A Pilot-Scale Supercritical Carbon Dioxide Extraction to Valorize Colombian Mango Seed Kernel
Abstract
1. Introduction
2. Results and Discussions
2.1. Total Extraction Yield Analysis
2.2. Influence of Solid Conditions on Extraction Yield
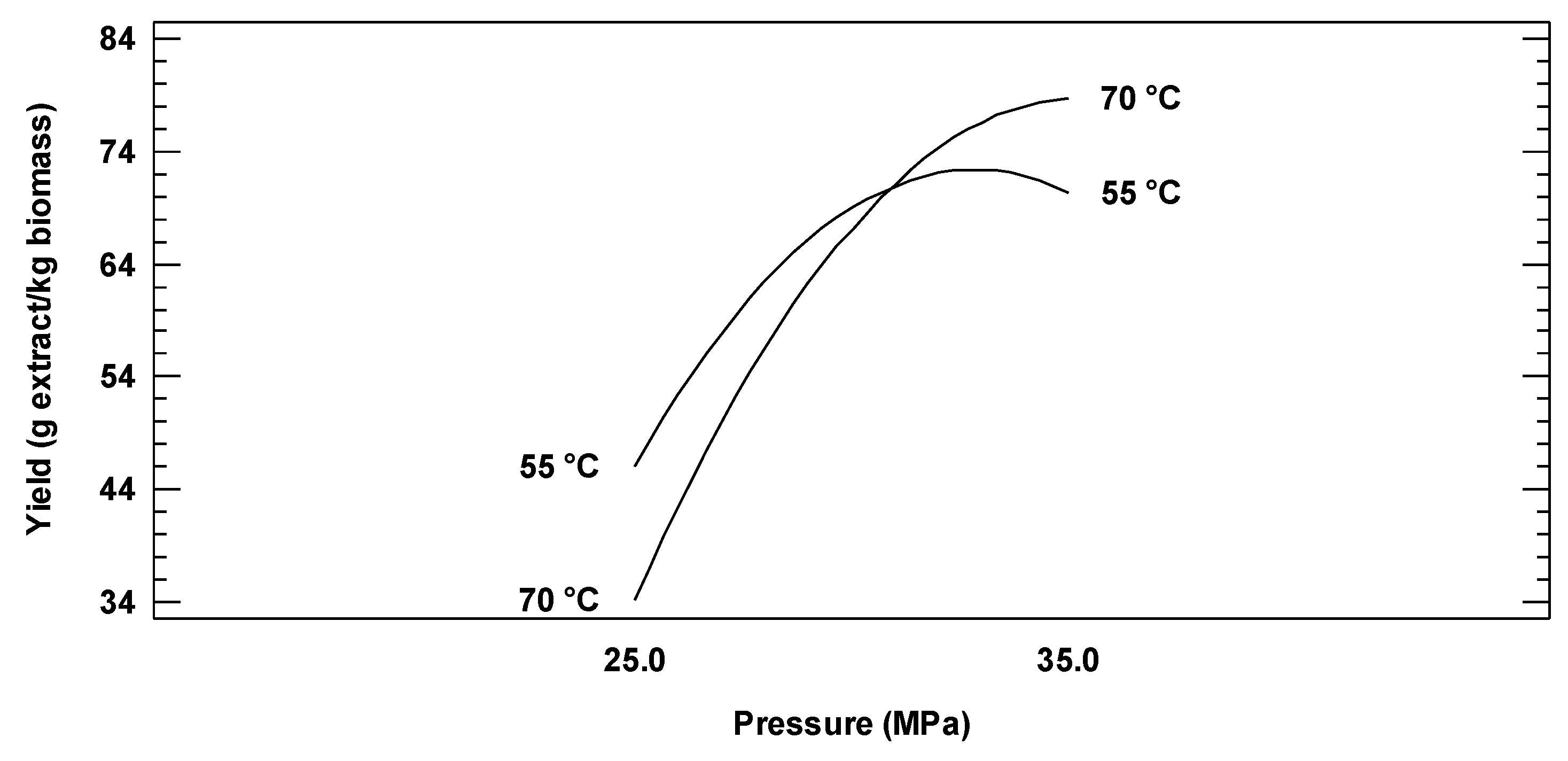
2.3. Effect of Process Parameters on Oil Extraction Yield
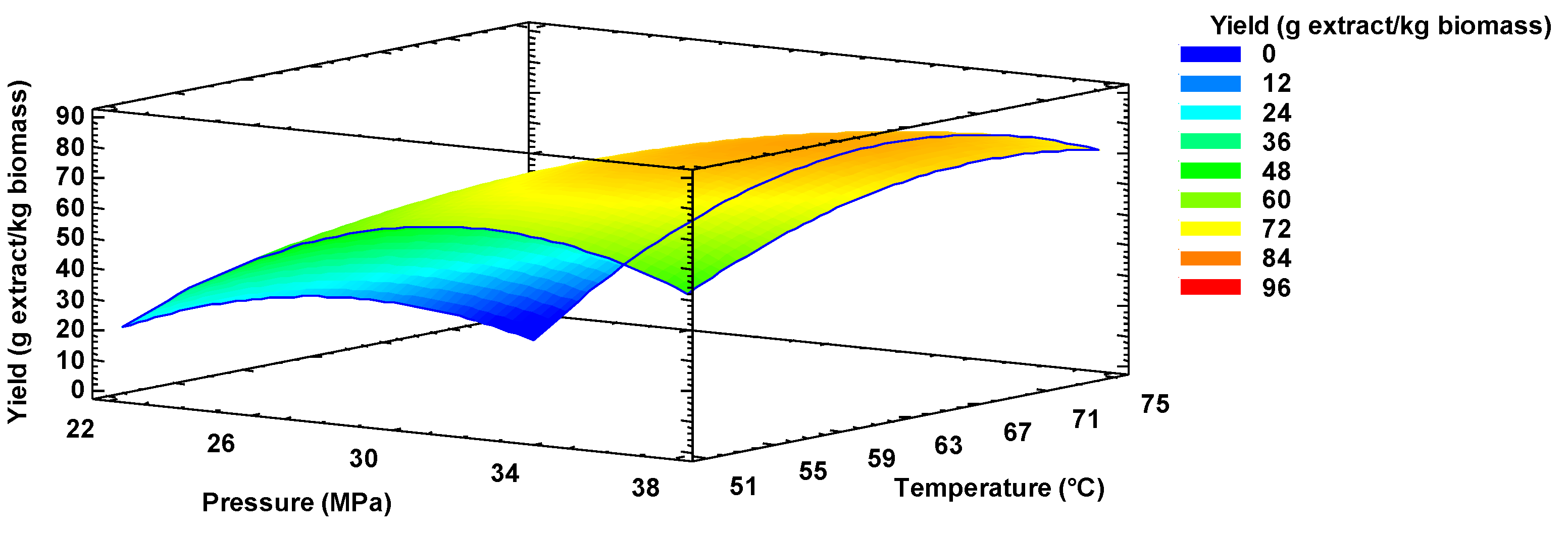
2.4. Oil Extraction Yield Optimization
2.5. Fatty Acid Profile
2.6. Extraction Optimization of Targeted Fatty Acids
3. Materials and Methods
3.1. Reagents
3.2. Biomass
3.3. Supercritical Fluid Extraction
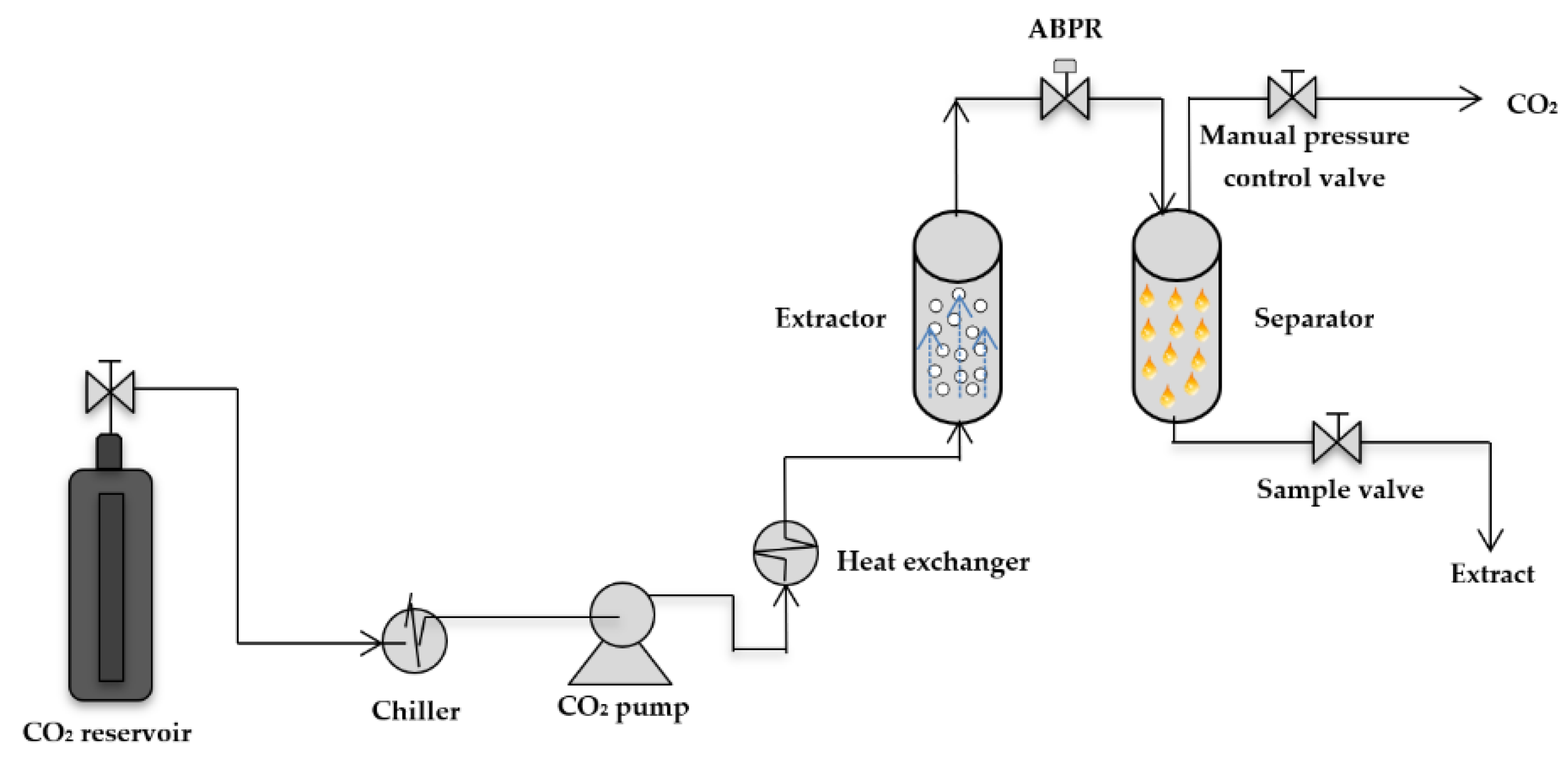
3.3.1. Experimental Installation
3.3.2. Operation Procedure
3.3.3. Optimization
3.4. Conventional Solvent Extraction
3.5. Fatty Acids Analysis
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Imbert, E. Food waste valorization options: Opportunities from the bioeconomy. Open Agric. 2017, 2, 195–204. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.; Gutiérrez, L.F.; Vargas, S.M.; Martinez-Correa, H.A.; Parada-Alfonso, F.; Narváez-Cuenca, C.E. Valorisation of mango peel: Proximate composition, supercritical fluid extraction of carotenoids, and application as an antioxidant additive for an edible oil. J. Supercrit. Fluids 2019, 152, 104574. [Google Scholar] [CrossRef]
- Otles, S.; Kartal, C. Food waste valorization. In Sustainable Food Systems from Agriculture to Industry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 371–399. [Google Scholar]
- Wang, H.W.; Liu, Y.Q.; Wei, S.L.; Yan, Z.J.; Lu, K. Comparison of microwave-assisted and conventional hydrodistillation in the extraction of essential oils from mango (Mangifera indica L.) flowers. Molecules 2010, 15, 7715–7723. [Google Scholar] [CrossRef]
