Clinical Perspectives of Theranostics
Abstract
1. Introduction
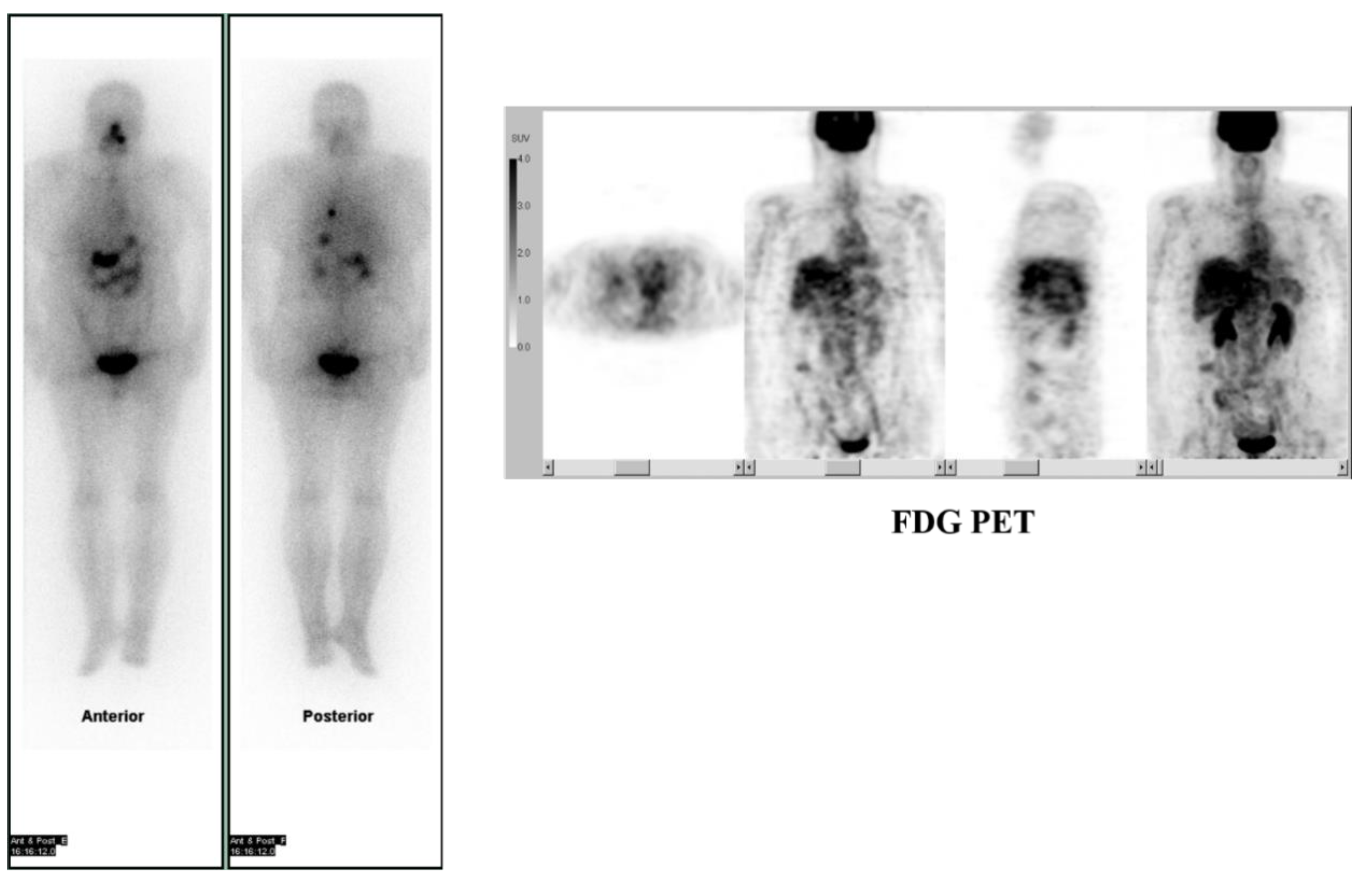
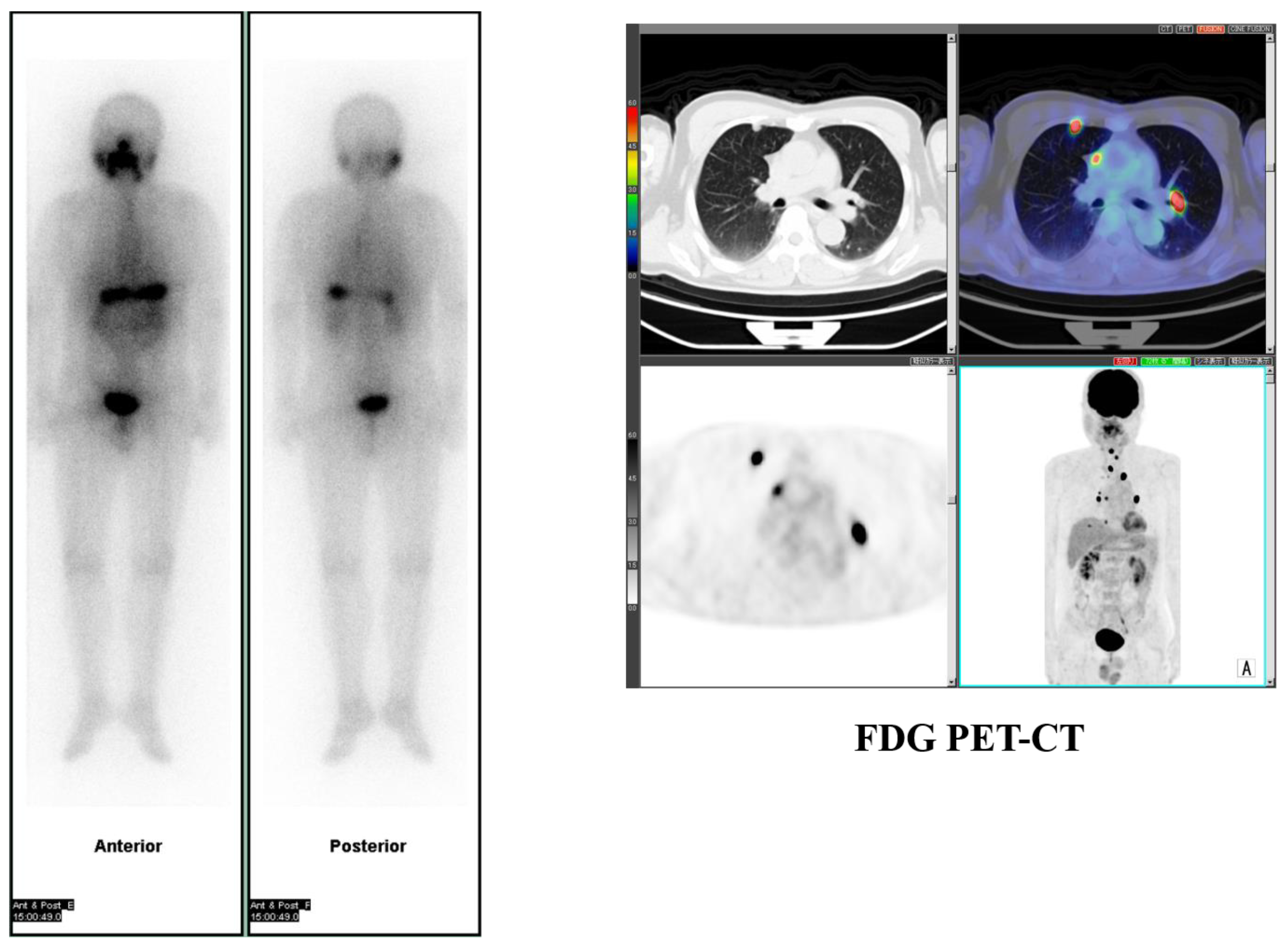
2. Prognostic Value of Theranostics for Thyroid Cancer
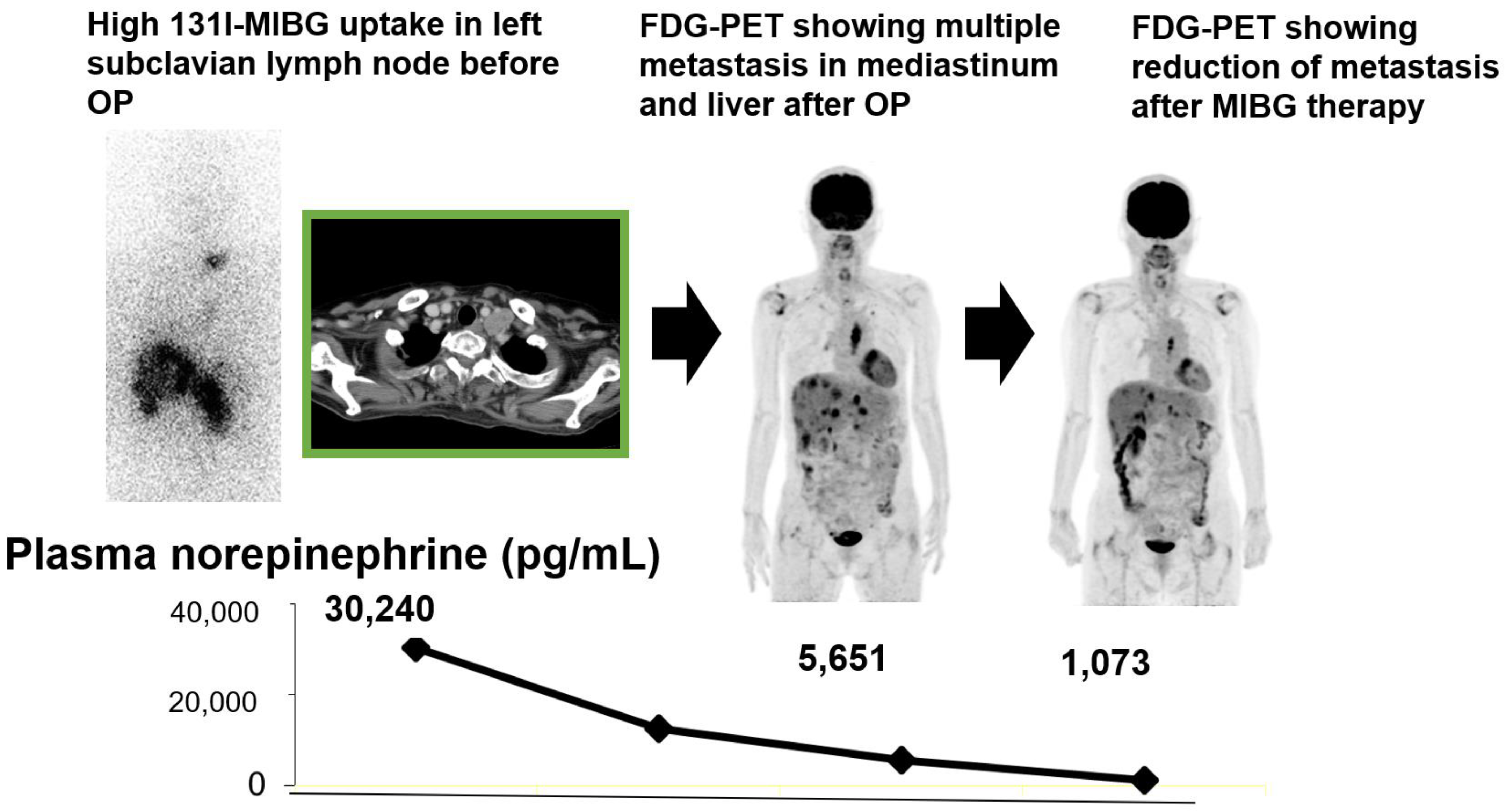
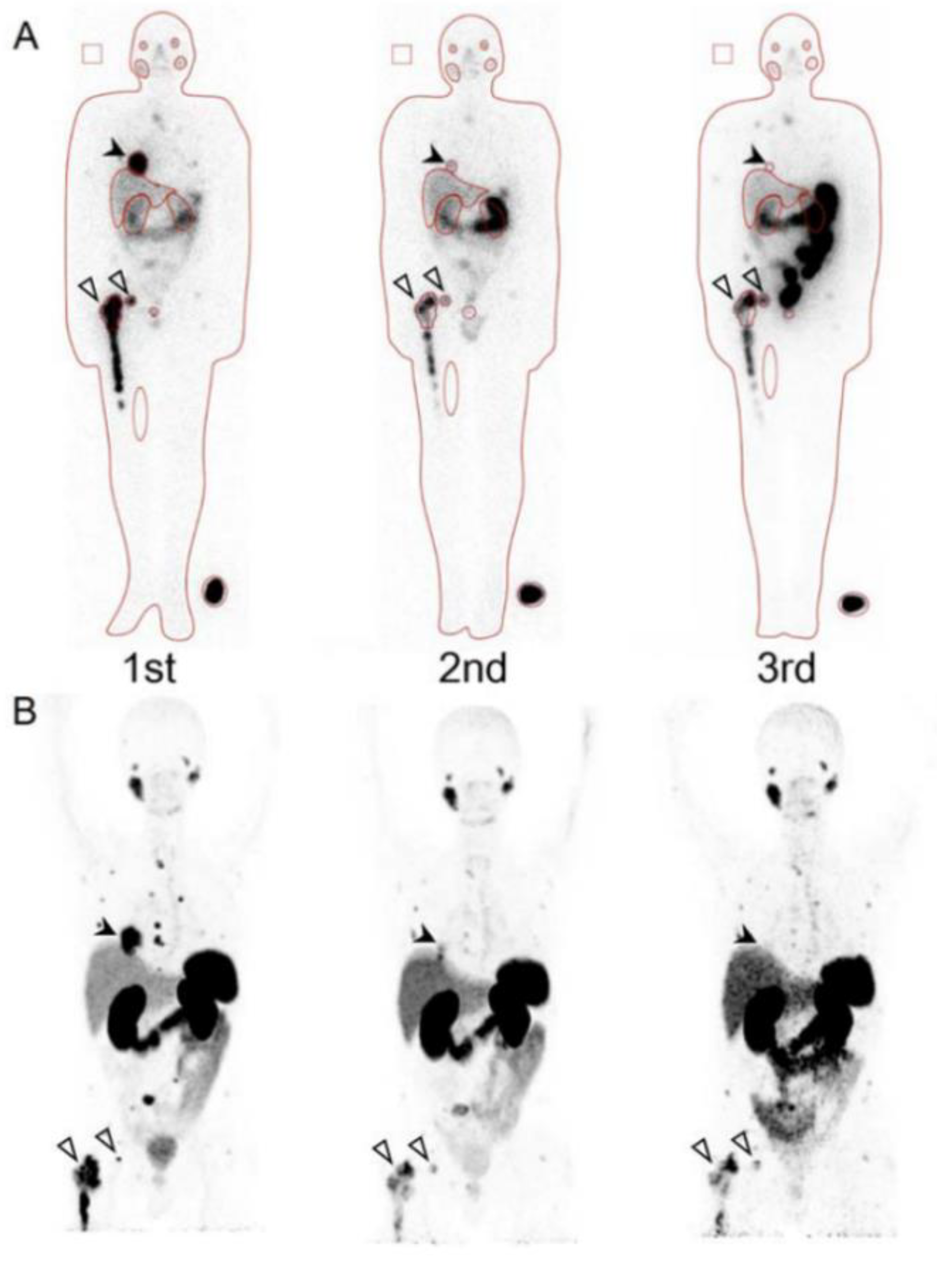
3. Prognostic Value of Theranostics for Neuroendocrine Tumor
4. Prognostic Value of Radioimmunotherapy of Non-Hodgkin Lymphoma
5. Prognostic Value of Theranostics for Bone Metastasis
6. Alpha vs. Beta-Emitting Isotope Radiochemistry
7. Future Perspectives of Theranostics
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilban, I. Theranostics—An emerging tool in drug discovery and commercialisation. Drug Discov. World. 2002, 1, 17–23. [Google Scholar]
- DeNardo, G.L.; DeNardo, S.J. Concepts, Consequences, and Implications of Theranosis. Semin. Nucl. Med. 2012, 42, 147–150. [Google Scholar] [CrossRef]
- Sharma, H.; Mishra, P.K.; Talegaonkar, S.; Vaidya, B. Metal nanoparticles: A theranostic nanotool against cancer. Drug Discov. Today 2015, 20, 1143–1151. [Google Scholar] [CrossRef]
- Feng, G.; Liu, B. Aggregation-Induced Emission (AIE) Dots: Emerging Theranostic Nanolights. Acc. Chem. Res. 2018, 51, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Ding, J.; Liu, J. Theranostic DNAzymes. Theranostics 2017, 7, 1010–1025. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Luster, M.; Clarke, S.E.; Dietlein, M.; Lassmann, M.; Lind, P.; Oyen, W.J.G.; Tennvall, J.; Bombardieri, E. Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1941–1959. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Mazzaferri, E.L.; Jhiang, S.M. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am. J. Med. 1994, 97, 418–428. [Google Scholar] [CrossRef]
- Neumann, H.P.H.; Young, W.F., Jr.; Eng, C. Pheochromocytoma and Paraganglioma. N. Engl. J. Med. 2019, 381, 552–565. [Google Scholar] [CrossRef]
- Kinuya, S.; Yoshinaga, K.; Higuchi, T.; Jinguji, M.; Kurihara, H.; Kawamoto, H. Guideline Drafting Committee for Radiotherapy with 131I-MIBG, Committee for Nuclear Oncology and Immunology, The Japanese Society of Nuclear Medicine. Ann. Nucl. Med. 2015, 29, 543–552. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Wagner, H.N., Jr.; Wiseman, G.A.; Marcus, C.S.; Nabi, H.A.; Nagle, C.E.; Fink-Bennett, D.M.; Lamonica, D.M.; Conti, P.S. Administration guidelines for radioimmunotherapy of non-Hodgkin’s lymphoma with (90)Y-labeled anti-CD20 monoclonal antibody. J. Nucl. Med. 2002, 43, 267–272. [Google Scholar] [PubMed]
