Sulfanyl Porphyrazines with Morpholinylethyl Periphery—Synthesis, Electrochemistry, and Photocatalytic Studies after Deposition on Titanium(IV) Oxide P25 Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
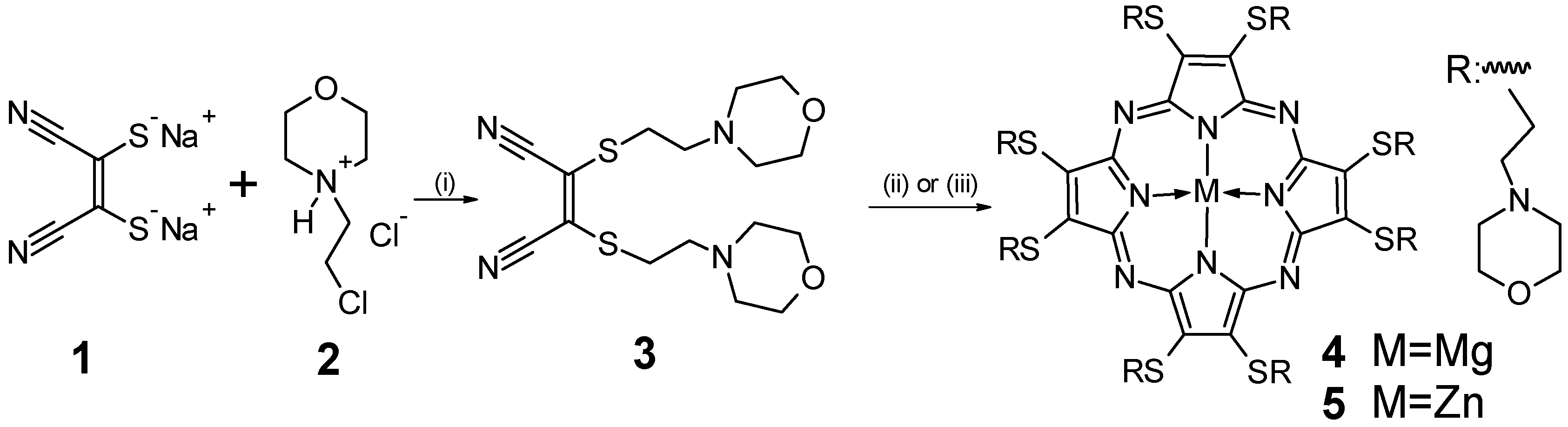
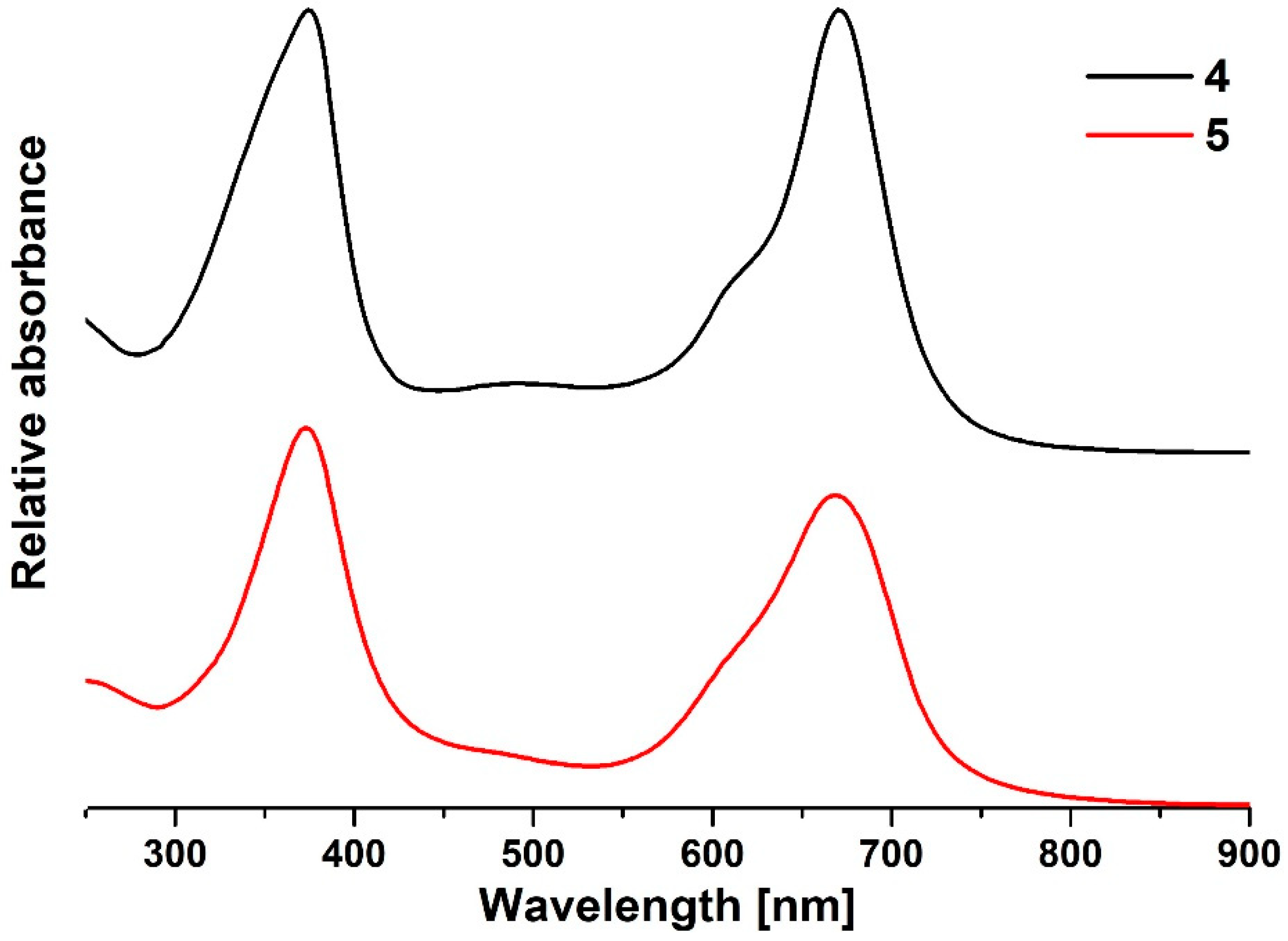
2.1. Synthesis and Physicochemical Characterization
2.2. Electrochemistry
2.3. TiO2 Deposition and Characterization
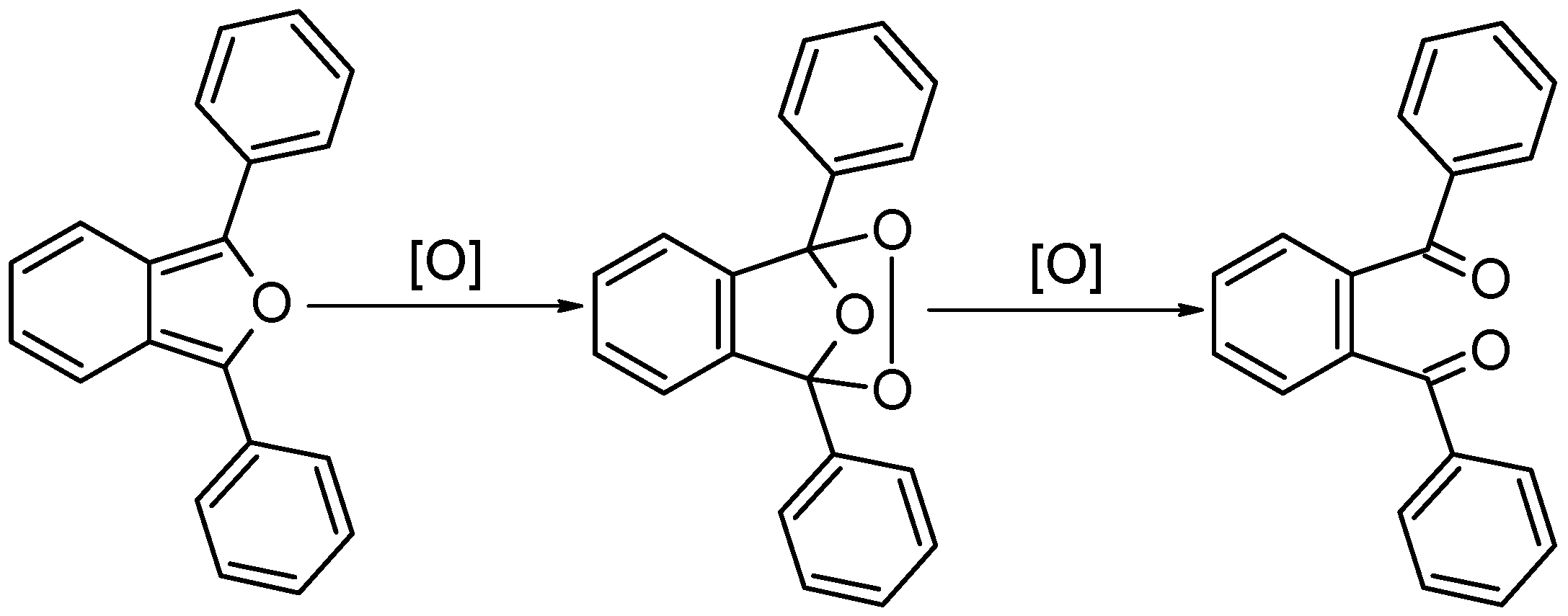
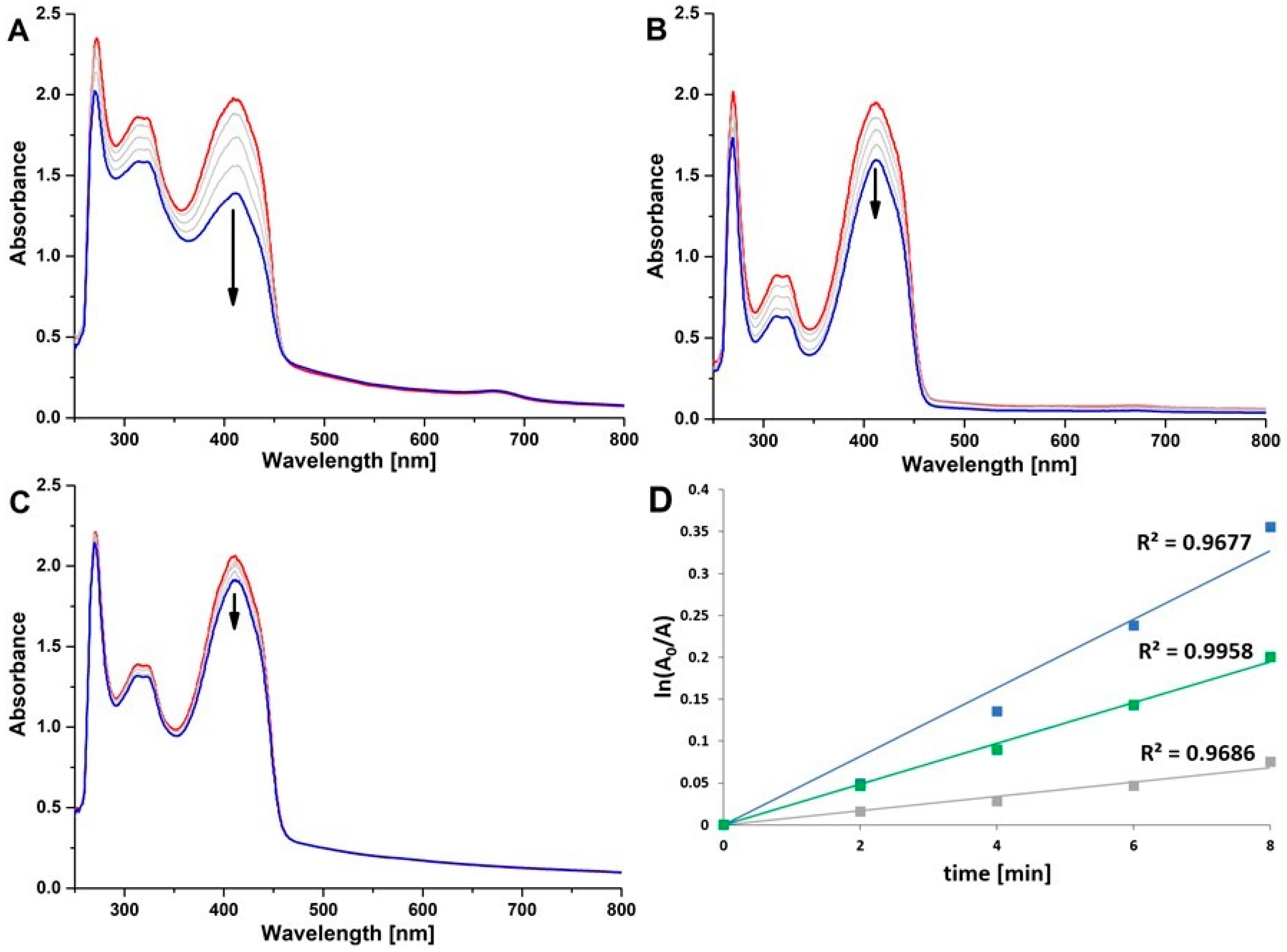
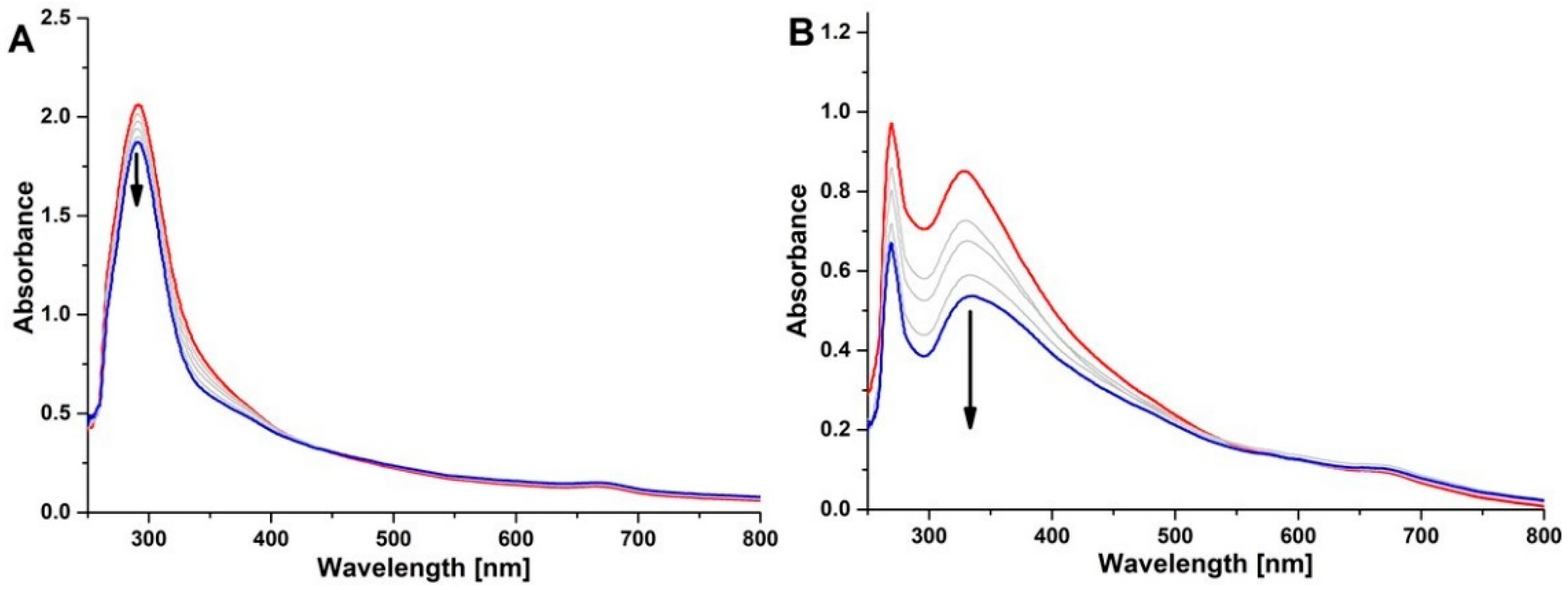
2.4. Photocatalysis
3. Materials and Methods
3.1. Materials and Instruments
3.2. Synthesis
3.3. Electrochemical Studies
3.4. Deposition of Porphyrazines on TiO2 P25 Nanoparticles
3.5. Photocatalytic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rodríguez-Morgade, M.S.; Stuzhin, P.A. The chemistry of porphyrazines: An overview. J. Porphyrins Phthalocyanines 2004, 8, 1129–1165. [Google Scholar] [CrossRef]
- Fuchter, M.J.; Zhong, C.; Zong, H.; Hoffman, B.M.; Barrett, A.G.M. Porphyrazines: Designer Macrocycles by Peripheral Substituent Change. Aust. J. Chem. 2008, 61, 235–255. [Google Scholar] [CrossRef]
- Belviso, S.; Ricciardi, G.; Lelj, F.; Scolaro, L.M.; Bencini, A.; Carbonera, C. Inducing asymmetry in free-base, MnIII, NiII and CuII (ethylsulfanyl)porphyrazines: Synthetic aspects and spectro-electrochemical implications. J. Chem. Soc. Dalton Trans. 2001, 1143–1150. [Google Scholar] [CrossRef]
- Belviso, S.; Giugliano, A.; Amati, M.; Ricciardi, G.; Lelj, F.; Scolaro, L.M. Two-electron reduction of alkyl(sulfanyl)porphyrazines: A route to free-base and peripherally metallated asymmetric porphyrazines. Dalton Trans. 2003, 305–312. [Google Scholar] [CrossRef]
- Belviso, S.; Capasso, A.; Santoro, E.; Najafi, L.; Lelj, F.; Superchi, S.; Casarini, D.; Villani, C.; Spirito, D.; Bellani, S.; et al. Thioethyl-Porphyrazine/Nanocarbon Hybrids for Photoinduced Electron Transfer. Adv. Funct. Mater. 2018, 28, 1705418. [Google Scholar] [CrossRef]
- Lochman, L.; Machacek, M.; Miletin, M.; Uhlířová, Š.; Lang, K.; Kirakci, K.; Zimcik, P.; Novakova, V. Red-Emitting Fluorescence Sensors for Metal Cations: The Role of Counteranions and Sensing of SCN– in Biological Materials. ACS Sens. 2019, 4, 1552–1559. [Google Scholar] [CrossRef]
- Cao, L.; Yang, C.; Zhang, B.; Lv, K.; Li, M.; Deng, K. Synergistic photocatalytic performance of cobalt tetra(2-hydroxymethyl-1,4-dithiin)porphyrazine loaded on zinc oxide nanoparticles. J. Hazard. Mater. 2018, 359, 388–395. [Google Scholar] [CrossRef]