- Severi, J.A.; Lima, Z.P.; Kushima, H.; Monteiro Souza Brito, A.R.; Campaner dos Santos, L.; Vilegas, W.; Hiruma-Lima, C.A. Polyphenols with antiulcerogenic action from aqueous decoction of mango leaves (Mangifera indica L.). Molecules 2009, 14, 1098–1110. [Google Scholar] [CrossRef] [PubMed]
- FAO. Medium-Term Outlook: Prospects for Global Production and Trade in Bananas and Tropical Fruits 2019 to 2028; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Sánchez-Camargo, A.; Ballesteros-Vivas, D.; Buelvas-Puello, L.M.; Martinez-Correa, H.A.; Parada-Alfonso, F.; Cifuentes, A.; Ferreira, S.R.S.; Gutiérrez, L.F. Microwave-assisted extraction of phenolic compounds with antioxidant and anti-proliferative activities from supercritical CO2 pre-extracted mango peel as valorization strategy. LWT 2021, 137, 110414. [Google Scholar] [CrossRef]
- Ballesteros-Vivas, D.; Álvarez-Rivera, G.; Morantes, S.J.; Sánchez-Camargo, A.; Ibáñez, E.; Parada-Alfonso, F.; Cifuentes, A. An integrated approach for the valorization of mango seed kernel: Efficient extraction solvent selection, phytochemical profiling and antiproliferative activity assessment. Food Res. Int. 2019, 126, 108616. [Google Scholar] [CrossRef]
- Minagricultura. Cadena del Mango: Indicadores e Instrumentos; Ministerio de Agricultura y Desarrollo Rural: Bogotá, Colombia, 2019.
- Ballesteros-Vivas, D.; Alvarez-Rivera, G.; García Ocampo, A.F.; Morantes, S.J.; Sánchez-Camargo, A.; Cifuentes, A.; Parada-Alfonso, F.; Ibánez, E. Supercritical antisolvent fractionation as a tool for enhancing antiproliferative activity of mango seed kernel extracts against colon cancer cells. J. Supercrit. Fluids 2019, 152, 104563. [Google Scholar] [CrossRef]
- Gasiński, A.; Kawa-Rygielska, J.; Szumny, A.; Czubaszek, A.; Gąsior, J.; Pietrzak, W. Volatile compounds content, physicochemical parameters, and antioxidant activity of beers with addition of mango fruit (Mangifera indica). Molecules 2020, 25, 3033. [Google Scholar] [CrossRef]
