The Role of Zinc(II) Ion in Fluorescence Tuning of Tridentate Pincers: A Review
Abstract
1. Introduction
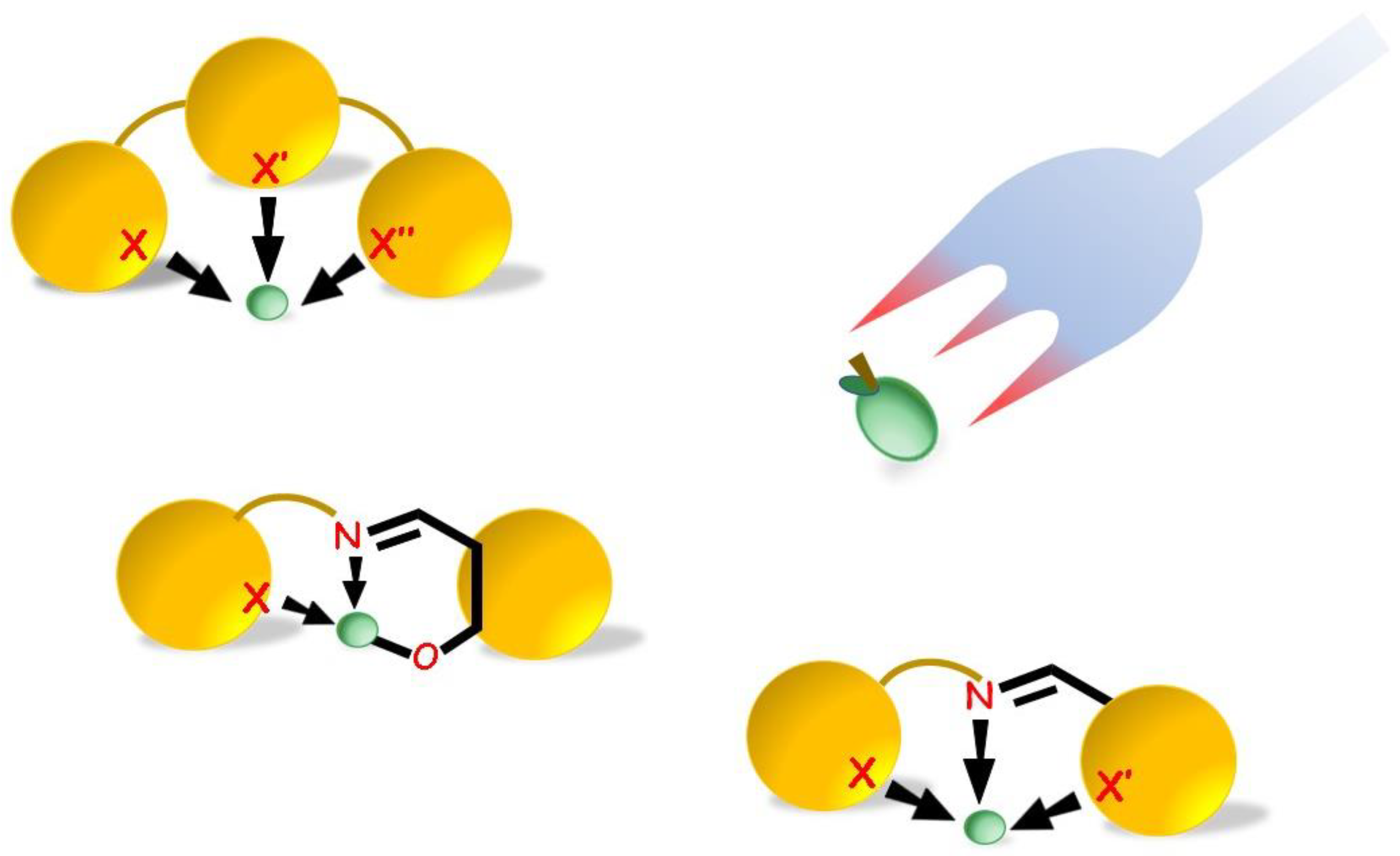
2. Nitrogen Binding Sites
2.1. Terpyridine-Type Ligands
2.2. N,N,N Schiff Base-Type Ligands
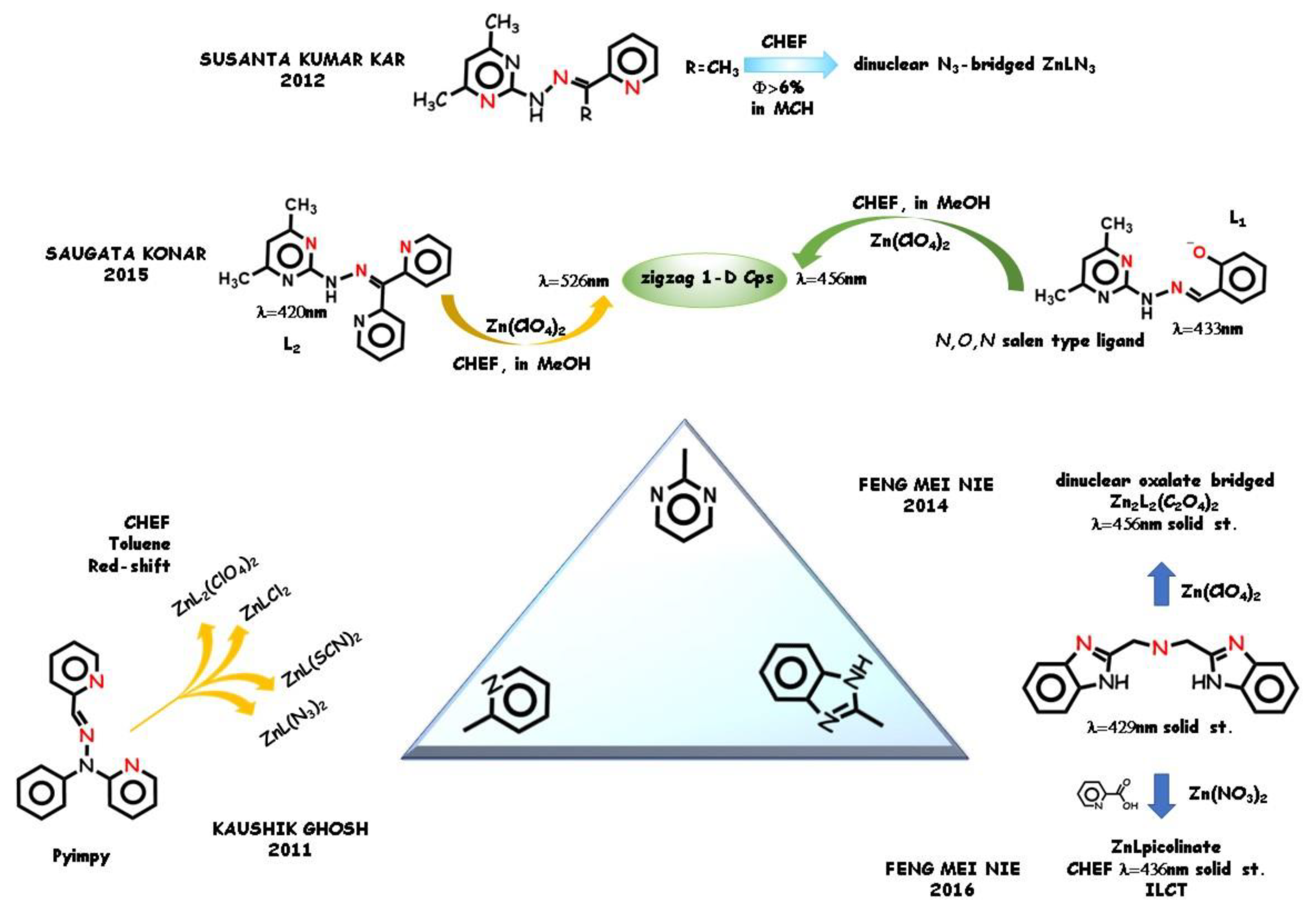
2.3. Ligands for Sensing Analysis and for Supramolecular Architecture Building
3. Nitrogen and Oxygen Binding Sites
3.1. N,N,O Ligands
3.1.1. Half-Salen-Type Ligands
3.1.2. Ligands for Sensing Analysis and for Biological Applications
3.2. O,N,O Ligands
4. Nitrogen, Oxygen, and Sulfur Binding Sites
4.1. N,N,S Ligands
4.2. N,S,O Ligands
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mautner, F.A.; Berger, C.; Gspan, C.; Sudy, B.; Fischer, R.C.; Massoud, S.S. Pyridyl and triazole ligands directing the assembling of zinc(II) into coordination polymers with different dimensionality through azides. Polyhedron 2017, 130, 136–144. [Google Scholar] [CrossRef]
- Caruso, U.; Panunzi, B.; Roviello, A.; Tuzi, A. Fluorescent metallopolymers with Zn(II) in a Schiff base/phenoxide coordination environment. Inorg. Chem. Commun. 2013, 29, 138–140. [Google Scholar] [CrossRef]
- Günay Sezer, G.; Zafer Yeşilel, O.; Şahin, O.; Burrows, A.D. Zinc(II) and cadmium(II) coordination polymers containing phenylenediacetate and bis(imidazol-1-ylmethyl)benzene linkers: The effect of ligand isomers on the solid state structures. J. Solid State Chem. 2017, 252, 8–21. [Google Scholar] [CrossRef]
- Gusev, A.; Shul’gin, V.; Braga, E.; Zamnius, E.; Kryukova, M.; Linert, W. Luminescent properties of Zn complexes based on tetradentate N2O2-donor pyrazolone schiff bases. Dye. Pigm. 2020, 183. [Google Scholar] [CrossRef]
- Caruso, U.; Panunzi, B.; Roviello, A.; Tingoli, M.; Tuzi, A. Two aminobenzothiazole derivatives for Pd(II) and Zn(II) coordination: Synthesis, characterization and solid state fluorescence. Inorg. Chem. Commun. 2011, 14, 46–48. [Google Scholar] [CrossRef]
- Matozzo, P.; Colombo, A.; Dragonetti, C.; Righetto, S.; Roberto, D.; Biagini, P.; Fantacci, S.; Marinotto, D. A Chiral Bis(salicylaldiminato)zinc(II) Complex with Second-Order Nonlinear Optical and Luminescent Properties in Solution. Inorganics 2020, 8, 25. [Google Scholar] [CrossRef]
- Barbieri, A.; Accorsi, G.; Armaroli, N. Luminescent complexes beyond the platinum group: The d10 avenue. Chem. Commun. (Camb.) 2008, 19, 2185–2193. [Google Scholar] [CrossRef]
- Alam, R.; Bhowmick, R.; Islam, A.S.M.; Chaudhuri, K.; Ali, M. A rhodamine based fluorescent trivalent sensor (Fe 3+, Al 3+, Cr 3+) with potential applications for live cell imaging and combinational logic circuits and memory devices. New J. Chem. 2017, 41, 8359–8369. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Arca, M.; Bencini, A.; Caltagirone, C.; Garau, A.; Isaia, F.; Light, M.E.; Lippolis, V.; Lodeiro, C.; Mameli, M. Zn 2+/Cd 2+ optical discrimination by fluorescent chemosensors based on 8-hydroxyquinoline derivatives and sulfur-containing macrocyclic units. Dalton Trans. 2013, 42, 14516–14530. [Google Scholar] [CrossRef]
- Lee, J.J.; Lee, S.A.; Kim, H.; Nguyen, L.; Noh, I.; Kim, C. A highly selective CHEF-type chemosensor for monitoring Zn2+ in aqueous solution and living cells. RSC Adv. 2015, 5, 41905–41913. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.S.; Reibenspies, J.H.; Hancock, R.D. Mechanism of “turn-on” fluorescent sensors for mercury(II) in solution and its implications for ligand design. Inorg. Chem. 2012, 51, 10904–10915. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.S.; Kim, S.J.; Kool, E.T. Differentiating between fluorescence-quenching metal ions with polyfluorophore sensors built on a DNA backbone. J. Am. Chem. Soc. 2011, 133, 2664–2671. [Google Scholar] [CrossRef] [PubMed]
- Deems, J.C.; Reibenspies, J.H.; Lee, H.-S.; Hancock, R.D. Strategies for a fluorescent sensor with receptor and fluorophore designed for the recognition of heavy metal ions. Inorg. Chim. Acta 2020, 499. [Google Scholar] [CrossRef]
- Lee, H.; Hancock, R.D.; Lee, H.S. Role of fluorophore-metal interaction in photoinduced electron transfer (PET) sensors: Time-dependent density functional theory (TDDFT) study. J. Phys. Chem. A 2013, 117, 13345–13355. [Google Scholar] [CrossRef] [PubMed]
