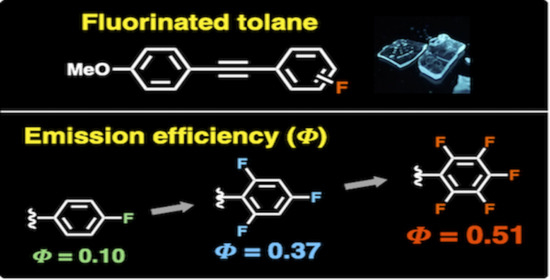
Systematic Studies on the Effect of Fluorine Atoms in Fluorinated Tolanes on Their Photophysical Properties
Abstract
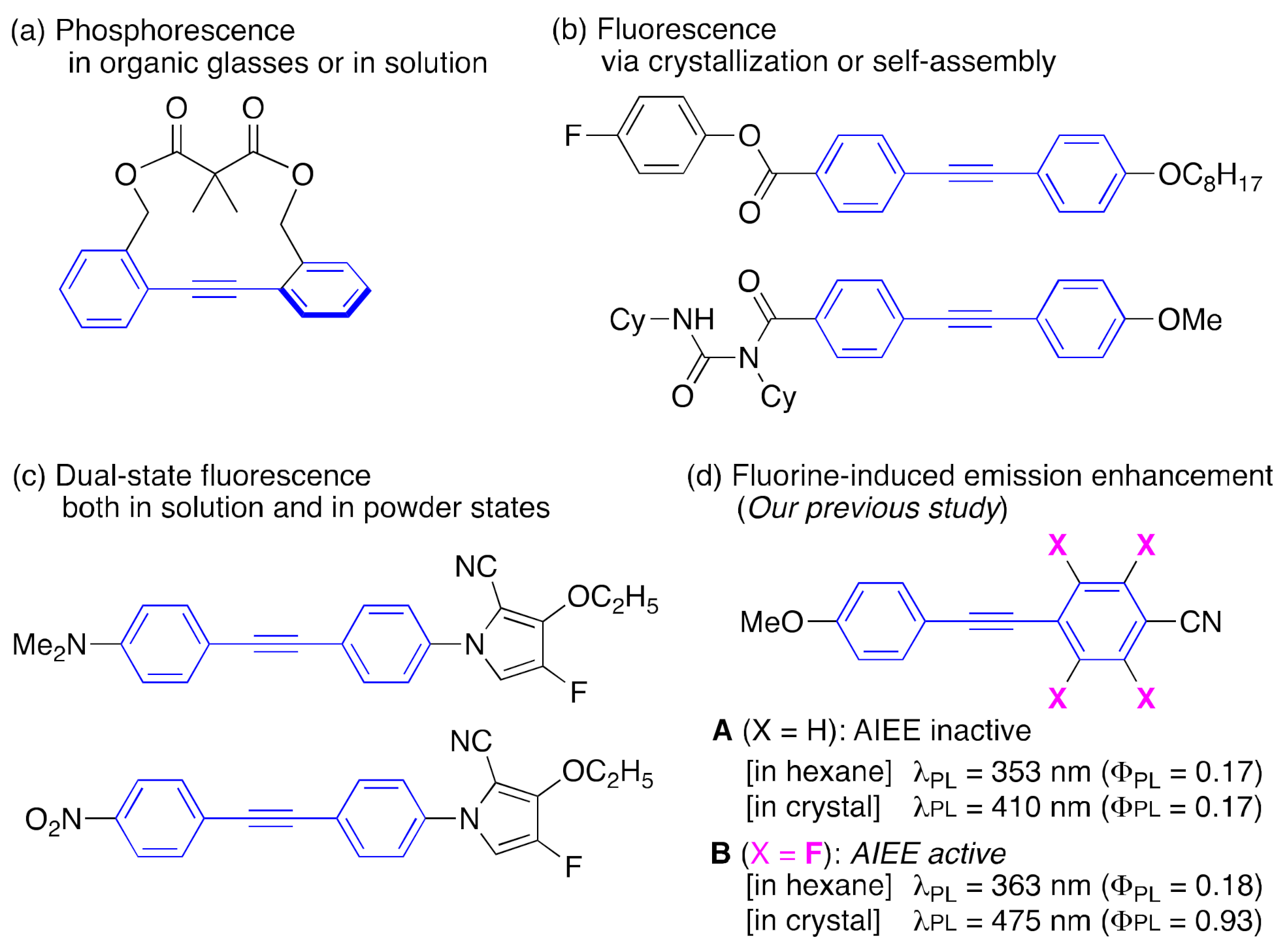
1. Introduction
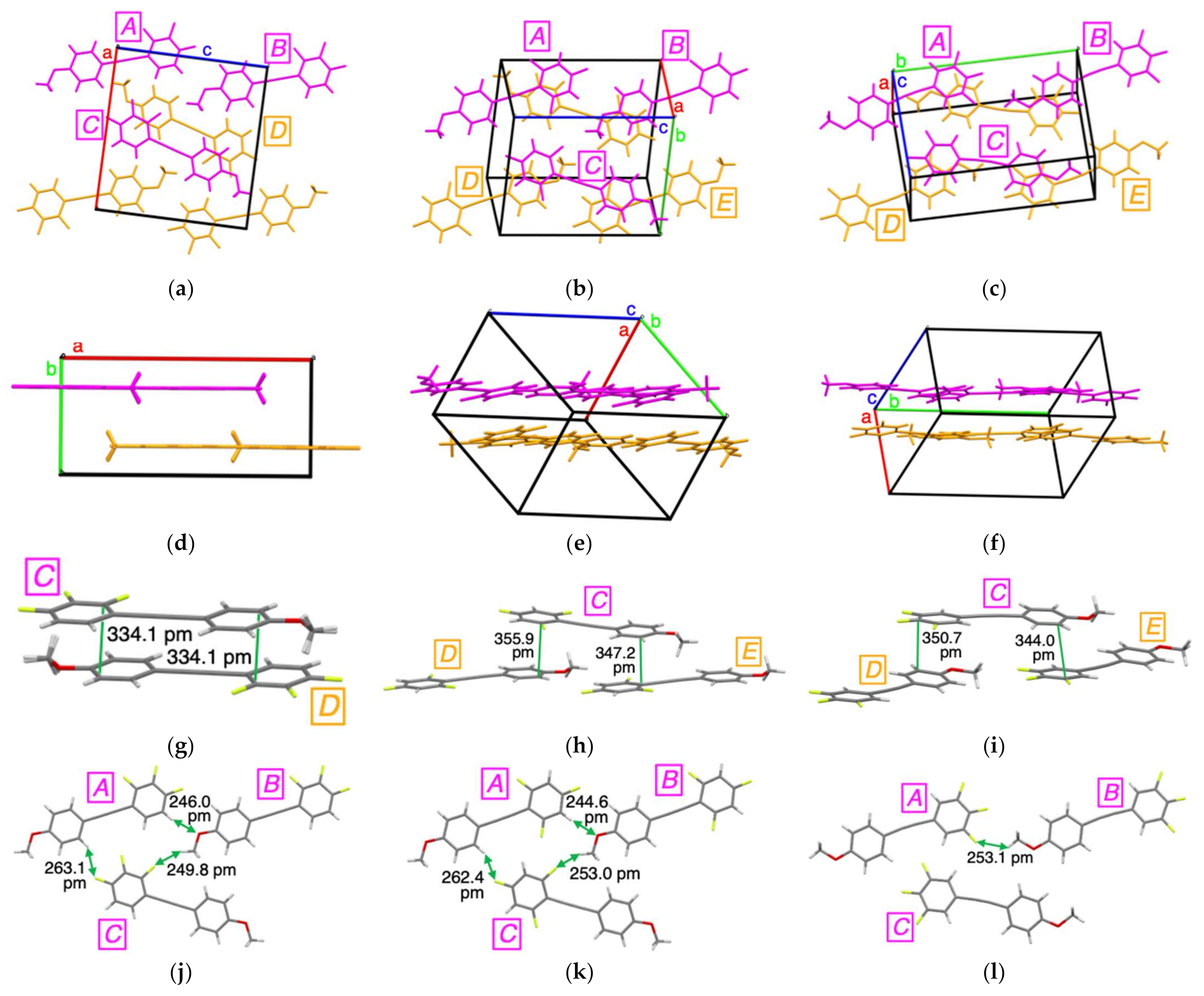
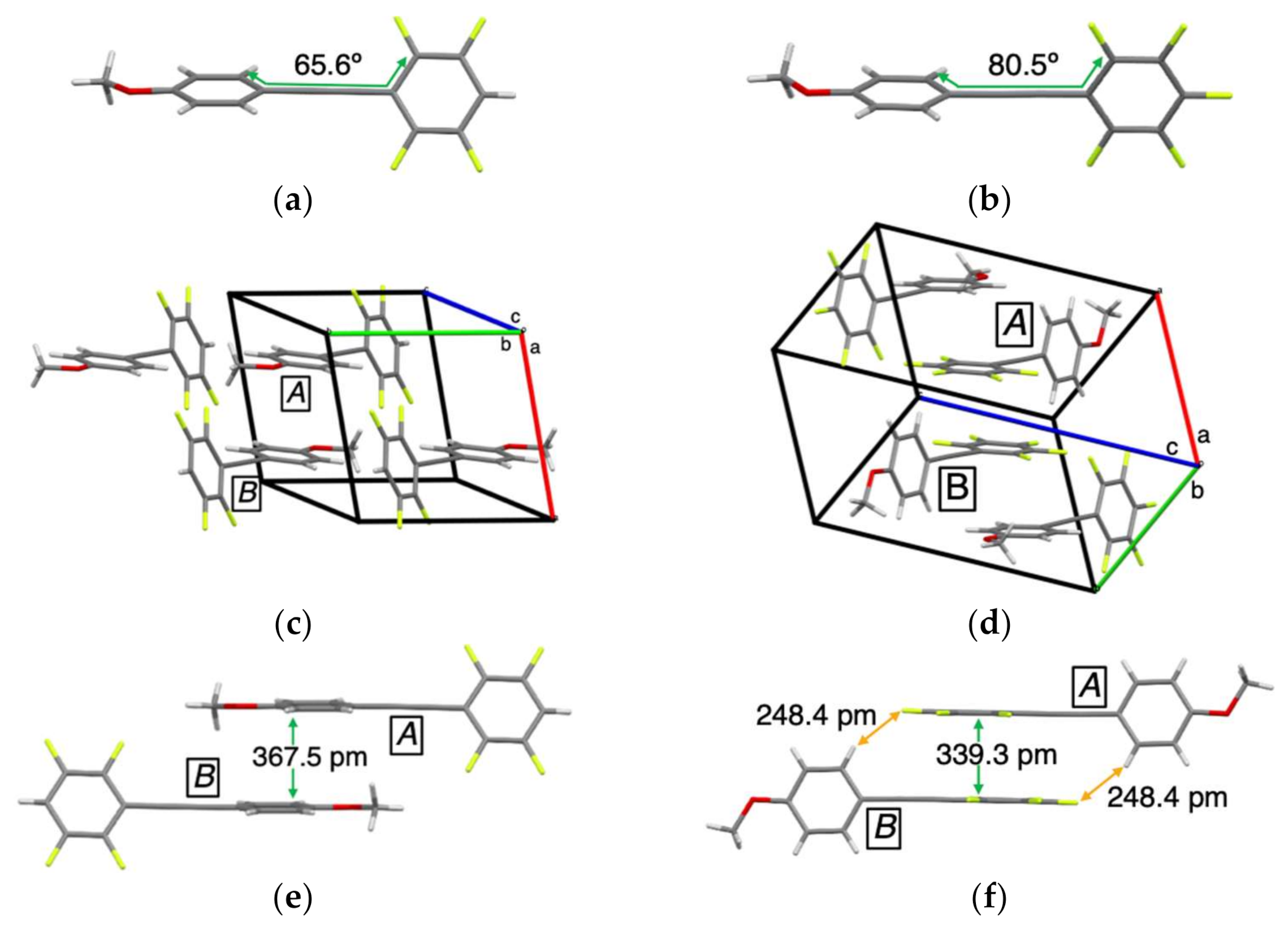
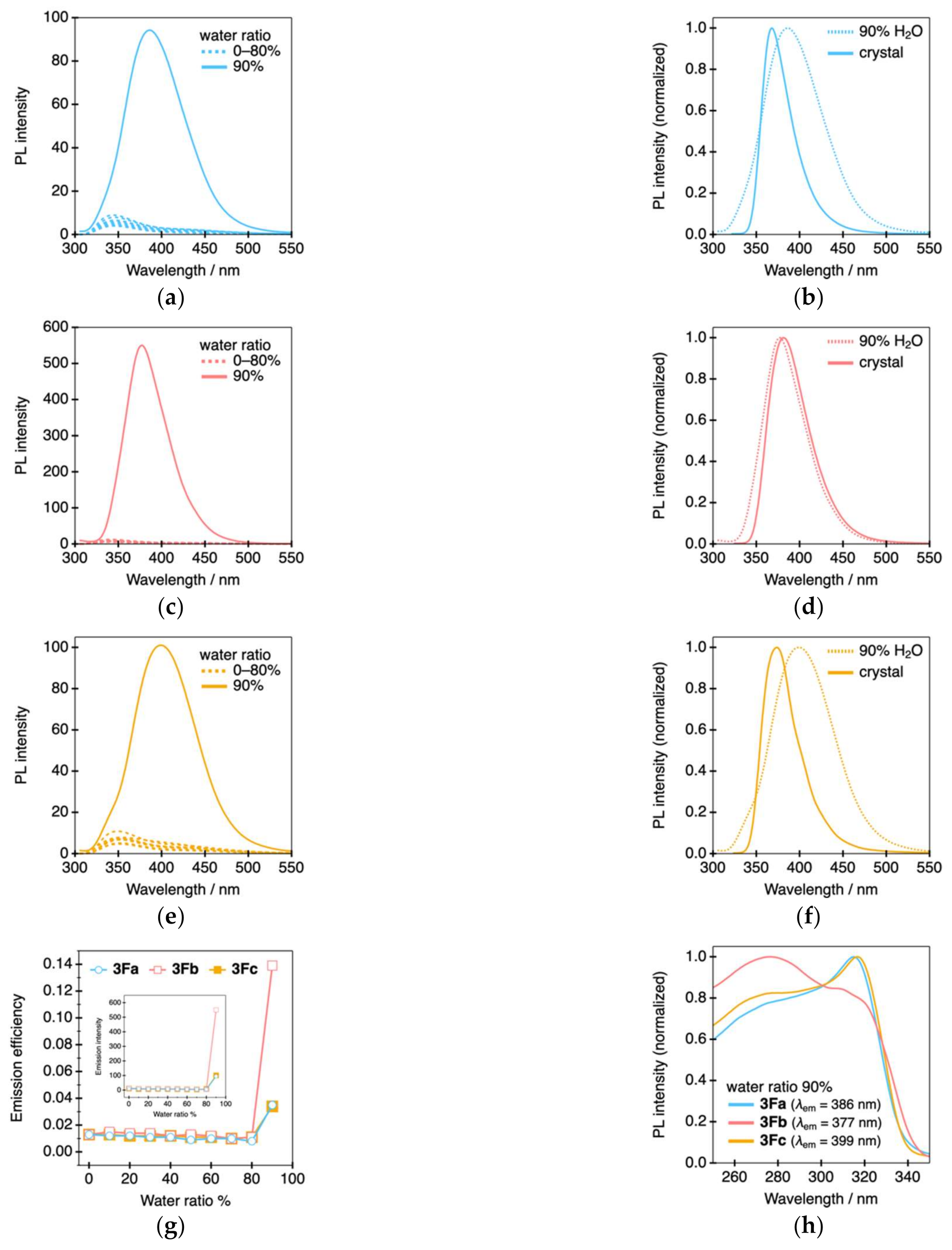
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. General Synthesis Procedure for the Pd(0)-Catalyzed Sonogashira Cross-Coupling Reaction
3.2.1. [2-(4-methoxyphenyl)ethyn-1-yl]benzene (0F)
3.2.2. 1-Fluoro-4-[2-(4-methoxyphenyl)ethyn-1-yl]benzene (1F)
3.2.3. 1,2,3-Trifluoro-4-[2-(4-methoxyphenyl)ethyn-1-yl]benzene (3Fa)
3.2.4. 1,3,5-Trifluoro-4-[2-(4-methoxyphenyl)ethyn-1-yl]benzene (3Fb)
3.2.5. 1,2,6-Trifluoro-4-[2-(4-methoxyphenyl)ethyn-1-yl]benzene (3Fc)
3.2.6. 2,3,5,6-Tetrafluoro-4-[2-(4-methoxyphenyl)ethyn-1-yl]benzene (4F)
3.3. Photophysical Measurements
3.4. Single Crystal X-ray Diffraction
3.5. Cyclic Voltammetry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gutmann, M.; Gudipati, M.; Schoenzart, P.F.; Hohlneicher, G. Electronic spectra of matrix-isolated tolan: Site selective one- and two-photon spectra. J. Phys. Chem. 1992, 96, 2433–2442. [Google Scholar] [CrossRef]
- Suzuki, T.; Nakamura, M.; Isozaki, T.; Ikoma, T. “Dark” Excited States of Diphenylacetylene Studied by Nonresonant Two-Photon Excitation Optical-Probing Photoacoustic Spectroscopy. Int. J. Thermophys. 2012, 33, 2046–2054. [Google Scholar] [CrossRef]