- Garrido-Suárez, B.B.; Garrido, G.; Delgado, R.; Bosch, F.; Rabí, M.d.C. A Mangifera indica L. extract could be used to treat neuropathic pain and implication of mangiferin. Molecules 2010, 15, 9035–9045. [Google Scholar] [CrossRef] [PubMed]
- Khaw, K.-Y.; Parat, M.-O.; Shaw, P.N.; Falconer, J.R. Solvent supercritical fluid technologies to extract bioactive compounds from natural sources: A review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent advances in supercritical fluid extraction of natural bioactive compounds from natural plant materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Tyśkiewicz, K.; Konkol, M.; Rój, E. The application of supercritical fluid extraction in phenolic compounds isolation from natural plant materials. Molecules 2018, 23, 2625. [Google Scholar] [CrossRef]
- Durante, M.; Ferramosca, A.; Treppiccione, L.; Di Giacomo, M.; Zara, V.; Montefusco, A.; Piro, G.; Mita, G.; Bergamo, P.; Lenucci, M.S. Application of response surface methodology (RSM) for the optimization of supercritical CO2 extraction of oil from patè olive cake: Yield, content of bioactive molecules and biological effects in vivo. Food Chem. 2020, 332, 127405. [Google Scholar] [CrossRef] [PubMed]
- Rustan, A.C.; Drevon, C.A. Fatty acids: Structures and properties. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Chichester, UK, 2005; pp. 1–7. [Google Scholar]
- Serafim, V.; Tiugan, D.-A.; Andreescu, N.; Mihailescu, A.; Paul, C.; Velea, I.; Puiu, M.; Niculescu, M. Development and validation of a LC–MS/MS-based assay for quantification of free and total omega 3 and 6 fatty acids from human plasma. Molecules 2019, 24, 360. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods—A review. J. Food Sci. Technol. 2014, 51, 2289–2303. [Google Scholar] [CrossRef]
