A Stretchable Pillararene-Containing Supramolecular Polymeric Material with Self-Healing Property
Abstract
1. Introduction
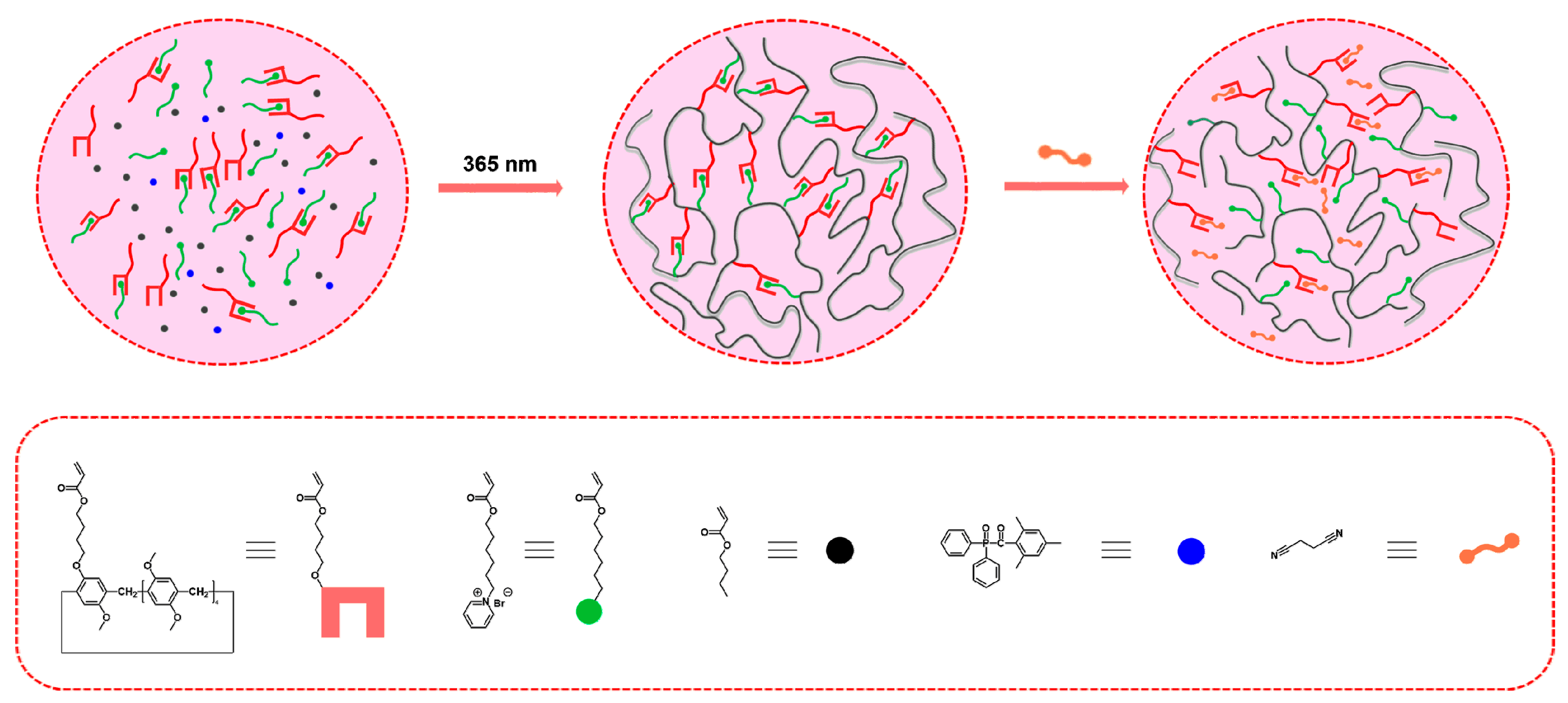
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Xia, D.; Wang, P.; Ji, X.; Khashab, N.M.; Sessler, J.L.; Huang, F. Functional supramolecular polymeric networks: The marriage of covalent polymers and macrocycle-based host-guest interactions. Chem. Rev. 2020, 120, 6070–6123. [Google Scholar] [CrossRef] [PubMed]
- Morsali, M.; Khan, M.T.A.; Ashirov, R.; Hollo, G.; Baytekin, H.T.; Lagzi, I.; Baytekin, B. Mechanical control of periodic precipitation in stretchable gels to retrieve information on elastic deformation and for the complex patterning of matter. Adv. Mater. 2020, 32, 1905779. [Google Scholar] [CrossRef]
- Li, W.; Gao, F.; Wang, X.; Zhang, N.; Ma, M. Strong and robust polyaniline-based supramolecular hydrogels for flexible supercapacitors. Angew. Chem. Int. Ed. Engl. 2016, 55, 9196–9201. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Saha, T.; Naskar, K.; Stamm, M.; Heinrich, G.; Das, A. A highly stretchable gel-polymer electrolyte for lithium-sulfur batteries. Polymer 2017, 112, 447–456. [Google Scholar] [CrossRef]
- Le, X.; Lu, W.; Zheng, J.; Tong, D.; Zhao, N.; Ma, C.; Xiao, H.; Zhang, J.; Huang, Y.; Chen, T. Stretchable supramolecular hydrogels with triple shape memory effect. Chem. Sci. 2016, 7, 6715–6720. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, Y.; Zhang, K.; Zhuo, S.; Fang, R.; Zhang, J.; Jiang, L.; Liu, M. Biphasic synergistic gel materials with switchable mechanics and self-healing capacity. Angew. Chem. Int. Ed. Engl. 2017, 56, 13464–13469. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Lee, W.; Dai, G.; Hong, Y. Highly elastic biodegradable single-network hydrogel for cell printing. ACS Appl. Mater. Inter. 2018, 10, 9969–9979. [Google Scholar] [CrossRef]
- Lu, W.; Le, X.; Zhang, J.; Huang, Y.; Chen, T. Supramolecular shape memory hydrogels: A new bridge between stimuli-responsive polymers and supramolecular chemistry. Chem. Soc. Rev. 2017, 46, 1284–1294. [Google Scholar] [CrossRef]
- Ye, D.; Yang, P.; Lei, X.; Zhang, D.; Li, L.; Chang, C.; Sun, P.; Zhang, L. Robust anisotropic cellulose hydrogels fabricated via strong self-aggregation forces for cardiomyocytes unidirectional growth. Chem. Mater. 2018, 30, 5175–5183. [Google Scholar] [CrossRef]
- Zhao, B.; Xu, S.; Zheng, S. Synthesis, self-assembly and self-healing properties of organic–inorganic ABA triblock copolymers with poly(POSS acrylate) endblocks. Polym. Chem. 2019, 10, 2424–2435. [Google Scholar] [CrossRef]
- Huang, J.; Liao, J.; Wang, T.; Sun, W.; Tong, Z. Super strong dopamine hydrogels with shape memory and bioinspired actuating behaviours modulated by solvent exchange. Soft. Matter. 2018, 14, 2500–2507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Jin, H.; Wang, S.; Song, W. Bioinspired supramolecular lubricating hydrogel induced by shear force. J. Am. Chem. Soc. 2018, 140, 3186–3189. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kushner, A.M.; Williams, G.A.; Guan, Z. Multiphase design of autonomic self-healing thermoplastic elastomers. Nat. Chem. 2012, 4, 467–472. [Google Scholar] [CrossRef]
