Abstract
In orderto synthesize new pyridazine derivatives anellated with different nitrogen heterocyclic moieties, spiro[cycloalkane]pyridazinones were transformed into the corresponding thioxo derivatives via a reaction with phosphorus pentasulfide. The reaction of the formed 2,3-diazaspiro[5.5]undec-3-ene-1-thiones with hydrazine provided the corresponding 1-hydrazono-2,3-diazaspiro[5.5]undec-3-ene, whose diazotization led to the desired spiro[cyclohexane-1,8′-tetrazolo[1,5-b]pyridazines. The reaction of dihydropyridazinethiones with benzhydrazide afforded the corresponding 7H-spiro[[1,2,4]triazolo[4,3-b]pyridazin-8,1′-cyclohexanes]. As a result of our work, seven new pyridazinethione intermediates were prepared, which served as starting materials for the synthesis of two kinds of new ring systems: tetrazolo-pyridazines and triazolo-pyridazines. The six new annulated derivatives were characterized by physicochemical parameters. The new N-heterocycles are valuable members of the large family of pyridazines.
1. Introduction
Pyridazine derivatives are representative members of N-heterocycles [1,2]. A large number of pyridazines show various types of bioactivity [3,4,5,6,7]. For example, sulfamethoxypyridazine is an old sulfonamide antibacterial drug [8], while hydralazine (Apresoline) is a well-known antihypertensive agent [9]. The calcium sensitizer levosimendan is useful in congestive heart failure [10]. The monoamine oxidase (MAO) inhibitor is induced by minaprine [11,12] and the tricyclic pipofezine (Azaphen) [13] are well-known antidepressants. The aldose-reductase inhibitor zopolrestat was developed for treating diabetic neuropathy [14]. The pyridazines under development are also noteworthy. The anaplastic lymphoma kinase (ALK) inhibitor ensartinib is in Phase II clinical trials [15]. The gonadotropin releasing hormone (GnRH) receptor antagonist relugolix (Relumina) was approved for the treatment of uterine fibroids [16]. Talazoparib (Talzenna) is a poly adenosine diphosphate (ADP) ribose polymerase (PARP) inhibitor, which was developed for treating advanced breast cancer [17]. Talazoparib has a similar phthalazin-1(2H)-one motif with that found in the other PARP inhibitor olaparib (Lynparza) [18] (Figure 1).
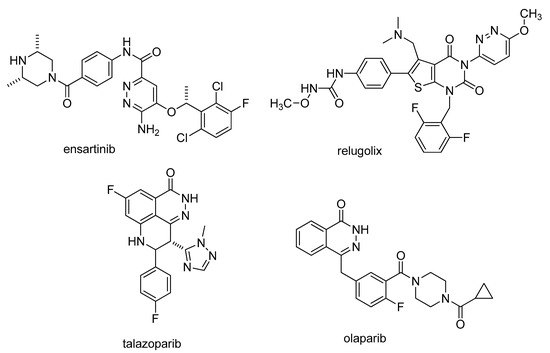
Figure 1.
Pyridazine derivatives under clinical development: ensartinib, relugolix, talazoparib, and olaparib.
The spirocycles have recently gained more attention, as they usually have a high Fsp3 character, and the three-dimensionality is better in the case of flat aromatic rings. The use of spiro building blocks better optimizes physicochemical parameters, structural novelty, and higher patentability. There are well-known examples among the spiro compounds, e.g., the anxiolytic agent buspirone, the angiotensin receptor blocker irbesartan, the antifungal natural product griseofulvin, or the spironolactone with sterane skeleton. The к opioid agonist enadoline seemed to be the most potent к selective analgesic. Most of the recently published spiro compounds contain five and six membered rings, but there are examples for 4-membered or 7-membered spirocyclic systems too [19,20,21,22,23].
Realizing that the use of the nitrogen containing heterocycles and the spiro motif may be advantageous, a few spiro[cycloalkane]pyridazinone derivatives were synthesized by us (Scheme 1) [24,25]. Depending on the substituents, the starting anhydrides (2-oxaspiro[4.4]nonane-1,3-dione (1a) and 2-oxaspiro[4.5]-1,3-dione (1b)) were converted to the desired keto-carboxylic acids (2 and 3) either by the Friedel–Crafts reaction or the Grignard reaction. This was followed by the ring closure with hydrazine hydrate in refluxing ethanol to give the corresponding pyridazinone derivatives (4a–d, 4e and 5a,b) in high yields [24,25].
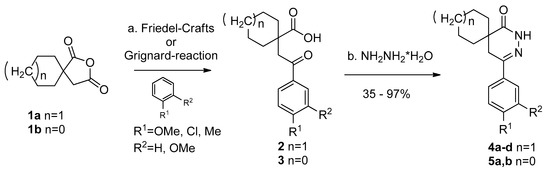
Scheme 1.
Synthesis of spiro[cycloalkane]pyridazinones (4a–d and 5a,b) [24,25]; (a) 1a,b (1.0 mmol), Friedel–Crafts reaction: AlCl3 (3.0 mmol), ArH derivative (3.0 mmol), 0 °C (30 min) -> room temperature (RT) (24 h); Grignardreaction: Grignard reagent (1.0 mmol), 0 °C (30 min) -> RT (24 h); (b) 2,3 (0.80 mmol), hydrazine hydrate (2.40 mmol), EtOH, reflux, (4 h); (c) 4a–d, 5a,b isolated yields after thin layer chromatography (TLC) purification: 35–97% [24,25].
In this work, we described the use of spiro[cycloalkane]pyridazinone derivatives (4a–d, 5a,b) in the synthesis of new triazolo-pyridazine and tetrazolo-pyridazine derivatives via the corresponding thioxo intermediates.
2. Results and Discussion
Our plan was to synthesize derivatives, where the pyridazine ring was fused with another N-heterocyclic moiety (9 and 10).
2.1. Synthesis of Pyridazinethiones
Phosphorus pentasulfide or the Lawesson reagent may be used for the thionation of an amide function [26]. The earlier described spiro[cycloalkane]pyridazinones (4a–d and 5a,b) reacted with phosphorus pentasulfide in the refluxing toluene. According to thin layer chromatography (TLC), the starting material was consumed after 6–8 h. After the workup comprising extraction, concentration, and preparative TLC purification, the products (6a–d and 7a,b) were isolated in yields of 40–89% (Scheme 2). The new compounds (6a–d and 7a,b) were characterized by 1H and 13C nuclear magnetic resonance spectroscopy (NMR), as well as high resolution mass spectrometry (HRMS).
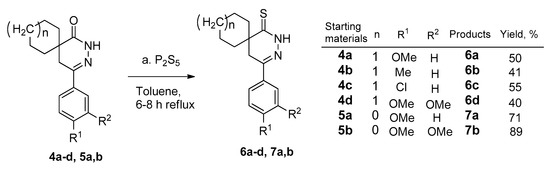
Scheme 2.