- Belviso, S.; Santoro, E.; Penconi, M.; Righetto, S.; Tessore, F. Thioethyl Porphyrazines: Attractive Chromophores for Second-Order Nonlinear Optics and DSSCs. J. Phys. Chem. C 2019, 123, 13074–13082. [Google Scholar] [CrossRef]
- Yuzhakova, D.V.; Lermontova, S.A.; Grigoryev, I.S.; Muravieva, M.S.; Gavrina, A.I.; Shirmanova, M.V.; Balalaeva, I.V.; Klapshina, L.G.; Zagaynova, E.V. In vivo multimodal tumor imaging and photodynamic therapy with novel theranostic agents based on the porphyrazine framework-chelated gadolinium (III) cation. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 3120–3130. [Google Scholar] [CrossRef]
- Kunt, H.; Gonca, E. Synthesis and characterization of novel metal-free and metallo-porphyrazines with eight 3-thiopropylpentafluorobenzoate units. Polyhedron 2012, 38, 218–223. [Google Scholar] [CrossRef]
- Gonca, E. Metallo-porphyrazines with eight [5-thiopentyl 3,4,5-tris(benzyloxy)benzoate] groups: Synthesis, characterization, aggregation, and solubility behavior. J. Mol. Struct. 2017, 1130, 10–18. [Google Scholar] [CrossRef]
- Gonca, E. New soluble porphyrazine derivatives containing electron-rich substituents. J. Coord. Chem. 2013, 66, 1720–1729. [Google Scholar] [CrossRef]
- Yoshida, T.; Furuyama, T.; Kobayashi, N. Synthesis and optical properties of tetraazaporphyrin phosphorus(V) complexes with electron-rich heteroatoms. Tetrahedron Lett. 2015, 56, 1671–1674. [Google Scholar] [CrossRef]
- Fernandez-Ariza, J.; Urbani, M.; Rodriguez-Morgade, M.S.; Torres, T. Panchromatic Photosensitizers Based on Push-Pull, Unsymmetrically Substituted Porphyrazines. Chem. A Eur. J. 2017, 24, 2618–2625. [Google Scholar] [CrossRef]
- Tuncer, S.; Koca, A.; Gul, A.; Avcıata, U. Synthesis, characterization, electrochemistry and spectroelectrochemistry of novel soluble porphyrazines bearing unsaturated functional groups. Dye Pigment. 2012, 92, 610–618. [Google Scholar] [CrossRef]
- Belviso, S.; Amati, M.; Rossano, R.; Crispini, A.; Lelj, F. Non-symmetrical aryl- and arylethynyl-substituted thioalkyl-porphyrazines for optoelectronic materials: Synthesis, properties, and computational studies. Dalton Trans. 2014, 44, 2191–2207. [Google Scholar] [CrossRef] [PubMed]
- Falkowski, M.; Rebis, T.; Kryjewski, M.; Popenda, L.; Lijewski, S.; Jurga, S.; Mielcarek, J.; Milczarek, G.; Goslinski, T. An enhanced electrochemical nanohybrid sensing platform consisting of reduced graphene oxide and sulfanyl metalloporphyrazines for sensitive determination of hydrogen peroxide and l-cysteine. Dye Pigment. 2017, 138, 190–203. [Google Scholar] [CrossRef]