- Bauman, G.; Charette, M.; Reid, R.; Sathya, J. Radiopharmaceuticals for the palliation of painful bone metastases—A systematic review. Radiother. Oncol. 2005, 75, 258.E1–258.E13. [Google Scholar] [CrossRef]
- Kuroda, I. Effective use of strontium-89 in osseous metastases. Ann. Nucl. Med. 2012, 26, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.; O’Sullivan, J.; Fosså, S.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef]
- Feine, U.; Lietzenmayer, R.; Hanke, J.P.; Wöhrle, H.; Müller-Schauenburg, W. 18FDG whole-body PET in differentiated thyroid carcinoma. Flipflop in uptake patterns of 18FDG and 131I. Nuklearmedizin 1995, 34, 127–134. [Google Scholar] [PubMed]
- Schoenberger, J.; Rüschoff, J.; Grimm, D.; Marienhagen, J.; Rümmele, P.; Meyringer, R.; Kossmehl, P.; Hofstaedter, F.; Eilles, C. Glucose Transporter 1 Gene Expression is Related to Thyroid Neoplasms with an Unfavorable Prognosis: An Immunohistochemical Study. Thyroid 2002, 12, 747–754. [Google Scholar] [CrossRef]
- Robbins, R.J.; Wan, Q.; Grewal, R.K.; Reibke, R.; Gonen, M.; Strauss, H.W.; Tuttle, R.M.; Drucker, W.; Larson, S.M. Real-Time Prognosis for Metastatic Thyroid Carcinoma Based on 2-[18F]Fluoro-2-Deoxy-d-Glucose-Positron Emission Tomography Scanning. J. Clin. Endocrinol. Metab. 2006, 91, 498–505. [Google Scholar] [CrossRef]
- Deandreis, D.; Al Ghuzlan, A.; Leboulleux, S.; Lacroix, L.; Garsi, J.P.; Talbot, M.; Lumbroso, J.; Baudin, E.; Caillou, B.; Bidart, J.M.; et al. Do histological, immunohistochemical, and metabolic (radioiodine and fluorodeoxyglucose uptakes) patterns of metastatic thyroid cancer correlate with patient outcome? Endocrine-Related Cancer 2011, 18, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Yoshio, K.; Sato, S.; Okumura, Y.; Katsui, K.; Takemoto, M.; Suzuki, E.; Katayama, N.; Kaji, M.; Kanazawa, S. The Local Efficacy of I-131 for F-18 FDG PET Positive Lesions in Patients With Recurrent or Metastatic Thyroid Carcinomas. Clin. Nucl. Med. 2011, 36, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Stokkel, M.P.M.; Duchateau, C.S.J.; Dragoiescu, C. The value of FDG-PET in the follow-up of differentiated thyroid cancer: A review of the literature. Q. J. Nucl. Med. Mol. Imaging 2006, 50, 78–87. [Google Scholar]
- Reske, S.N.; Kotzerke, J. FDG-PET for clinical use. Eur. J. Nucl. Med. Mol. Imaging 2001, 28, 1707–1723. [Google Scholar] [CrossRef] [PubMed]