Thionation reaction of the spiro[cycloalkane]pyridazinones (4a–d and 5a,b); (a) 4a–d, 5a,b (1.0 mmol), P2S5 (3.0 mmol), PhMe, reflux, (6–8 h); (b) 6a–d, 7a,b isolated yields after TLC purification: 40–89%.
During the preparation of the p-methoxyphenyl[spirocyclohexane]pyridazinone (4a), the isomeric pyridazinone derivative (4e) was isolated as a byproduct. The formation of the two isomers was explained earlier [24]. The thionation reaction with phosphorus pentasulfide led to the corresponding pyridazinethione (6e) in a yield of 14% (Scheme 3).
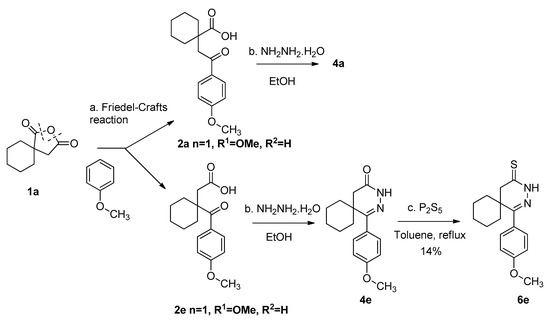
Scheme 3.
Preparation of 1-(4-methoxyphenyl)-2,3-diazaspiro[5.5]undec-1-ene-4-thione (6e): (a) 1a (1.0 mmol), Friedel–Crafts reaction: AlCl3 (3.0 mmol), anizole (3.0 mmol), 0 °C (30 min) -> RT (24 h); (b) 2a,e (0.80 mmol), hydrazine hydrate (2.40 mmol), EtOH, reflux, (4 h); (c) 2a,e (1.0 mmol), P2S5 (3.0 mmol), PhMe, reflux, (6–8 h); (d) 2e isolated yield after TLC purification: 14%.
2.2. Preparing the Tetrazole Derivatives through Hydrazones
After reacting the thioxo derivatives 6a–c with hydrazine hydrate, the corresponding hydrazones 8a–c were isolated in 48–61% yields. The 4-(phenyl)-2,3-diazaspiro[5.5]undec-3-ene-1-thiones (6a–c) and hydrazine hydrate in THF had to be stirred at reflux for 5 h. It was observed that compounds (8a–c) were unstable when standing at room temperature. After a week, they began decomposing. However, after preparation, intermediates 8a–c were immediately reacted further in a diazotization reaction. The tetrazolo-pyridazinones (9a–c) were obtained in 45–77% yields (Scheme 4) after a preparative TLC purification. Compounds 8a–c and 9a–c were new and were characterized by 1H and 13C NMR, as well as HRMS. A similar method was applied in the sphere of benzodiazepines, where the extended conjugation helped the transformation [27]. In the case of spiro compounds 8a–c, the reaction was new.
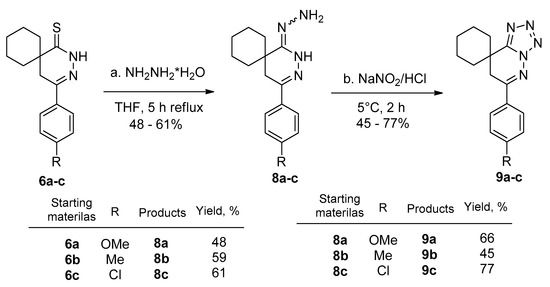
Scheme 4.
Synthesis of the hydrazono-pyridazinone (8a–c) and tetrazole derivatives (9a–c) (a) 6a–c (0.40 mmol), hydrazine monohydrate (1.20 mmol), tetrahydrofuran (THF), reflux, (5 h); (b) 8a–c (0.34 mmol), NaNO2 (0.51 mmol), 0.5 N HCl, 5 °C, (2 h); (c) 8a–c isolated yields after TLC purification: 48–61%; (d) 9a–c isolated yields after TLC purification: 45–77%.
Table 1 summarizes the most important physicochemical parameters of the tetrazolo-pyridazinones (9a–c). All compounds revealed excellent Fsp3 character values: 0.5 ± 0.05. The logP (3.1–3.7) and topological polar surface area (TPSA) values (52.7–61.9) were satisfactory according to the Lipinski rules and Lovering studies [28,29,30,31,32]. For comparison, the launched pyridazinone derivatives sometimes have lower Fsp3 characters (e.g., hydralazine: 0, zopolrestate: 0.16, levosimendan: 0.21), while pipofezine and minaprine have higher Fsp3 characters (e.g., 0.38 and 0.41, respectively). The drugs under development showed similar Fsp3 values (i.e., talazoparib: 0.16, relugolix: 0.21, ensartinib: 0.27, olaparib: 0.34 (Table 2)).

Table 1.
The physicochemical parameters of the hydrazone derivatives (8a–c and 9a–c).

Table 2.
The physicochemical parameters of drugs and drugs under development a.
2.3. Preparing the Triazole Derivatives from Thioxo Compounds with Acid Hydrazides
Our next purpose was to synthesize triazole derivatives starting from the thioxo compounds (6a–c). The thioxo compounds (6a–c) were refluxed with benzoic acid hydrazide (11) in n-butanol. After evaporation and chromatographic purification, the triazole-pyridazinone derivatives (10a–c) were isolated in yields of 14–42% (Scheme 5). This annulation technique was also applied in the sphere of benzodiazepines [33], where the extended aromatic conjugation may be advantageous for the reaction. Similarly to the preparation of tetrazoles, this reaction is new in case of spiro derivatives 10a–c. After the preparative TLC chromatographic separation, the three new products (10a–c) were characterized by 1H and 13C NMR, as well as HRMS. Table 3 summarizes the physicochemical parameters of the triazolo-pyridazinones (10a–c). The introduction of an additional aromatic ring increased the log P and clogP values, and decreased the Fsp3 character.
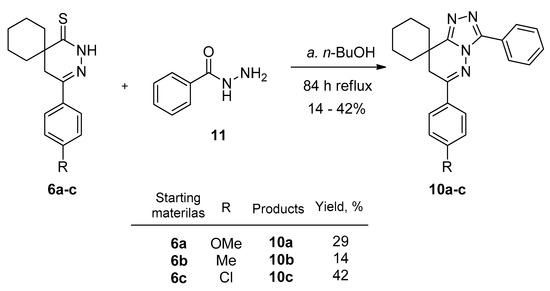
Scheme 5.