- Liu, H.; Chung, H. Self-healing properties of lignin-containing nanocomposite: Synthesis of lignin-graft-poly(5-acetylaminopentyl acrylate) via RAFT and click chemistry. Macromolecules 2016, 49, 7246–7256. [Google Scholar] [CrossRef]
- Ju, H.; Zhu, F.; Xing, H.; Wu, Z.L.; Huang, F. Ultrastiff hydrogels prepared by schiff’s base reaction of bis(p-formylphenyl) sebacate and pillar[5]arene appended with multiple hydrazides. Macromol. Rapid. Commun. 2017, 38, 1–6. [Google Scholar] [CrossRef]
- Yan, X.; Liu, Z.; Zhang, Q.; Lopez, J.; Wang, H.; Wu, H.C.; Niu, S.; Yan, H.; Wang, S.; Lei, T.; et al. Quadruple H-bonding cross-linked supramolecular polymeric materials as substrates for stretchable, antitearing, and self-healable thin film electrodes. J. Am. Chem. Soc. 2018, 140, 5280–5289. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, B.; Wang, H.; Shangguan, L.; Li, Z.; Zhang, M.; Huang, F. Construction of metallacage-cored supramolecular gel by hierarchical self-assembly of metal coordination and pillar[5]arene-based host-guest recognition. Macromol. Rapid. Commun. 2018, 39, 1800655. [Google Scholar] [CrossRef]
- Han, L.; Yan, L.; Wang, K.; Fang, L.; Zhang, H.; Tang, Y.; Ding, Y.; Weng, L.-T.; Xu, J.; Weng, J.; et al. Tough, self-healable and tissue-adhesive hydrogel with tunable multifunctionality. NPG. Asia. Mater. 2017, 9, 1–12. [Google Scholar] [CrossRef]
- Wu, Y.; Li, H.; Yan, Y.; Shan, X.; Zhao, M.; Zhao, Q.; Liao, X.; Xie, M. Pillararene-containing polymers with tunable conductivity based on host–guest complexations. ACS Macro. Lett. 2019, 8, 1588–1593. [Google Scholar] [CrossRef]
- Huang, F.H.; Gibson, H.W.; Bryant, W.S.; Nagvekar, D.S.; Fronczek, F.R. First pseudorotaxane-like [3]complexes based on cryptands and paraquat: Self-assembly and crystal structures. J. Am. Chem. Soc. 2003, 125, 9367–9371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-M.; Zhu, W.; Huang, X.-J.; Qu, W.-J.; He, J.-X.; Fang, H.; Yao, H.; Wei, T.-B.; Lin, Q. Supramolecular aggregation-induced emission gels based on pillar[5]arene for pltrasensitive detection and separation of multianalytes. ACS Sustain. Chem. Eng. 2018, 6, 16597–16606. [Google Scholar] [CrossRef]
- Zafrani, Y.; Kaizerman, D.; Hadar, M.; Bigan, N.; Granot, E.; Ghosh, M.; Adler-Abramovich, L.; Patolsky, F.; Cohen, Y. Pillararene-based two-component thixotropic supramolecular organogels: Complementarity and multivalency as prominent motifs. Chem. Eur. J. 2018, 24, 15750–15755. [Google Scholar] [CrossRef]
- Jia, Y.-G.; Zhang, M.; Zhu, X.X. CO2-switchable self-healing host–guest hydrogels. Macromolecules, 2017, 50, 9696–9701. [Google Scholar] [CrossRef]