- BCC Research. Oleochemical Fatty Acids: Global Markets to 2023; BCC Research: Wellesley, MA, USA, 2019. [Google Scholar]
- Awolu, O.O.; Manohar, B. Quantitative and qualitative characterization of mango kernel seed oil extracted using supercritical CO2 and solvent extraction techniques. Heliyon 2019, 5, e03068. [Google Scholar] [CrossRef]
- Jahurul, M.H.A.; Zaidul, I.S.M.; Norulaini, N.N.A.; Sahena, F.; Jaffri, J.M.; Omar, A.K.M. Supercritical carbon dioxide extraction and studies of mango seed kernel for cocoa butter analogy fats. CyTA J. Food 2014, 12, 97–103. [Google Scholar] [CrossRef]
- Jahurul, M.H.A.; Zaidul, I.S.M.; Norulaini, N.; Ferdosh, S.; Rahman, M.M.; Mohd, A.K. Optimization of supercritical carbon dioxide extraction parameters of cocoa butter analogy fat from mango seed kernel oil using response surface methodology. J. Food Sci. Technol. 2015, 52, 319–326. [Google Scholar] [CrossRef]
- Tirado, D.F.; de la Fuente, E.; Calvo, L. A selective extraction of hydroxytyrosol rich olive oil from alperujo. J. Food Eng. 2019, 263, 409–416. [Google Scholar] [CrossRef]
- Grodowska, K.; Parczewski, A. Organic solvents in the pharmaceutical industry. Acta Pol. Pharm. 2010, 67, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Viguera, M.; Marti, A.; Masca, F.; Prieto, C.; Calvo, L. The process parameters and solid conditions that affect the supercritical CO2 extraction of the lipids produced by microalgae. J. Supercrit. Fluids 2016, 113, 16–22. [Google Scholar] [CrossRef]
- Brunner, G. Gas Extraction; Topics in Physical Chemistry; Steinkopff: Heidelberg, Germany, 1994; Volume 4, ISBN 978-3-662-07382-7. [Google Scholar]
- Del Valle, J.M.; De La Fuente, J.C. Supercritical CO2 extraction of oilseeds: Review of kinetic and equilibrium models. Crit. Rev. Food Sci. Nutr. 2006, 46, 131–160. [Google Scholar] [CrossRef]