- Nanni, C.; Rubello, D.; Fanti, S.; Farsad, M.; Ambrosini, V.; Rampin, L.; Banti, E.; Carpi, A.; Muzzio, P.; Franchi, R. Role of 18F-FDG-PET and PET/CT imaging in thyroid cancer. Biomed. Pharmacother. 2006, 60, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Shiga, T.; Tsukamoto, E.; Nakada, K.; Morita, K.; Kato, T.; Mabuchi, M.; Yoshinaga, K.; Katoh, C.; Kuge, Y.; Tamaki, N. Comparison of (18)F-FDG, (131)I-Na, and (201)Tl in diagnosis of recurrent or metastatic thyroid carcinoma. J. Nucl. Med. 2001, 42, 414–419. [Google Scholar] [PubMed]
- Gaertner, F.C.; Okamoto, S.; Shiga, T.; Ito, Y.M.; Uchiyama, Y.; Manabe, O.; Hattori, N.; Tamaki, N. FDG PET Performed at Thyroid Remnant Ablation Has a Higher Predictive Value for Long-Term Survival of High-Risk Patients with Well-Differentiated Thyroid Cancer Than Radioiodine Uptake. Clin. Nucl. Med. 2015, 40, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Ramirez, M.; Feng, L.; Johnson, M.M.; Ejaz, S.; Habra, M.A.; Rich, T.; Busaidy, N.; Cote, G.J.; Perrier, N.D.; Phan, A.; et al. Clinical Risk Factors for Malignancy and Overall Survival in Patients with Pheochromocytomas and Sympathetic Paragangliomas: Primary Tumor Size and Primary Tumor Location as Prognostic Indicators. J. Clin. Endocrinol. Metab. 2011, 96, 717–725. [Google Scholar] [CrossRef]
- Lenders, J.W.; Eisenhofer, G.; Mannelli, M.; Pacak, K. Phaeochromocytoma. Lancet 2005, 366, 665–675. [Google Scholar] [CrossRef]
- Jimenez, P.; Tatsui, C.; Jessop, A.; Thosani, S.; Jimenez, C. Treatment for Malignant Pheochromocytomas and Paragangliomas: 5 Years of Progress. Curr. Oncol. Rep. 2017, 19, 83. [Google Scholar] [CrossRef] [PubMed]
- Björklund, P.; Pacak, K.; Crona, J. Precision medicine in pheochromocytoma and paraganglioma: Current and future concepts. J. Intern. Med. 2016, 280, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Taieb, D.; Jha, A.; Treglia, G.; Pacak, K. Molecular imaging and radionuclide therapy of paraganglioma and pheochromocytoma. Endocr. Relat. Cancer 2019, 26, R627–R652. [Google Scholar] [CrossRef]
- Jimenez, C.; Erwin, W.D.; Chasen, B. Targeted Radionuclide Therapy for Patients with Metastatic Pheochromocytoma and Paraganglioma: From Low-Specific-Activity to High-Specific-Activity Iodine-131 Metaiodobenzylguanidine. Cancers 2019, 11, 1018. [Google Scholar] [CrossRef]
