Surface Modification Effect and Electrochemical Performance of LiOH-High Surface Activated Carbon as a Cathode Material in EDLC
Abstract
1. Introduction
2. Results
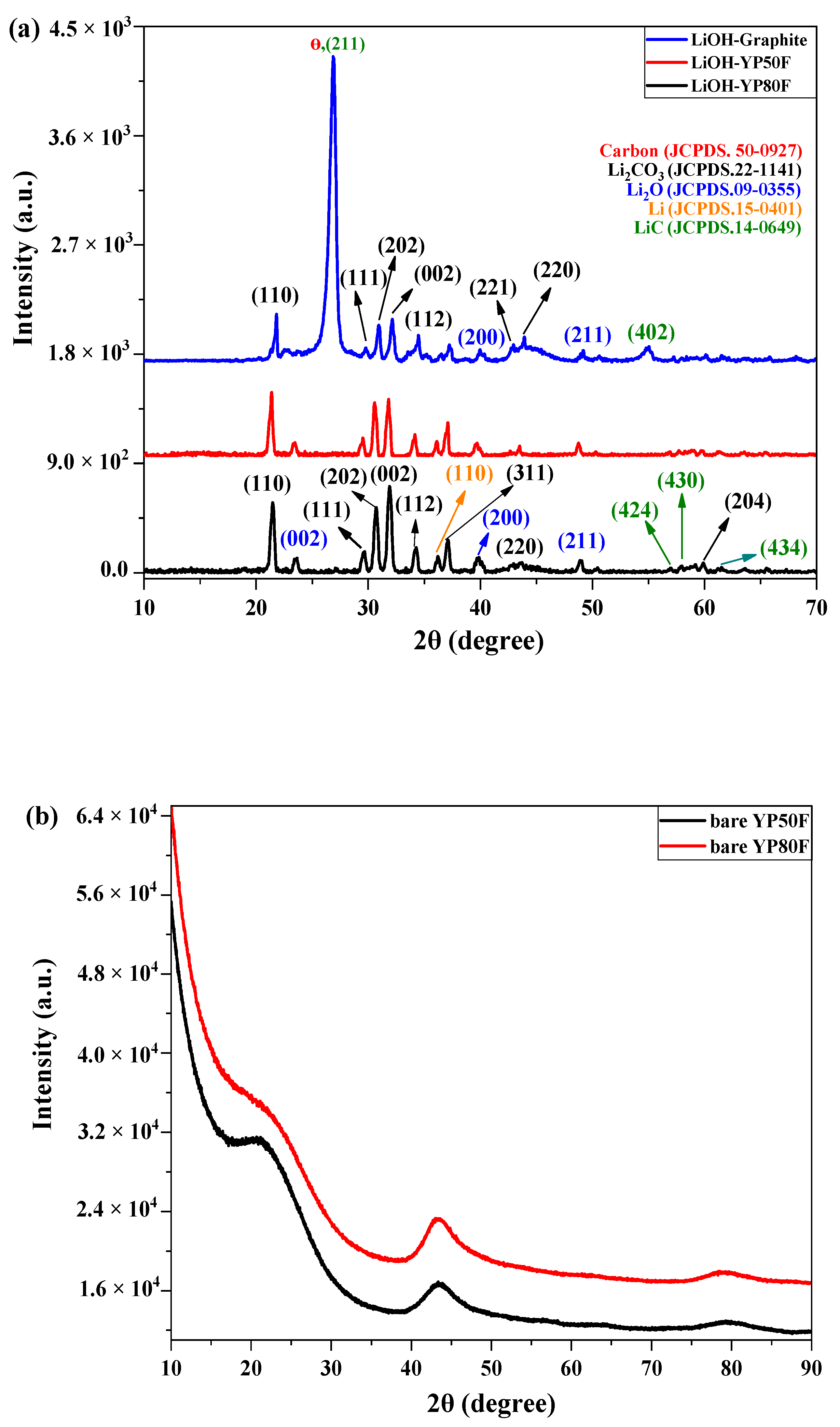
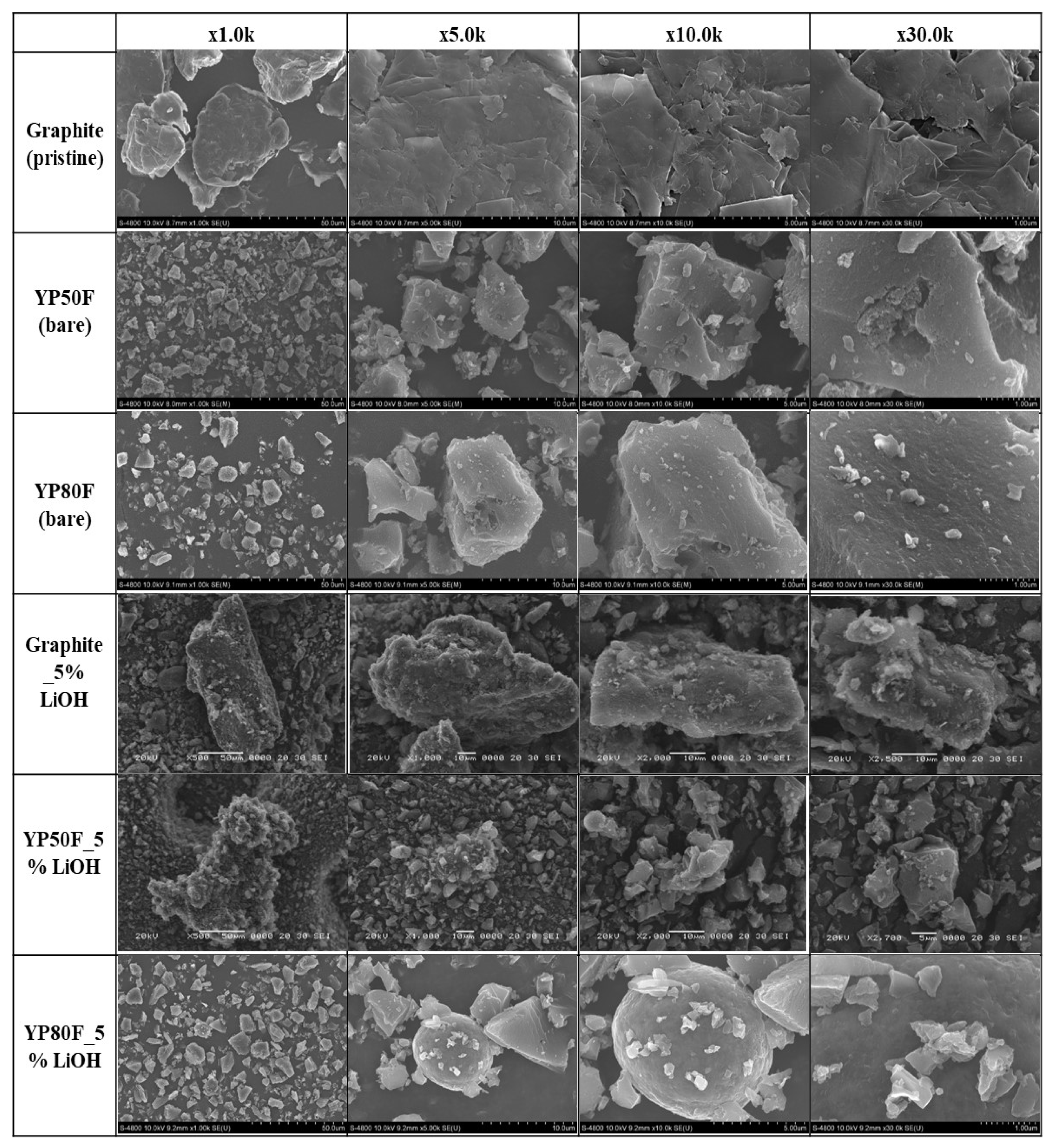
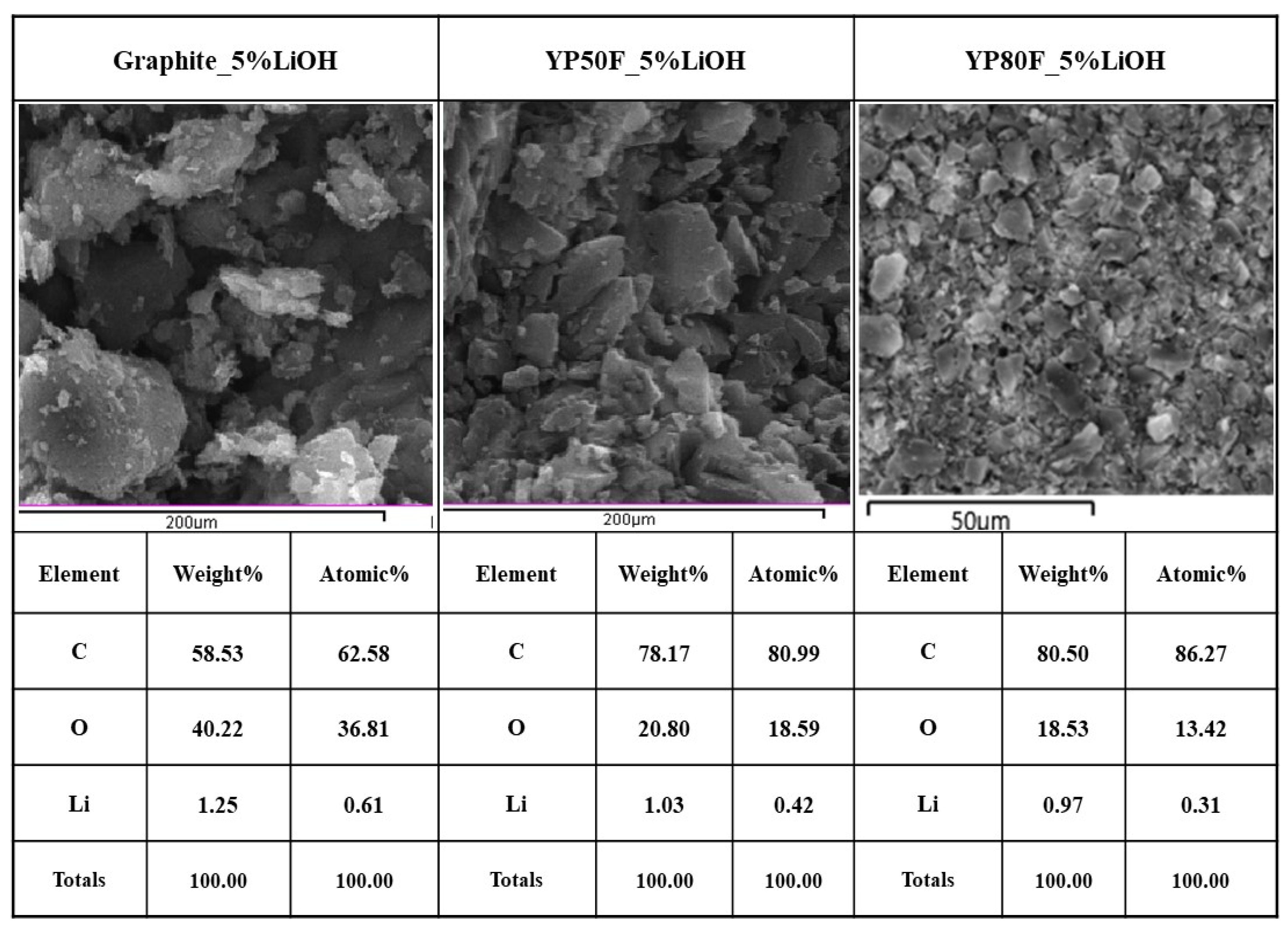
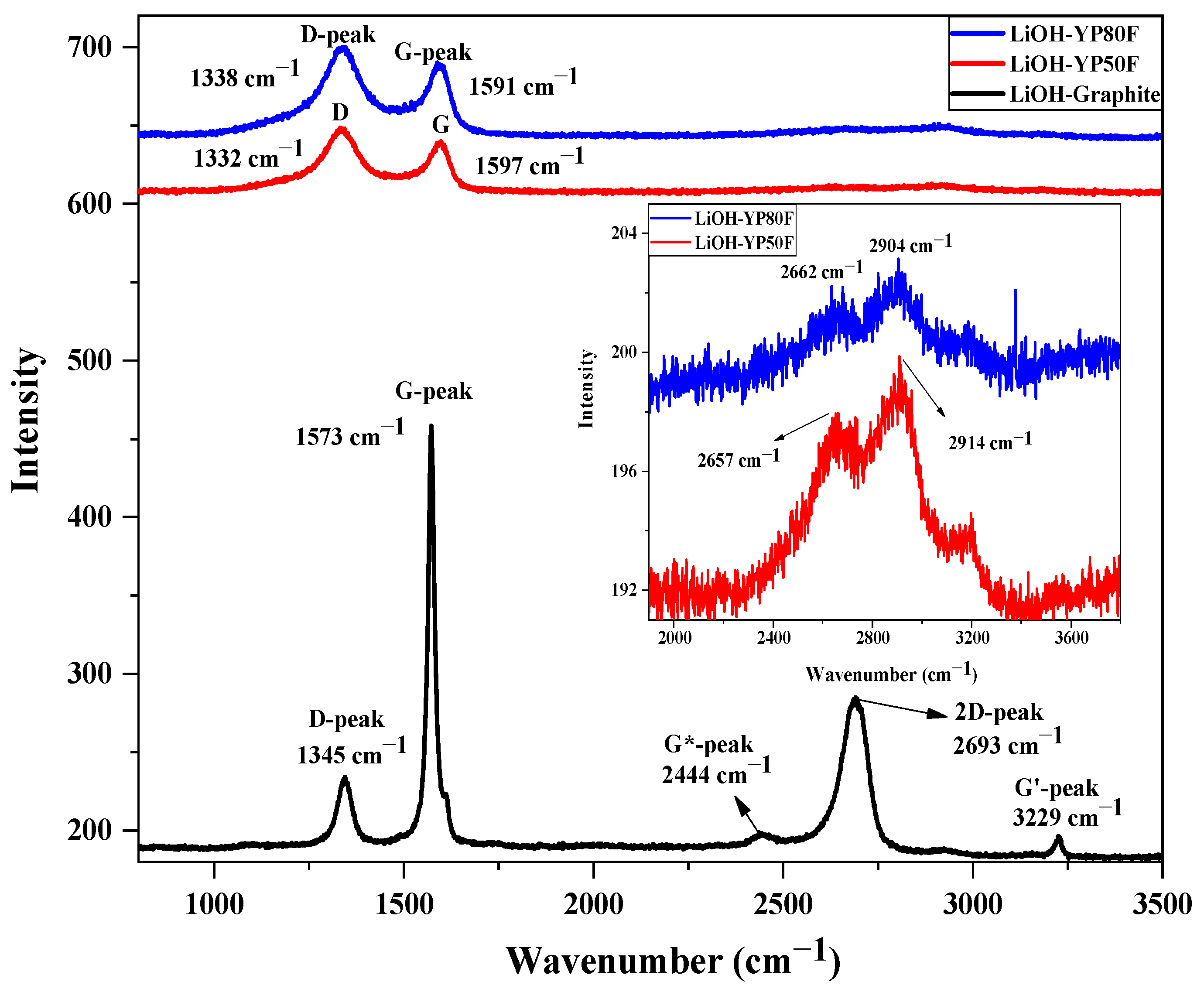
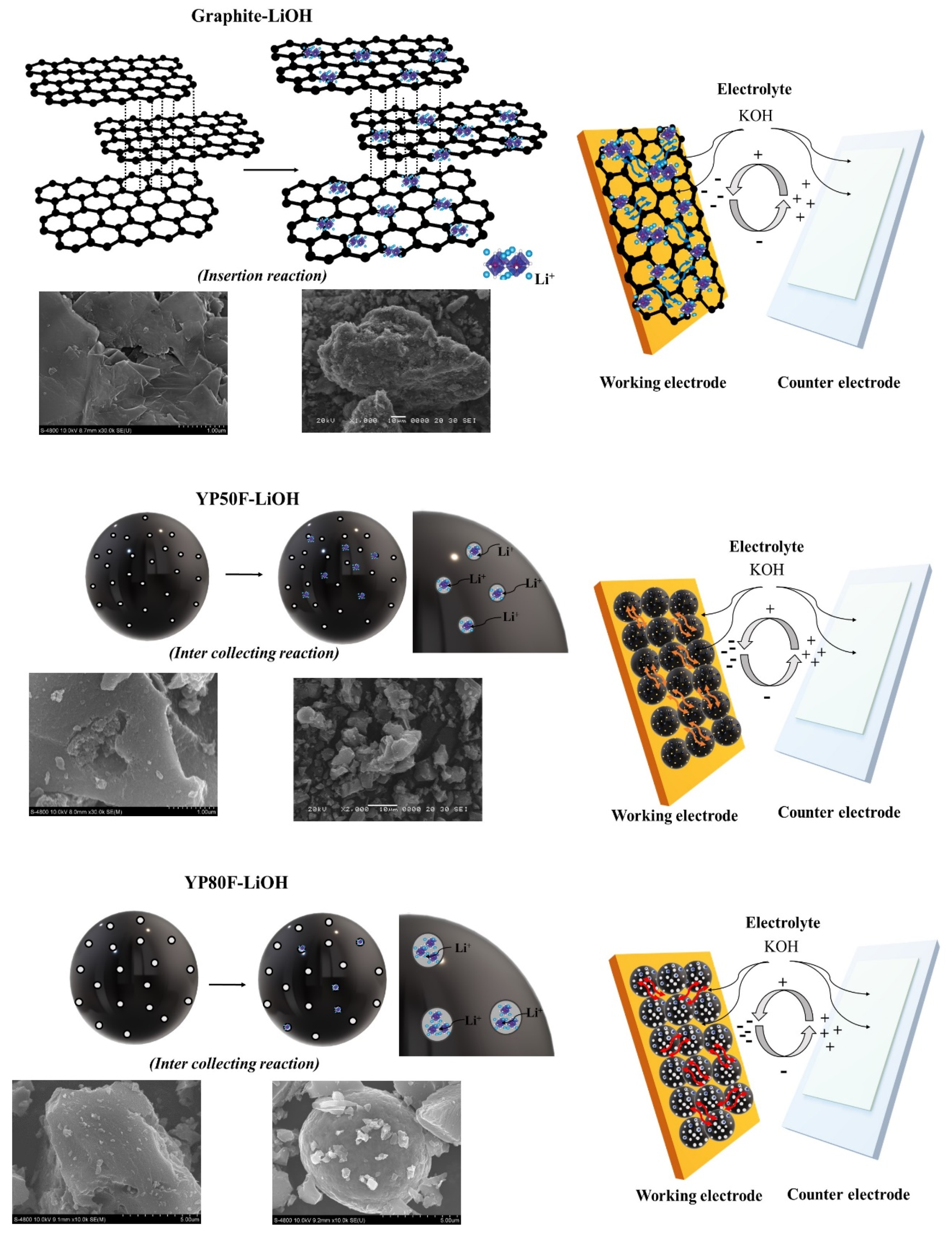
2.1. Crystallography and Morphology Analysis
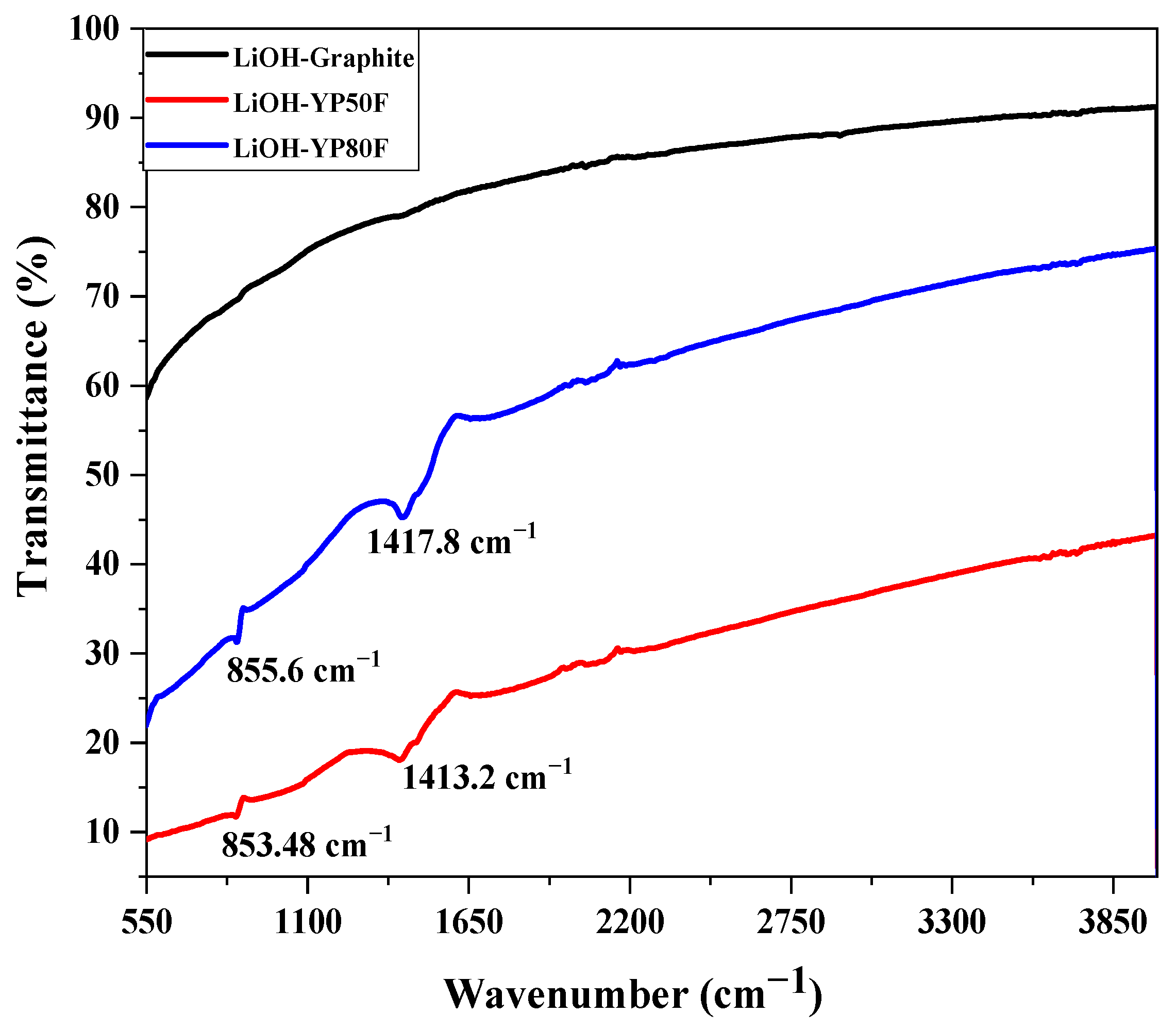
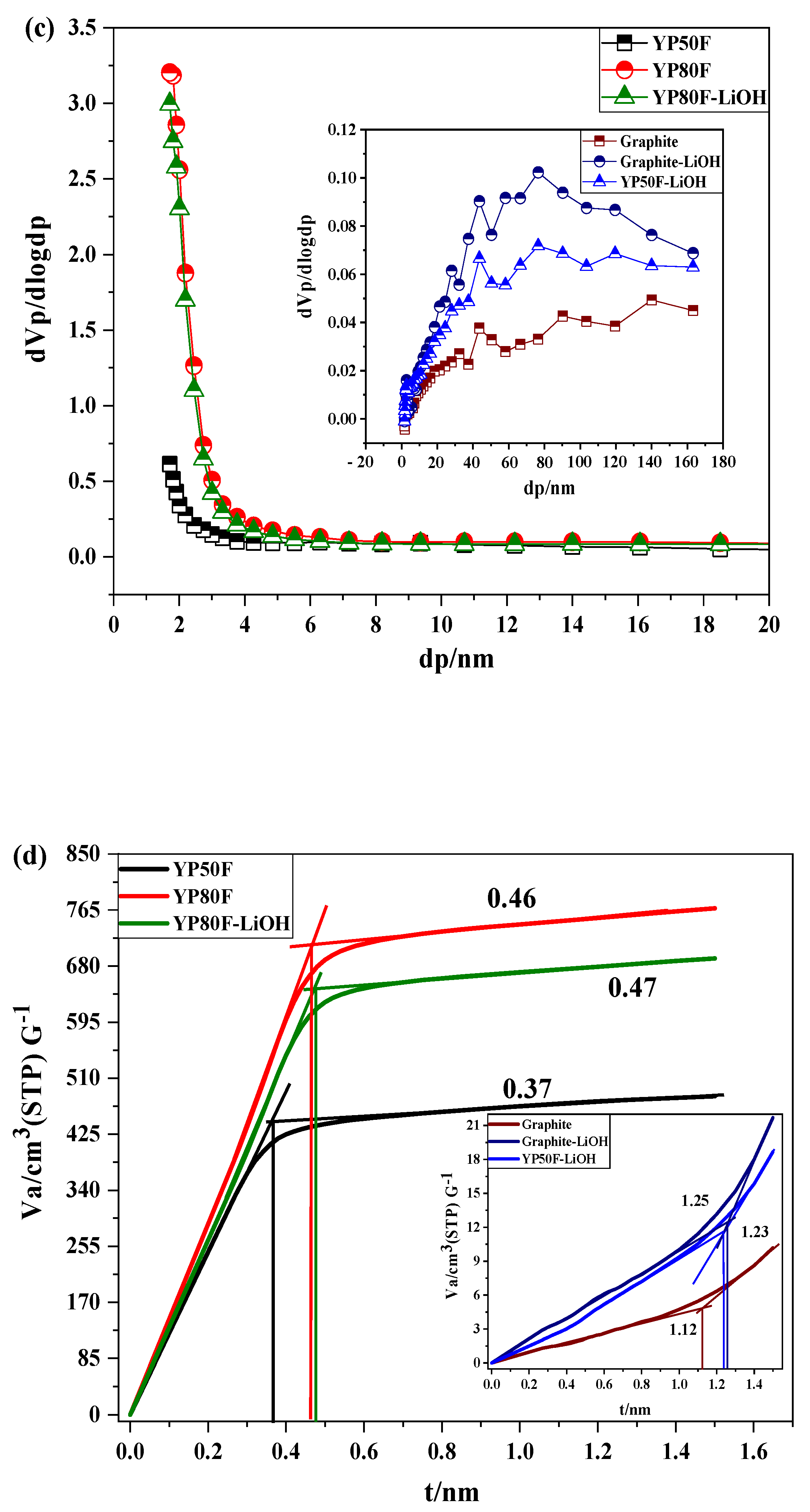
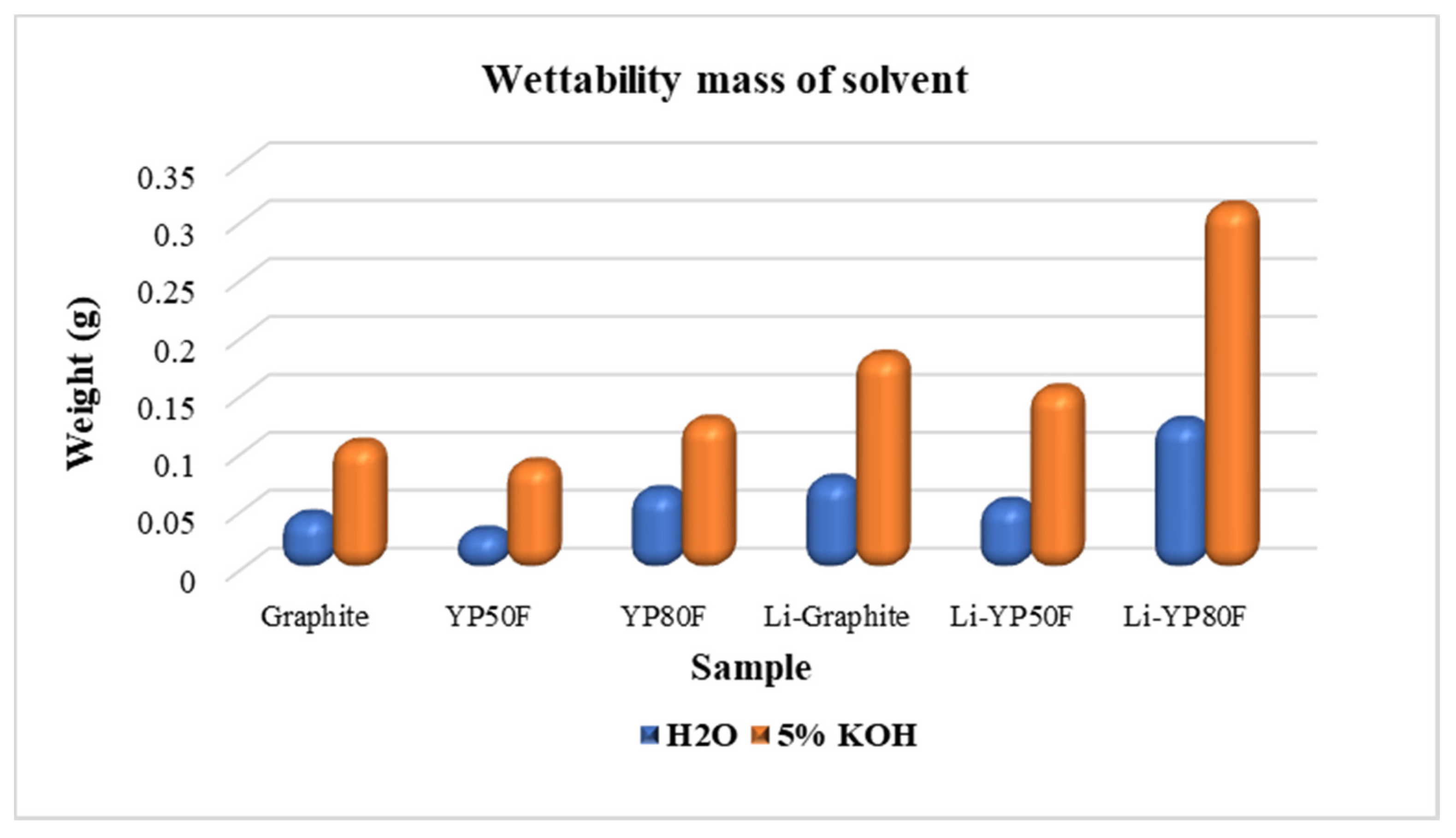
2.2. The Functional Group and Surface Area Analysis
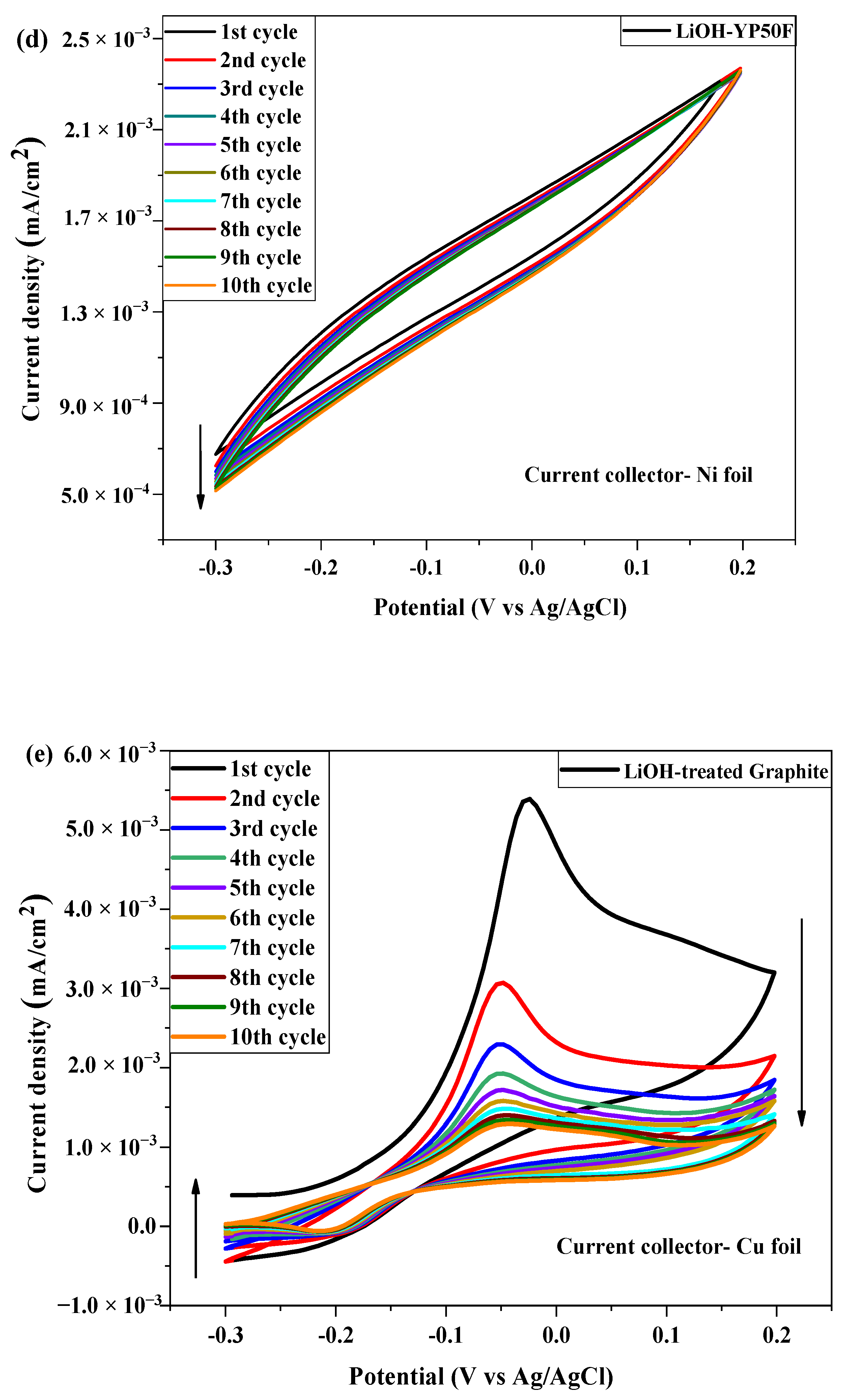
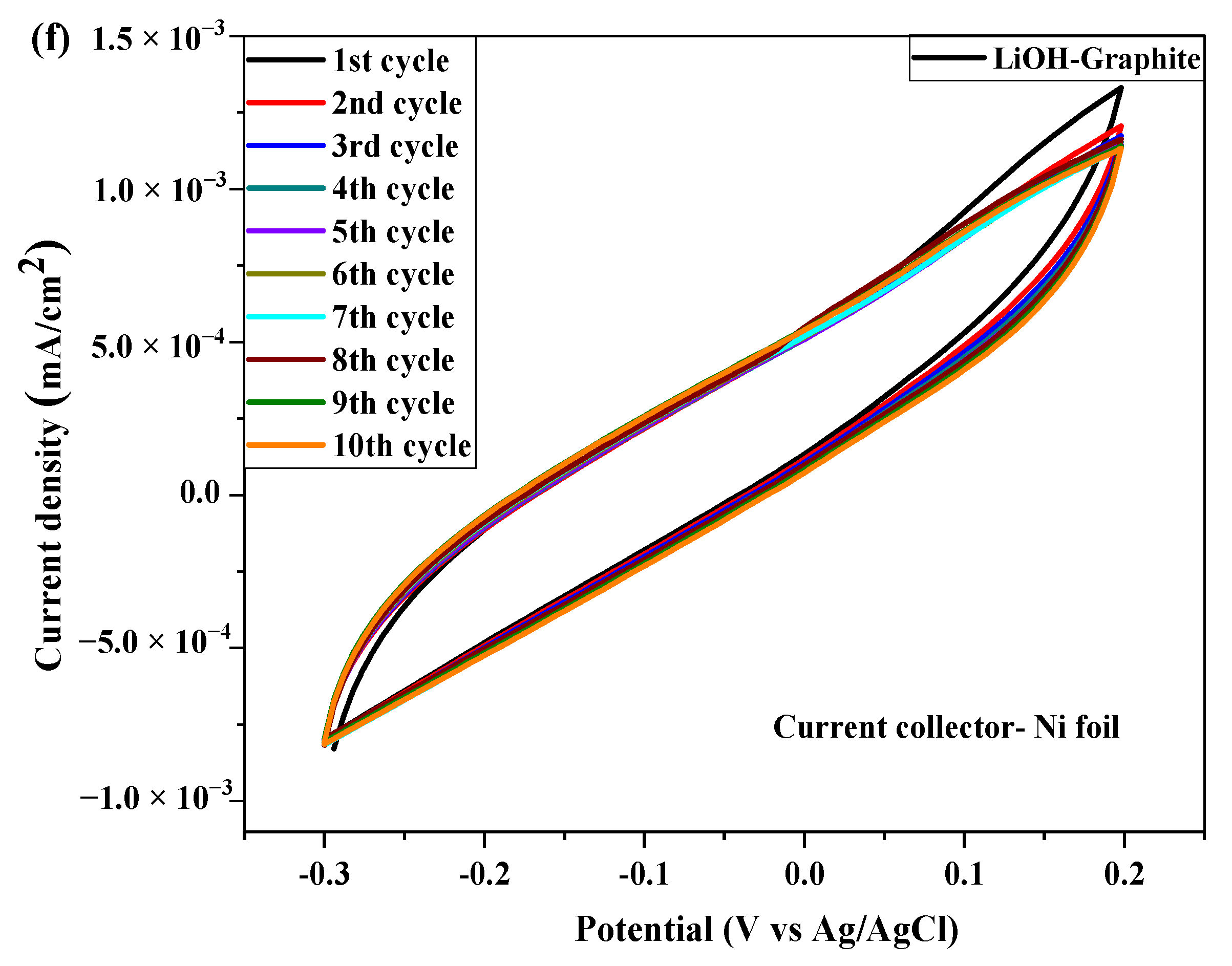
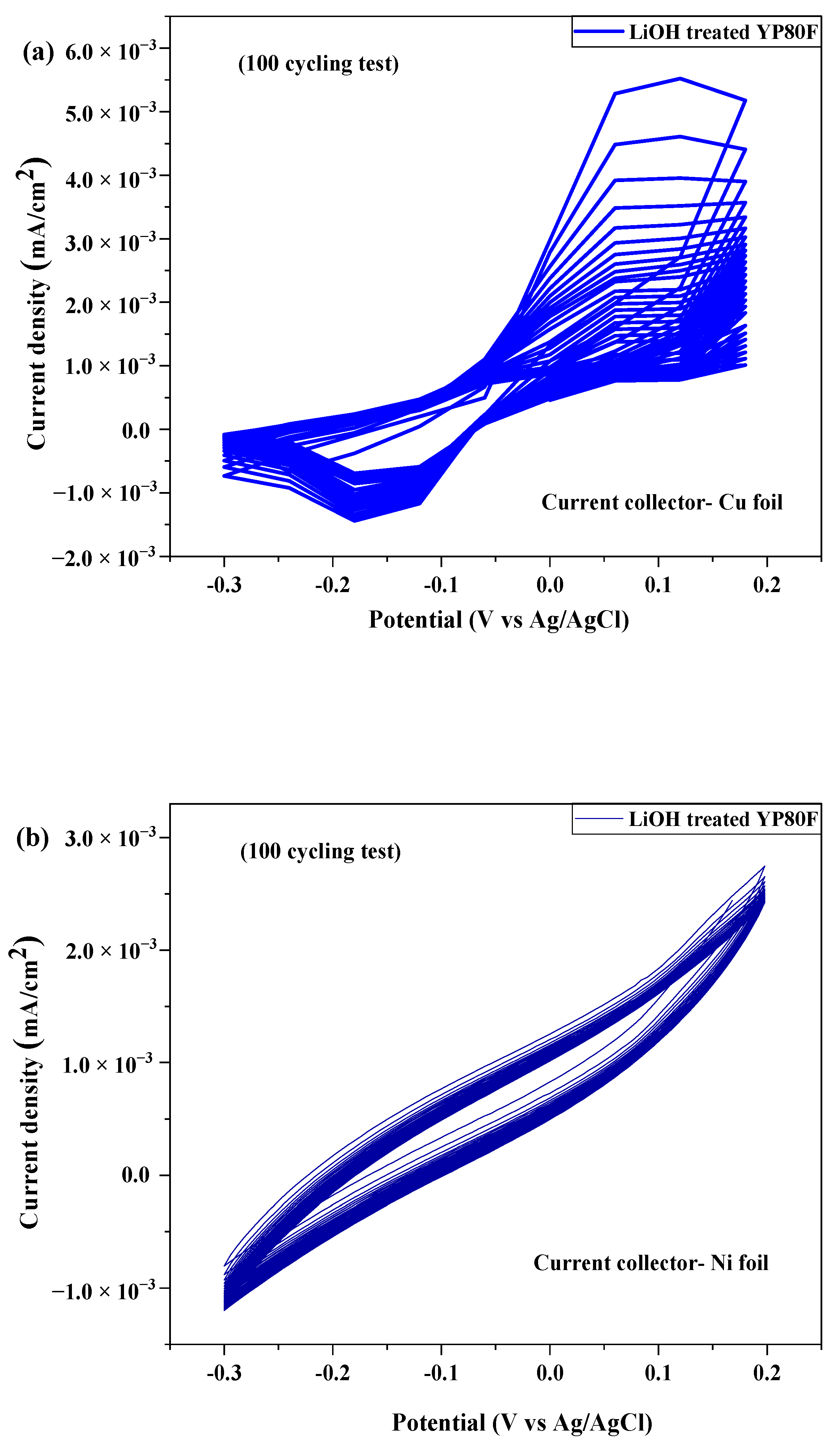
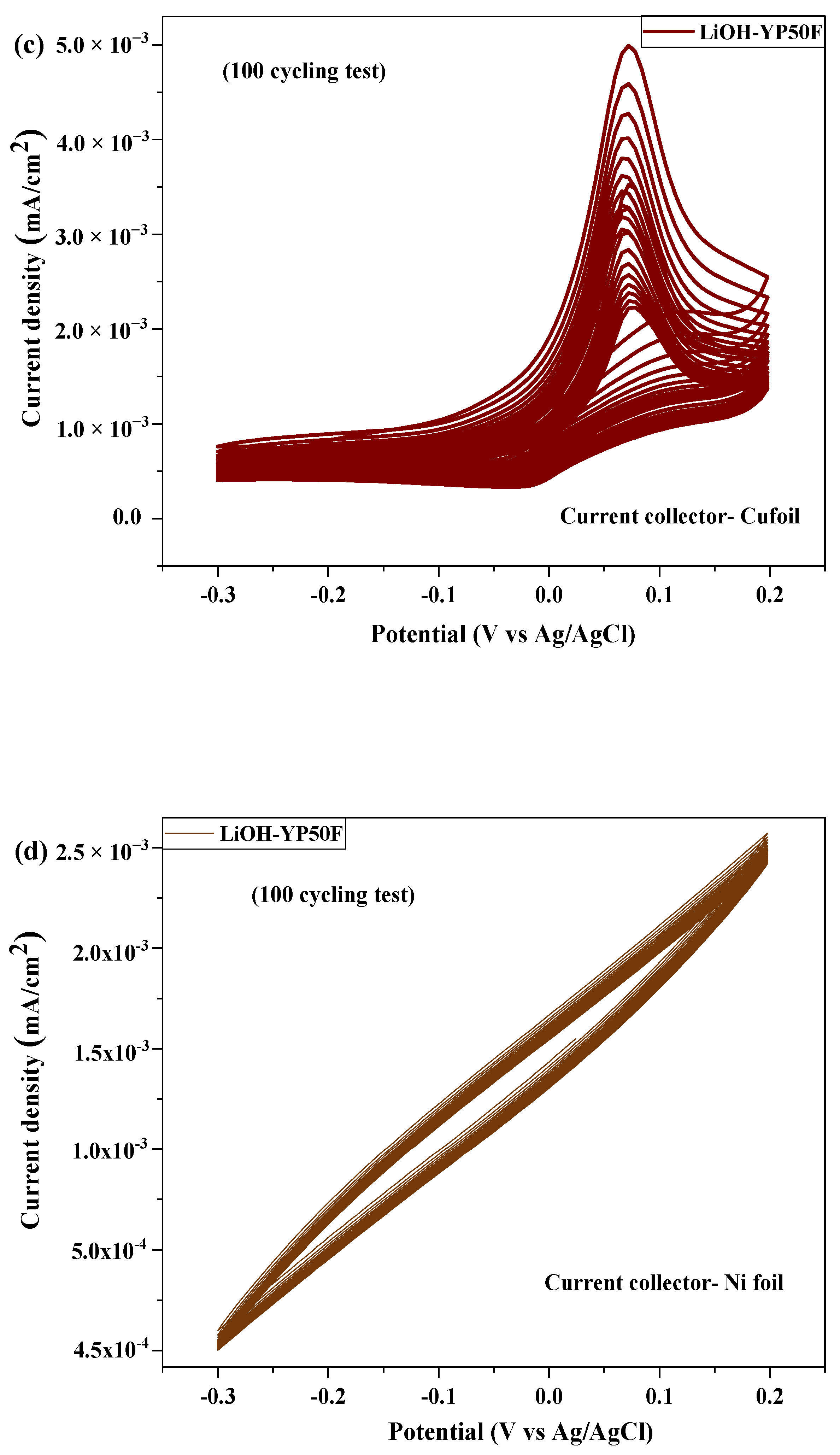
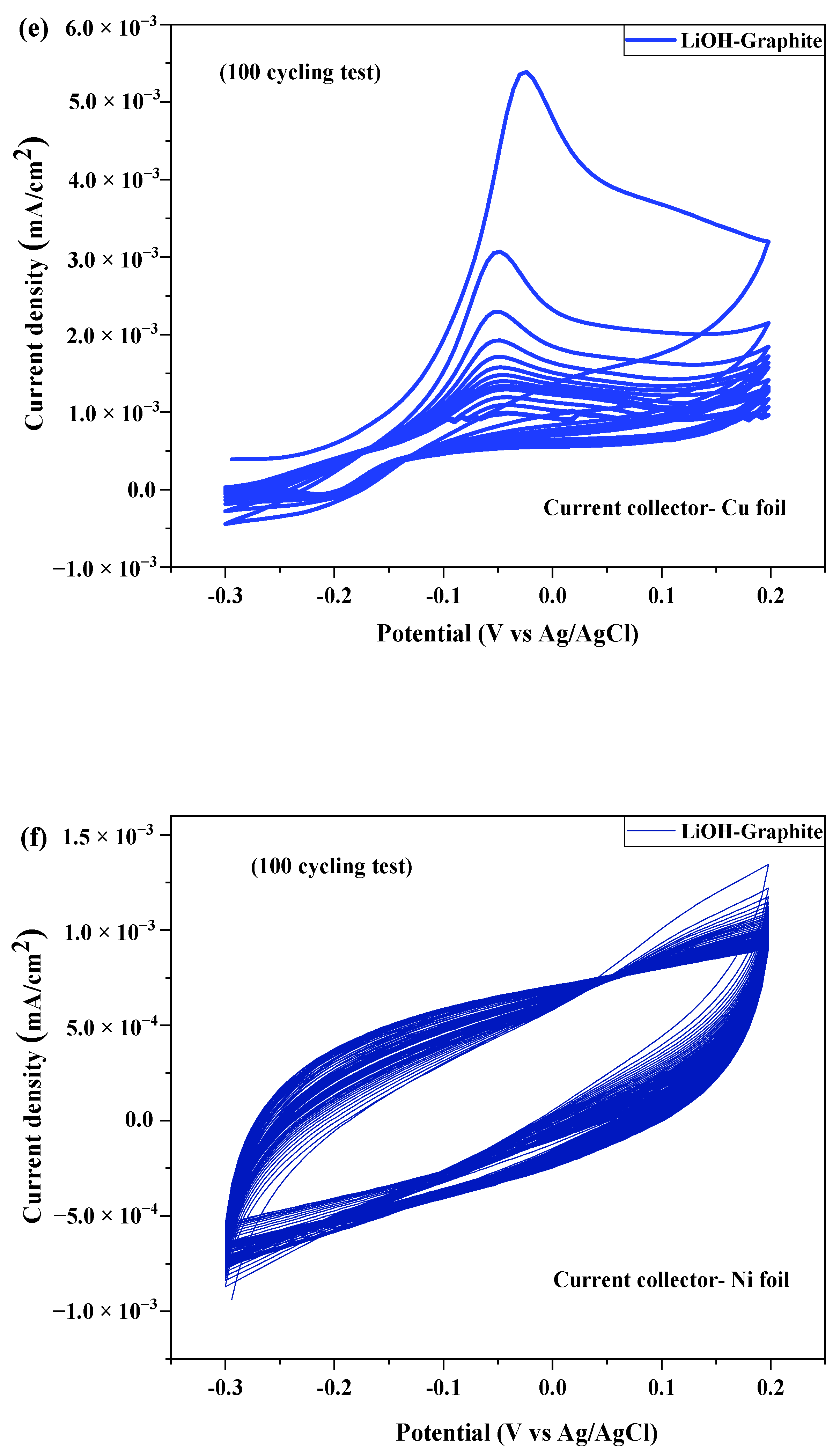
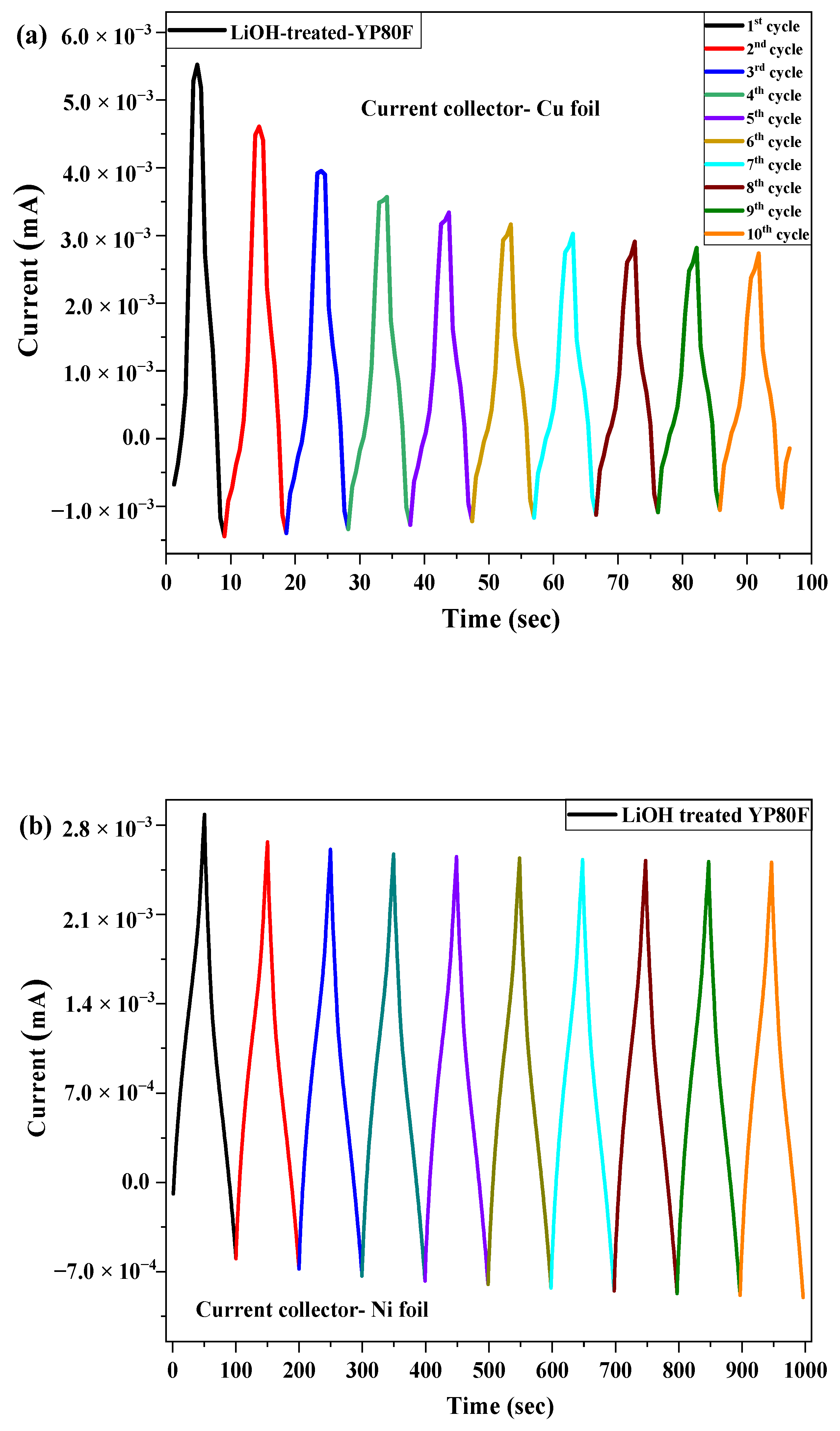
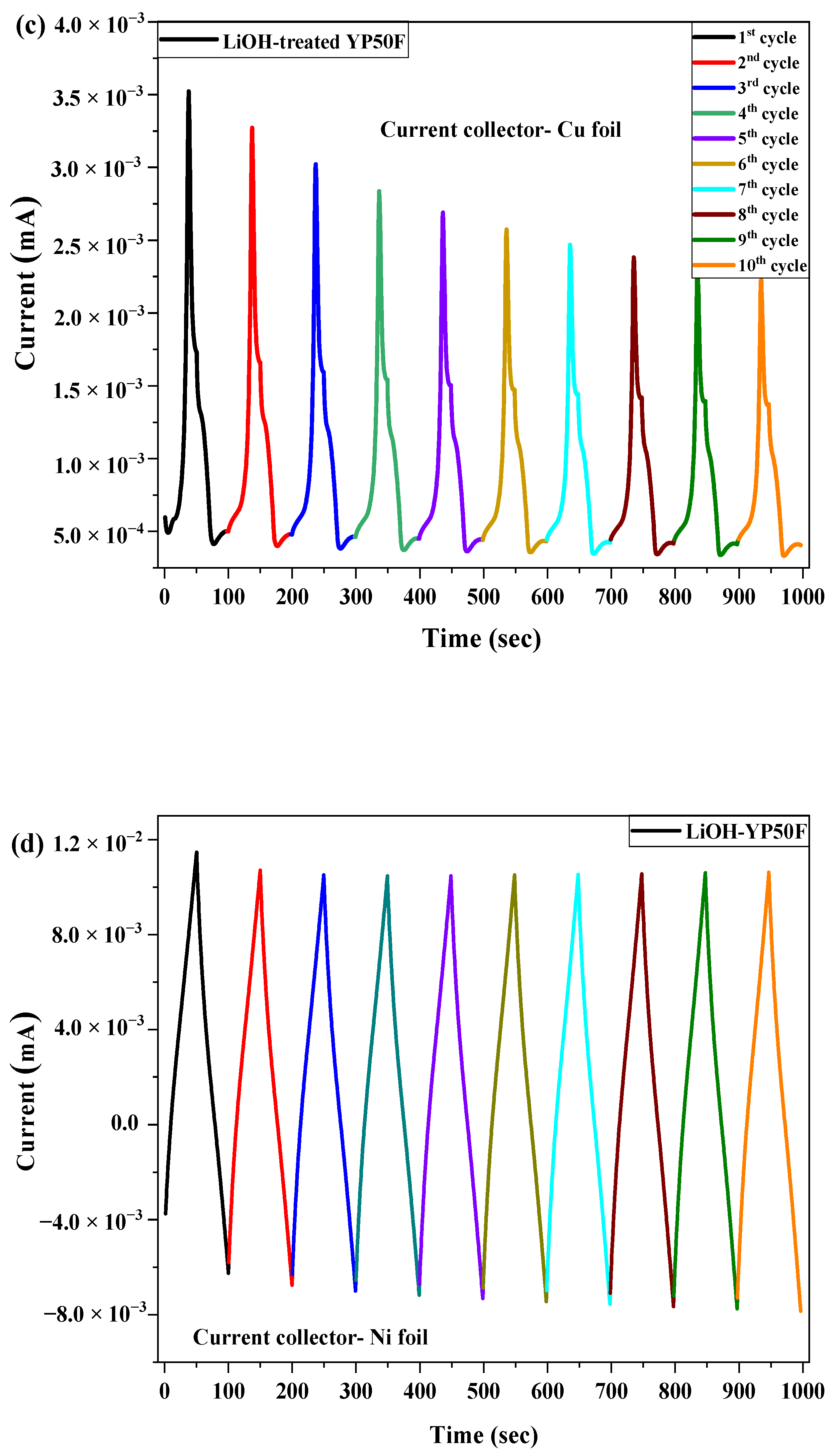
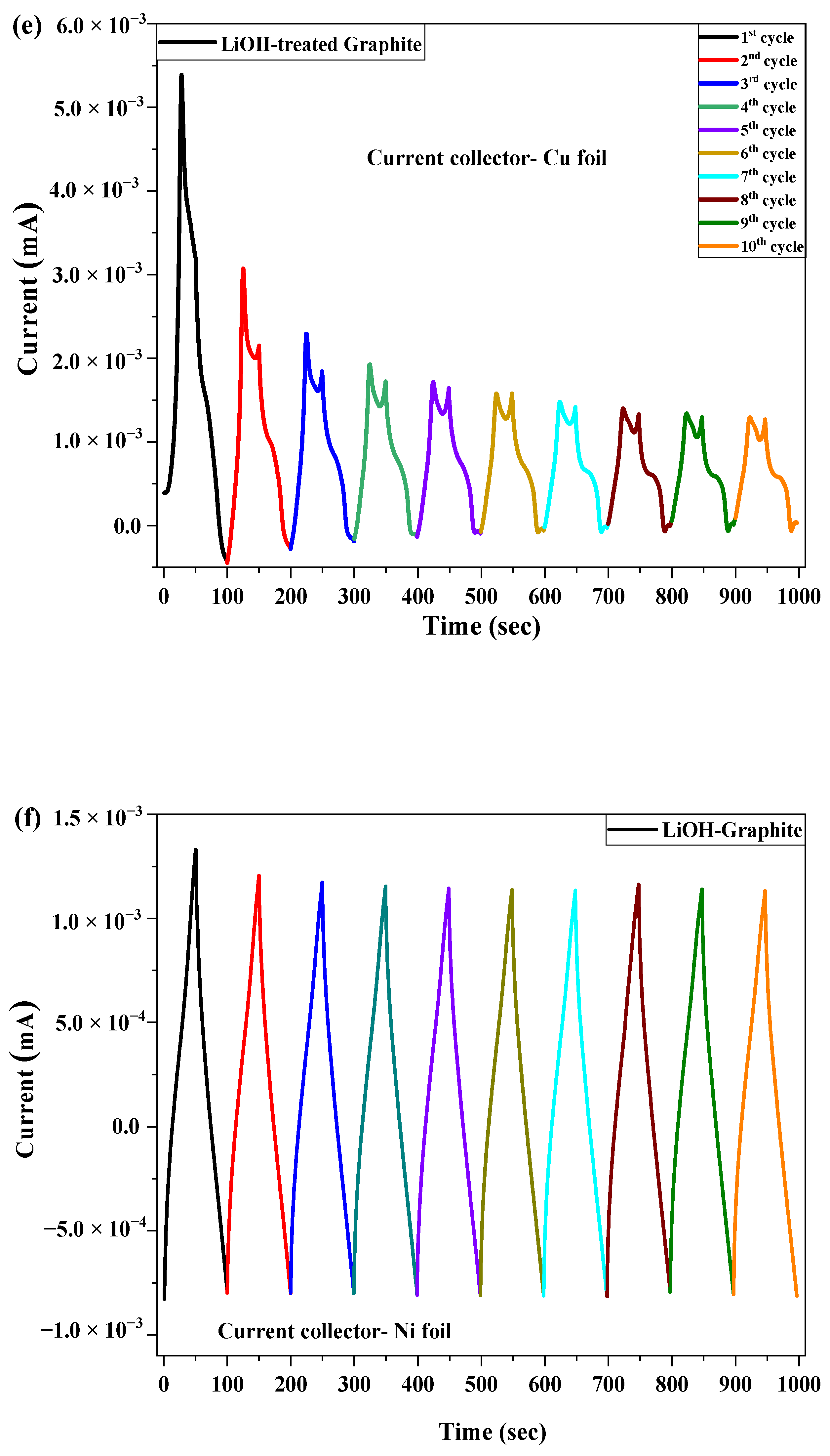
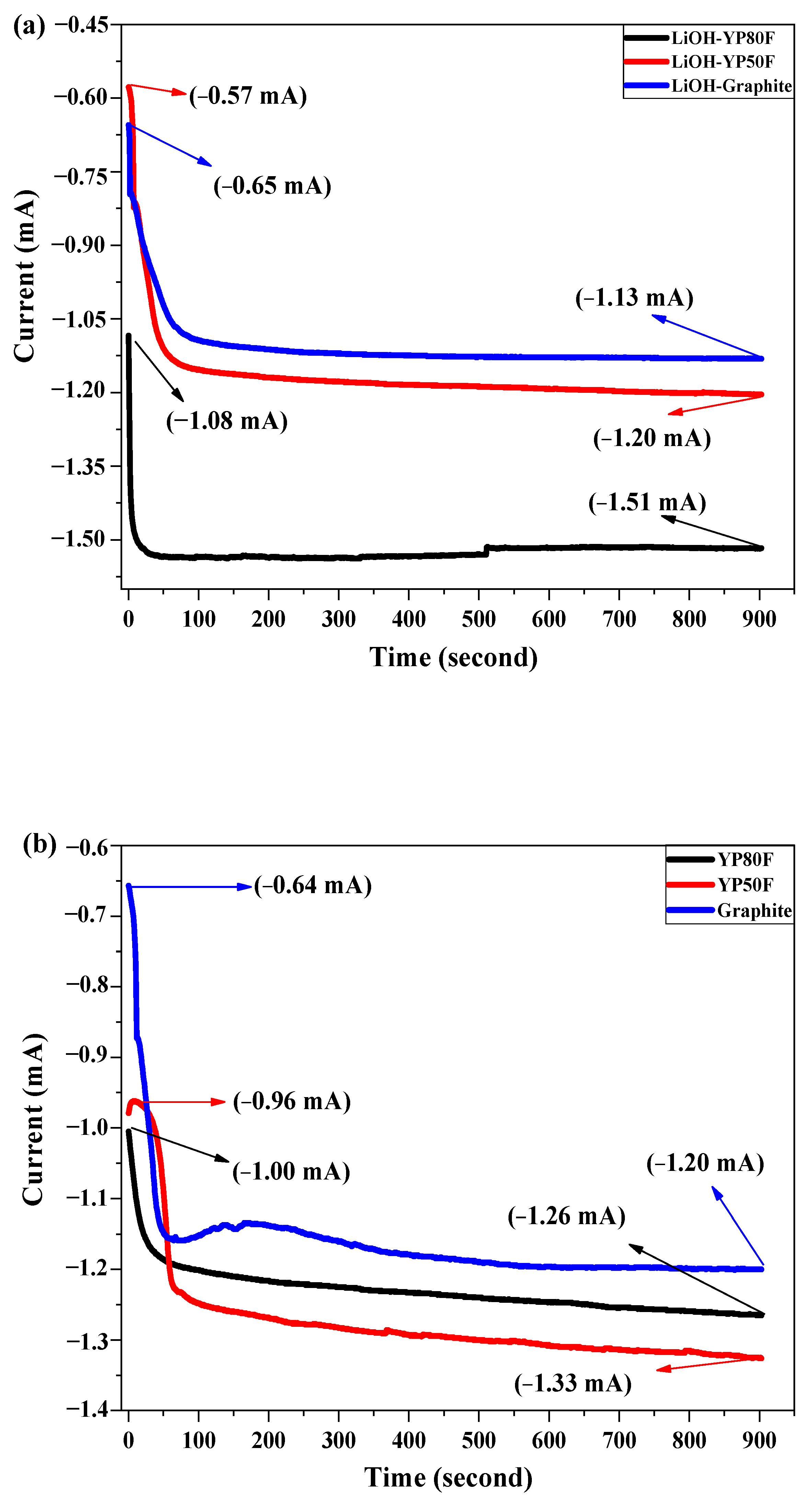
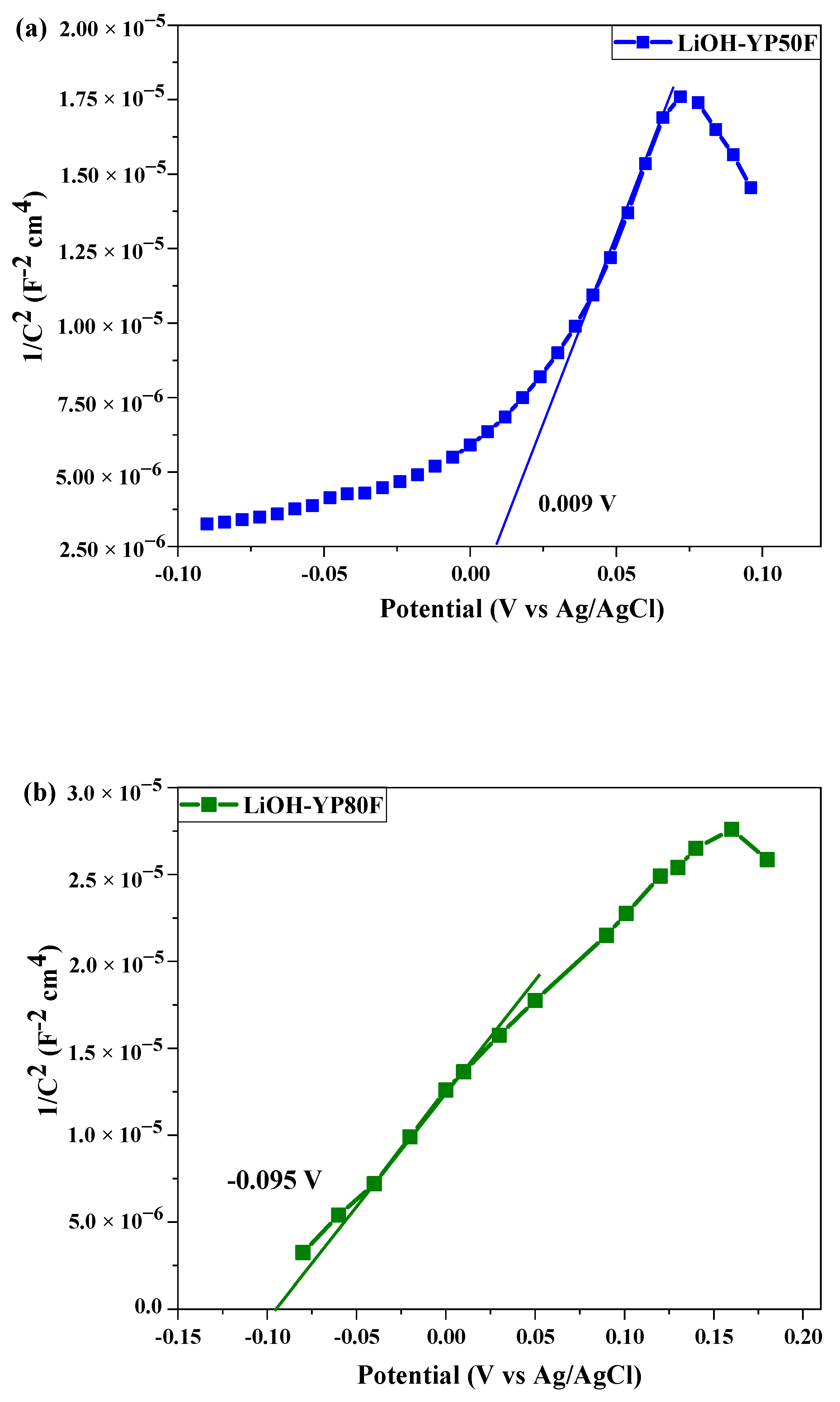
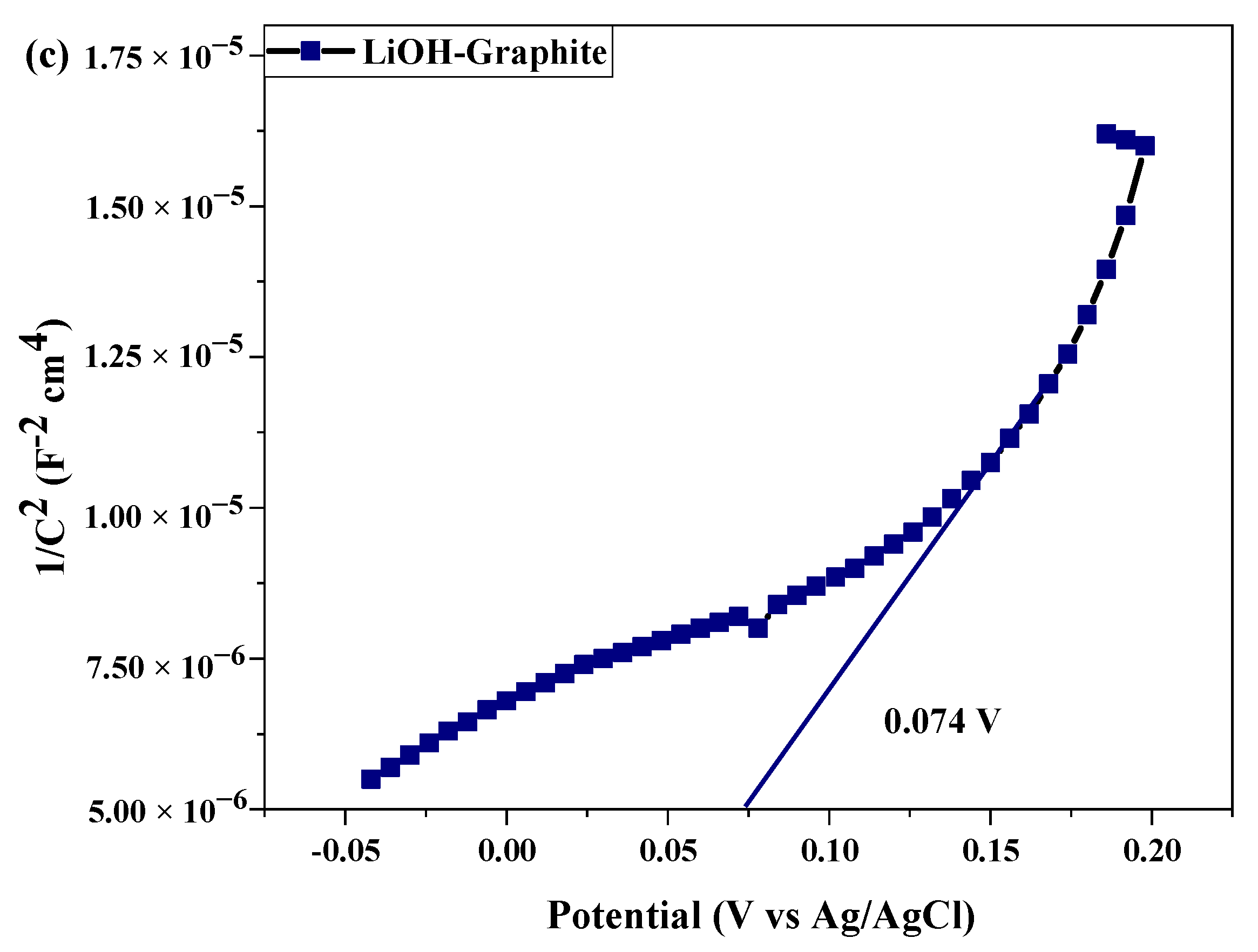
2.3. Electrochemical Test
3. Materials and Methods
3.1. Chemical Reagents
3.2. Preparation of Hydrophilic Carbon
3.3. Characterization
3.4. Boehm Titration
3.5. Cyclic Voltammetry (CV) Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jiang, W.; Yu, D.; Zhang, Q.; Goh, K.; Wei, L.; Yong, Y.; Jiang, R.; Wei, J.; Chen, Y. Ternary hybrids of amorphous nickel hydroxide–carbon nanotube-conducting polymer for supercapacitors with high energy density, excellent rate capability, and long cycle life. Adv. Funct. Mater. 2015, 25, 1063–1073. [Google Scholar] [CrossRef]
- Zhou, S.Z.; Zhou, G.Y.; Jiang, S.H.; Fan, P.C.; Hou, H.Q. Flexible and refractory tantalum carbide-carbon electro spun nanofibers with high modulus and electric conductivity. Mater. Lett. 2017, 200, 97–100. [Google Scholar] [CrossRef]