Reaction of thioxo-derivatives (6a–c) with benzoic acid hydrazide (11) (a) 6a–c (0.73 mmol), 11 (2.20 mmol), n-BuOH, reflux, (84 h); (b) (c) 10a–c isolated yields after TLC purification: 14–42%.

Table 3.
The physicochemical parameters of the triazole derivatives (10a–c).
3. Materials and Method
3.1. LC-MS Analysis, TLC, and Preparative TLC
Liquid chromatography-mass spectrometry (LC-MS) spectra were recorded on an Agilent 1100/ZQMSD (Santa Clara, CA, USA) instrument equipped with UV (220 nm and 254 nm) and MSAP+ and PL-ELS detectors. TLC was carried out using Kieselgel 60 F254 (Merck 1.05554.0001, Merck KGaA, Darmstadt, Germany). The analytical samples for NMR and HRMS studies were purified by preparative TLC using Kieselgel 60 F254 (Merck 1.07748.1000, Merck KGaA, Darmstadt, Germany) coated glass plates.
3.2. NMR Spectroscopy
NMR measurements were performed on a Varian VNMRS 400 MHz (Varian, Palo Alto, CA, USA) NMR spectrometer equipped with a 15N-31P(1H-19F) 5 mm one NMR room temperature probe, a Varian VNMRS 500 MHz NMR spectrometer equipped with a 1H (13C/15N) 5 mm pulsed field gradient (PFG) Triple Resonance 13C Enhanced Cold Probe, a Varian VNMRS 800 MHz NMR spectrometer equipped with a 1H(13C/15N) Triple Resonance 13C Enhanced Salt Tolerant Cold Probe (Varian, Inc., Palo Alto, CA, USA), a Bruker Avance III HDX 500 MHz NMR spectrometer equipped with a 1H (13C/15N) 5 mm TCI CryoProbe, and a Bruker Avance III HDX 800 MHz NMR spectrometer equipped with a 1H-19F(13C/15N) 5 mm TCI CryoProbe (Bruker Corporation, Billerica, MA, USA). 1H and 13C chemical shifts are given on the delta scale as parts per million (ppm) with tetramethylsilane (TMS) (1H, 13C) or dimethylsulfoxide-d6(13C) as the internal standard (0.00 ppm and 39.4 ppm, respectively). 1H-1H, direct 1H-13C, and long-range 1H-13C scalar spin-spin connectivity were established from 2D. correlation spectroscopy, total correlation spectroscopy, heteronuclear single quantum coherence and heteronuclear multiple bond correlation (COSY, TOCSY, HSQC, and HMBC) experiments. 1H-1H spatial proximities were determined using two-dimensional nuclear Overhauser effect spectroscopy or rotating-frame Overhauser effect spectroscopy (NOESY or ROESY) experiments. 15N Chemical shifts were referenced to nitromethane (0.0 ppm) and were obtained from 1H-15N HMBC measurements. All pulse sequences were applied using the standard spectrometer software package. All experiments were performed at 298 K. NMR spectra were processed using VnmrJ 2.2 Revision C (Varian, Inc., Palo Alto, CA, USA), Bruker TopSpin 3.5 pl 6 (Bruker Corporation, Billerica, MA, USA) and ACD/Spectrus Processor version 2017.1.3 (Advanced Chemistry Development, Inc., Toronto, ON, Canada).
3.3. Mass Spectrometry
HRMS and MS-MS analyses were performed on a Thermo Velos Pro Orbitrap Elite (Thermo Fisher Scientific, Bremen, Germany) system. The ionization method was ESI operated in a positive ion mode. The protonated molecular ion peaks were fragmented by collisison-induced dissociation (CID) at a normalized collision energy of 35%. For the CID experiment helium was used as the collision gas. The samples were dissolved in methanol. Data acquisition and analysis were accomplished with Xcalibur software version 2.0 (Thermo Fisher Scientific, Bremen, Germany).
3.4. Synthesis of the Starting Materials
The preparation of the starting pyridazinone derivatives (4a–e and 5a,b) was described earlier [24,25].
3.5. General Procedure for the Synthesis of the Pyridazine-Thiones (6a–e and 7a,b)
The pyridazinone derivatives (4a–e and 5a,b) (1.0 mmol) were dissolved in toluene (60 mL) and phosphorus pentasulfide (0.67 g, 3.0 mmol) was added. The reaction mixture was stirred at reflux for 6–8 h. After the completion of the reaction, the mixture was washed with 5% NaHCO3 solution (80 mL), and then with distilled water (80 mL). The organic layer was dried over MgSO4 and then concentrated. The crude product was purified by preparative thin layer chromatography (eluent: heptane:dichloromethane:methanol/5:5:1) to give the thioxo derivatives as a yellow solids (6a–e and 7a,b).
4-(4-Methoxyphenyl)-2,3-diazaspiro[5.5]undec-3-ene-1-thione (6a). Yield = 74%, Rf(heptane:dichloromethane:methanol/5:5:1) = 0.60, 1H-NMR (499.9 MHz; CDCl3) δ = 1.33–2.04 (m, 10H, cyclohexyl); 2.79 (s, 2H; H2-5); 3.86 (s, 3H; C(4′)-OCH3); 6.97 (m, 2H; H-3′, H-5′); 7.78 (m, 2H; H-2′, H-6′); 10.28 (br s, 1H; NH-2) ppm; 13C-NMR (125.7 MHz; CDCl3) δ = 20.2 (C-8, C-10); 25.4 (C-9); 27.6 (C-5); 33.44 (C-7, C-11); 41.14 (C-6); 55.45 (C(4′)-OCH3); 114.22 (C-3′, C-5′); 127.74 (C-2′, C-6′); 127.97 (C-1′); 154.54 (C-4), 161.61 (C-4′); 204.34 (C-1) ppm; HRMS: M + H = 289.13657 (delta = −1.2 ppm; C16H21ON2S). HR-ESI-MS-MS (CID = 35%; rel. int. %): 272(4); 255(100); 255(46); 241(4); 166(8); 156(10); 134(6); 121(6).
1-(4-Metoxyphenyl)-2,3-diazaspiro[5.5]undec-1-ene-4-thione (6e). Yield = 14%, Rf(heptane:dichloromethane:methanol/5:5:1) = 0.40, 1H NMR (399.8 MHz; CDCl3) δ = 1.10–1.24 (m; 1H; Hax-9); 1.45–1.60 (m; 4H; H2-8, H2-10); (br s; 1H; NH-3); 1.61–1.68 (m; 4H; H2-7, H2-11); 1.69–1.75 (m; 1H; Heq-9); 3.08 (s; 2H; H2-5); 3.84 (s; 3H; C(4′)-OCH3); 6.90–6.95 (m; 2H; H-3′, H-5′); 7.30–7.35 (m; 2H; H-2′, H-6′); 10.05 (br s; 1H; NH-3) ppm; 13C NMR (100.5 MHz; CDCl3) δ = 20.2 (C-8, C-10); 25.4 (C-9); 31.4 (C-7, C-11); 36.8 (C-6); 42.7 (C-5); 55.4 (C(4′)-OCH3); 113.7 (C-3′, C-5′); 127.5 (C-1′); 129.5 (C-2′, C-6′); 160.4 (C-4′); 165.8 (C-1); 193.9 (C-4) ppm; HRMS: M + H = 289.13769 (delta = 2.7 ppm; C16H21ON2S). MS-MS (CID = 45%; rel. int. %): 272(6); 257(6); 255(11); 189(38); 181(39); 121(100); 113(12).