- Patra, L.; Das, S.; Gharami, S.; Aich, K.; Mondal, T.K. A new multi-analyte fluorogenic sensor for efficient detection of Al3+ and Zn2+ ions based on ESIPT and CHEF features. New J. Chem. 2018, 42, 19076–19082. [Google Scholar] [CrossRef]
- Hancock, R.D. The pyridyl group in ligand design for selective metal ion complexation and sensing. Chem. Soc. Rev. 2013, 42, 1500–1524. [Google Scholar] [CrossRef]
- Panunzi, B.; Diana, R.; Concilio, S.; Sessa, L.; Tuzi, A.; Piotto, S.; Caruso, U. Fluorescence pH-dependent sensing of Zn(II) by a tripodal ligand. A comparative X-ray and DFT study. J. Lumin. 2019, 212, 200–206. [Google Scholar] [CrossRef]
- Wu, M.X.; Filley, S.J.; Hill, K.A. Cooperative binding of zinc to an aminoacyl-tRNA synthetase. Biochem. Biophys. Res. Commun. 1994, 201, 1079–1083. [Google Scholar] [CrossRef]
- Shafaatian, B.; Mousavi, S.S.; Afshari, S. Synthesis, characterization, spectroscopic and theoretical studies of new zinc(II), copper(II) and nickel(II) complexes based on imine ligand containing 2-aminothiophenol moiety. J. Mol. Struct. 2016, 1123, 191–198. [Google Scholar] [CrossRef]
- Bi, X.; Pang, Y. Optical Response of Terpyridine Ligands to Zinc Binding: A Close Look at the Substitution Effect by Spectroscopic Studies at Low Temperature. J. Phys. Chem. B 2016, 120, 3311–3317. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Zhou, S.; Ma, Z. Synthesis, structure, and thermal and photoluminescent properties of a zinc(II) sulfate 4′-phenyl-terpyridine compound. Inorg. Nano-Met. Chem. 2017, 47, 876–880. [Google Scholar] [CrossRef]
- Mandal, H.; Chakrabartty, S.; Ray, D. Isothiocyanato and azido coordination induced structural diversity in zinc(ii) complexes with Schiff base containing tetrahydrofuran group: Synthesis, characterization, crystal structure and fluorescence study. RSC Adv. 2014, 4, 65044–65055. [Google Scholar] [CrossRef]
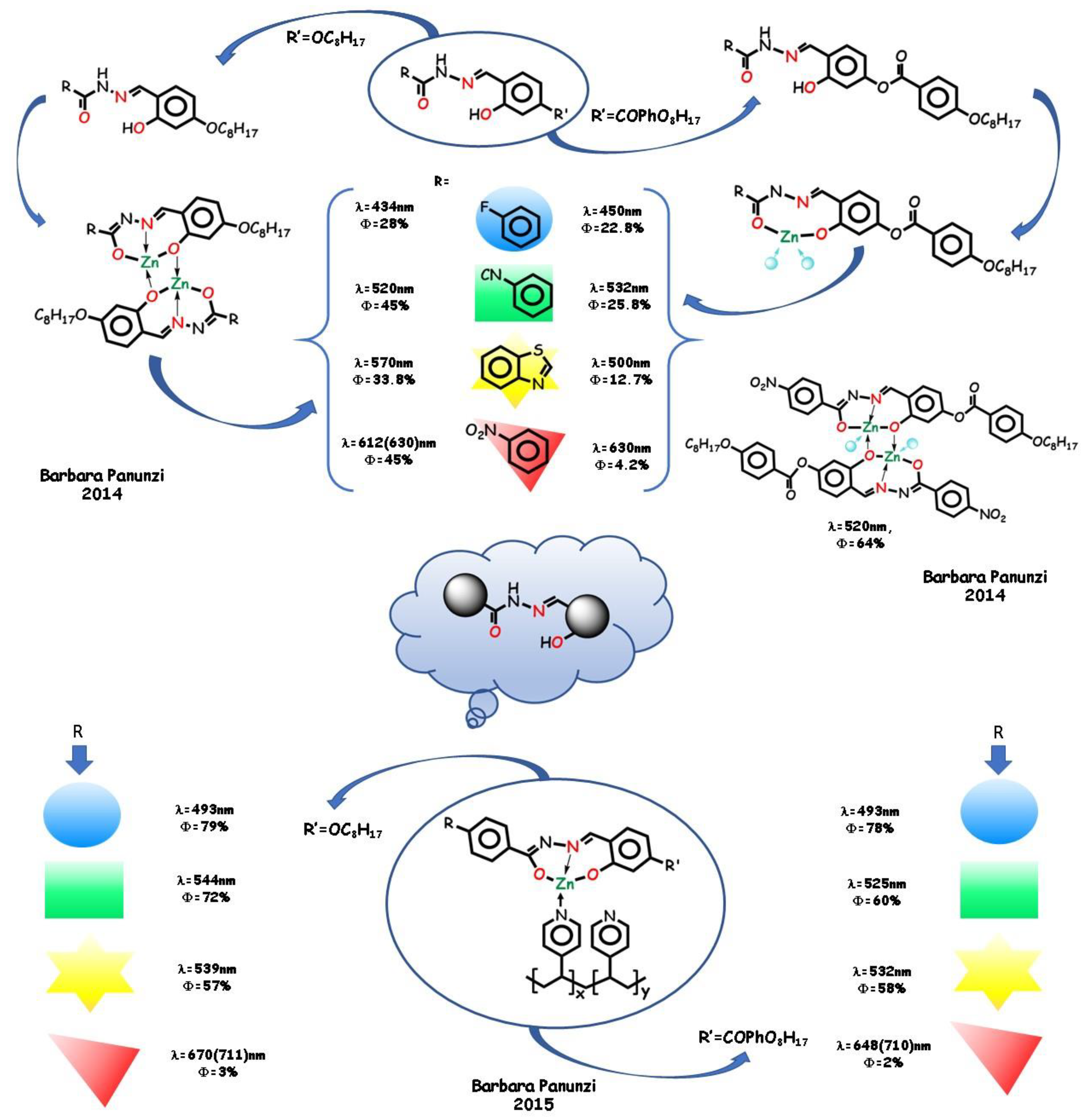
- Borbone, F.; Caruso, U.; Causà, M.; Fusco, S.; Panunzi, B.; Roviello, A.; Shikler, R.; Tuzi, A. Series of O,N,O-tridentate ligands zinc(II) complexes with high solid-state photoluminescence quantum yield. Eur. J. Inorg. Chem. 2014, 16, 2695–2703. [Google Scholar] [CrossRef]
- Borbone, F.; Caruso, U.; Concilio, S.; Nabha, S.; Panunzi, B.; Piotto, S.; Shikler, R.; Tuzi, A. Mono-, Di-, and Polymeric Pyridinoylhydrazone ZnII Complexes: Structure and Photoluminescent Properties. Eur. J. Inorg. Chem. 2016, 2016, 818–825. [Google Scholar] [CrossRef]
- Panunzi, B.; Concilio, S.; Diana, R.; Shikler, R.; Nabha, S.; Piotto, S.; Sessa, L.; Tuzi, A.; Caruso, U. Photophysical Properties of Luminescent Zinc(II)‒Pyridinyloxadiazole Complexes and their Glassy Self-Assembly Networks. Eur. J. Inorg. Chem. 2018, 2018, 2709–2716. [Google Scholar] [CrossRef]
- Borbone, F.; Carella, A.; Caruso, U.; Roviello, G.; Tuzi, A.; Dardano, P.; Lettieri, S.; Maddalena, P.; Barsella, A. Large second-order NLO activity in poly(4-vinylpyridine) grafted with PdII and CuII chromophoric complexes with tridentate bent ligands containing heterocycles. Eur. J. Inorg. Chem. 2008, 11, 1846–1853. [Google Scholar] [CrossRef]
- Caruso, U.; Centore, R.; Panunzi, B.; Roviello, A.; Tuzi, A. Grafting poly(4-vinylpyridine) with a second-order nonlinear optically active nickel(II) chromophore. Eur. J. Inorg. Chem. 2005, 13, 2747–2753. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Wu, L.; Guo, Z.; Zhao, J.; Liu, Y.; Bai, R.; Yan, X. Woven Polymer Networks via the Topological Transformation of a [2]Catenane. J. Am. Chem. Soc. 2020, 142, 14343–14349. [Google Scholar] [CrossRef]
- Diana, R.; Panunzi, B.; Piotto, S.; Caruso, T.; Caruso, U. Solid-state fluorescence of two zinc coordination polymers from bulky dicyano-phenylenevinylene and bis-azobenzene cores. Inorg. Chem. Commun. 2019, 110. [Google Scholar] [CrossRef]
- Diana, R.; Panunzi, B.; Shikler, R.; Nabha, S.; Caruso, U. Highly efficient dicyano-phenylenevinylene fluorophore as polymer dopant or zinc-driven self-assembling building block. Inorg. Chem. Commun. 2019, 104, 145–149. [Google Scholar] [CrossRef]
- Cannizzo, A.; Blanco-Rodriguez, A.M.; El Nahhas, A.; Sebera, J.; Zalis, S.; Vlcek, A., Jr.; Chergui, M. Femtosecond fluorescence and intersystem crossing in rhenium(I) carbonyl-bipyridine complexes. J. Am. Chem. Soc. 2008, 130, 8967–8974. [Google Scholar] [CrossRef] [PubMed]