- Falkowski, M.; Rebis, T.; Piskorz, J.; Popenda, L.; Jurga, S.; Mielcarek, J.; Milczarek, G.; Goslinski, T. Multiwalled carbon nanotube/sulfanyl porphyrazine hybrids deposited on glassy carbon electrode—Effect of nitro peripheral groups on electrochemical properties. J. Porphyrins Phthalocyanines 2017, 21, 295–301. [Google Scholar] [CrossRef]
- Falkowski, M.; Rebis, T.; Piskorz, J.; Popenda, L.; Jurga, S.; Mielcarek, J.; Milczarek, G.; Goslinski, T. Improved electrocatalytic response toward hydrogen peroxide reduction of sulfanyl porphyrazine/multiwalled carbon nanotube hybrids deposited on glassy carbon electrodes. Dye Pigment 2016, 134, 569–579. [Google Scholar] [CrossRef]
- Koczorowski, T.; Rębiś, T.; Szczolko, W.; Antecka, P.; Teubert, A.; Milczarek, G.; Goslinski, T. Reduced graphene oxide/iron(II) porphyrazine hybrids on glassy carbon electrode for amperometric detection of NADH and L-cysteine. J. Electroanal. Chem. 2019, 848. [Google Scholar] [CrossRef]
- Rębiś, T.; Falkowski, M.; Milczarek, G.; Goslinski, T. Electrocatalytic NADH Sensing using Electrodes Modified with 2-[2-(4-Nitrophenoxy)ethoxy]ethylthio-Substituted Porphyrazine/Single-Walled Carbon Nanotube Hybrids. ChemElectroChem 2020, 7, 2838–2850. [Google Scholar] [CrossRef]
- Piskorz, J.; Lijewski, S.; Gierszewski, M.; Gorniak, K.; Sobotta, L.; Wicher, B.; Tykarska, E.; Düzgüneş, N.; Konopka, K.; Sikorski, M.; et al. Sulfanyl porphyrazines: Molecular barrel-like self-assembly in crystals, optical properties and in vitro photodynamic activity towards cancer cells. Dye Pigment 2017, 136, 898–908. [Google Scholar] [CrossRef]
- Piskorz, J.; Mlynarczyk, D.T.; Szczolko, W.; Konopka, K.; Düzgüneş, N.; Mielcarek, J. Liposomal formulations of magnesium sulfanyl tribenzoporphyrazines for the photodynamic therapy of cancer. J. Inorg. Biochem. 2018, 184, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Sobotta, L.; Skupin-Mrugalska, P.; Piskorz, J.; Mielcarek, J. Porphyrinoid photosensitizers mediated photodynamic inactivation against bacteria. Eur. J. Med. Chem. 2019, 175, 72–106. [Google Scholar] [CrossRef] [PubMed]
- Kabay, N.; Baygu, Y.; Alpoguz, H.K.; Kaya, A.; Gök, Y. Synthesis and characterization of porphyrazines as novel extractants for the removal of Ag(I) and Hg(II) from aqueous solution. Dye Pigment 2013, 96, 372–376. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, X.; Zhang, Z.; Zhang, B.; Deng, K. Effect of carrier and axial ligand on the photocatalytic activity of cobalt thioporphyrazine. Chin. J. Catal. 2017, 38, 330–336. [Google Scholar] [CrossRef]
