni) Clusters. Inorg. Chem. 2006, 45, 10058–10065. [Google Scholar] [CrossRef]
- Beltran, L.M.C.; Long, J.R. Directed Assembly of Metal−Cyanide Cluster Magnets. Acc. Chem. Res. 2005, 38, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Rebilly, J.N.; Mallah, T. Synthesis of Single-Molecule Magnets Using Metallocyanates. Single-Mol. Magn. Relat. Phenom. 2006, 103–131. [Google Scholar] [CrossRef]
- Evangelisti, M.; Brechin, E.K. Recipes for enhanced molecular cooling. Dalton Trans. 2010, 39, 4672–4676. [Google Scholar] [CrossRef] [PubMed]
- Schnack, J. Effects of frustration on magnetic molecules: A survey from Olivier Kahn until today. Dalton Trans. 2010, 39, 4677–4686. [Google Scholar] [CrossRef] [PubMed]
- Milios, C.J.; Winpenny, R.E.P. Cluster-Based Single-Molecule Magnets. Mol. Nanomagnets Relat. Phenom. 2015, 1–109. [Google Scholar] [CrossRef]
- Coulon, C.; Pianet, V.; Urdampilleta, M.; Clérac, R. Single-Chain Magnets and Related Systems. Mol. Nanomagnets Relat. Phenom. 2015, 143–184. [Google Scholar] [CrossRef]
- Gaita-Ariño, A.; Luis, F.; Hill, S.; Coronado, E. Molecular spins for quantum computation. Nat. Chem. 2019, 11, 301–309. [Google Scholar] [CrossRef]
- Larsen, F.K.; McInnes, E.J.L.; Mkami, H.E.; Overgaard, J.; Piligkos, S.; Rajaraman, G.; Rentschler, E.; Smith, A.A.; Boote, V.; Jennings, M.; et al. Synthesis and Characterization of Heterometallic {Cr7M} Wheels. Angew. Chem. Int. Ed. 2003, 115, 105–109. [Google Scholar] [CrossRef]
- Garlatti, E.; Guidi, T.; Ansbro, S.; Santini, P.; Amoretti, G.; Ollivier, J.; Mutka, H.; Timco, G.; Vitorica-Yrezabal, I.J.; Whitehead, G.F.S.; et al. Portraying entanglement between molecular qubits with four-dimensional inelastic neutron scattering. Nat. Commun. 2017, 8, 14543. [Google Scholar] [CrossRef]
- Timco, G.A.; McInnes, E.J.L.; Pritchard, R.G.; Tuna, F.; Winpenny, R.E.P. Heterometallic Rings Made From Chromium Stick Together Easily. Angew. Chem. Int. Ed. 2008, 47, 9681–9684. [Google Scholar] [CrossRef]
- Timco, G.A.; Batsanov, A.S.; Larsen, F.K.; Muryn, C.A.; Overgaard, J.; Teat, S.J.; Winpenny, R.E.P. Influencing the nuclearity and constitution of heterometallic rings via templates. Chem. Commun. 2005, 3649–3651. [Google Scholar] [CrossRef] [PubMed]
- Fraser, H.W.L.; Nichol, G.S.; Uhrín, D.; Nielsen, U.G.; Evangelisti, M.; Schnack, J.; Brechin, E. Order in disorder: Solution and solid-state studies of [MIII2MII5] wheels (MIII = Cr, Al; MII = Ni, Zn). Dalton Trans. 2018, 47, 11834–11842. [Google Scholar] [CrossRef] [PubMed]
- Kakaroni, F.E.; Collet, A.; Sakellari, E.; Tzimopoulos, D.I.; Siczek, M.; Lis, T.; Murri, M.; Milios, C.J. Constructing CrIII-centered heterometallic complexes: [NiII6CrIII] and [CoII6CrIII] wheels. Dalton Trans. 2018, 47, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Manole, O.S.; Batsanov, A.S.; Struchkov, Y.T.; Timko, G.A.; Synzheryan, L.D.; Gerbeleu, N.V. Synthesis and crystalline-structure of pentanuclear heterometallic pivaltoacetylacetonate complexes. Koord. Khim. 1994, 20, 231–237. [Google Scholar]
- Heath, S.L.; Laye, R.H.; Muryn, C.A.; Lima, N.; Sessoli, R.; Shaw, R.; Teat, S.J.; Timco, G.A.; Winpenny, R.E.P. Templating Open- and Closed-Chain Structures around Metal Complexes of Macrocycles. Angew. Chem. Int. Ed. 2004, 43, 6132–6135. [Google Scholar] [CrossRef]
- Sanz, S.; O’Connor, H.M.; Pineda, E.M.; Pedersen, K.S.; Nichol, G.S.; Mønsted, O.; Weihe, H.; Piligkos, S.; McInnes, E.J.L.; Lusby, P.J.; et al. [CrIII8MII6]12+Coordination Cubes (MII=Cu, Co). Angew. Chem. Int. Ed. 2015, 54, 6761–6764. [Google Scholar] [CrossRef]
- Sanz, S.; O’Connor, H.M.; Comar, P.; Baldansuren, F.T.A.; Pitak, M.B.; Coles, S.J.; Weihe, H.; Chilton, N.F.; McInnes, E.J.L.; Lusby, P.J.; et al. Modular [FeIII8MII6]n+ (MII = Pd, Co, Ni, Cu) Coordination Cages. Inorg. Chem. 2018, 57, 3500–3506. [Google Scholar] [CrossRef]