- Isozaki, T.; Oba, H.; Ikoma, T.; Suzuki, T. Simultaneous two-photon absorption to gerade excited singlet states of diphe-nylacetylene and diphenylbutadiyne using optical-probing photoacoustic spectroscopy. J. Phys. Chem. A 2016, 120, 6137–6145. [Google Scholar] [CrossRef]
- Young, D.D.; Scharrer, E.; Yoa, M.V. Synthesis and phase behavior of liquid crystalline diphenylacetylene derivatives possessing high clearing temperatures. Mol. Cryst. Liq. Cryst. 2004, 408, 21–31. [Google Scholar] [CrossRef]
- Cheng, Z.; Zang, Y.; Li, Y.; Li, B.; Hu, C.; Li, H.; Yang, Y. A chiral luminescent liquid crystal with a tolane unit. Liq. Cryst. 2016, 43, 777–782. [Google Scholar] [CrossRef]
- Arakawa, Y.; Inui, S.; Tsuji, H. Novel diphenylacetylene-based room-temperature liquid crystalline molecules with alkylthio groups, and investigation of the role for terminal alkyl chains in mesogenic incidence and tendency. Liq. Cryst. 2017, 45, 811–820. [Google Scholar] [CrossRef]
- Yang, W.-Y.; Roy, S.; Phrathep, B.; Rengert, Z.; Kenworthy, R.; Zorio, D.A.R.; Alabugin, I.V. Engineering pH-gated transi-tions for selective and efficient double-strand DNA photocleavage in hypoxic tumors. J. Med. Chem. 2011, 54, 8501–8516. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-Y.; Marrone, S.A.; Minors, N.; Zorio, D.A.R.; Alabugin, I.V. Fine-tuning alkyne cycloadditions: Insights into photochemistry responsible for the double-strand DNA cleavage via structural perturbations in diaryl alkyne conjugates. Beilstein J. Org. Chem. 2011, 7, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Do, T.T.; Chavhan, S.; Subbiah, J.; Ou, T.