- Stufkens, D. Ligand-dependent excited state behaviour of Re(I) and Ru(II) carbonyl–diimine complexes. Coord. Chem. Rev. 1998, 177, 127–179. [Google Scholar] [CrossRef]
- Vlček, A., Jr. Ultrafast excited-state processes in Re(I) carbonyl-diimine complexes: From excitation to photochemistry. In Topics in Organometallic Chemistry; Lees, A.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 29, pp. 73–114. [Google Scholar]
- Zalis, S.; Milne, C.J.; El Nahhas, A.; Blanco-Rodriguez, A.M.; van der Veen, R.M.; Vlcek, A., Jr. Re and Br X-ray absorption near-edge structure study of the ground and excited states of [ReBr(CO)3(bpy)] interpreted by DFT and TD-DFT calculations. Inorg. Chem. 2013, 52, 5775–5785. [Google Scholar] [CrossRef]
- Baková, R.; Chergui, M.; Daniel, C.; Vlček, A., Jr.; Záliš, S. Relativistic effects in spectroscopy and photophysics of heavy-metal complexes illustrated by spin–orbit calculations of [Re(imidazole)(CO)3(phen)]+. Coord. Chem. Rev. 2011, 255, 975–989. [Google Scholar] [CrossRef]
- Świtlicka-Olszewska, A.; Klemens, T.; Nawrot, I.; Machura, B.; Kruszynski, R. Novel Re(I) tricarbonyl coordination compound of 5-amino-1,10-phenanthroline – Synthesis, structural, photophysical and computational studies. J. Lumin. 2016, 171, 166–175. [Google Scholar] [CrossRef]
- Kurtz, D.A.; Brereton, K.R.; Ruoff, K.P.; Tang, H.M.; Felton, G.A.N.; Miller, A.J.M.; Dempsey, J.L. Bathochromic Shifts in Rhenium Carbonyl Dyes Induced through Destabilization of Occupied Orbitals. Inorg. Chem. 2018, 57, 5389–5399. [Google Scholar] [CrossRef]
- Zarkadoulas, A.; Koutsouri, E.; Kefalidi, C.; Mitsopoulou, C.A. Rhenium complexes in homogeneous hydrogen evolution. Coord. Chem. Rev. 2015, 304, 55–72. [Google Scholar] [CrossRef]
- Nganga, J.K.; Samanamu, C.R.; Tanski, J.M.; Pacheco, C.; Saucedo, C.; Batista, V.S.; Grice, K.A.; Ertem, M.Z.; Angeles-Boza, A.M. Electrochemical Reduction of CO2 Catalyzed by Re(pyridine-oxazoline)(CO)3Cl Complexes. Inorg. Chem. 2017, 56, 3214–3226. [Google Scholar] [CrossRef]
- Martinez, J.F.; La Porte, N.T.; Wasielewski, M.R. Electron Transfer from Photoexcited Naphthalene Diimide Radical Anion to Electrocatalytically Active Re(bpy)(CO)3Cl in a Molecular Triad. J. Phys. Chem. C 2018, 122, 2608–2617. [Google Scholar] [CrossRef]
- Hostachy, S.; Policar, C.; Delsuc, N. Re(I) carbonyl complexes: Multimodal platforms for inorganic chemical biology. Coord. Chem. Rev. 2017, 351, 172–188. [Google Scholar] [CrossRef]
- Gabr, M.T.; Pigge, F.C. Rhenium tricarbonyl complexes of AIE active tetraarylethylene ligands: Tuning luminescence properties and HSA-specific binding. Dalton Trans. 2017, 46, 15040–15047. [Google Scholar] [CrossRef]
- Ramdass, A.; Sathish, V.; Babu, E.; Velayudham, M.; Thanasekaran, P.; Rajagopal, S. Recent developments on optical and electrochemical sensing of copper(II) ion based on transition metal complexes. Coord. Chem. Rev. 2017, 343, 278–307. [Google Scholar] [CrossRef]
- Ma, Z.; Lu, W.; Liang, B.; Pombeiro, A.J.L. Synthesis, characterization, photoluminescent and thermal properties of zinc(ii) 4′-phenyl-terpyridine compounds. New J. Chem. 2013, 37. [Google Scholar] [CrossRef]
- Ma, Z.; Wei, L.; Alegria, E.C.; Martins, L.M.; Guedes da Silva, M.F.; Pombeiro, A.J. Synthesis and characterization of copper(II) 4′-phenyl-terpyridine compounds and catalytic application for aerobic oxidation of benzylic alcohols. Dalton Trans. 2014, 43, 4048–4058. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, B.; Guedes da Silva, M.F.; Silva, J.; Mendo, A.S.; Baptista, P.V.; Fernandes, A.R.; Pombeiro, A.J. Synthesis, characterization, thermal properties and antiproliferative potential of copper(II) 4′-phenyl-terpyridine compounds. Dalton Trans. 2016, 45, 5339–5355. [Google Scholar] [CrossRef]
- Winter, A.; Friebe, C.; Chiper, M.; Schubert, U.S.; Presselt, M.; Dietzek, B.; Schmitt, M.; Popp, J. Synthesis, characterization, and electro-optical properties of Zn(II) complexes with pi-conjugated terpyridine ligands. ChemPhysChem 2009, 10, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.N.; Topic, F.; Sahoo, P.K.; Mal, P.; Linnera, J.; Kalenius, E.; Tuononen, H.M.; Rissanen, K. Synthesis, structure and photophysical properties of a highly luminescent terpyridine-diphenylacetylene hybrid fluorophore and its metal complexes. Dalton Trans. 2015, 44, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Nakagawa, T.; Kawai, T. Recent progress of luminescent metal complexes with photochromic units. Coord. Chem. Rev. 2010, 254, 2643–2651. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Lo, K.K.-W. Luminescent polynuclear d10 metal complexes. Chem. Soc. Rev. 1999, 28, 323–334. [Google Scholar] [CrossRef]