4-(p-Tolyl)-2,3-diazaspiro[5.5]undec-3-ene-1-thione (6b). Yield = 41%, Rf(heptane:dichloromethane:methanol/5:5:1) = 0.60, LC-MS: UV (220 nm) (M + H = 273): 100%, UV (254 nm) (M + H = 273): 100%, 1H NMR (499.9 MHz; DMSO-d6) δ = 1.16–1.84 (m, 10H, cyclohexyl); 2.35 (s, 3H; C(4′)-CH3); 2.83 (s, 2H; H2-5); 7.29 (m, 2H; H-3′, H-5′); 7.82 (m, 2H; H-2′, H-6′); 12.69 (br s, 1H; NH-2) ppm; 13C-NMR (125.7 MHz; DMSO-d6) δ = 20.35 (C-8, C-10); 20.86 (C(4′)-CH3); 25.26 (C-9); 26.39 (C-5); 32.75 (C-7, C-11); 40.05 (C-6); 125.93 (C-2′, C-6′); 129.28 (C-3′, C-5′); 132.68 (C-1′); 140.12 (C-4′); 154.26 (C-4); 202.78 (C-1) ppm; HRMS: M + H = 273.14235 (delta = −1.6 ppm; C16H21N2S). MS-MS (CID = 45%; rel. int. %): 256(5); 241(53); 240(100); 239(75); 225(4); 186(4); 171(5); 166(11); 159(18); 156(12); 133(6); 118(5).
4-(4-Chlorophenyl)-2,3-diazaspiro[5.5]undec-3-ene-1-thione (6c). Yield = 55%, Rf(heptane:dichloromethane:methanol/5:5:1) = 0.66, LC-MS: UV (220 nm) (M + H = 293): 100%, UV (254 nm) (M + H = 293): 100%, 1H NMR (499.9 MHz; DMSO-d6) δ = 1.16–1.84 (m, 10H, cyclohexyl); 2.86 (s, 2H; H2-5); 7.54 (m, 2H; H-3′, H-5′); 7.95 (m, 2H; H-2′, H-6′); 12.78 (brbr s, 1H; NH-2) ppm; 13C-NMR (125.7 MHz; DMSO-d6) δ = 20.28 (C-8, C-10); 25.23 (C-9); 26.33 (C-5); 32.85 (C-7, C-11); 40.05 (C-6) 127.76 (C-2′, C-6′); 128.72 (C-3′, C-5′); 134.31 (C-1′); 134.87 (C-4′); 153.11 (C-4); 203.20 (C-1) ppm; HRMS: M + H = 293.08752 (delta = 0.5 ppm; C15H18N2ClS). HR-ESI-MS-MS (CID = 45%; rel. int. %): 276(5); 259(100); 191(23); 179(28); 166(6); 154(5).
4-(3,4-Dimethoxyphenyl)-2,3-diazaspiro[5.5]undec-3-ene-1-thione (6d). Yield = 40%, Rf(heptane:dichloromethane:methanol/5:5:1) = 0.40, 1H NMR (499.9 MHz; CDCl3) δ = 1.27–1.35 (m; 1H; Hax-9); 1.35–1.45 (m; 2H; Hax-8, Hax-10); 1.50–1.56 (m; 2H; Heq-7, Heq-11); 1.67–1.75 (m; 3H; Heq-8, Heq-10, Heq-9); 2.01–2.08 (m; 2H; Hax-7, Hax-11); 2.79 (s; 2H; H2-5); 3.94 (s; 3H; C(4′)-OCH3); 3.95 (s; 3H; C(3′)-OCH3); 6.91 (d, J = 8,4 Hz; 1H; H-5′); 7.29 (dd, J = 8.4, 2.1 Hz; 1H; H-6′); 7.50 (d, J = 2.1 Hz; 1H; H-2′); 10.11 (br s; 1H; NH-2) ppm; 13C-NMR (125.7 MHz; CDCl3) δ = 21.2 (C-8, C-10); 25.4 (C-9); 27.4 (C-5); 33.5 (C-7, C-11); 41.2 (C-6); 55.99, 56.02 (C(3′)-OCH3, C(4′)-OCH3); 108.1 (C-2′); 110.5 (C-5′); 119.8 (C-6′); 128.2 (C-1′); 149.4 (C-3′); 151.5 (C-4′); 154.4 (C-4); 204.6 (C-1) ppm; HRMS: M + H = 319.14735 (delta = −0.39 ppm; C17H23O2N2S). MS-MS (CID = 65%; rel. int. %): 304(5); 302(5); 287(100); 286(75); 285(45); 271(10).
9-(4-Methoxyphenyl)-7,8-diazaspiro[4.5]dec-8-ene-6-thione (7a). Yield = 71%, Rf(heptane:dichloromethane:methanol/5:5:1) = 0.40, 1H NMR (499.9 MHz; CDCl3) δ = 1.56–1.62 (m; 2H; Hx-1, Hx-4); 1.68–1.77 (m; 2H; Hx-2, Hx-3); 1.87–1.96 (m; 2H; Hy-2, Hy-3); 2.28–2.35 (m; 2H; Hy-1, Hy-4); 2.68 (s; 2H; H2-10); 3.86 (s; 3H; C(4′)-OCH3); 6.93–6.97 (m; 2H; H-3′, H-5′); 7.71–7.75 (m; 2H; H-2′, H-6′); 10.13 (br s; 1H; NH-7) ppm; 13C-NMR (125.7 MHz; CDCl3) δ = 25.5 (C-2, C-3); 32.9 (C-10); 38.8 (C-1, C-4); 48.9 (C-5); 55.5 (C(4′)-OCH3); 114.2 (C-3′, C-5′); 127.7 (C-2′, C-6′); 128.0 (C-1′); 155.2 (C-9); 161.6 (C-4′); 204.2 (C-6) ppm; HRMS: M + H = 275.12072 (delta = −1.9 ppm; C15H19ON2S). MS-MS (CID = 45%; rel. int. %): 259(3); 242(100); 227(5); 201(6); 152(7); 142(7); 134(3).