-H.; Manzhos, S.; Jones, D.J.; Bell, J.M.; Jou, J.-H.; Sonar, P.M. Naphthalimide end-capped diphenylacetylene: A versatile organic semiconductor for blue light emitting diodes and a donor or an acceptor for solar cells. New J. Chem. 2019, 43, 9243–9254. [Google Scholar] [CrossRef]
- Ferrante, C.; Kensy, U.; Dick, B. Does diphenylacetylene (tolan) fluorescence from its second excited singlet state? Sem-iempirical MO calculations and fluorescence quantum yield measurements. J. Phys. Chem. 1993, 97, 13457–13463. [Google Scholar] [CrossRef]
- Zgierski, M.Z.; Lim, E.C. Nature of the ’dark’ state in diphenylacetylene and related molecules: State switch from the linear ππ* state to the bent πσ*state. Chem. Phys. Lett. 2004, 387, 352–355. [Google Scholar] [CrossRef]
- Saltiel, J.; Kumar, V.K.R. Photophysics of diphenylacetylene: Light from the “dark state”. J. Phys. Chem. A 2012, 116, 10548–10558. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, V.; Prabhu, D.D.; Das, S. Optical investigation of self-aggregation of a tetrazole-substituted diphenylacety-lene derivative: Steady and excited state dynamics in solid and solution state. J. Phys. Chem. C 2013, 117, 9404–9415. [Google Scholar] [CrossRef]