- Ghosh, B.N.; Puttreddy, R.; Rissanen, K. Synthesis and structural characterization of new transition metal complexes of a highly luminescent amino-terpyridine ligand. Polyhedron 2020, 177. [Google Scholar] [CrossRef]
- Medlycott, E.A.; Hanan, G.S. Designing tridentate ligands for ruthenium(II) complexes with prolonged room temperature luminescence lifetimes. Chem. Soc. Rev. 2005, 34, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Goodall, W.; Williams, J.A.G. A new, highly fluorescent terpyridine which responds to zinc ions with a large red-shift in emissionElectronic supplementary information (ESI) available: Details of synthetic details and characterisation and plots of: (i) the decrease in fluorescence as a function of pH for the two ligands L1 and L2, and (ii) the increase in the intensity of fluorescence of L1 at 620 nm as a function of added zinc, including the method used for determination of Kass. Chem. Commun. 2001, 23, 2514–2515. [Google Scholar] [CrossRef]
- Diana, R.; Caruso, U.; Di Costanzo, L.; Bakayoko, G.; Panunzi, B. A novel DR/NIR T-shaped aiegen: Synthesis and x-ray crystal structure study. Crystals 2020, 10, 269. [Google Scholar] [CrossRef]
- Kozhevnikov, V.N.; Shabunina, O.V.; Sharifullina, A.R.; Rusinov, V.L.; Chupakhin, O.N.; König, B. Aminomethyl bi- and terpyridines as luminescent probes for Zn2+ ions. Mendeleev Commun. 2005, 15, 8–9. [Google Scholar] [CrossRef]
- Feng, Z.; Li, D.; Zhang, M.; Shao, T.; Shen, Y.; Tian, X.; Zhang, Q.; Li, S.; Wu, J.; Tian, Y. Enhanced three-photon activity triggered by the AIE behaviour of a novel terpyridine-based Zn(ii) complex bearing a thiophene bridge. Chem. Sci. 2019, 10, 7228–7232. [Google Scholar] [CrossRef]
- Jiang, T.; Lu, N.; Hang, Y.; Yang, J.; Mei, J.; Wang, J.; Hua, J.; Tian, H. Dimethoxy triarylamine-derived terpyridine–zinc complex: A fluorescence light-up sensor for citrate detection based on aggregation-induced emission. J. Mater. Chem. C 2016, 4, 10040–10046. [Google Scholar] [CrossRef]
- Ghosh, K.; Kumar, P.; Tyagi, N. Synthesis, crystal structure and DNA interaction studies on mononuclear zinc complexes. Inorg. Chim. Acta 2011, 375, 77–83. [Google Scholar] [CrossRef]
- Diana, R.; Caruso, U.; Concilio, S.; Piotto, S.; Tuzi, A.; Panunzi, B. A real-time tripodal colorimetric/fluorescence sensor for multiple target metal ions. Dye. Pigm. 2018, 155, 249–257. [Google Scholar] [CrossRef]
- Otsuka, M.; Fujita, M.; Sugiura, Y.; Ishii, S.; Tsutomu, A.; Yamamoto, T.; Inoue, J.I. Novel zinc chelators which inhibit the binding of HIV-EP1 (HIV enhancer binding protein) to NF-kappa B recognition sequence. J. Med. Chem. 1994, 37, 4267–4269. [Google Scholar] [CrossRef]
- Zamora, F.; Kunsman, M.; Sabat, M.; Lippert, B. Metal-Stabilized Rare Tautomers of Nucleobases. 6. Imino Tautomer of Adenine in a Mixed-Nucleobase Complex of Mercury(II). Inorg Chem. 1997, 36, 1583–1587. [Google Scholar] [CrossRef]
- Ray, S.; Konar, S.; Jana, A.; Jana, S.; Patra, A.; Chatterjee, S.; Golen, J.A.; Rheingold, A.L.; Mandal, S.S.; Kar, S.K. Three new pseudohalide bridged dinuclear Zn(II), Cd(II) complexes of pyrimidine derived Schiff base ligands: Synthesis, crystal structures and fluorescence studies. Polyhedron 2012, 33, 82–89. [Google Scholar] [CrossRef]
- Konar, S.; Jana, A.; Das, K.; Ray, S.; Chatterjee, S.; Kar, S.K. Complexes of a functionally modified pyrazole derived ligand—Mononuclear zinc(II), dinuclear nickel(II) and a rare pentanuclear cadmium(II) complex with a TBP core and their photoluminescence studies. Polyhedron 2012, 47, 143–150. [Google Scholar] [CrossRef]
- Paira, M.K.; Dinda, J.; Lu, T.H.; Paital, A.R.; Sinha, C. Zn(II), Cd(II) and Hg(II) complexes of 8-aminoquinoline. Polyhedron 2007, 26, 4131–4140. [Google Scholar] [CrossRef]