9-(3,4-Dimethoxyphenyl)-7,8-diazaspiro[4.5]dec-8-ene-6-thione (7b). Yield = 89%, Rf(heptane:dichloromethane:methanol/5:5:1) = 0.40, 1H-NMR (499.9 MHz; CDCl3) δ = 1.57–1.63 (m; 2H; Hx-1, Hx-4); 1.69–1.78 (m; 2H; Hx-2, Hx-3); 1.87–1.96 (m; 2H; Hy-2, Hy-3); 2.29–2.36 (m; 2H; Hy-1, Hy-4); 2.69 (s; 2H; H2-10); 3.93 (s; 3H), 3.95 (s; 3H): C(3′)-OCH3, C(4′)-OCH3; 6.89 (d, J = 8.4 Hz; 1H; H- 5′); 7.23 (dd, J = 8.4, 2.0 Hz; 1H; H-6′); 7.47 (d, J = 2.0 Hz; 1H; H-2′); 10.23 (br s; 1H; NH-7) ppm; 13C-NMR (125.7 MHz; CDCl3) δ = 25.5 (C-2, C-3); 32.8 (C-10); 38.8 (C-1, C-4); 49.0 (C-5); 55.98, 56.02 (C(3′)-OCH3, C(4′)-OCH3); 108.1 (C-2′); 110.5 (C-5′); 119.8 (C- 6′); 128.2 (C-1′); 149.3 (C-3′); 151.4 (C-4′); 155.1 (C-9); 204.2 (C-6) ppm; HRMS:M + H = 305.13149 (delta = −1.10 ppm; C16H21O2N2S). MS-MS (CID = 35%; rel. int. %): 288(5); 273(100); 272(80); 271(38); 257(12); 231(18); 164(8); 138(7).
3.6. General Procedure for the Preparation of Hydrazones (8a–c)
A solution of hydrazine monohydrate (0.10 mL, 1.2 mmol) in THF (5 mL) was added to the corresponding pyridazinone derivatives (6a–c) (0.40 mmol) in THF (15 mL) dropwise. The reaction mixture was stirred at reflux for 5 h, then the solvent was evaporated. The residue was dissolved in dichloromethane (20 mL) and washed with distilled water (2 × 10 mL). The organic layer was dried over MgSO4, filtered and evaporated. The crude product was purified by preparative thin layer chromatography (eluent: heptane:dichloromethane:methanol/5:5:1) to give the hydrazones (8a–c).
1-Hydrazono-4-(4-Methoxyphenyl)-2,3-diazaspiro[5.5]undec-3-ene (8a). Yield: 48%, Rf(heptane:dichloromethane:methanol/5:5:1) = 0.30, 1H NMR (499.9 MHz; DMSO-d6) δ = 1.20–1.64 (m, 10H, cyclohexyl); 2.62 (s, 2H, H-5); 3.78 (s, 3H, C(4′)-OCH3); 5.01 (br s, 2H, NH2-2″); 6.95 (m, 2H, H-3′, H-5′); 7.69 (m, 2H, H-2′, H-6′); 9.32 (br s, 1H, NH-2) ppm; 13C-NMR (125.7 MHz; DMSO-d6) δ = 20.7 (C(4′)-CH3); 21.1 (C-8, C-10); 25.7(C-9); 31.0 (C-5); 32.6 (C-6); 33.0 (C-7, C-11); 55.1 (C(4′)-OCH3); 113.7 (C-3′, C-5′); 126.0 (C-2′, C-6′);130.2 (C-1′); 142.8 (C-4); 144.8 (C-1); 159.2 (C-4′) ppm; HRMS: M + H = 287.18646 (delta = −0.6 ppm; C16H23ON4). HR-ESI-MS-MS (CID = 35%; rel. int. %): 270(99); 255(21); 242(47); 228(22); 216(13); 186(21); 164(100); 148(10); 133(19); 121(23).
1-Hydrazono-4-(p-tolyl)-2,3-diazaspiro[5.5]undec-3-ene (8b). Yield: 59%, Rf(heptane:dichloromethane:methanol/5:5:1) = 0.27. 1H-NMR (499.9 MHz; DMSO-d6) δ = 1.18–1.72 (m, 10H, cyclohexyl); 2.31 (s, 3H, C(4′)-CH3); 2.62 (s, 2H, H-5); 5.07 (br s, 2H, NH2-2″); 7.19 (m, 2H, H-3′, H-5′); 7.64 (m, 2H, H-2′, H-6′); 9.39 (br s, 1H, NH-2) ppm; 13C NMR (125.7 MHz; DMSO-d6) δ = 21.1 (C-8, C-10); 25.7(C-9); 31.1 (C-5); 32.5 (C-6); 33.0 (C-7, C-11); 124.6 (C-2′, C-6′); 128.9 (C-3′, C-5′); 134.9 (C-1′); 137.3 (C-4′); 142.8 (C-4); 144.6 (C-1) ppm; HRMS: M + H = 271.19165 (delta = −0.3 ppm; C16H23N4). HR-ESI-MS-MS (CID = 35%; rel. int. %): 254(70); 239(45); 226(54); 212(40); 181(29); 164(100); 147(67); 131(27); 117(59); 105(24).
4-(4-Chlorophenyl)-1-hydrazono-2,3-diazaspiro[5.5]undec-3-ene (8c). Yield = 61%, Rf(heptane:dichloromethane:methanol/5:5:1) = 0.50, 1H-NMR (499.9 MHz; DMSO-d6) δ = 1.25–1.58 (m, 10H, cyclohexyl); 2.64 (s, 2H, H-5); 5.12 (br s, 2H, NH2-2″); 7.43 (m, 2H, H-3′, H-5′); 7.77 (m, 2H, H-2′, H-6′); 9.57 (br s, 1H, NH-2) ppm; 13C-NMR (125.7 MHz; DMSO-d6) δ = 21.1 (C-8, C-10); 25.7(C-9); 31.0 (C-5); 32.4 (C-6); 33.0 (C-7, C-11); 126.3 (C-2′, C-6′); 128.3 (C-3′, C-5′); 132.4 (C-4′); 136.5 (C-1′); 141.4 (C-4); 144.0 (C-1) ppm; HRMS: M + H = 291.13630 (delta = −2.8 ppm; C15H20N4Cl). HR-ESI-MS-MS (CID = 45%; rel. int. %): 274(38); 261(42); 259(46); 246(26); 232(35); 220(21); 204(24); 201(22); 190(100); 167(26); 164(52); 152(22); 137(82); 125(29).