- Wierzbicka, M.; Bylińska, I.; Czaplewski, C.; Wiczk, W. Experimental and theoretical studies of the spectroscopic properties of simple symmetrically substituted diphenylacetylene derivatives. RSC Adv. 2015, 5, 29294–29303. [Google Scholar] [CrossRef]
- Kozhemyakin, Y.; Krämer, M.; Rominger, F.; Dreuw, A.; Bunz, U.H.F. A Tethered Tolane: Twisting the Excited State. Chem. A Eur. J. 2018, 24, 15219–15222. [Google Scholar] [CrossRef]
- Menning, S.; Krämer, M.; Duckworth, A.; Rominger, F.; Beeby, A.; Dreuw, A.; Bunz, U.H.F. Bridged Tolanes: A Twisted Tale. J. Org. Chem. 2014, 79, 6571–6578. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Wang, Y.J.; Wang, Z.; Sun, J.Z.; Tang, B.Z. Crystallization-Induced Emission Enhancement of a Simple Tolane-Based Mesogenic Luminogen. J. Phys. Chem. C 2015, 119, 21875–21881. [Google Scholar] [CrossRef]
- Zang, Y.; Li, Y.; Li, B.; Li, H.; Yang, Y. Light emission properties and self-assembly of a tolane-based luminogen. RSC Adv. 2015, 5, 38690–38695. [Google Scholar] [CrossRef]
- Bu, X.; Zhu, D.; Liu, T.; Li, Y.; Cai, S.; Wang, H.; Zeng, Z. Approach to tuned emitting color of luminescent liquid crystals with substituted fluoropyrrole acceptor unit. Dye. Pigment. 2017, 145, 324–330. [Google Scholar] [CrossRef]
- Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2013; pp. 7–21. [Google Scholar]
- O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2007, 37, 308–319. [Google Scholar] [CrossRef]