- Gurr, M.I.; Harwood, J.L. Fatty acid structure and metabolism. In Lipid Biochemistry; Springer: Boston, MA, USA, 1991; pp. 23–118. [Google Scholar]
- Simopoulos, A.P. Essential fatty acids in health and chronic disease. Am. J. Clin. Nutr. 1999, 70, 560s–569s. [Google Scholar] [CrossRef] [PubMed]
- Lieb, V.M.; Schuster, L.K.; Kronmüller, A.; Schmarr, H.-G.; Carle, R.; Steingass, C.B. Fatty acids, triacylglycerols, and thermal behaviour of various mango (Mangifera indica L.) kernel fats. Food Res. Int. 2019, 116, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Torres-León, C.; Rojas, R.; Contreras-Esquivel, J.C.; Serna-Cock, L.; Belmares-Cerda, R.E.; Aguilar, C.N. Mango seed: Functional and nutritional properties. Trends Food Sci. Technol. 2016, 55, 109–117. [Google Scholar] [CrossRef]
- López-Padilla, A.; Ruiz-Rodriguez, A.; Reglero, G.; Fornari, T. Study of the diffusion coefficient of solute-type extracts in supercritical carbon dioxide: Volatile oils, fatty acids and fixed oils. J. Supercrit. Fluids 2016, 109, 148–156. [Google Scholar] [CrossRef]
- Tirado, D.F.; Rousset, A.; Calvo, L. The selective supercritical extraction of high-value fatty acids from Tetraselmis suecica using the Hansen solubility theory. Chem. Eng. Trans. 2019, 75, 133–138. [Google Scholar] [CrossRef]
- Tirado, D.F.; Tenorio, M.J.; Cabañas, A.; Calvo, L. Prediction of the best cosolvents to solubilise fatty acids in supercritical CO2 using the Hansen solubility theory. Chem. Eng. Sci. 2018, 190, 14–20. [Google Scholar] [CrossRef]
- Tirado, D.F.; Calvo, L. The Hansen theory to choose the best cosolvent for supercritical CO2 extraction of β-carotene from Dunaliella salina. J. Supercrit. Fluids 2019, 145, 211–218. [Google Scholar] [CrossRef]