- Jackson, M.R.; Falzone, N.; Vallis, K.A. Advances in Anticancer Radiopharmaceuticals. Clin. Oncol. 2013, 25, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Giammarile, F.; Chiti, A.; Lassmann, M.; Brans, B.; Flux, G. EANM procedure guidelines for 131I-meta-iodobenzylguanidine (131I-mIBG) therapy. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Tokue, A.; Higuchi, T.; Oriuchi, N.; Arisaka, Y.; Endo, K. Clinical significance of 2-[18F]fluoro-2-deoxy-d-glucose positron emission tomography for the assessment of 131I-metaiodobenzylguanidine therapy in malignant phaeochromocytoma. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1869–1875. [Google Scholar] [CrossRef]
- Yoshinaga, K.; Abe, T.; Okamoto, S.; Uchiyama, Y.; Manabe, O.; Ito, Y.M.; Tamura, N.; Ito, N.; Yoshioka, N.; Washino, K.; et al. Effects of Repeated 131I-meta-iodobenzylguanidine Radiotherapy on Tumor Size and Tumor Metabolic Activities in Patients with Metastatic Neuroendocrine Tumors. J. Nucl. Med. 2020. Online ahead of print. [Google Scholar] [CrossRef]
- Leahy, M.F.; Turner, J.H. Radioimmunotherapy of relapsed indolent non-Hodgkin lymphoma with 131I-rituximab in routine clinical practice: 10-year single-institution experience of 142 consecutive patients. Blood 2011, 117, 45–52. [Google Scholar] [CrossRef][Green Version]
- McQuillan, A.D.; Macdonald, W.B.; Turner, J.H. Phase II study of first-line (131)I- rituximab radioimmunotherapy in follicular non-Hodgkin lymphoma and prognostic (18)F-fluorodeoxyglucose positron emission tomography. Leuk. Lymphoma 2015, 56, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Kesavan, M.; McQuillan, A.D.; Macdonald, W.B.G.; Turner, J.H. First-line radioimmunotherapy of advanced follicular non-Hodgkin lymphoma with 131-I-rituximab; Ten year follow-up. Leuk. Lymphoma 2017, 35, 366–367. [Google Scholar]
- Forrer, F.; Oechslin-Oberholzer, C.; Campana, B.; Herrmann, R.; Maecke, H.R.; Mueller-Brand, J.; Lohri, A. Radioimmunotherapy with 177Lu-DOTA-rituximab: Final results of a phase I/II Study in 31 patients with relapsing follicular, mantle cell, and other indolent B-cell lymphomas. J. Nucl. Med. 2013, 54, 1045–1052. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pandit-Taskar, N.; Batraki, M.; Divgi, C.R. Radiopharmaceutical therapy for palliation of bone pain from osseous metastases. J. Nucl. Med. 2004, 45, 1358–1365. [Google Scholar]
- Du, Y.; Carrio, I.; De Vincentis, G.; Fanti, S.; Ilhan, H.; Mommsen, C.; Nitzsche, E.; Sundram, F.; Vogel, W.; Oyen, W.; et al. Practical recommendations for radium-223 treatment of metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1671–1678. [Google Scholar] [CrossRef]