- Yamada, S.; Uto, E.; Agou, T.; Kubota, T.; Konno, T. Fluorinated tolane dyads with alkylene linkage: Synthesis and eval-uation of photophysical characteristics. Crystals 2020, 10, 711. [Google Scholar] [CrossRef]
- Morita, M.; Yamada, S.; Konno, T. Fluorine-induced emission enhancement of tolanes via formation of tight molecular aggregates. New J. Chem. 2020, 44, 6704–6708. [Google Scholar] [CrossRef]
- Yamada, S.; Mitsuda, A.; Miyano, K.; Tanaka, T.; Morita, M.; Agou, T.; Kubota, T.; Konno, T. Development of novel sol-id-state light-emitting materials based on pentafluorinated tolane fluorophores. ACS Omega 2018, 3, 9105–9113. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, A.R.; Davydova, M.P.; Sherin, P.S.; Korolev, V.V.; Stepanov, A.A.; Kalneus, E.V.; Benassi, E.; Vasilevsky, S.F.; Stass, D.V. X-Ray Generated Recombination Exciplexes of Substituted Diphenylacetylenes with Tertiary Amines: A Ver-satile Experimental Vehicle for Targeted Creation of Deep-Blue Electroluminescent Systems. J. Phys. Chem. A 2018, 122, 1235–1252. [Google Scholar] [CrossRef]
- Hirata, Y.; Okada, T.; Nomoto, T. Higher excited singlet state of diphenylacetylene in solution phase. Chem. Phys. Lett. 1993, 209, 397–402. [Google Scholar] [CrossRef]
- Khundkar, L.R.; Stiegman, A.E.; Perry, J.W. Solvent-tuned intramolecular charge-recombination rates in a conjugated donor-acceptor molecule. J. Phys. Chem. 1990, 94, 1224–1226. [Google Scholar] [CrossRef]