3.7. Preparation of Tetrazolo-Pyridazines (9a–c)
A solution of sodium nitrite (35.2 mg, 0.51 mmol) in water (3.8 mL) was added dropwise to a suspension of (8a–c) (100 mg, 0.34 mmol) in 0.5 N HCI (1.22 mL) at 5 °C. After stirring for 2 h, the reaction mixture was neutralized with a saturated solution of NaHCO3 (0.40 mL) and the precipitate was filteredand it was purified by preparative thin layer chromatography (eluent: heptane:dichloromethane:methanol/5:5:1) to give the tetrazolo-pyridazines (9a–c).
6′-(4-Methoxyphenyl)-7′H-spiro[cyclohexane-1,8′-tetrazolo[1,5-b]pyridazine] (9a). Yield = 66%, LC-MS: UV (220 nm) (M + H = 298): 100%, UV (254 nm) (M + H = 298): 100%, Rf(heptane:dichloromethane:methanol/5:5:1) = 0.42, 1H NMR (499.9 MHz; DMSO-d6) δ = 1.36–1.85 (m, 10H, cyclohexyl); 3.27 (s, 2H, H-7′); 3.87 (s, 3H, C(4″)-OCH3); 7.12 (m, 2H, H-3″, H-5″); 8.08 (m, 2H, H-2″, H-6″) ppm; 13C NMR (125.7 MHz; DMSO-d6) δ = 20.98 (C-2, C-5); 24.85 (C-4); 32.28 (C-1 = C-8′); 33.44 (C-2, C-6); 34.98 (C-7′); 114.34 (C-3″, C-5″); 125.73 (C-1″); 129.44 (C-2″, C-6″); 149.62 (C-9′);162.59 (C-4″); 164.16 (C-6′) ppm; HRMS: M + H = 298.16613 (delta = −0.4 ppm; C16H20ON5). HR-ESI-MS-MS (CID = 40%; rel. int. %): 270(100); 242(73); 227(6); 215(8); 200(4); 174(19); 162(27); 160(18); 133(5); 121(4).
6′-(p-Tolyl)-7′H-spiro[cyclohexane-1,8′-tetrazolo[1,5-b]pyridazine] (9b). Yield = 45%, LC-MS: UV (220 nm) (M + H = 282): 100%, UV (254 nm) (M + H = 282): 100% Rf(heptane:dichloromethane:methanol/5:5:1) = 0.42, 1H-NMR (499.9 MHz; DMSO-d6) δ = 1.36–1.86 (m, 10H, cyclohexyl); 2.41 (s, 3H, C(4″)-CH3); 3.29 (s, 2H, H-7′); 7.40 (m, 2H, H-3″, H-5″); 8.00 (m, 2H, H-2″, H-6″) ppm; 13C-NMR (125.7 MHz; DMSO-d6) δ = 20.97 (C-2, C-5, C(4″)-CH3); 24.83 (C-4); 32.28 (C-1 = C-8′); 33.48 (C-2, C-6); 35.13 (C-7′); 129.51 (C-3″, C-5″); 127.44 (C-2″, C-6″); 130.80 (C-1″); 142.69 (C-4″); 149.73 (C-9′); 164.72 (C-6′) ppm; HRMS: M + H = 282.17121 (delta = −0.4 ppm; C16H20N5). HR-ESI-MS-MS (CID = 40%; rel. int. %): 254(100); 226(64); 209(7); 184(4); 170(12); 158(9); 146(17); 144(14); 117(6).
6′-(4-Chlorophenyl)-7′H-spiro[cyclohexane-1,8′-tetrazolo[1,5-b]pyridazine] (9c). Yield = 77%, LC-MS: UV (220 nm) (M + H = 302): 97%, UV (254 nm) (M + H = 302): 98% Rf(heptane:dichloromethane:methanol/5:5:1) = 0.42, 1H NMR (499.9 MHz; DMSO-d6) δ = 1.40–1.82 (m, 10H, cyclohexyl); 3.22 (s, 2H, H-7′); 7.60 (m, 2H, H-3″, H-5″); 8.12 (m, 2H, H-2″, H-6″) ppm; 13C NMR (125.7 MHz; DMSO-d6) δ = 20.91 (C-2, C-5); 24.80 (C-4); 32.29 (C-1 = C-8′); 33.56 (C-2, C-6); 35.13 (C-7′); 129.01 (C-3″, C-5″); 129.29 (C-2″, C-6″); 132.40 (C-1″); 137.19 (C-4″); 149.70 (C-9′); 163.95 (C-6′) ppm; HRMS: M + H = 302.11716 (delta = 1.5 ppm; C15H17N5Cl). MS-MS (CID = 40%; rel. int. %): 274(100); 246(44); 229(6); 211(13); 190(23); 178(8); 166(13); 164(21); 137(6).
3.8. General Procedure for the Preparation of 6-(4-Substituted-phenyl)-3-phenyl-7H-spiro[[1,2,4]triazolo[4,3-b]pyridazin-8,1′-cyclohexanes] (10a–c)
Benzhydrazide (11) (300 mg, 2.2 mmol) was added to the appropriate thioxo derivative (6a–c) (0.73 mmol) in n-BuOH (25 mL). The reaction mixture was refluxed until the starting material disappeared (84 h). The solvent was evaporated, then the residue was taken up in dichloromethane (15 mL). It was washed with 1% (w/w) aqueous HCl solution (2 × 10 mL), and then with water (10 mL). The organic layer was dried over MgSO4, then filtered and evaporated. The crude product was purified by preparative thin layer chromatography (eluent: heptane:dichloromethane:methanol/5:5:1) to give the triazole derivatives (10a–c).
6-(4-Methoxyphenyl)-3-phenyl-7H-spiro[[1,2,4]triazolo[4,3-b]pyridazin-8,1′-cyclohexane] (10a). Yield = 29%, LC-MS: UV (220 nm) (M + H = 373): 93%, UV (254 nm) (M + H = 373): 98%, Rf(heptane:dichloromethane:methanol/5:5:1) = 0.32, 1H-NMR (799.7 MHz; DMSO-d6) δ = 1.45–1.85 (m, 10H, cyclohexyl); 3.14 (s, 2H, H-7); 3.85 (s, 3H, C(4″)-CH3); 7.10 (m, 2H, H-3″, H-5″); 7.53 (m, 1H, H-4‴); 7.57 (m, 2H, H-3‴, H-5‴); 8.01 (m, 2H, H-2″, H-6″); 8.12 (m, 2H, H-2‴, H-6‴) ppm; 13C-NMR (201.1 MHz; DMSO-d6) δ = 21.14 (C-3′, C-5′); 25.17 (C-4′); 32.65 (C-1′ = C-8); 33.63 (C-2′, C-6′); 34.07 (C-7); 55.39 (C(4″)-CH3); 114.25 (C-3″, C-5″); 126.59 (C-1‴); 127.03 (C-1″); 127.79 (C-2‴, C-6‴); 128.58 (C-3‴, C-5‴); 128.80 (C-2″, C-6″); 129.70 (C-4‴); 148.97 (C-3); 151.18 (C-9); 161.23 (C-6); 161.86 (C-4″) ppm; HRMS: M + H = 373.20153 (delta = −2.0 ppm; C23H25ON4). HR-ESI-MS-MS (CID = 40%; rel. int. %): 226(100); 148(11).