- Yang, C.; Gao, L.; Zhang, B.; Zhang, Z.; Deng, K. Uniform zinc thioporphyrazine nanosphere by self-assembly and the photocatalytic performance. J. Porphyrins Phthalocyanines 2018, 22, 868–876. [Google Scholar] [CrossRef]
- Zhang, Z.; Wen, X.; Deng, K.; Zhang, B.; Lv, K.; Sun, J. Photodegradation of rhodamine B with molecular oxygen catalyzed by a novel unsymmetrical iron porphyrazine under simulated sunlight. Catal. Sci. Technol. 2013, 3, 1415–1422. [Google Scholar] [CrossRef]
- Yang, C.; Sun, J.; Deng, K.; Wang, D. Synthesis and photocatalytic properties of iron(II)tetramethyl- tetra(1,4-dithiin)porphyrazine. Catal. Commun. 2008, 9, 321–326. [Google Scholar] [CrossRef]
- Su, R.; Sun, J.; Sun, Y.; Deng, K.; Cha, D.; Wang, D. Oxidative degradation of dye pollutants over a broad pH range using hydrogen peroxide catalyzed by FePz(dtnCl2)4. Chemosphere 2009, 77, 1146–1151. [Google Scholar] [CrossRef]
- Tang, J.; Chen, L.; Sun, J.; Lv, K.; Deng, K. Synthesis and properties of iron(II) tetra(1,4-dithiin)porphyrazine bearing peripheral long-chain alkyl group of active end-bromine. Inorg. Chem. Commun. 2010, 13, 236–239. [Google Scholar] [CrossRef]
- Wieczorek, E.; Mlynarczyk, D.T.; Kucinska, M.; Dlugaszewska, J.; Piskorz, J.; Popenda, L.; Szczolko, W.; Jurga, S.; Murias, M.; Mielcarek, J.; et al. Photophysical properties and photocytotoxicity of free and liposome-entrapped diazepinoporphyrazines on LNCaP cells under normoxic and hypoxic conditions. Eur. J. Med. Chem. 2018, 150, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Mlynarczyk, D.T.; Lijewski, S.; Falkowski, M.; Piskorz, J.; Szczolko, W.; Sobotta, L.; Stolarska, M.; Popenda, L.; Jurga, S.; Konopka, K.; et al. Dendrimeric Sulfanyl Porphyrazines: Synthesis, Physico-Chemical Characterization, and Biological Activity for Potential Applications in Photodynamic Therapy. ChemPlusChem 2016, 81, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Mlynarczyk, D.T.; Dlugaszewska, J.; Falkowski, M.; Popenda, L.; Kryjewski, M.; Szczolko, W.; Jurga, S.; Mielcarek, J.; Goslinski, T. Tribenzoporphyrazines with dendrimeric peripheral substituents and their promising photocytotoxic activity against Staphylococcus aureus. J. Photochem. Photobiol. B Biol. 2020, 204, 111803. [Google Scholar] [CrossRef] [PubMed]
- Mlynarczyk, D.T.; Piskorz, J.; Popenda, L.; Stolarska, M.; Szczolko, W.; Konopka, K.; Jurga, S.; Sobotta, L.; Mielcarek, J.; Düzgüneş, N.; et al. S-seco-porphyrazine as a new member of the seco-porphyrazine family—Synthesis, characterization and photocytotoxicity against cancer cells. Bioorg. Chem. 2020, 96, 103634. [Google Scholar] [CrossRef]