- Sun, S.; Geng, M.; Huang, L.; Chen, Y.; Cen, M.; Lu, D.; Wang, A.; Wang, Y.; Shi, Y.; Yao, Y. A new amphiphilic pillar[5]arene: Synthesis and controllable self-assembly in water and application in white-light-emitting systems. Chem. Commun. 2018, 54, 13006–13009. [Google Scholar] [CrossRef]
- Liu, X.Y.; Xu, H.; Zhang, L.Q.; Zhong, M.; Xie, X.M. Homogeneous and real super tough multi-bond network hydrogels created through a controllable metal ion permeation strategy. ACS Appl. Mater. Inter. 2019, 11, 42856–42864. [Google Scholar] [CrossRef]
- Yang, L.; Tan, X.; Wang, Z.; Zhang, X. Supramolecular polymers: Historical development, preparation, characterization, and functions. Chem. Rev. 2015, 115, 7196–7239. [Google Scholar] [CrossRef] [PubMed]
- Hiroshige, S.; Kureha, T.; Aoki, D.; Sawada, J.; Aoki, D.; Takata, T.; Suzuki, D. Formation of tough films by evaporation of water from dispersions of elastomer microspheres crosslinked with rotaxane supramolecules. Chem. Eur. J. 2017, 23, 8405–8408. [Google Scholar] [CrossRef]
- Tan, M.; Cui, Y.; Zhu, A.; Han, H.; Guo, M.; Jiang, M. Ultraductile, notch and stab resistant supramolecular hydrogels via host–guest interactions. Polym. Chem. 2015, 6, 7543–7549. [Google Scholar] [CrossRef]
- Nomimura, S.; Osaki, M.; Park, J.; Ikura, R.; Takashima, Y.; Yamaguchi, H.; Harada, A. Self-healing alkyl acrylate-based supramolecular elastomers cross-linked via host–guest interactions. Macromolecules 2019, 52, 2659–2668. [Google Scholar] [CrossRef]
- Ogoshi, T.; Fujinami, S.; Yamagishi, T.A.; Nakamoto, Y. para-Bridged symmetrical pillar[5]arenes: Their lewis acid catalyzed synthesis and host–guest property. J. Am. Chem. Soc. 2008, 130, 5022–5023. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.Y.; Li, Y.P.; Yang, Y.W. Gated materials: Installing macrocyclic arenes-based supramolecular nanovalves on porous nanomaterials for controlled cargo release. Biotechnol. J. 2019, 14, 1800354. [Google Scholar] [CrossRef] [PubMed]
- Ogoshi, T.; Takashima, S.; Yamagishi, T.A. Photocontrolled reversible guest uptake, storage, and release by azobenzene-modified microporous multilayer films of pillar[5]arenes. J. Am. Chem. Soc. 2018, 140, 1544–1548. [Google Scholar] [CrossRef]
- Chen, Y.; Srikala, P.; Sun, B.; Qian, C.; Sun, G.; Cheng, M.; Lin, C.; Lu, X.; Jiang, J.; Wang, L. Stoichiometry-controlled chirality induced by co-assembly of tetraphenylethylene derivative, γ-CD, and water-soluble pillar[5]arene. ACS Appl. Bio. Mater. 2020, 4, 2066–2072. [Google Scholar] [CrossRef]
- Zhang, D.; Cheng, J.; Wei, L.; Song, W.; Wang, L.; Tang, H.; Cao, D. Host-guest complexation of monoanionic and dianionic guests with a polycationic pillararene host: Same two-step mechanism but striking difference in rate upon inclusion. J. Phys. Chem. Lett. 2020, 11, 2021–2026. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-F.; Lin, Q.; Yao, H.; Zhang, Y.-M.; Wei, T.-B. Pillar[5]arene-based multifunctional supramolecular hydrogel: Multistimuli responsiveness, self-healing, fluorescence sensing, and conductivity. Mater. Chem. Front. 2018, 2, 999–1003. [Google Scholar] [CrossRef]