- Chen, L.; Li, D.; Zheng, X.; Chen, L.; Zhang, Y.; Liang, Z.; Feng, J.; Si, P.; Lou, J.; Ci, L. Integrated nanocomposite of LiMn2O4/graphene/carbon nanotubes with pseudocapacitive properties as superior cathode for aqueous hybrid capacitors. J. Electroanal. Chem. 2019, 842, 74–81. [Google Scholar] [CrossRef]
- Liu, C.; Ren, Q.Q.; Zhang, S.W.; Yin, B.S.; Que, L.F.; Zhao, L.; Sui, X.L.; Yu, F.D.; Li, X.F.; Gu, D.M.; et al. High energy and power lithium-ion capacitors based on Mn3O4/3D-graphene as anode and activated polyaniline-derived carbon nanorods as cathode. Chem. Eng. J. 2019, 370, 1485–1492. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Liao, H.J.; Yang, T.Y.; Chen, Z.; Yan, X.; Zhou, Z.; Lv, H.; Liu, W.W.; Chueh, Y.L. Hierarchical Mn-doped Fe2O3@rGO hollow core-shell spheres for high-performance hybrid capacitor. Mater. Today Energy 2020, 17, 100388. [Google Scholar] [CrossRef]
- Ji, H.M.; Liu, X.L.; Liu, Z.J.; Yan, B.; Chen, L.; Xie, Y.F.; Liu, C.; Hou, W.H.; Yang, G. In Situ Preparation of Sandwich MoO3/C Hybrid Nanostructures for High-Rate and UltralongLife Supercapacitors. Adv. Funct. Mater. 2015, 25, 1886–1894. [Google Scholar] [CrossRef]
- Yu, M.H.; Zeng, Y.; Han, Y.; Cheng, X.Y.; Zhao, W.X.; Liang, C.L.; Tong, Y.X.; Tang, H.L.; Lu, X.H. Valence-Optimized Vanadium Oxide Supercapacitor Electrodes Exhibit Ultrahigh Capacitance and Super-Long Cyclic Durability of 100 000 Cycles. Adv. Funct. Mater. 2015, 25, 3534–3540. [Google Scholar] [CrossRef]
- Yang, C.C.; Sun, W.C.; Kumar, A.; Pattanayak, B.; Tseng, T.Y. Templating synthesis of nickel cobaltite nanoflakes and their nanocomposites for making high-performance symmetric supercapacitors. Mater. Today Energy 2019, 14, 100356. [Google Scholar] [CrossRef]