- Lijewski, S.; Gierszewski, M.; Sobotta, L.; Piskorz, J.; Kordas, P.; Kucinska, M.; Baranowski, D.; Gdaniec, Z.; Murias, M.; Karolczak, J.; et al. Photophysical properties and photochemistry of a sulfanyl porphyrazine bearing isophthaloxybutyl substituents. Dye Pigment 2015, 113, 702–708. [Google Scholar] [CrossRef]
- Gierszewski, M.; Falkowski, M.; Sobotta, L.; Stolarska, M.; Popenda, L.; Lijewski, S.; Wicher, B.; Burdzinski, G.; Karolczak, J.; Jurga, S.; et al. Porphyrazines with peripheral isophthaloxyalkylsulfanyl substituents and their optical properties. J. Photochem. Photobiol. A Chem. 2015, 307–308, 54–67. [Google Scholar] [CrossRef]
- Falkowski, M.; Kucinska, M.; Piskorz, J.; Wieczorek-Szweda, E.; Popenda, L.; Jurga, S.; Sikora, A.; Mlynarczyk, D.T.; Murias, M.; Marszall, M.P.; et al. Synthesis of sulfanyl porphyrazines with bulky peripheral substituents—Evaluation of their photochemical properties and biological activity. J. Photochem. Photobiol. A Chem. 2021, 405, 112964. [Google Scholar] [CrossRef]
- Kucinska, M.; Skupin-Mrugalska, P.; Szczolko, W.; Sobotta, L.; Sciepura, M.; Tykarska, E.; Wierzchowski, M.; Teubert, A.; Fedoruk-Wyszomirska, A.; Wyszko, E.; et al. Phthalocyanine Derivatives Possessing 2-(Morpholin-4-yl)ethoxy Groups As Potential Agents for Photodynamic Therapy. J. Med. Chem. 2015, 58, 2240–2255. [Google Scholar] [CrossRef] [PubMed]
- Dlugaszewska, J.; Szczolko, W.; Koczorowski, T.; Skupin-Mrugalska, P.; Teubert, A.; Konopka, K.; Kucinska, M.; Murias, M.; Düzgüneş, N.; Mielcarek, J.; et al. Antimicrobial and anticancer photodynamic activity of a phthalocyanine photosensitizer with N -methyl morpholiniumethoxy substituents in non-peripheral positions. J. Inorg. Biochem. 2017, 172, 67–79. [Google Scholar] [CrossRef]
- Kucinska, M.; Plewinski, A.; Szczolko, W.; Kaczmarek, M.; Goslinski, T.; Murias, M. Modeling the photodynamic effect in 2D versus 3D cell culture under normoxic and hypoxic conditions. Free Radic. Biol. Med. 2021, 162, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Rak, J.; Pouckova, P.; Benes, J.; Vetvicka, D. Drug Delivery Systems for Phthalocyanines for Photodynamic Therapy. Anticancer Res. 2019, 39, 3323–3339. [Google Scholar] [CrossRef]
- Ghosh, S.; Carter, K.A.; Lovell, J.F. Liposomal formulations of photosensitizers. Biomaterials 2019, 218, 119341. [Google Scholar] [CrossRef]