- O’Connor, H.M.; Sanz, S.; Pitak, M.B.; Coles, S.J.; Nichol, G.S.; Piligkos, S.; Lusby, P.J.; Brechin, E.K. [CrIII8MII6]n+ (MII = Cu, Co) face-centred, metallosupramolecular cubes. CrystEngComm 2016, 18, 4914–4920. [Google Scholar] [CrossRef]
- Sanz, S.; O’Connor, H.M.; Martí-Centelles, V.; Comar, P.; Pitak, M.B.; Coles, S.J.; Lorusso, G.; Palacios, E.; Evangelisti, M.; Baldansuren, F.T.A.; et al. [MIII2MII3]n+ trigonal bipyramidal cages based on diamagnetic and paramagnetic metalloligands. Chem. Sci. 2017, 8, 5526–5535. [Google Scholar] [CrossRef]
- Chakrabarty, R.; Mukherjee, P.S.; Stang, P.J. Supramolecular Coordination: Self-Assembly of Finite Two- and Three-Dimensional Ensembles. Chem. Rev. 2011, 111, 6810–6918. [Google Scholar] [CrossRef]
- Wu, H.-B.; Wang, Q.-M. Construction of Heterometallic Cages with Tripodal Metalloligands. Angew. Chem. Int. Ed. 2009, 48, 7343–7345. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Lesher, G.Y.; Pluncket, K.C.; Pagani, E.D.; Bode, D.C.; Bentley, R.G.; Connell, M.J.; Hamel, L.T.; Silver, P.J. Novel cAMP PDE III inhibitors: 1,6-naphthyridin-2(1H)-ones. J. Med. Chem. 1992, 35, 4858–4865. [Google Scholar] [CrossRef] [PubMed]
- Coles, S.J.; Gale, P.A. Changing and challenging times for service crystallography. Chem. Sci. 2011, 3, 683–689. [Google Scholar] [CrossRef]
- Rigaku, O.; CrysAlis, P.R.O.; Rigaku Oxford Diffraction. CrysAlisPro. 2016. Available online: https://www.rigaku.com/zh-hans/products/smc/crysalis (accessed on 18 January 2021).
- Kottke, T.; Stalke, D. Crystal handling at low temperatures. J. Appl. Crystallogr. 1993, 26, 615–619. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Blake, A.J.; Champness, N.R.; Schröder, M. OLEX: New software for visualization and analysis of extended crystal structures. J. Appl. Crystallogr. 2003, 36, 1283–1284. [Google Scholar] [CrossRef]
- Wong, S.S.M. Nuclear Statistical Spectroscopy; Oxford University Press: Oxford, UK; Clarendon Press: Oxford, UK, 1986. [Google Scholar]
- Otieno, T.; Thompson, R.C. Antiferromagnetism and metamagnetism in 1,4-diazine and pyridine complexes of nickel(II). Can. J. Chem. 1995, 73, 275–283. [Google Scholar] [CrossRef]
- Krzystek, J.; Ozarowski, A.; Telser, J. Multi-frequency, high-field EPR as a powerful tool to accurately determine zero-field splitting in high-spin transition metal coordination complexes. Coord. Chem. Rev. 2006, 250, 2308–2324. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Connor, H.M.; Sanz, S.; Scott, A.J.; Pitak, M.B.; Klooster, W.T.; Coles, S.J.; Chilton, N.F.; McInnes, E.J.L.; Lusby, P.J.; Weihe, H.; et al. [CrIII8NiII6]n+ Heterometallic Coordination Cubes. Molecules 2021, 26, 757. https://doi.org/10.3390/molecules26030757
O’Connor HM, Sanz S, Scott AJ, Pitak MB, Klooster WT, Coles SJ, Chilton NF, McInnes EJL, Lusby PJ, Weihe H, et al. [CrIII8NiII6]n+ Heterometallic Coordination Cubes. Molecules. 2021; 26(3):757. https://doi.org/10.3390/molecules26030757
Chicago/Turabian StyleO’Connor, Helen M., Sergio Sanz, Aaron J. Scott, Mateusz B. Pitak, Wim T. Klooster, Simon J. Coles, Nicholas F. Chilton, Eric J. L. McInnes, Paul J. Lusby, Høgni Weihe, and et al. 2021. "[CrIII8NiII6]n+ Heterometallic Coordination Cubes" Molecules 26, no. 3: 757. https://doi.org/10.3390/molecules26030757
APA StyleO’Connor, H. M., Sanz, S., Scott, A. J., Pitak, M. B., Klooster, W. T., Coles, S. J., Chilton, N. F., McInnes, E. J. L., Lusby, P. J., Weihe, H., Piligkos, S., & Brechin, E. K. (2021). [CrIII8NiII6]n+ Heterometallic Coordination Cubes. Molecules, 26(3), 757. https://doi.org/10.3390/molecules26030757