- Shoyebmohamad, F.; Shaikh Fouzia, F.M.; Shaikh, A.V.; Shaikh Mohd, U.; Abdullah, M.; Al-Enizi Habib, M.P. Electrodeposited more-hydrophilic nano-nest polyaniline electrodes for supercapacitor application. J. Phys. Chem. Solids 2021, 149, 109774. [Google Scholar] [CrossRef]
- Long, C.; Chen, X.; Jiang, L. Porous layer-stacking carbon derived from in-built template in biomass for high volumetric performance supercapacitors. Nano Energy 2015, 12, 141–151. [Google Scholar] [CrossRef]
- Mousavi-Kamazani, M.; Rahmatolahzadeh, R.; Shobeiri, S.A.; Beshkar, F. Sonochemical synthesis, formation mechanism, and solar cell application of tellurium nanoparticles. Ultrason. Sonochem. 2017, 39, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Asakura, Y.; Okada, N.; Koda, S.; Yasuda, K. Effect of ultrasonic cavitation on measurement of sound pressure using hydrophone. Jpn. J. Appl. Phys. 2017, 56. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, C. State-of-the-art on ultrasonic oil production technique for EOR in China. Ultrason. Sonochem. 2017, 38, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Cao, C.; Ma, X. From rice bran to high energy density supercapacitors: A new route to control porous structure of 3D carbon. Sci. Rep. 2014, 4, 7260. [Google Scholar] [CrossRef]
- Tzanakis, I.; Lebon, G.S.B.; Eskin, D.G.; Pericleous, K.A. Characterizing the cavitation development and acoustic spectrum in various liquids. Ultrason. Sonochem. 2017, 34, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.P.; Rao, P.N.; Reddy, M.C.S.; Rao, B.A.; Veeraiah, N. Second harmonic generation and spectroscopic characteristics of TiO2 doped Li2O–Al2O3–B2O3 glass matrix. Appl. Phys. A 2020, 126. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, L.; Cui, Y.; Liu, W.; Liu, X.; Yang, Y.Z. A hydrophilic surface molecularly imprinted polymer on a spherical porous carbon support for selective phenol removal from coking wastewater. New Carbon Mater. 2020, 34, 220–231. [Google Scholar] [CrossRef]
- Zambaga, O.; Kamrun, N.F.; Rafat, M.N.; Ryu, J.; Yang, S.H.; Kim, I.J.; Oh, W.C. Surface Modification and Electrochemical Performance of KMnO4-Ultra-surface Area Activated Carbon (USAC) Composites as Cathode Material. J. Korean Ceram. Soc. 2020, 6. [Google Scholar] [CrossRef]
- Schönherr, J.; Buchheim, J.R.; Scholz, P.; Adelhelm, P. Boehm Titration Revisited (Part I): Practical Aspects for Achieving a High Precision in Quantifying Oxygen-Containing Surface Groups on Carbon Materials. J. Carbon Res. 2018, 4, 21. [Google Scholar] [CrossRef]
- Zhang, D.D.; Wang, J.L.; He, C.; Wang, Y.; Guan, T.; Zhao, J.; Qiao, J.; Li, K. Rational surface tailoring oxygen functional groups on carbon spheres for capacitive mechanistic study. ACS Appl. Mater. Interfaces 2019, 11, 13214–13224. [Google Scholar] [CrossRef]
- Miao, Z.Y.; Huang, Y.; Xin, J.P.; Su, X.; Sang, Y.; Liu, H.; Wang, J.J. High-performance symmetric supercapacitor constructed using carbon cloth boosted by engineering oxygen-containing functional groups. ACS Appl. Mater. Interfaces 2019, 11, 18044–18050. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.L.; Sheng, L.Z.; Long, C.L.; Wei, T.; Fan, Z. Functional pillared graphene frameworks for ultrahigh volumetric performance supercapacitors. Adv. Energy Mater. 2015, 5, 1500771. [Google Scholar] [CrossRef]