- Szyszkowska, M.; Bylińska, I.; Wiczk, W. Influence of an electron-acceptor substituent type on the photophysical properties of unsymmetrically substituted diphenylacetylene. J. Photochem. Photobiol. A Chem. 2016, 326, 76–88. [Google Scholar] [CrossRef]
- Soloshonok, V.A.; Hayashi, T. Gold(I)-catalyzed asymmetric aldol reaction of methyl isocyanoacetate with fluorinated benzaldehydes. Tetrahedron Lett. 1994, 35, 2713–2716. [Google Scholar] [CrossRef]
- Soloshonok, V.A.; Hayashi, T. Gold(I)-catalyzed asymmetric aldol reactions of fluorinated benzaldehydes with an α-isocyanoacetamide. Tetrahedron Asymmetry 1994, 5, 1091–1094. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Turley, A.T.; Wang, L.; McGonigal, P.R.; Tu, Y.; Li, Y.; Wang, Z.; Kwok, R.T.K.; Lam, J.W.Y.; et al. Aggregate Science: From Structures to Properties. Adv. Mater. 2020, 32, 2001457. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.-T.; Liu, C.; Li, Q.; Zhang, H.; Lam, J.W.Y.; Tang, B.Z. Structure, Assembly, and Function of (Latent)-Chiral AIEgens. ACS Mater. Lett. 2019, 1, 192–202. [Google Scholar] [CrossRef]
- He, Z.; Ke, C.; Tang, B.Z. Journey of Aggregation-Induced Emission Research. ACS Omega 2018, 3, 3267–3277. [Google Scholar] [CrossRef]
- Nielsen, A.; Kuzmanich, G.; Garcia-Garibay, M.A. Quantum Chain Reaction of Tethered Diarylcyclopropenones in the Solid State and Their Distance-Dependence in Solution Reveal a Dexter S2–S2Energy-Transfer Mechanism. J. Phys. Chem. A 2014, 118, 1858–1863. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.L.N.; Jadhav, D.N.; Dasgupta, P. Pd-Catalyzed Domino Synthesis of Internal Alkynes Using Triarylbismuths as Multicoupling Organometallic Nucleophiles. Org. Lett. 2010, 12, 2048–2051. [Google Scholar] [CrossRef] [PubMed]