- Cerón, L.J.; Hurtado, A.M.; Ayala, A.A. Efecto de la presión y la temperatura de extracción con CO2 supercrítico sobre el rendimiento y composición de aceite de semillas de guayaba (Psidium guajava). Inf. Tecnológica 2016, 27, 249–258. [Google Scholar] [CrossRef][Green Version]
- AOAC Fatty acid in oils and fats preparation of methyl ester boron trifluoride method. In Official Methods of Analysis of AOAC International; AOAC International: Arlington, VA, USA, 1995; pp. 17–22.
- Torres-León, C.; Ascacio-Valdés, J.A.; Chávez-González, M.L.; Serna-Cock, L.; Ramirez-Guzman, N.; Cintra, A.; López-Badillo, C.; Rojas, R.; Belmares-Cerda, R.; Aguilar, C.N. Valorization of Ataulfo mango seed byproduct based on its nutritional and functional properties. In Bioprocessing of Agri-Food Residues for Production of Bioproducts; Flores-Gallegos, A.C., Rodriguez-Jasso, R.M., Aguilar, C.N., Eds.; Apple Academic: Orlando, FL, USA, 2020; p. 19. ISBN 9781771889162. [Google Scholar]

| Pressure (MPa) | Temperature (°C) | Extraction Yield (g Extract/kg Biomass) |
|---|---|---|
| 25 (−1) | 55 (−1) | 50 ± 3 b |
| 25 (−1) | 70 (+1) | 35 ± 3 a |
| 35 (+1) | 55 (−1) | 69 ± 1 cd |
| 35 (+1) | 70 (+1) | 74 ± 2 cd |
| 23 (−1.41) | 63 (0) | 24 ± 1 a |
| 37 (+1.41) | 63 (0) | 83 ± 2 de |
| 30 (0) | 52 (−1.41) | 63 ± 2 bc |
| 30 (0) | 73 (+1.41) | 65 ± 1 bc |
| 30 (0) | 63 (0) | 73 ± 3 cd |
| Pressure (MPa) | Temperature (°C) | g Fatty Acid/kg Extract | SFA/UFA | ||||
|---|---|---|---|---|---|---|---|
| C16:0 | C18:0 | C18:1 | C18:2 | C18:3 | |||
| 25 | 55 | 32 ± 1 abc | 61 ± 2 ab | 93 ± 1 ab | 24 ± 1 ab | 3 ± 0 bcd | 1.29 a |
| 25 | 70 | 38 ± 2 cd | 63 ± 1 ab | 105 ± 1 abc | 31 ± 1 ab | 4 ± 0 de | 1.41 a |
| 35 | 55 | 28 ± 1 abc | 45 ± 1 a | 139 ± 1 bc | 25 ± 0 ab | 2 ± 0 abc | 2.45 a |
| 35 | 70 | 23 ± 1 ab | 54 ± 1 ab | 69 ± 0 a | 16 ± 1 a | 1 ± 0 a | 1.12 a |