- Lou, X.Y.; Song, N.; Yang, Y.W. Fluorescence resonance energy transfer systems in supramolecular macrocyclic chemistry. Molecules 2017, 22, 1640. [Google Scholar] [CrossRef]
- Ogoshi, T.; Sueto, R.; Yagyu, M.; Kojima, R.; Kakuta, T.; Yamagishi, T.; Doitomi, K.; Tummanapelli, A.K. Molecular weight fractionation by confinement of polymer in one-dimensional pillar[5]arene channels. Nat. Commun. 2019, 10, 479. [Google Scholar] [CrossRef]
- Shao, L.; Pan, Y.T.; Hua, B.; Xu, S.D.; Yu, G.C.; Wand, M.B.; Liu, B.; Huang, F.H. Constructing adaptive photosensitizers via supramolecular modification based on pillararene host-guest interactions. Angew. Chem. Int. Ed. 2020, 59, 11779–11783. [Google Scholar] [CrossRef]
- Xia, W.; Ni, M.; Yao, C.; Wang, X.; Chen, D.; Lin, C.; Hu, X.-Y.; Wang, L. Responsive gel-like supramolecular network based on pillar[6]arene−ferrocenium recognition motifs in polymeric matrix. Macromolecules 2015, 48, 4403–4409. [Google Scholar] [CrossRef]
- Chang, J.; Zhao, Q.; Kang, L.; Li, H.; Xie, M.; Liao, X. Multiresponsive supramolecular gel based on pillararene-containing polymers. Macromolecules 2016, 49, 2814–2820. [Google Scholar] [CrossRef]
- Chen, J.-F.; Chen, P. Pillar[5]arene-based resilient supramolecular gel with dual-stimuli responses and self-healing properties. ACS Appl. Polym. Mater. 2019, 1, 2224–2229. [Google Scholar] [CrossRef]
- Li, C.; Xu, Q.; Li, J.; Yao, F.; Jia, X. Complex interactions of pillar[5]arene with paraquats and bis(pyridinium) derivatives. Org. Biomol. Chem. 2010, 8, 1568–1576. [Google Scholar] [CrossRef]
- Park, J.; Choi, Y.; Lee, S.S.; Jung, J.H. Critical role of achiral guest molecules in planar chirality inversion of alanine-appended pillar[5]arenes. Org. Lett. 2019, 21, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.D.; Yu, G.C.; Ji, X.F.; Li, Y.; Tang, G.P.; Huang, F. Redox-responsive amphiphilic macromolecular [2]pseudorotaxane constructed from a water-soluble pillar[5]arene and a paraquat-containing homopolymer. ACS Macro. Lett. 2015, 4, 996–999. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, G.; Du, G.; Cheng, Y.; Fu, J. Super tough, ultrastretchable, and thermoresponsive hydrogels with functionalized triblock copolymer micelles as macro-cross-linkers. ACS Macro. Lett. 2014, 3, 496–500. [Google Scholar] [CrossRef]
- Sawada, J.; Aoki, D.; Kuzume, M.; Nakazono, K.; Otsuka, H.; Takata, T. A vinylic rotaxane cross-linker for toughened network polymers from the radical polymerization of vinyl monomers. Poly. Chem. 2017, 8, 1878–1881. [Google Scholar] [CrossRef]
- Baddi, S.; Palanisamy, A. Thermoreversible gelation of poly(urethane acyl-semicarbazides) carrying cycloaliphatic moieties and studies on selective adsorption of dyes from wastewater. Eur. Poly. J. 2018, 99, 90–101. [Google Scholar] [CrossRef]