- Lee, J.; Abbas, M.A.; Bang, J.H. Exploring the capacitive behavior of carbon functionalized with cyclic ethers: A rational strategy to exploit oxygen functional groups for enhanced capacitive performance. ACS Appl. Mater. Interfaces 2019, 11, 19056–19065. [Google Scholar] [CrossRef]
- Fan, L.Z.; Qiao, S.Y.; Song, W.L.; Wu, M.; He, X.; Qu, X. Effects of the functional groups on the electrochemical properties of ordered porous carbon for supercapacitors. Electrochim. Acta 2013, 105, 299–304. [Google Scholar] [CrossRef]
- Xi, L.; Yang, J.; Pei, W.; Yan, M.; Wen, L.; Zheng, L.; Ru, Y.; Yu, D.; Xin, Z.; Yong, C. Effect of the oxygen functional groups of activated carbon on its electrochemical performance for supercapacitors. New Carbon Mater. 2020, 35, 232–243. [Google Scholar] [CrossRef]
- Yanan, X.; Xiaofeng, W.; Cuihua, A.; Yijing, W.; Lifang, J.; Huatang, Y. Effect of the length and surface area on electrochemical performance of cobalt oxide nanowires for alkaline secondary battery application. J. Power Source 2014, 272, 703–710. [Google Scholar] [CrossRef]
- Venezuela, P.; Lazzeri, M.; Mauri, F. Theory of double-resonant Raman spectra in graphene: Intensity and line shape of defect-induced and two-phonon bands. Phys. Rev. B 2011, 84, 035433. [Google Scholar] [CrossRef]
- Kumar, B.; Thomas, D.; Kumar, J. Space-Charge-Mediated Superionic Transport in Lithium Ion Conducting Glass–Ceramics. J. Electrochem. Soc. 2009, 156, A506–A513. [Google Scholar] [CrossRef]
- Neetu, S.; Neha, G.; Rama, K. Experimental corroboration of the theory of chronoamperometry at high roughness electrode for reversible charge transfer. J. Electroanal. Chem. 2017, 788, 83–90. [Google Scholar] [CrossRef]