- Maffioli, L.S.; Florimonte, L.; Costa, D.C.; Castanheira, J.C.; Grana, C.; Luster, M.; Bodei, L.; Chinol, M. New radiopharmaceutical agents for the treatment of castration-resistant prostate cancer. Q J. Nucl. Med. Mol. Imaging 2015, 59, 420–438. [Google Scholar]
- Hosono, M.; Ikebuchi, H.; Nakamura, Y.; Yanagida, S.; Kinuya, S. Introduction of the targeted alpha therapy (with Radium-223) into clinical practice in Japan: Learnings and implementation. Ann. Nucl. Med. 2019, 33, 211–221. [Google Scholar] [CrossRef]
- Poty, S.; Francescpni, L.C.; McDevitt, M.R.; Morris, M.J.; Lewis, J.S. α-emisstiers for radiotherapy: From basic radiochemistry to clinical studies. Part 1. J. Nucl. Med. 2018, 59, 878–884. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Haberkorn, U.; Eder, M.; Eisenhut, M.; Zechmann, C.M. [68Ga]Gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: Comparison with 18F-FECH. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1085–1086. [Google Scholar] [CrossRef]
- Culp, M.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef]
- Center, M.M.; Jemal, A.; Lortet-Tieulent, J.; Ward, E.; Ferlay, J.; Brawley, O.W.; Bray, F. International Variation in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2012, 61, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Komura, K.; Sweeney, C.J.; Inamoto, T.; Ibuki, N.; Azuma, H.; Kantoff, P.W. Current treatment strategies for advanced prostate cancer. Int. J. Urol. 2018, 25, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Eiber, M.; Maurer, T.; Souvatzoglou, M.; Beer, A.J.; Ruffani, A.; Haller, B.; Graner, F.P.; Kübler, H.; Haberkorn, U.; Eisenhut, M.; et al. Evaluation of Hybrid ⁶⁸Ga-PSMA Ligand PET/CT in 248 Patients with Biochemical Recurrence After Radical Prostatectomy. J. Nucl. Med. 2015, 56, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Pyka, T.; Okamoto, S.; Dahlbender, M.; Tauber, R.; Retz, M.; Heck, M.; Tamaki, N.; Schwaiger, M.; Maurer, T.; Eiber, M. Comparison of bone scintigraphy and 68Ga-PSMA PET for skeletal staging in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 2114–2121. [Google Scholar] [CrossRef]
- Calais, J.; Ceci, F.; Eiber, M.; Hope, T.A.; Hofman, M.S.; Rischpler, C.; Bach-Gansmo, T.; Nanni, C.; Savir-Baruch, B.; Elashoff, D.; et al. 18F-fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: A prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019, 20, 1286–1294. [Google Scholar] [CrossRef]