- Konar, S. Dicynamide bridged two new zig-zag 1-D Zn(II) coordination polymers of pyrimidine derived Schiff base ligands: Synthesis, crystal structures and fluorescence studies. J. Mol. Struct. 2015, 1092, 34–43. [Google Scholar] [CrossRef]
- Blackman, A.G. The coordination chemistry of tripodal tetraamine ligands. Polyhedron 2005, 24, 1–39. [Google Scholar] [CrossRef]
- Contreras, R.; Flores-Parra, A.; Mijangos, E.; Téllez, F.; López-Sandoval, H.; Barba-Behrens, N. From mono to polydentate azole and benzazole derivatives, versatile ligands for main group and transition metal atoms. Coord. Chem. Rev. 2009, 253, 1979–1999. [Google Scholar] [CrossRef]
- Boča, M.; Jameson, R.F.; Linert, W. Fascinating variability in the chemistry and properties of 2,6-bis-(benzimidazol-2-yl)-pyridine and 2,6-bis-(benzthiazol-2-yl)-pyridine and their complexes. Coord. Chem. Rev. 2011, 255, 290–317. [Google Scholar] [CrossRef]
- Li, L.-Q.; Li, M.; Zhang, H.; Li, S.; Nie, F.-M. Synthesis, structures, and fluorescent properties of two oxalato-bridged dinuclear zinc(II) complexes with tridentate and tetradentate polybenzimidazole ligands. J. Coord. Chem. 2014, 67, 847–856. [Google Scholar] [CrossRef]
- Feng, R.; Huang, F.-F.; Yuan, J.-L.; Lu, Z.; Fang, T.; Nie, F.-M. Structures and fluorescent properties of picolinato zinc(II) and cadmium(II) complexes based on tridentate and tetradentate benzimidazole ligands. J. Coord. Chem. 2016, 69, 3776–3791. [Google Scholar] [CrossRef]
- Concilio, S.; Bugatti, V.; Neitzert, H.C.; Landi, G.; De Sio, A.; Parisi, J.; Piotto, S.; Iannelli, P. Zn-complex based on oxadiazole/carbazole structure: Synthesis, optical and electric properties. Thin Solid Film. 2014, 556, 419–424. [Google Scholar] [CrossRef]
- Borbone, F.; Tuzi, A.; Panunzi, B.; Piotto, S.; Concilio, S.; Shikler, R.; Nabha, S.; Centore, R. On-Off Mechano-Responsive Switching of ESIPT Luminescence in Polymorphic N-salicylidene-4-amino-2-methylbenzotriazole. Cryst. Growth Des. 2017, 17, 5517. [Google Scholar] [CrossRef]
- Diana, R.; Caruso, U.; Di Costanzo, L.; Gentile, F.S.; Panunzi, B. Colorimetric recognition of multiple first-row transition metals: A single water-soluble chemosensor in acidic and basic conditions. Dye. Pigm. 2021, 184. [Google Scholar] [CrossRef]
- Panunzi, B.; Diana, R.; Caruso, U. A highly efficient white luminescent zinc (II) based metallopolymer by RGB approach. Polymers 2019, 11, 1712. [Google Scholar] [CrossRef] [PubMed]
- Diana, R.; Panunzi, B.; Tuzi, A.; Caruso, U. Two tridentate pyridinyl-hydrazone zinc(II) complexes as fluorophores for blue emitting layers. J. Mol. Struct. 2019, 1197, 672–680. [Google Scholar] [CrossRef]
- Diana, R.; Panunzi, B.; Concilio, S.; Marrafino, F.; Shikler, R.; Caruso, T.; Caruso, U. The effect of bulky substituents on two π-conjugated mesogenic fluorophores. Their organic polymers and zinc-bridged luminescent networks. Polymers 2019, 11, 1379. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zuo, T.; Guo, F.; Sun, J.; Liu, Z.; Diao, G. Perylene dye-functionalized silver nanoparticles serving as pH-dependent metal sensor systems. RSC Adv. 2017, 7, 24215–24220. [Google Scholar] [CrossRef]
- Li, C.; Krautler, B. Transition metal complexes of phyllobilins—A new realm of bioinorganic chemistry. Dalton Trans. 2015, 44, 10116–10127. [Google Scholar] [CrossRef]
- Gil-Ramirez, G.; Leigh, D.A.; Stephens, A.J. Catenanes: Fifty years of molecular links. Angew. Chem. Int. Ed. Engl. 2015, 54, 6110–6150. [Google Scholar] [CrossRef]
- Niu, Z.; Gibson, H.W. Polycatenanes. Chem. Rev. 2009, 109, 6024–6046. [Google Scholar] [CrossRef]
- Wu, Q.; Rauscher, P.M.; Lang, X.; Wojtecki, R.J.; de Pablo, J.J.; Hore, M.J.A.; Rowan, S.J. Poly[n]catenanes: Synthesis of molecular interlocked chains. Science 2017, 358, 1434–1439. [Google Scholar] [CrossRef]
- Li, H.; Zhu, Z.; Fahrenbach, A.C.; Savoie, B.M.; Ke, C.; Barnes, J.C.; Lei, J.; Zhao, Y.L.; Lilley, L.M.; Marks, T.J.; et al. Mechanical bond-induced radical stabilization. J. Am. Chem. Soc. 2013, 135, 456–467. [Google Scholar] [CrossRef]
- Wang, X.Q.; Li, W.J.; Wang, W.; Wen, J.; Zhang, Y.; Tan, H.; Yang, H.B. Construction of Type III-C Rotaxane-Branched Dendrimers and Their Anion-Induced Dimension Modulation Feature. J. Am. Chem. Soc. 2019, 141, 13923–13930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Stephens, A.J.; Nussbaumer, A.L.; Lemonnier, J.F.; Jurcek, P.; Vitorica-Yrezabal, I.J.; Leigh, D.A. Stereoselective synthesis of a composite knot with nine crossings. Nat. Chem. 2018, 10, 1083–1088. [Google Scholar] [CrossRef]