- Zang, X.N.; Shen, C.W.; Sanghadasa, M.; Lin, L.W. High-Voltage Supercapacitors Based on Aqueous Electrolytes. Chemelectrochem 2019, 6, 976–988. [Google Scholar] [CrossRef]
- See, D.M.; White, R.E. Temperature and Concentration Dependence of the Specific Conductivity of Concentrated Solutions of Potassium Hydroxide. J. Chem. Eng. Data 1997, 42, 1266–1268. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, Q.; Wei, T.; Fan, Z. Supercapacitors: Recent Advances in Design and Fabrication of Electrochemical Supercapacitors with High Energy Densities. Adv. Energy Mater. 2014, 4, 1300816. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Jiang, L.; Wu, H.; Wu, C.; Su, J. Effect of aqueous electrolytes on the electrochemical behaviors of supercapacitors based on hierarchically porous carbons. J. Power Source 2012, 216, 290–296. [Google Scholar] [CrossRef]
- Mao, Z.X.; Zhang, W.; Wang, M.J.; Peng, L.; Hong, W.; Deng, J.; Li, J.; Wei, Z. Enhancing rate performances of carbon-based supercapacitors. Chem. Select 2019, 4, 6827–6832. [Google Scholar] [CrossRef]
- Zhi, M.J.; Yang, F.; Meng, F.K.; Li, M.; Manivannan, A.; Wu, N. Effects of pore structure on performance of an activated-carbon supercapacitor electrode recycled from scrap waste tires. ACS Sustain. Chem. Eng. 2014, 2, 1592–1598. [Google Scholar] [CrossRef]
- Xie, Y.B.; Qiao, W.M.; Zhang, W.Y.; Sun, G.W.; Ling, L.C. Effect of the surface chemistry of activated carbon on its electrochemical properties in electric double layer capacitors. New Carbon Mater. 2010, 25, 248–254. [Google Scholar] [CrossRef]
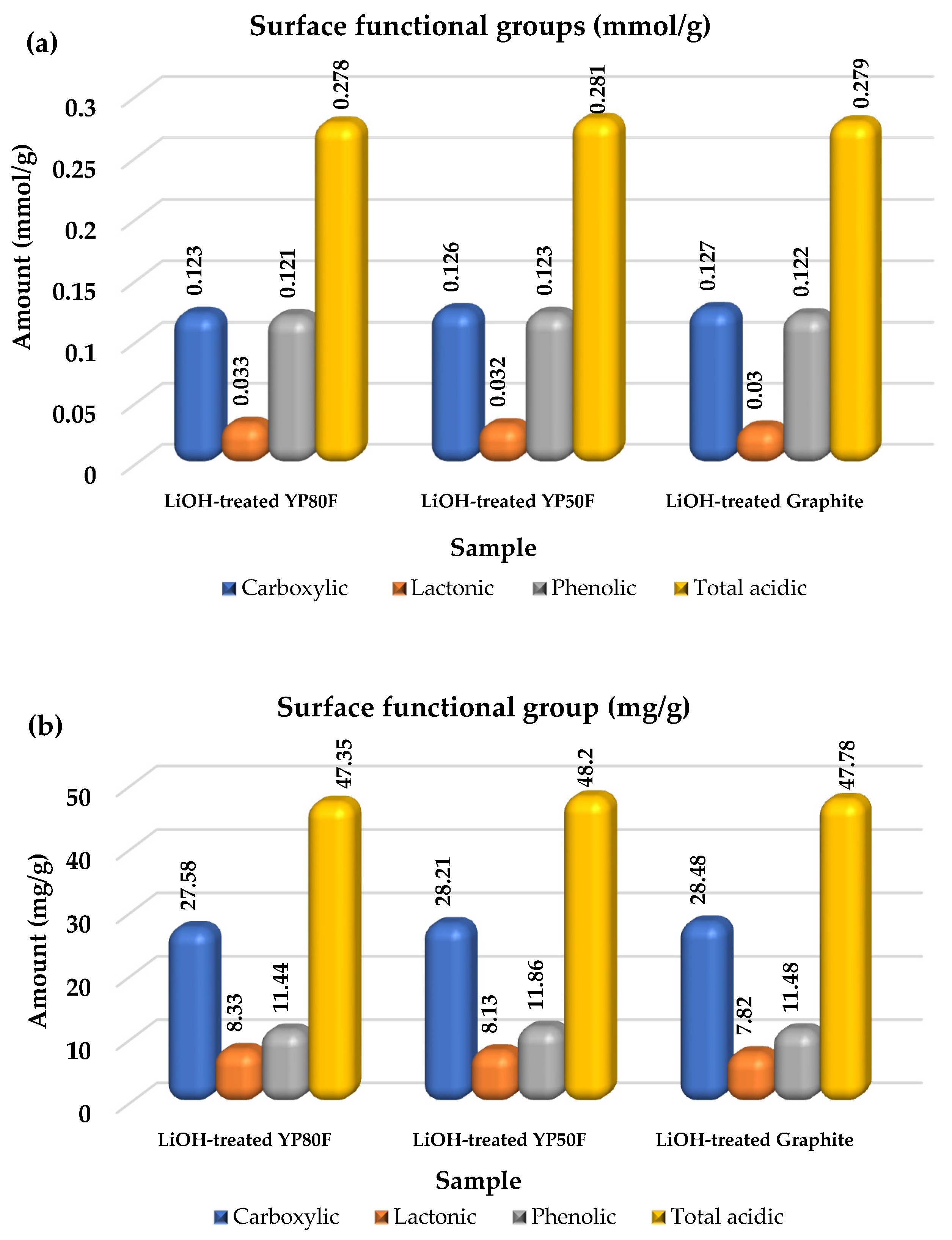



| Surface Functional Group (mg/g) | Surface Functional Group (mmol/g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Carboxylic | Lactonic | Phenolic | Total acidic | Carboxylic | Lactonic | Phenolic | Total Acidic | ||