- Fendler, W.P.; Calais, J.; Eiber, M.; Flavell, R.R.; Mishoe, A.; Feng, F.Y.; Nguyen, H.G.; Reiter, R.E.; Rettig, M.B.; Okamoto, S.; et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol. 2019, 5, 856–863. [Google Scholar] [CrossRef]
- Okamoto, S.; Thieme, A.; Allmann, J.; D’Alessandria, C.; Maurer, T.; Retz, M.; Tauber, R.; Heck, M.M.; Wester, H.-J.; Tamaki, N.; et al. Radiation Dosimetry for 177 Lu-PSMA I&T in Metastatic Castration-Resistant Prostate Cancer: Absorbed Dose in Normal Organs and Tumor Lesions. J. Nucl. Med. 2017, 58, 445–450. [Google Scholar] [CrossRef]
- Violet, J.; Sandhu, S.; Iravani, A.; Ferdinandus, J.; Thang, S.-P.; Kong, G.; Kumar, A.R.; Akhurst, T.; Pattison, D.A.; Beaulieu, A.; et al. Long-Term Follow-up and Outcomes of Retreatment in an Expanded 50-Patient Single-Center Phase II Prospective Trial of 177Lu-PSMA-617 Theranostics in Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2020, 61, 857–865. [Google Scholar] [CrossRef]
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schäfers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German Multicenter Study Investigating 177 Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2017, 58, 85–90. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Wegen, S.; Yordanova, A.; Fimmers, R.; Kürpig, S.; Eppard, E.; Wei, X.; Schlenkhoff, C.; Hauser, S.; Essler, M. Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using [177Lu]Lu-PSMA-617. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1448–1454. [Google Scholar] [CrossRef]
- Bräuer, A.; Grubert, L.S.; Roll, W.; Schrader, A.J.; Schäfers, M.; Bögemann, M.; Rahbar, K. 177Lu-PSMA-617 radioligand therapy and outcome in patients with metastasized castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Kulkarni, H.R.; Schuchardt, C.; Singh, A.; Wirtz, M.; Wiessalla, S.; Schottelius, M.; Mueller, D.; Klette, I.; Wester, H.-J. 177Lu-Labeled Prostate-Specific Membrane Antigen Radioligand Therapy of Metastatic Castration-Resistant Prostate Cancer: Safety and Efficacy. J. Nucl. Med. 2016, 57, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Hohenfellner, M.; Giesel, F.L.; Haberkorn, U.; Morgenstern, A. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with 225Ac-PSMA-617: Swimmer-Plot Analysis Suggests Efficacy Regarding Duration of Tumor Control. J. Nucl. Med. 2018, 59, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Tripathi, M.; Seth, A.; Bal, C. Efficacy and safety of 225Ac-PSMA-617 targeted alpha therapy in metastatic castration-resistant Prostate Cancer patients. Theranostics 2020, 10, 9364–9377. [Google Scholar] [CrossRef] [PubMed]