3-Phenyl-6-(p-tolyl)-7H-spiro[[1,2,4]triazolo[4,3-b]pyridazin-8,1′-cyclohexane] (10b). Yield = 14%, LC-MS: UV (220 nm) (M + H = 357): 87%, UV (254 nm) (M + H = 357): 85%, Rf(heptane:dichloromethane:methanol/5:5:1) = 0.32, 1H-NMR (499.9 MHz; DMSO-d6) δ = 1.41–1.89 (m, 10H, cyclohexyl); 2.39 (s, 3H, C(4″)-CH3); 3.16 (s, 2H, H-7); 7.37 (m, 2H, H-3″, H-5″); 7.54 (m, 1H, H-4‴); 7.58 (m, 2H, H-3‴, H-5‴); 7.93 (m, 2H, H-2″, H-6″); 8.12 (m, 2H, H-2‴, H-6‴) ppm; 13C NMR (125.7 MHz; DMSO-d6) δ = 20.95 (C(4″)-CH3); 21.14 (C-3′, C-5′); 25.18 (C-4′); 32.65 (C-1′ = C-8); 33.67 (C-2′, C-6′); 34.21 (C-7); 126.52 (C-1‴); 126.98 (C-2″, C-6″); 127.85 (C-2‴, C-6‴); 128.60 (C-3‴, C-5‴); 129.44 (C-3″, C-5″); 129.75 (C-4‴); 132.09 (C-1″); 141.58 (C-4″); 149.09 (C-3); 151.21 (C-9); 161.73 (C-6) ppm; HRMS: M + H = 357.20934 (delta = 5.5 ppm; C23H25N4). HR-ESI-MS-MS (CID = 35%; rel. int. %): 226(100); 132(8).
6-(4-Chlorophenyl)-3-phenyl-7H-spiro[[1,2,4]triazolo[4,3-b]pyridazin-8,1′-cyclohexane] (10c). Yield = 42%, LC-MS: UV (220 nm) (M + H = 377): 100%, UV (254 nm) (M + H = 377): 99%, Rf(heptane:dichloromethane:methanol/5:5:1) = 0.36, 1H NMR (499.9 MHz; DMSO-d6) δ = 1.41–1.90 (m, 10H, cyclohexyl); 3.18 (s, 2H, H-7); 7.51–7.59 (m, 3H, H-3‴, H-4‴, H-5‴); 7.63 (m, 2H, H-3″, H-5″); 8.05 (m, 2H, H-2″, H-6″); 8.09 (m, 2H, H-2‴, H-6‴) ppm; 13C-NMR (125.7 MHz; DMSO-d6) δ = 21.10 (C-3′, C-5′); 25.15 (C-4′); 32.65 (C-1′ = C-8); 33.71 (C-2′, C-6′); 34.20 (C-7); 126.38 (C-1‴); 127.90 (C-2‴, C-6‴); 128.64 (C-3‴, C-5‴); 128.83 (C-2″, C-6″); 128.94 (C-3‴, C-5‴); 129.83 (C-4‴); 133.65 (C-1″); 136.25 (C-4″); 149.24 (C-3); 151.04 (C-9); 160.90 (C-6) ppm; HRMS: M + H = 377.15255 (delta = −0.5 ppm; C22H22N4Cl). HR-ESI-MS-MS (CID = 35%; rel. int. %): 226(100).
4. Conclusions
Herein, we soughtto expand the family of spiro[cycloalkane]pyridazinones with further derivatives by anellating the spiro[cyclohexane]pyridazinones and spiro[cyclopentane]pyridazinones with tetrazole or triazole rings. To this end, seven new 4-substituted phenyl-2,3-diazaspiro[5.5]undec-3-ene-1-thiones and nine substituted phenyl-7,8-diazaspiro[4.5]dec-8-ene-6-thiones were prepared by the reaction of the spiro[cycloalkane]pyridazinones with phosphorus pentasulfide. By treating the p-substituted 4-phenyl-2,3-diazaspiro[5.5]undec-3-ene-1-thiones (6a–c) with hydrazine hydrate, the desired hydrazones were obtained (8a–c) to give the tetrazolo-pyridazine after diazotization (9a–c). The reaction of the p-substituted phenyldiazaspiroundecene-thiones (6a–c) with benzhydrazide (11) yielded the triazolo-pyridazines (10a–c). Eventually two kinds of new ring systems were prepared: a tetrazolo-pyridazine and triazolo-pyridazines.
Supplementary Materials
The followings are available, Figure: NMR and HRMS spectrum.
Author Contributions
C.S.F. and H.B. planned the experiments. C.S.F. and G.R. carried out the experimental work. H.B. managed the project and wrote the paper. Á.S. measured the NMR spectra, M.D. performed the HRMS measurements. L.H. was the consultant. G.K. refined the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
C.S.F. is grateful for the support of the Gedeon Richter’s Centenárium Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in Supplementary information.
Acknowledgments
The authors are grateful to Zoltán Urbán and Klára Szlifka-Pethő for the LC-MS studies.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds prepared are available from the authors.