- Severin, R.; Reimer, J.; Doye, S. One-Pot Procedure for the Synthesis of Unsymmetrical Diarylalkynes. J. Org. Chem. 2010, 75, 3518–3521. [Google Scholar] [CrossRef]
- CrysAlisPro 1.171.39.43a. Rigaku Oxford Diffraction; Rigaku Corporation: Akishima, Japan, 2015. [Google Scholar]
- Sheldrick, G.M. SHELXT– Integrated space-group and crystal-structure determination. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]

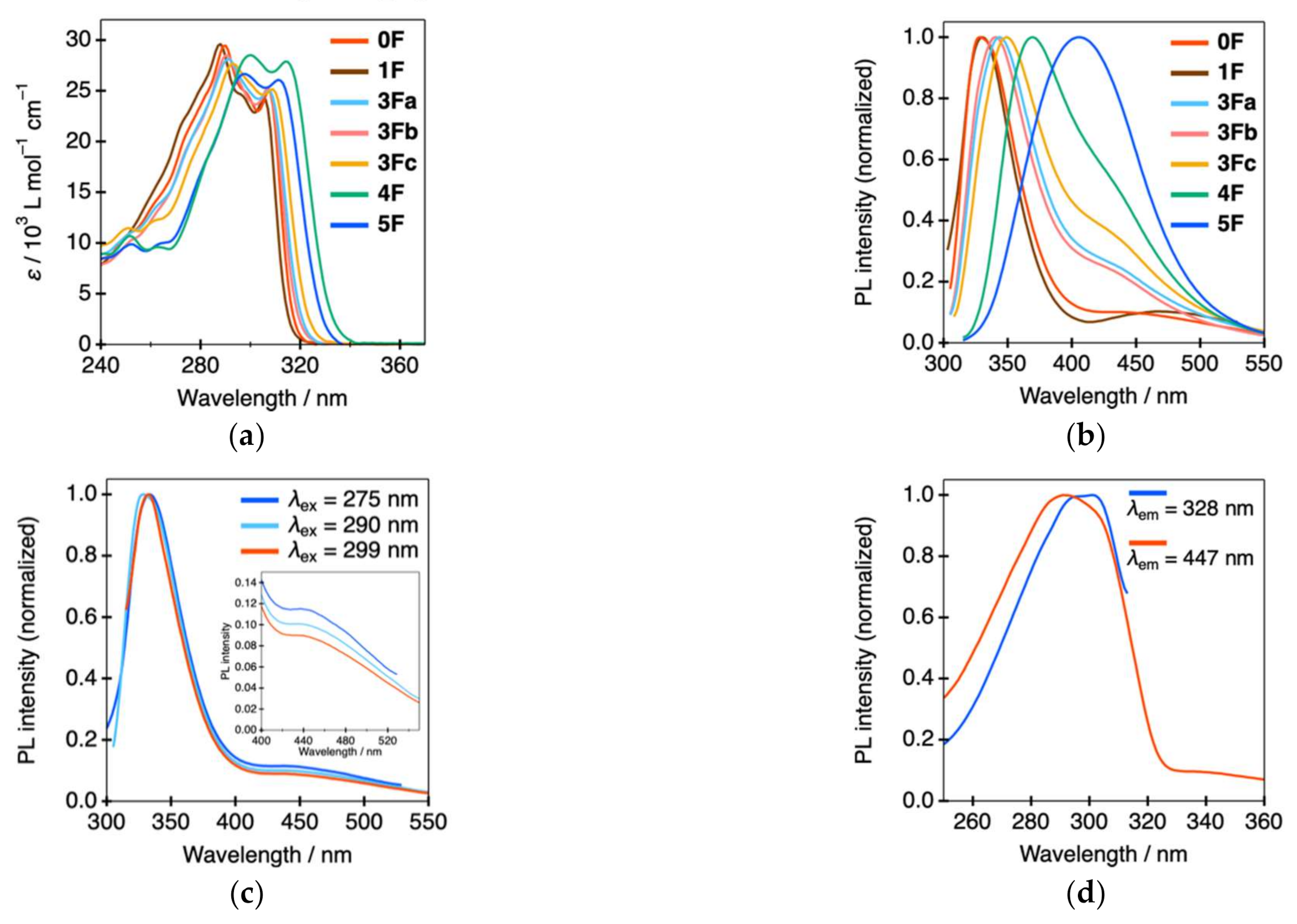
| Compound | EHOMO (eV) a | ELUMO (eV) a | ΔE (eV) a | λabs (nm) [ε, 103 L·mol–1·cm–1] | λPL (nm) b | ФPL c | τave (ns) d | τ1 (ns) d | τ2 (ns) d |
|---|---|---|---|---|---|---|---|---|---|
| 0F | −5.81 | –2.09 | 3.72 | 290 [29.5], 299 [24.6], 307 [25.0] | 328, 447 | <0.01 | 2.63 | 0.82 | 5.08 |
| 1F | –5.81 | –2.06 | 3.75 | 288 [29.6], 297 [24.5], 305 [24.2] | 330, 469 | <0.01 | 2.86 | 0.78 | 5.23 |
| 3Fa | –5.96 | –2.33 | 3.63 | 291 [28.2], 307 [25.0] | 343, 431 | 0.01 | 2.55 | 0.86 | 4.84 |
| 3Fb | –5.96 | –2.27 | 3.69 | 290 [28.5], 307 [25.3] | 340, 433 | 0.01 | 2.12 | 0.75 | 4.92 |
| 3Fc | –5.96 | –2.38 | 3.58 | 293 [27.7], 309 [25.2] | 349, 436 | 0.01 | 2.37 | 0.86 | 5.93 |
| 4F | –6.07 | –2.50 | 3.57 | 300 [28.5], 314 [27.9] | 369, 432 | 0.04 | 3.38 | 1.12 | 5.68 |