- Chui, S.S.; Lo, S.M.; Charmant, J.P.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M. “Let’s twist again” double-stranded, triple-stranded, and circular helicates. Chem. Rev. 2001, 101, 3457–3497. [Google Scholar] [CrossRef] [PubMed]
- Akbar Ali, M.; Livingstone, S.E. Metal complexes of sulphur-nitrogen chelating agents. Coord. Chem. Rev. 1974, 13, 101–132. [Google Scholar] [CrossRef]
- Katsuki, T. Catalytic asymmetric oxidations using optically active (salen)manganese(III) complexes as catalysts. Coord. Chem. Rev. 1995, 140, 189–214. [Google Scholar] [CrossRef]
- Singh, R.; Banerjee, A.; Colacio, E.; Rajak, K.K. Enantiopure tetranuclear iron(III) complexes using chiral reduced Schiff base ligands: Synthesis, structure, spectroscopy, magnetic properties, and DFT studies. Inorg. Chem. 2009, 48, 4753–4762. [Google Scholar] [CrossRef]
- Bagai, R.; Datta, S.; Betancur-Rodriguez, A.; Abboud, K.A.; Hill, S.; Christou, G. Diversity of new structural types in polynuclear iron chemistry with a tridentate N,N,O ligand. Inorg. Chem. 2007, 46, 4535–4547. [Google Scholar] [CrossRef]
- Naiya, S.; Wang, H.S.; Drew, M.G.; Song, Y.; Ghosh, A. Structural and magnetic studies of Schiff base complexes of nickel(II) nitrite: Change in crystalline state, ligand rearrangement and a very rare mu-nitrito-1kappaO:2kappaN:3kappaO’ bridging mode. Dalton Trans. 2011, 40, 2744–2756. [Google Scholar] [CrossRef]
- Mukherjee, P.; Drew, M.G.; Gomez-Garcia, C.J.; Ghosh, A. The crucial role of polyatomic anions in molecular architecture: Structural and magnetic versatility of five nickel(II) complexes derived from A N,N,O-donor Schiff base ligand. Inorg. Chem. 2009, 48, 5848–5860. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Sen, S.; Banerjee, S.; Mitra, S.; Rosair, G.; Rodriguez, M.T.G. Three new pseudohalide bridged dinuclear Zn(II) Schiff base complexes: Synthesis, crystal structures and fluorescence studies. Polyhedron 2007, 26, 5104–5112. [Google Scholar] [CrossRef]
- Ruiz, E.; Alemany, P.; Alvarez, S.; Cano, J. Toward the Prediction of Magnetic Coupling in Molecular Systems: Hydroxo- and Alkoxo-Bridged Cu(II) Binuclear Complexes. J. Am. Chem. Soc. 1997, 119, 1297–1303. [Google Scholar] [CrossRef]
- Jelfs, K.E.; Wu, X.; Schmidtmann, M.; Jones, J.T.; Warren, J.E.; Adams, D.J.; Cooper, A.I. Large self-assembled chiral organic cages: Synthesis, structure, and shape persistence. Angew. Chem. Int. Ed. Engl. 2011, 50, 10653–10656. [Google Scholar] [CrossRef]
- Diedrich, C.; Deeth, R.J. On the performance of ligand field molecular mechanics for model complexes containing the peroxido-bridged [Cu2O2]2+ center. Inorg. Chem. 2008, 47, 2494–2506. [Google Scholar] [CrossRef]
- Holm, R.H.; Kennepohl, P.; Solomon, E.I. Structural and Functional Aspects of Metal Sites in Biology. Chem. Rev. 1996, 96, 2239–2314. [Google Scholar] [CrossRef] [PubMed]
- Canali, L.; Sherrington, D.C. Utilisation of homogeneous and supported chiral metal(salen) complexes in asymmetric catalysis. Chem. Soc. Rev. 1999, 28, 85–93. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Tang, H.-Y.; Lin, C.-C. Ring-Opening Polymerization of Lactides Initiated by Zinc Alkoxides Derived from NNO-Tridentate Ligands. Macromolecules 2006, 39, 3745–3752. [Google Scholar] [CrossRef]
- Morris, G.A.; Zhou, H.; Stern, C.L.; Nguyen, S.T. A general high-yield route to bis(salicylaldimine) zinc(II) complexes: Application to the synthesis of pyridine-modified salen-type zinc(II) complexes. Inorg. Chem. 2001, 40, 3222–3227. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Sano, T.; Fujita, M.; Fujii, T.; Nishio, Y.; Shibata, K. Blue Electroluminescence in Thin Films of Azomethin-Zinc Complexes. Jpn. J. Appl. Phys. 1993, 32, L511–L513. [Google Scholar] [CrossRef]
- De La Durantaye, L.; McCormick, T.; Liu, X.Y.; Wang, S. Interaction of 2-(2′-pyridyl)benzimidazolyl derivative ligands with group 12 metal ions: Coordination, structures and luminescence. Dalton Trans. 2006, 48, 5675–5682. [Google Scholar] [CrossRef] [PubMed]