| 1 | LiOH-treated YP80F | 27.58 | 8.33 | 11.44 | 47.35 | 0.123 | 0.033 | 0.121 | 0.278 |
| 2 | LiOH-treated Graphite | 28.48 | 7.82 | 11.48 | 47.78 | 0.127 | 0.030 | 0.122 | 0.279 |
| 3 | LiOH-treated YP50F | 28.21 | 8.13 | 11.86 | 48.20 | 0.126 | 0.032 | 0.123 | 0.281 |
| BET Analysis | ||||||
| Samples | Surface Area (m2/g) | Total Pore Volume (cm3/g) | Mean Pore Diameter (nm) | |||
| Graphite | 6.29 | 0.042 | 26.6 | |||
| YP50F | 1676.1 | 0.776 | 1.9 | |||
| YP80F | 2374.8 | 1.227 | 2.1 | |||
| Graphite-LiOH | 15.2 | 0.094 | 24.6 | |||
| YP50F-LiOH | 11.7 | 0.074 | 25.3 | |||
| YP80F-LiOH | 2155.7 | 1.104 | 2.0 | |||
| BJH Analysis | ||||||
| Samples | Mesopore Pore Diameter (nm) | Micropore Surface Area (m2/g) | Mesopore Surface Area (m2/g) | Micropore Volume (cm3/g) | Mesopore Volume (cm3/g) | Micropore Vol. Percent (%) |
| Graphite | 140.0 | −0.38 | 6.7 | 0.002 | 0.040 | 4 |
| YP50F | 1.7 | 1471.35 | 204.8 | 0.586 | 0.191 | 75 |
| YP80F | 1.7 | 1345.30 | 1029.5 | 0.571 | 0.656 | 47 |
| Graphite-LiOH | 76.49 | 0.11 | 15.11 | 0.004 | 0.090 | 4 |
| YP50F-LiOH | 76.5 | −3.28 | 15.0 | 0.003 | 0.072 | 3 |
| YP80F-LiOH | 1.7 | 1238.64 | 917.1 | 0.526 | 0.578 | 48 |
| T-Plot | ||||||
| Samples | Total Surface Area (m2/g) | Micropore Surface Area (m2/g) | External Surface (m2/g) | Micropore Volume (cm3/g) | Mesopore Volume (cm3/g) | Micropore Vol. Percent (%) |
| Graphite | 7.40 | 2.44 | 23.96 | −0.021 | - | |
| YP50F | 1898.4 | 1639.4 | 36.7 | 0.693 | 0.084 | 89 |
| YP80F | 2226.9 | 2304.4 | 70.4 | 1.084 | 0.100 | 88 |
| Graphite-LiOH | 1.665 | 2.30 | 21.283 | 0.023 | - | |
| YP50F-LiOH | 43.5 | 11.59 | 11.61 | 0.037 | 0.111 | 50 |