- Guo, M.; Pitet, L.M.; Wyss, H.M.; Vos, M.; Dankers, P.Y.; Meijer, E.W. Tough stimuli-responsive supramolecular hydrogels with hydrogen-bonding network junctions. J. Am. Chem. Soc. 2014, 136, 6969–6977. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Zhou, Z.; Ni, X.; Kam, W.L. Formation of supramolecular hydrogels induced by inclusion complexation between pluronics and r-cyclodextrin. Macromolecules 2001, 34, 7236–7237. [Google Scholar] [CrossRef]
- Zhang, T.; Guo, Q. Continuous preparation of polyHIPE monoliths from ionomer-stabilized high internal phase emulsions (HIPEs) for efficient recovery of spilled oils. Chem. Eng. J. 2017, 307, 812–819. [Google Scholar] [CrossRef]
- Guan, X.; Jiang, H.; Ngai, T. Pickering high internal phase emulsions templated super-hydrophobic–oleophilic elastic foams for highly efficient oil/water separation. ACS Appl. Polym. Mater. 2020, 2, 5664–5673. [Google Scholar] [CrossRef]
- Lai, J.C.; Jia, X.Y.; Wang, D.P.; Deng, Y.B.; Zheng, P.; Li, C.H.; Zuo, J.L.; Bao, Z. Thermodynamically stable whilst kinetically labile coordination bonds lead to strong and tough self-healing polymers. Nat. Commun. 2019, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-Y.; Zhang, Y.; Zhang, C.-W.; Chen, L.-J.; Wang, C.; Tan, H.; Yu, Y.; Li, X.; Yang, H.-B. Cross-linked supramolecular polymer gels constructed from discrete multi-pillar[5]arene metallacycles and their multiple stimuli-responsive behavior. J. Am. Chem. Soc. 2014, 136, 8577–8589. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Cao, W.T.; Ma, M.G.; Wan, P. Ultrasensitive wearable soft strain sensors of conductive, self-healing, and elastic hydrogels with synergistic “soft and hard” hybrid networks. ACS Appl. Mater. Inter. 2017, 9, 25559–25570. [Google Scholar]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Li, C.; Shan, X.; Han, H.; Zhao, Q.; Xie, M.; Chen, J.; Liao, X. A Stretchable Pillararene-Containing Supramolecular Polymeric Material with Self-Healing Property. Molecules 2021, 26, 2191. https://doi.org/10.3390/molecules26082191
Zhao M, Li C, Shan X, Han H, Zhao Q, Xie M, Chen J, Liao X. A Stretchable Pillararene-Containing Supramolecular Polymeric Material with Self-Healing Property. Molecules. 2021; 26(8):2191. https://doi.org/10.3390/molecules26082191
Chicago/Turabian StyleZhao, Meng, Changjun Li, Xiaotao Shan, Huijing Han, Qiuhua Zhao, Meiran Xie, Jianzhuang Chen, and Xiaojuan Liao. 2021. "A Stretchable Pillararene-Containing Supramolecular Polymeric Material with Self-Healing Property" Molecules 26, no. 8: 2191. https://doi.org/10.3390/molecules26082191
APA StyleZhao, M., Li, C., Shan, X., Han, H., Zhao, Q., Xie, M., Chen, J., & Liao, X. (2021). A Stretchable Pillararene-Containing Supramolecular Polymeric Material with Self-Healing Property. Molecules, 26(8), 2191. https://doi.org/10.3390/molecules26082191