| 5F | –6.02 | –2.61 | 3.41 | 297 [26.7], 311 [26.1] | 406 | 0.08 | 2.82 | 1.41 | 5.42 |
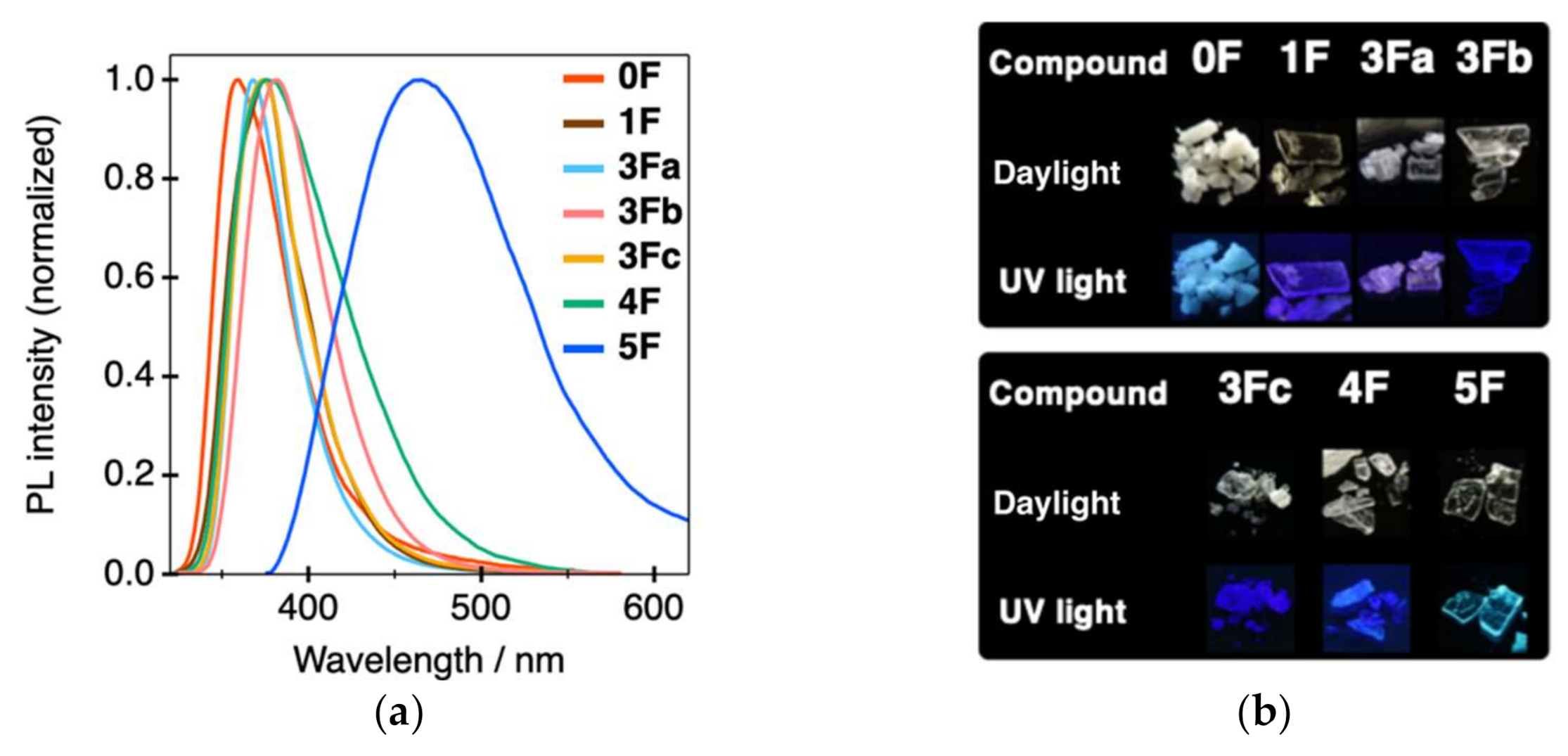
| Compound | λPL (nm) a | ФPL b | τPL (ns) c | kr (108 s−1) d | knr (108 s−1) e |
|---|---|---|---|---|---|
| 0F | 359 | 0.04 | 0.76 | 0.53 | 12.6 |
| 1F | 375 | 0.10 | 2.21 | 0.45 | 4.07 |
| 3Fa | 368 | 0.31 | 2.12 | 1.46 | 3.25 |
| 3Fb | 381 | 0.37 | 3.81 | 0.97 | 1.65 |
| 3Fc | 374 | 0.14 | 1.13 | 1.24 | 7.61 |
| 4F | 375 | 0.04 | 0.77 | 0.52 | 12.5 |
| 5F | 465 | 0.51 | 2.37 | 2.15 | 2.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morita, M.; Yamada, S.; Konno, T. Systematic Studies on the Effect of Fluorine Atoms in Fluorinated Tolanes on Their Photophysical Properties. Molecules 2021, 26, 2274. https://doi.org/10.3390/molecules26082274
Morita M, Yamada S, Konno T. Systematic Studies on the Effect of Fluorine Atoms in Fluorinated Tolanes on Their Photophysical Properties. Molecules. 2021; 26(8):2274. https://doi.org/10.3390/molecules26082274
Chicago/Turabian StyleMorita, Masato, Shigeyuki Yamada, and Tsutomu Konno. 2021. "Systematic Studies on the Effect of Fluorine Atoms in Fluorinated Tolanes on Their Photophysical Properties" Molecules 26, no. 8: 2274. https://doi.org/10.3390/molecules26082274
APA StyleMorita, M., Yamada, S., & Konno, T. (2021). Systematic Studies on the Effect of Fluorine Atoms in Fluorinated Tolanes on Their Photophysical Properties. Molecules, 26(8), 2274. https://doi.org/10.3390/molecules26082274