| YP80F-LiOH | 1063.2 | 1094.1 | 46.0 | 0.511 | 0.143 | 84 |
| № | Sample | Current Collector | Area of Integration | Specific Capacitance (Cp) (F/g) | Energy Density (Wh/kg) | Power Density (kW/kg) |
|---|---|---|---|---|---|---|
| 1 | Li-YP80F | Cu foil | 9.828 × 10−4 | 1.196 × 10−4 | 14.95 | 66.44 |
| Ni foil | 3.918 × 10−4 | 2.118 × 10−5 | 2.65 | 13.81 | ||
| 2 | Li-YP50F | Cu foil | 7.601 × 10−4 | 1.169 × 10−4 | 14.61 | 59.77 |
| Ni foil | 2.21 × 10−4 | 1.21 × 10−5 | 1.51 | 7.26 | ||
| 3 | Li-Graphite | Cu foil | 6.559 × 10−4 | 1.17 × 10−4 | 14.62 | 57.83 |
| Ni foil | 2.158 × 10−4 | 1.192 × 10−5 | 1.49 | 6.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otgonbayar, Z.; Yang, S.; Kim, I.-J.; Oh, W.-C. Surface Modification Effect and Electrochemical Performance of LiOH-High Surface Activated Carbon as a Cathode Material in EDLC. Molecules 2021, 26, 2187. https://doi.org/10.3390/molecules26082187
Otgonbayar Z, Yang S, Kim I-J, Oh W-C. Surface Modification Effect and Electrochemical Performance of LiOH-High Surface Activated Carbon as a Cathode Material in EDLC. Molecules. 2021; 26(8):2187. https://doi.org/10.3390/molecules26082187
Chicago/Turabian StyleOtgonbayar, Zambaga, Sunhye Yang, Ick-Jun Kim, and Won-Chun Oh. 2021. "Surface Modification Effect and Electrochemical Performance of LiOH-High Surface Activated Carbon as a Cathode Material in EDLC" Molecules 26, no. 8: 2187. https://doi.org/10.3390/molecules26082187
APA StyleOtgonbayar, Z., Yang, S., Kim, I.-J., & Oh, W.-C. (2021). Surface Modification Effect and Electrochemical Performance of LiOH-High Surface Activated Carbon as a Cathode Material in EDLC. Molecules, 26(8), 2187. https://doi.org/10.3390/molecules26082187