- Zacherl, M.J.; Gildehaus, F.J.; Mittlmeier, L.; Boening, G.; Gosewisch, A.; Wenter, V.; Schmidt-Hegemann, N.-S.; Belka, C.; Kretschmer, A.; Casuscelli, J.; et al. First clinical results for PSMA targeted alpha therapy using 225Ac-PSMA-I&T in advanced mCRPC patients. J. Nucl. Med. 2020. [Google Scholar] [CrossRef]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.C.; Goéré, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, v56–v68. [Google Scholar] [CrossRef]
- Körner, M.; Waser, B.; Strobel, O.; Büchler, M.W.; Reubi, J.C. Neurotensin receptors in pancreatic ductal carcinomas. EJNMMI Res. 2015, 5, 17. [Google Scholar] [CrossRef]
- Schulz, J.; Rohracker, M.; Stiebler, M.; Goldschmidt, J.; Stöber, F.; Noriega, M.; Pethe, A.; Lukas, M.; Osterkamp, F.; Reineke, U.; et al. Proof of therapeutic efficacy of a novel 177Lu-labeled neurotensin receptor 1 antagonist in a colon carcinoma xenograft model. J. Nucl. Med. 2017, 58, 936–941. [Google Scholar] [CrossRef]
- Baum, R.P.; Singh, A.; Schuchardt, C.; Kulkarni, H.R.; Klette, I.; Wiessalla, S.; Osterkamp, F.; Reineke, U.; Smerling, C. 177Lu-3BP-227 for Neurotensin Receptor 1–Targeted Therapy of Metastatic Pancreatic Adenocarcinoma: First Clinical Results. J. Nucl. Med. 2018, 59, 809–814. [Google Scholar] [CrossRef]
- Chiesa, C.; Sjogreen Gleisner, K.; Flux, G.; Gear, J.; Walrand, S.; Bacher, K.; Eberlein, U.; Visser, E.P.; Chouin, N.; Ljungberg, M.; et al. The conflict between treatment optimization and registration of radiopharmaceuticals with fixed activity posology in oncological nuclear medicine therapy. Eur. J. Nucl. Med. Mol. Imaging 2017, 1783–1786. [Google Scholar] [CrossRef] [PubMed]
- Svensson, J.; Hagmarker, L.; Magnander, T.; Wängberg, B.; Bernhardt, P. Radiation exposure of the spleen during (177)Lu-DOTATATE treatment and its correlation with haematological toxicity and spleen volume. EJNMMI Phys. 2016, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Bergsma, H.; Konijnenberg, M.W.; Kam, B.L.; Teunissen, J.J.; Kooij, P.P.; de Herder, W.W.; Franssen, G.J.; van Eijck, C.H.; Krenning, E.P.; Kwekkeboom, D.J.; et al. Subacute haematotoxicity after PRRT with (177)Lu-DOTA-octreotate: Prognostic factors, incidence and course. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Van den Hoven, A.F.; Rosenbaum, C.E.; Elias, S.G.; de Jong, H.W.; Koopman, M.; Verkooijen, H.M.; Alavi, A.; van den Bosch, M.A.; Lam, M.G. Insights into the Dose-Response Relationship of Radioembolization with Resin 90Y-Microspheres: A Prospective Cohort Study in Patients with Colorectal Cancer Liver Metastases. J. Nucl. Med. 2016, 57, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Garske-Román, U.; Sandström, M.; Fröss Baron, K.; Lundin, L.; Hellman, P.; Welin, S.; Johansson, S.; Khan, T.; Lundqvist, H.; Eriksson, B.; et al. Prospective observational study of 177Lu-DOTA-octreotate therapy in 200 patients with advanced metastasized neuroendocrine tumours (NETs): Feasibility and impact of a dosimetry-guided study protocol on outcome and toxicity. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 970–988. [Google Scholar] [CrossRef] [PubMed]
| Target Disease | Target | Companion Diagnostics for Theranostics | Radionuclide Therapy |
|---|---|---|---|
| Differentiated thyroid cancer | Iodine affinity for hormone synthesis | I-131 | I-131 [8,9,10] |
|
Malignant pheochromocytoma, Paraganglioma | Norepinephrine transporter |
I-123 MIBG I-131 MIBG | I-131 MIBG [11,12] |
| Neuroendocrine tumor | Somatostatine receptor |
In-111 Pentetreotide Ga-68 DOTATE Ga-68 DOTATOC | Lu-177 DOTATATE [13] Lu-177 DOTATOC Y-90 DOTATATE Y-90 DOTATOC |
| B-cell non-Hodgkin malignant lymphoma | Anti-CD20 receptor |
In-111
Ibritumomab Tiuxetan I-131 rituximab | Y-90 Ibritumomab Tiuxetan [14] Lu-177 rituximab |
| Bone metastasis | Bone turnover | Tc-99m bone scan | Sr-89 [15,16] Sm-153 EDTMP [16] Ra-223 [17] |
| Castration-resistant prostatic cancer | Prostate specific membrane antigen |
Ga-68 PSMA F-18 PSMA | Lu-177 PSMA [18] Ac-225 PSMA [19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okamoto, S.; Shiga, T.; Tamaki, N. Clinical Perspectives of Theranostics. Molecules 2021, 26, 2232. https://doi.org/10.3390/molecules26082232
Okamoto S, Shiga T, Tamaki N. Clinical Perspectives of Theranostics. Molecules. 2021; 26(8):2232. https://doi.org/10.3390/molecules26082232
Chicago/Turabian StyleOkamoto, Shozo, Tohru Shiga, and Nagara Tamaki. 2021. "Clinical Perspectives of Theranostics" Molecules 26, no. 8: 2232. https://doi.org/10.3390/molecules26082232
APA StyleOkamoto, S., Shiga, T., & Tamaki, N. (2021). Clinical Perspectives of Theranostics. Molecules, 26(8), 2232. https://doi.org/10.3390/molecules26082232