- Maiti, M.; Thakurta, S.; Sadhukhan, D.; Pilet, G.; Rosair, G.M.; Nonat, A.; Charbonnière, L.J.; Mitra, S. Thermally stable luminescent zinc–Schiff base complexes: A thiocyanato bridged 1D coordination polymer and a supramolecular 1D polymer. Polyhedron 2013, 65, 6–15. [Google Scholar] [CrossRef]
- Shit, S.; Nandy, M.; Saha, D.; Zhang, L.; Schmitt, W.; Rizzoli, C.; Row, T.N.G. Synthesis, crystal structure and fluorescence properties of two dinuclear zinc(II) complexes incorporating tridentate (NNO) Schiff bases. J. Coord. Chem. 2016, 69, 2403–2414. [Google Scholar] [CrossRef]
- Konar, S.; Jana, A.; Das, K.; Ray, S.; Golen, J.A.; Rheingold, A.L.; Kar, S.K. A rare pentanuclear cadmium(II) complex and two new mononuclear zinc(II) complexes of pyrazole derived ditopic ligands—Synthesis, crystal structures and spectral studies. Inorg. Chim. Acta 2013, 397, 144–151. [Google Scholar] [CrossRef]
- Caruso, U.; Panunzi, B.; Diana, R.; Concilio, S.; Sessa, L.; Shikler, R.; Nabha, S.; Tuzi, A.; Piotto, S. AIE/ACQ effects in two DR/NIR emitters: A structural and DFT comparative analysis. Molecules 2018, 23, 1947. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Chakraborty, M.; Mondal, A.; Pakhira, B.; Mukhopadhyay, S.K.; Banik, A.; Sengupta, S.; Chattopadhyay, S.K. Crystal structure, spectroscopic, DNA binding studies and DFT calculations of a Zn(ii) complex. New J. Chem. 2019, 43, 5466–5474. [Google Scholar] [CrossRef]
- Jayendran, M.; Begum, P.M.S.; Kurup, M.R.P. Structural, spectral and biological investigations on Cu(II) and Zn(II) complexes derived from NNO donor tridentate Schiff base: Crystal structure of a 1D Cu(II) coordination polymer. J. Mol. Struct. 2020, 1206. [Google Scholar] [CrossRef]
- Li, M.; Xing, Y.; Zou, Y.; Chen, G.; You, J.; Yu, F. Imaging of the mutual regulation between zinc cation and nitrosyl via two-photon fluorescent probes in cells and in vivo. Sens. Actuators B Chem. 2020, 309. [Google Scholar] [CrossRef]
- Phatangare, K.R.; Lanke, S.K.; Sekar, N. Fluorescent coumarin derivatives with viscosity sensitive emission--synthesis, photophysical properties and computational studies. J. Fluoresc. 2014, 24, 1263–1274. [Google Scholar] [CrossRef]
- Li, D.; Zhao, W.; Sun, X.; Zhang, J.; Anpo, M.; Zhao, J. Photophysical properties of coumarin derivatives incorporated in MCM-41. Dye. Pigm. 2006, 68, 33–37. [Google Scholar] [CrossRef]
- Ray, D.; Bharadwaj, P.K. A coumarin-derived fluorescence probe selective for magnesium. Inorg. Chem. 2008, 47, 2252–2254. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Savani, C.; Singh, V.K. Synthesis, Photophysical, Thermal and Crystallographic Studies of 3-Aminocoumarin Based Monobasic κ3-O,N,O-tridentate/κ2-N,O-bidentate Schiff Base Divalent Complexes. ChemistrySelect 2019, 4, 14244–14252. [Google Scholar] [CrossRef]
- Aslkhademi, S.; Noshiranzadeh, N.; Sadjadi, M.S.; Mehrani, K.; Farhadyar, N. Synthesis, crystal structure and investigation of the catalytic and spectroscopic properties of a Zn(II) complex with coumarin-hydrazone ligand. Polyhedron 2019, 160, 115–122. [Google Scholar] [CrossRef]
- Gusev, A.N.; Braga, E.V.; Kryukova, M.A.; Lyubomirskii, N.V.; Zamnius, E.A.; Shul’gin, V.F. Photoluminescence of the Coordination Zinc Compounds with 3-Methyl-4-Formyl-1-Phenylpyrazol-5-one Acylhydrazones. Russ. J. Coord. Chem. 2020, 46, 251–259. [Google Scholar] [CrossRef]
- Argeri, M.; Borbone, F.; Caruso, U.; Causà, M.; Fusco, S.; Panunzi, B.; Roviello, A.; Shikler, R.; Tuzi, A. Color tuning and noteworthy photoluminescence quantum yields in crystalline mono-/dinuclear ZnII complexes. Eur. J. Inorg. Chem. 2014, 2014, 5916–5924. [Google Scholar] [CrossRef]
- Costa, R.D.; Orti, E.; Bolink, H.J.; Monti, F.; Accorsi, G.; Armaroli, N. Luminescent ionic transition-metal complexes for light-emitting electrochemical cells. Angew. Chem. Int. Ed. Engl. 2012, 51, 8178–8211. [Google Scholar] [CrossRef]
- Dianu, M.L.; Kriza, A.; Musuc, A.M. Synthesis, spectral characterization, and thermal behavior of mononuclear Cu(II), Co(II), Ni(II), Mn(II), and Zn(II) complexes with 5-bromosalycilaldehyde isonicotinoylhydrazone. J. Therm. Anal. Calorim. 2012, 112, 585–593. [Google Scholar] [CrossRef]