- Chelminiak-Dudkiewicz, D.; Rybczynski, P.; Smolarkiewicz-Wyczachowski, A.; Mlynarczyk, D.T.; Wegrzynowska-Drzymalska, K.; Ilnicka, A.; Goslinski, T.; Marszałł, M.P.; Ziegler-Borowska, M. Photosensitizing potential of tailored magnetite hybrid nanoparticles functionalized with levan and zinc (II) phthalocyanine. Appl. Surf. Sci. 2020, 524, 146602. [Google Scholar] [CrossRef] [PubMed]
- Aksenova, N.A.; Savko, M.A.; Uryupina, O.Y.; Roldugin, V.I.; Timashev, P.S.; Kuz’Min, P.G.; Shafeev, G.A.; Solov’Eva, A.B. Effect of the preparation method of silver and gold nanoparticles on the photosensitizing properties of tetraphenylporphyrin–amphiphilic polymer—nanoparticle systems. Russ. J. Phys. Chem. A 2017, 91, 124–129. [Google Scholar] [CrossRef]
- Bera, K.; Maiti, S.; Maity, M.; Mandal, C.; Maiti, N.C. Porphyrin–Gold Nanomaterial for Efficient Drug Delivery to Cancerous Cells. ACS Omega 2018, 3, 4602–4619. [Google Scholar] [CrossRef] [PubMed]
- Setaro, F.; Wennink, J.W.H.; Mäkinen, P.I.; Holappa, L.; Trohopoulos, P.N.; Ylä-Herttuala, S.; Van Nostrum, C.F.; De La Escosura, A.; Torres, T. Amphiphilic phthalocyanines in polymeric micelles: A supramolecular approach toward efficient third-generation photosensitizers. J. Mater. Chem. B 2020, 8, 282–289. [Google Scholar] [CrossRef]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef]
- Musial, J.; Krakowiak, R.; Mlynarczyk, D.T.; Goslinski, T.; Stanisz, B.J. Titanium Dioxide Nanoparticles in Food and Personal Care Products—What Do We Know about Their Safety? Nanomaterials 2020, 10, 1110. [Google Scholar] [CrossRef]
- Lü, X.-F.; Li, J.; Wang, C.; Duan, M.-Y.; Luo, Y.; Yao, G.-P.; Wang, J.-L. Enhanced photoactivity of CuPp-TiO2 photocatalysts under visible light irradiation. Appl. Surf. Sci. 2010, 257, 795–801. [Google Scholar] [CrossRef]
- Forsyth, T.P.; Williams, D.B.G.; Montalban, A.G.; Stern, C.L.; Barrett, A.G.M.; Hoffman, B.M. A Facile and Regioselective Synthesis of Trans-Heterofunctionalized Porphyrazine Derivatives. J. Org. Chem. 1998, 63, 331–336. [Google Scholar] [CrossRef]
- Linstead, R.P.; Whalley, M. 944. Conjugated macrocylces. Part XXII. Tetrazaporphin and its metallic derivatives. J. Chem. Soc. 1952, 1952, 4839–4846. [Google Scholar] [CrossRef]
- Roncucci, G.; Dei, D.; De Filippis, M.P.; Fantetti, L.; Masini, I.; Cosimelli, B.; Jori, G. Zinc-Phthalocyanines and Corresponding Conjugates, Their Preparation and Use in Photodynamic Therapy and as Diagnostic Agents. U.S. Patent 5,965,598, 12 October 1999. [Google Scholar]