| 23 | 63 | 50 ± 1 d | 71 ± 1 ab | 155 ± 1 c | 37 ± 2 b | 4 ± 0 e | 1.62 a |
| 37 | 63 | 23 ± 1 ab | 55 ± 2 ab | 69 ± 1 a | 16 ± 1 ab | 2 ± 0 ab | 1.09 a |
| 30 | 52 | 35 ± 1 bc | 84 ± 1 b | 111 ± 1 abc | 24 ± 1 ab | 2 ± 0 ab | 1.14 a |
| 30 | 73 | 41 ± 1 cd | 64 ± 1 ab | 111 ± 2 abc | 33 ± 1 ab | 3 ± 0 cde | 1.41 a |
| 30 | 63 | 30 ± 1 abc | 62 ± 1 ab | 93 ± 1 abc | 22 ± 1 ab | 2 ± 0 abc | 1.25 a |
| Soxhlet extraction | 21 ± 1 a | 47 ± 1 a | 61 ± 2 a | 17 ± 1 ab | 2 ± 0 ab | 1.15 a | |
| Factor | Level | ||
|---|---|---|---|
| Low (−1) | Central (0) | High (+1) | |
| Pressure (MPa, X1) | 25 | 30 | 35 |
| Temperature (°C, X2) | 55 | 63 | 70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerón-Martínez, L.J.; Hurtado-Benavides, A.M.; Ayala-Aponte, A.; Serna-Cock, L.; Tirado, D.F. A Pilot-Scale Supercritical Carbon Dioxide Extraction to Valorize Colombian Mango Seed Kernel. Molecules 2021, 26, 2279. https://doi.org/10.3390/molecules26082279
Cerón-Martínez LJ, Hurtado-Benavides AM, Ayala-Aponte A, Serna-Cock L, Tirado DF. A Pilot-Scale Supercritical Carbon Dioxide Extraction to Valorize Colombian Mango Seed Kernel. Molecules. 2021; 26(8):2279. https://doi.org/10.3390/molecules26082279
Chicago/Turabian StyleCerón-Martínez, Leidy J., Andrés M. Hurtado-Benavides, Alfredo Ayala-Aponte, Liliana Serna-Cock, and Diego F. Tirado. 2021. "A Pilot-Scale Supercritical Carbon Dioxide Extraction to Valorize Colombian Mango Seed Kernel" Molecules 26, no. 8: 2279. https://doi.org/10.3390/molecules26082279
APA StyleCerón-Martínez, L. J., Hurtado-Benavides, A. M., Ayala-Aponte, A., Serna-Cock, L., & Tirado, D. F. (2021). A Pilot-Scale Supercritical Carbon Dioxide Extraction to Valorize Colombian Mango Seed Kernel. Molecules, 26(8), 2279. https://doi.org/10.3390/molecules26082279