- Borbone, F.; Caruso, U.; Palma, S.D.; Fusco, S.; Nabha, S.; Panunzi, B.; Shikler, R. High solid state photoluminescence quantum yields and effective color tuning in polyvinylpyridine based zinc(II) metallopolymers. Macromol. Chem. Phys. 2015, 216, 1516–1522. [Google Scholar] [CrossRef]
- Diana, R.; Panunzi, B.; De Simone, B.; Borbone, F.; Tuzi, A.; Caruso, U. RGB emission of three charged O,N,O-chelate zinc (II) complexes in pyridine solution. Inorg. Chem. Commun. 2020, 113. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Lei, Y. Fluorescence based explosive detection: From mechanisms to sensory materials. Chem. Soc. Rev. 2015, 44, 8019–8061. [Google Scholar] [CrossRef]
- Taraba, L.; Krizek, T.; Kozlik, P.; Hodek, O.; Coufal, P. Protonation of polyaniline-coated silica stationary phase affects the retention behavior of neutral hydrophobic solutes in reversed-phase capillary liquid chromatography. J. Sep. Sci. 2018, 41, 2886–2894. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.-S.; Fan, N.-S.; Tseng, Y.-C.; Jan, J.-S. Self-Assembly and Hydrogelation of Coil–Sheet Poly(l-lysine)-block-poly(l-threonine) Block Copolypeptides. Macromolecules 2018, 51, 8054–8063. [Google Scholar] [CrossRef]
- Miecznikowski, J.R.; Lo, W.; Lynn, M.A.; Jain, S.; Keilich, L.C.; Kloczko, N.F.; O’Loughlin, B.E.; DiMarzio, A.P.; Foley, K.M.; Lisi, G.P.; et al. Syntheses, characterization, density functional theory calculations, and activity of tridentate SNS zinc pincer complexes based on bis-imidazole or bis-triazole precursors. Inorg. Chim. Acta 2012, 387, 25–36. [Google Scholar] [CrossRef]
- Li, M.X.; Zhang, L.Z.; Chen, C.L.; Niu, J.Y.; Ji, B.S. Synthesis, crystal structures, and biological evaluation of Cu(II) and Zn(II) complexes of 2-benzoylpyridine Schiff bases derived from S-methyl- and S-phenyldithiocarbazates. J. Inorg. Biochem. 2012, 106, 117–125. [Google Scholar] [CrossRef]
- Bharti, A.; Bharati, P.; Singh, N.K.; Bharty, M.K. NNS tridentate thiosemicarbazide and 1,3,4-thiadiazole-2-amine complexes of some transition metal ions: Syntheses, structure and fluorescence properties. J. Coord. Chem. 2016, 69, 1258–1271. [Google Scholar] [CrossRef]
- Kowol, C.R.; Trondl, R.; Arion, V.B.; Jakupec, M.A.; Lichtscheidl, I.; Keppler, B.K. Fluorescence properties and cellular distribution of the investigational anticancer drug triapine (3-aminopyridine-2-carboxaldehyde thiosemicarbazone) and its zinc(II) complex. Dalton Trans. 2010, 39, 704–706. [Google Scholar] [CrossRef]
- Vlasenko, V.G.; Garnovskii, D.A.; Aleksandrov, G.G.; Makarova, N.I.; Levchenkov, S.I.; Trigub, A.L.; Zubavichus, Y.V.; Uraev, A.I.; Koshchienko, Y.V.; Burlov, A.S. Electrochemical synthesis, structural, spectral studies and DFT calculations of heteroleptic metal-chelates bearing N, N, S tridentate tosylamino functionalized pyrazole containing Schiff base and 1,10-phenathroline. Polyhedron 2019, 157, 6–17. [Google Scholar] [CrossRef]
- Bharati, P.; Bharti, A.; Chaudhari, U.K.; Bharty, M.K.; Kashyap, S.; Singh, U.P.; Singh, N.K. Trinuclear supramolecular Zn(II) complexes derived from N′-(pyridine carbonyl) hydrazine carboperthioates: Synthesis, structural characterization, luminescent properties and metalloaromaticity. Inorg. Chim. Acta 2015, 425, 100–107. [Google Scholar] [CrossRef]
- Tyagi, P.; Tyagi, M.; Agrawal, S.; Chandra, S.; Ojha, H.; Pathak, M. Synthesis, characterization of 1,2,4-triazole Schiff base derived 3d-metal complexes: Induces cytotoxicity in HepG2, MCF-7 cell line, BSA binding fluorescence and DFT study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 171, 246–257. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diana, R.; Panunzi, B. The Role of Zinc(II) Ion in Fluorescence Tuning of Tridentate Pincers: A Review. Molecules 2020, 25, 4984. https://doi.org/10.3390/molecules25214984
Diana R, Panunzi B. The Role of Zinc(II) Ion in Fluorescence Tuning of Tridentate Pincers: A Review. Molecules. 2020; 25(21):4984. https://doi.org/10.3390/molecules25214984
Chicago/Turabian StyleDiana, Rosita, and Barbara Panunzi. 2020. "The Role of Zinc(II) Ion in Fluorescence Tuning of Tridentate Pincers: A Review" Molecules 25, no. 21: 4984. https://doi.org/10.3390/molecules25214984
APA StyleDiana, R., & Panunzi, B. (2020). The Role of Zinc(II) Ion in Fluorescence Tuning of Tridentate Pincers: A Review. Molecules, 25(21), 4984. https://doi.org/10.3390/molecules25214984