- Koczorowski, T.; Ber, J.; Sokolnicki, T.; Teubert, A.; Szczolko, W.; Goslinski, T. Electrochemical and catalytic assessment of peripheral bromoaryl-substituted manganese and iron porphyrazines. Dye Pigment 2020, 178, 108370. [Google Scholar] [CrossRef]
- Tuncer, S.; Koca, A.; Gül, A.; Avciata, U. 1,4-Dithiaheterocycle-fused porphyrazines: Synthesis, characterization, voltammetric and spectroelectrochemical properties. Dye Pigment 2009, 81, 144–151. [Google Scholar] [CrossRef]
- Fuchter, M.J.; Beall, L.S.; Baum, S.M.; Montalban, A.G.; Sakellariou, E.G.; Mani, N.S.; Miller, T.; Vesper, B.J.; White, A.J.; Williams, D.J.; et al. Synthesis of porphyrazine-octaamine, hexamine and diamine derivatives. Tetrahedron 2005, 61, 6115–6130. [Google Scholar] [CrossRef]
- Kryjewski, M.; Tykarska, E.; Rębiś, T.; Dlugaszewska, J.; Ratajczak, M.; Teubert, A.; Gapiński, J.; Patkowski, A.; Piskorz, J.; Milczarek, G.; et al. Porphyrazine with bulky 2-(1-adamantyl)-5-phenylpyrrol-1-yl periphery tuning its spectral and electrochemical properties. Polyhedron 2015, 98, 217–223. [Google Scholar] [CrossRef]
- Rębiś, T.; Lijewski, S.; Nowicka, J.; Popenda, L.; Sobotta, L.; Jurga, S.; Mielcarek, J.; Milczarek, G.; Goslinski, T. Electrochemical properties of metallated porphyrazines possessing isophthaloxybutylsulfanyl substituents: Application in the electrocatalytic oxidation of hydrazine. Electrochim. Acta 2015, 168, 216–224. [Google Scholar] [CrossRef]
- Hajri, A.; Touaiti, S.; Jamoussi, B. Preparation of Organic Zn-Phthalocyanine-Based Semiconducting Materials and Their Optical and Electrochemical Characterization. Adv. Optoelectron. 2013, 2013, 321563. [Google Scholar] [CrossRef]
- Pommerehne, J.; Vestweber, H.; Guss, W.; Mahrt, R.F.; Bässler, H.; Porsch, M.; Daub, J. Efficient two layer leds on a polymer blend basis. Adv. Mater. 1995, 7, 551–554. [Google Scholar] [CrossRef]
- Sakamoto, K.; Furuya, N.; Soga, H.; Yoshino, S. Cyclic voltammetry of non-peripheral thioaryl substituted phthalocyanines. Dye Pigment 2013, 96, 430–434. [Google Scholar] [CrossRef]
- Sułek, A.; Pucelik, B.; Kuncewicz, J.; Dubin, G.; Dąbrowski, J.M. Sensitization of TiO2 by halogenated porphyrin derivatives for visible light biomedical and environmental photocatalysis. Catal. Today 2019, 335, 538–549. [Google Scholar] [CrossRef]
- Oliveira, D.F.; Batista, P.S.; Muller, P.S.; Velani, V.; França, M.D.; De Souza, D.R.; Machado, A.E. Evaluating the effectiveness of photocatalysts based on titanium dioxide in the degradation of the dye Ponceau 4R. Dye Pigment 2012, 92, 563–572. [Google Scholar] [CrossRef]