References
- Wermuth, C.G. Are pyridazines privileged structures? Med. Chem. Comm. 2011, 2, 935–941. [Google Scholar] [CrossRef]
- Castle, R.N. (Ed.) The Chemistry of Heterocyclic Compounds Pyridazines; Wiley: Hoboken, NJ, USA, 2009; Volume 28, ISBN 978-0-470-18849-1. [Google Scholar]
- Heinisch, G.; Holzer, W.; Kunz, F.; Langer, T.; Lukavsky, P.; Pechlaner, C.; Weissenberger, H. On the Bioisosteric Potential of Diazines: Diazine Analogues of the Combined Thromboxane A2 Receptor Antagonist and Synthetase Inhibitor Ridogrel. J. Med. Chem. 1996, 39, 4058–4064. [Google Scholar] [CrossRef] [PubMed]
- Asif, M. The Pharmacological Importance of Some Diazine Containing Drug Molecules. Sci. Online Pub. Trans. Org. Chem. 2014, 1, 1–17. [Google Scholar]
- Akhtar, W.; Shaquiquzzaman, M.; Akhter, M.; Verma, G.; Khan, M.F.; Alam, M.M. The therapeutic journey of pyridazinone. Eur. J. Med. Chem. 2016, 123, 256–281. [Google Scholar] [CrossRef]
- Seth, S.; Sharma, A.; Raj, D. Pyridazinones: A wonder nucleus with scaffold of pharmacological activities. Am. J. Biol. Pharm. Res. 2014, 1, 105–116. [Google Scholar]
- Dubey, S.; Bhosle, P.A. Pyridazinone: An important element of pharmacophore possessing broad spectrum of activity. Med. Chem. Res. 2015, 24, 3579–3598. [Google Scholar] [CrossRef]
- Bartlett, M.S.; Shaw, M.M.; Smith, J.W.; Meshnick, S.R. Efficacy of Sulfamethoxypyridazine in a Murine Model of Pneumocystiscarinii Pneumonia. Antimic. Agents Chemother. 1998, 42, 934–935. [Google Scholar] [CrossRef]
- Vigil-De Gracia, P.; Lasso, M.; Ruiz, E.; Vega-Malek, J.C.; De Mena, F.T.; Lopez, J.C. Severe hypertension in pregnancy: Hydralazine or labetalol: A randomized clinical trial. Eur. J. Obstet. Gynecol. Reproduc. Biol. 2006, 128, 157–162. [Google Scholar] [CrossRef]
- Papp, Z.; Édes, I.; Fruhwald, S.; De Hert, S.G.; Salmenperä, M.; Leppikangas, H.; Mebazaa, A.; Landoni, G.; Grossini, E.; Caimmi, P.; et al. Levosimendan: Molecular Mechanisms and Clinical Implications: Consensus of Experts on the Mechanisms of Action of Levosimendan. Int. J. Cardiol. 2012, 159, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Kan, J.P.; Mouget-Goniot, C.; Worms, P.; Biziere, K. Effect of the antidepressant minaprine on both forms of monoamine oxidase in the rat. Biochem. Pharm. 1986, 35, 973–978. [Google Scholar] [CrossRef]
- Contreras, J.M.; Rival, Y.M.; Chayer, S.; Bourguignon, J.J.; Wermuth, C.G. Aminopyridazines as acetylcholinesterase inhibitors. J. Med. Chem. 1999, 42, 730–741. [Google Scholar] [CrossRef]
- Aleeva, G.N.; Molodavkin, G.M.; Voronina, T.A. Comparison of antidepressant effects of azafan, tianeptine, and paroxetine. Bul. Exp. Biol. Med. 2009, 148, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Inskeep, P.B.; Reed, A.E.; Ronfeld, R.A. Pharmacokinetics of zopolrestat, a carboxylicacid aldose reductase inhibitor in normal and diabetic rats. Pharm. Res. 1991, 8, 1511–1515. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Infante, J.R.; Reckamp, K.L.; Blumenschein, G.R.; Leal, T.A.; Waqar, S.N.; Gitlitz, B.J.; Sanborn, R.E.; Whisenant, J.G.; Du, L.; et al. Ensartinib (X-396) in ALK-Positive Non-Small Cell Lung Cancer: Results from a First-in-Human PhaseI/II, Multicenter Study. Clin. Cancer Res. 2018, 24, 2771–2779. [Google Scholar] [CrossRef] [PubMed]
- Barra, F.; Seca, M.; Della Corte, L.; Giampaolino, P.; Ferrero, S. Relugolix for the treatment of uterine fibroids. Drugs Today 2019, 55, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S.; Kaye, S.B.; Yap, T.A. PARP inhibitors: The race is on. Br. J. Cancer 2016, 114, 713–715. [Google Scholar] [CrossRef]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Eng. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef]
- Li, D.B.; Rogers-Evans, M.; Carreira, E.M. Synthesis of novel azaspiro[3.4]octanes as multifunctional modules in drug discovery. Org. Lett. 2011, 13, 6134–6136. [Google Scholar] [CrossRef]
- Burkhard, J.A.; Guérot, C.; Knust, H.; Carreira, E.M. Expanding the Azaspiro[3.3]heptanes Family: Synthesis of Novel HighlyFunctionalized Building Blocks. Org. Lett. 2012, 14, 66–69. [Google Scholar] [CrossRef]
- Li, D.B.; Rogers-Evans, M.; Carreira, E.M. Construction of multifunctional modules for drug discovery: Synthesis of novelthia/oxa-azaspiro [3.4] octanes. Org. Lett. 2013, 15, 4766–4769. [Google Scholar] [CrossRef]
- Carreira, E.M.; Fessard, T.C. Four-membered ring-containing spirocycles: Synthetic strategies and opportunities. Chem. Rev. 2014, 144, 8257–8322. [Google Scholar] [CrossRef]
- Zheng, Y.; Tice, C.M.; Singh, S.B. The use of spirocyclic scaffolds in drug discovery. Bioorg. Med. Chem. Lett. 2014, 24, 3673–3682. [Google Scholar] [CrossRef]
- Sepsey Für, C.; Riszter, G.; Gerencsér, J.; Szigetvári, A.; Dékány, M.; Hazai, L.; Keglevich, G.; Bölcskei, H. Synthesis of Spiro[Cycloalkane-pyridazinones] with High Fsp3 Character. Lett. Drug Des. Discov. 2020, 17, 731–744. [Google Scholar]
- Sepsey Für, C.; Horvát, E.J.; Szigetvári, A.; Dékány, M.; Hazai, L.; Keglevich, G.; Bölcskei, H. Synthesis of Spiro[Cycloalkane-pyridazinones] with High Fsp3 Character Part 2. Lett. Org. Chem. 2021, 18, 373–381. [Google Scholar]
- Ozturk, T.; Ertas, E.; Mert, O. A Berzelius Reagent, Phosphorus Decasulfide (P4S10), in Organic Syntheses. Chem. Rev. 2010, 110, 3419–3478. [Google Scholar] [CrossRef]
- Chimirri, A.; Zappala, M.; Gitto, R.; Quartarone, S.; Bevacqua, F. Synthesis and structural features of 11H-Tetrazolo[1,5-c][2,3]benzodiazepines. Heterocycles 1999, 51, 1303–1309. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of five revolution. Drug Discov. Today Tech. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the OralBioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Lovering, F.; Bikker, J.; Humblet, C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756. [Google Scholar] [CrossRef]
- Lovering, F. Escape from Flatland 2: Complexity and promiscuity. Med. Chem. Commun. 2013, 4, 515–519. [Google Scholar] [CrossRef]
- Chimirri, A.; Bevacqua, F.; Gitto, R.; Quartarone, S.; Zappala, M.; De Sarro, A.; Maciocco, L.; Biggio, G.; De Sarro, G. Synthesis and anticonvulsant activity of new 11H-triazolo[4,5-c][2,3]benzodiazepines. Med. Chem. Res. 1999, 9, 203–212. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).