- Kozlov, N.K.; Natashina, U.A.; Tamarov, K.P.; Gongalsky, M.B.; Solovyev, V.V.; Kudryavtsev, A.A.; Sivakov, V.; Osminkina, L.A. Recycling of silicon: From industrial waste to biocompatible nanoparticles for nanomedicine. Mater. Res. Express 2017, 4, 095026. [Google Scholar] [CrossRef]
- Sobotta, L.; Fita, P.; Szczolko, W.; Wrotynski, M.; Wierzchowski, M.; Goslinski, T.; Mielcarek, J. Functional singlet oxygen generators based on porphyrazines with peripheral 2,5-dimethylpyrrol-1-yl and dimethylamino groups. J. Photochem. Photobiol. A Chem. 2013, 269, 9–16. [Google Scholar] [CrossRef]
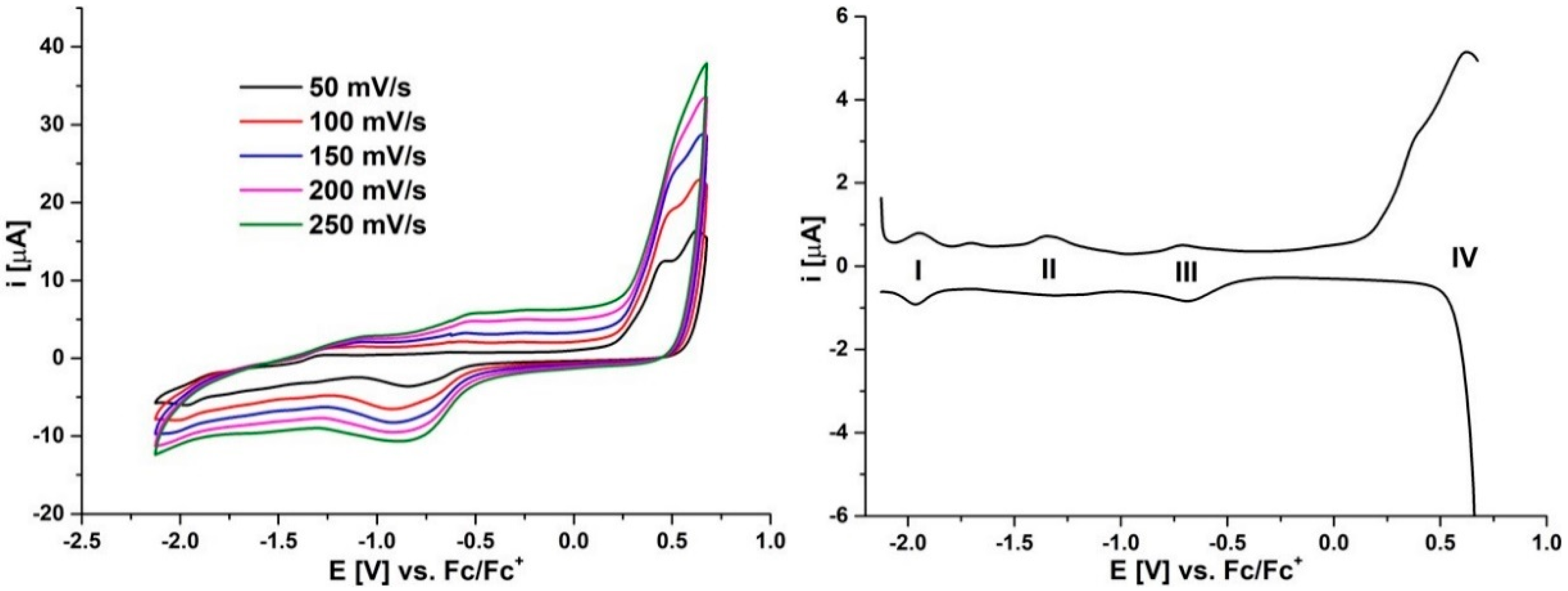
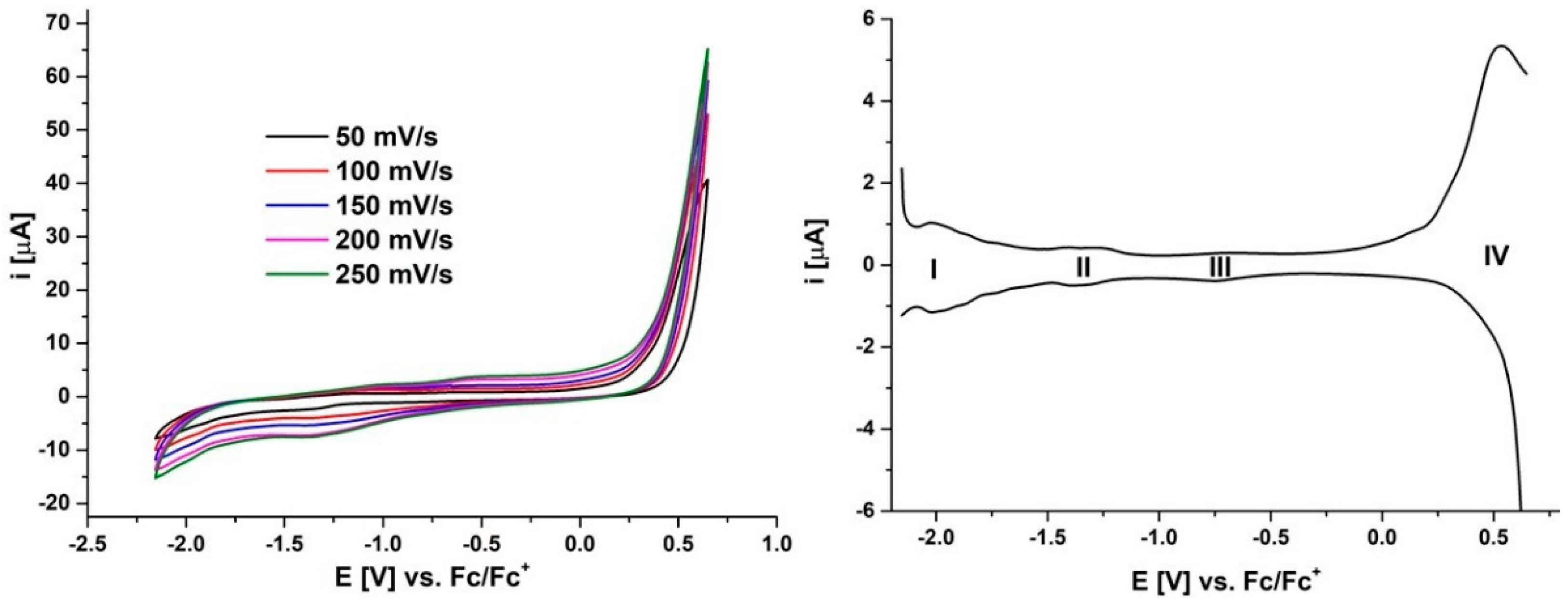

| Pz | MPz(−2)/ MPz(−3) I | MPz(−1)/ MPz(−2) II | MPz(0)/ MPz(−1) III | MPz(0)/ MPz(+1) IV | |
|---|---|---|---|---|---|
| 4 | E1/2 [V] vs. Fc/Fc+ | −1.95 | −1.33 | −0.70 | 0.62 |
| 5 | E1/2 [V] vs. Fc/Fc+ | −2.02 | −1.34 | −0.74 | 0.53 |
| λmax [nm] | λonset [nm] | Optical Band Gap Egap opt [eV] | Electrochemical Band Gap Egap el [eV] | ||
|---|---|---|---|---|---|
| Soret | Q Band | ||||
| 4 | 375 | 671 | 820 | 1.51 | 1.32 |
| 5 | 373 | 668 | 850 | 1.46 | 1.26 |
| Material | Measured Particle Size (nm) | Polydispersity Index a |
|---|---|---|
| P25 | 74.8 ± 7.7 | 0.17 |
| 4@P25 | 327.4 ± 15.5 | 0.13 |
| 5@P25 | 322.5 ± 28.2 | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koczorowski, T.; Szczolko, W.; Teubert, A.; Goslinski, T. Sulfanyl Porphyrazines with Morpholinylethyl Periphery—Synthesis, Electrochemistry, and Photocatalytic Studies after Deposition on Titanium(IV) Oxide P25 Nanoparticles. Molecules 2021, 26, 2280. https://doi.org/10.3390/molecules26082280
Koczorowski T, Szczolko W, Teubert A, Goslinski T. Sulfanyl Porphyrazines with Morpholinylethyl Periphery—Synthesis, Electrochemistry, and Photocatalytic Studies after Deposition on Titanium(IV) Oxide P25 Nanoparticles. Molecules. 2021; 26(8):2280. https://doi.org/10.3390/molecules26082280
Chicago/Turabian StyleKoczorowski, Tomasz, Wojciech Szczolko, Anna Teubert, and Tomasz Goslinski. 2021. "Sulfanyl Porphyrazines with Morpholinylethyl Periphery—Synthesis, Electrochemistry, and Photocatalytic Studies after Deposition on Titanium(IV) Oxide P25 Nanoparticles" Molecules 26, no. 8: 2280. https://doi.org/10.3390/molecules26082280
APA StyleKoczorowski, T., Szczolko, W., Teubert, A., & Goslinski, T. (2021). Sulfanyl Porphyrazines with Morpholinylethyl Periphery—Synthesis, Electrochemistry, and Photocatalytic Studies after Deposition on Titanium(IV) Oxide P25 Nanoparticles. Molecules, 26(8), 2280. https://doi.org/10.3390/molecules26082280






