Antimicrobial Peptide Brevinin-1RL1 from Frog Skin Secretion Induces Apoptosis and Necrosis of Tumor Cells
Abstract
1. Introduction
2. Results
2.1. Synthesis, Purification and Characterization of Peptides
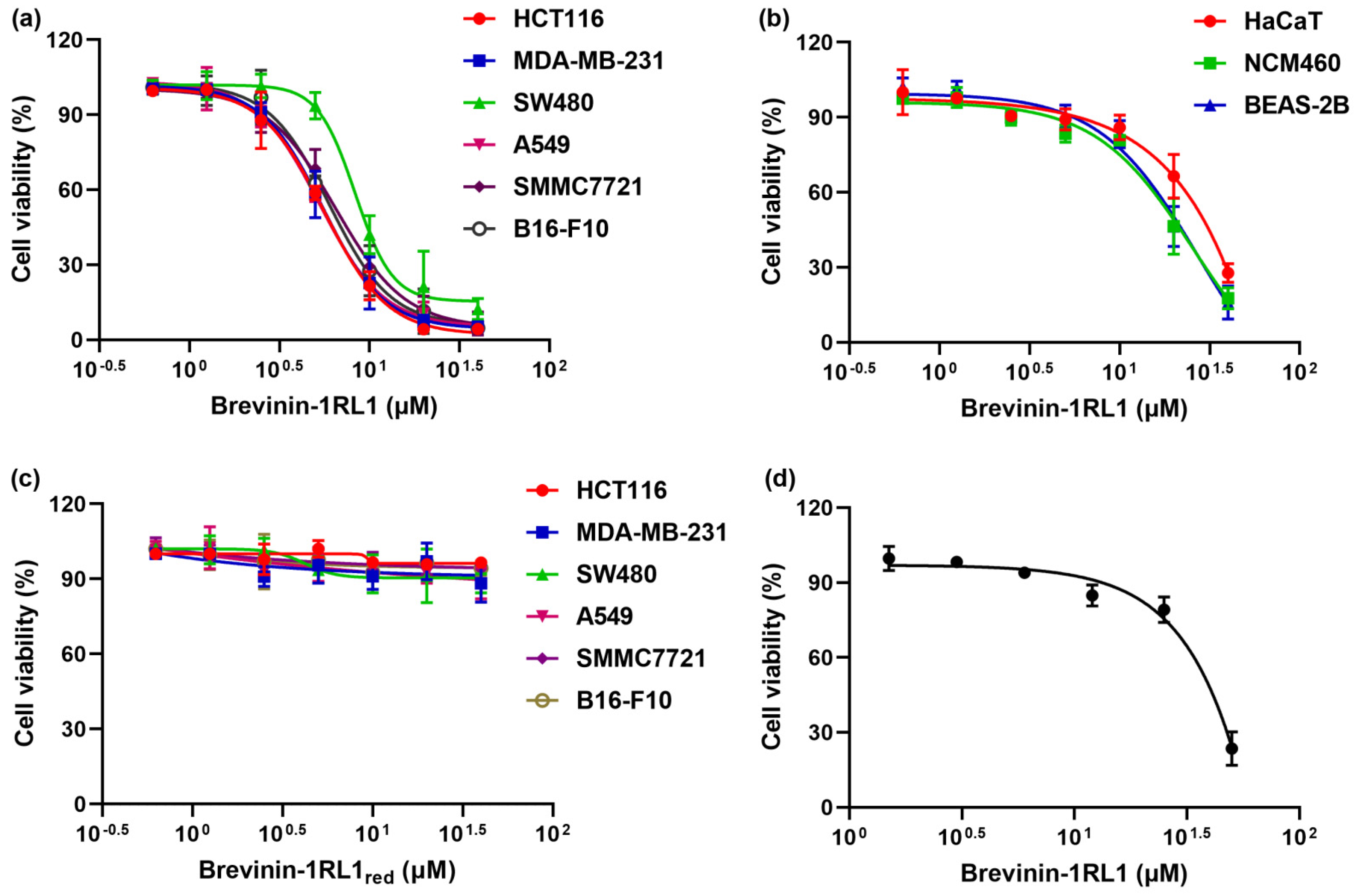
2.2. Brevinin-1RL1 Displays Cytotoxicity towards Tumor Cells with Moderate Hemolysis
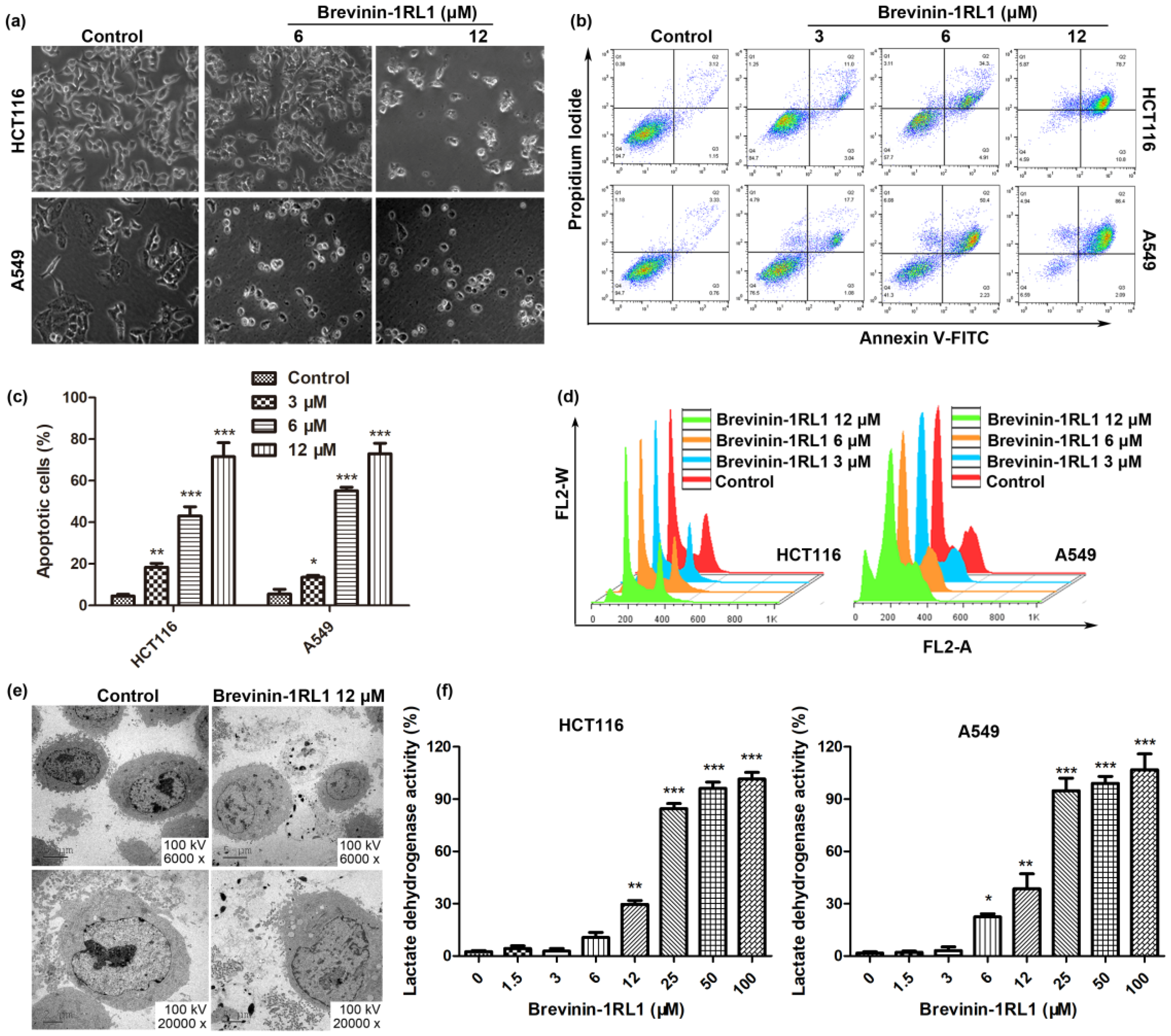
2.3. Brevinin-1RL1 Induces Cell Apoptosis and Necrosis
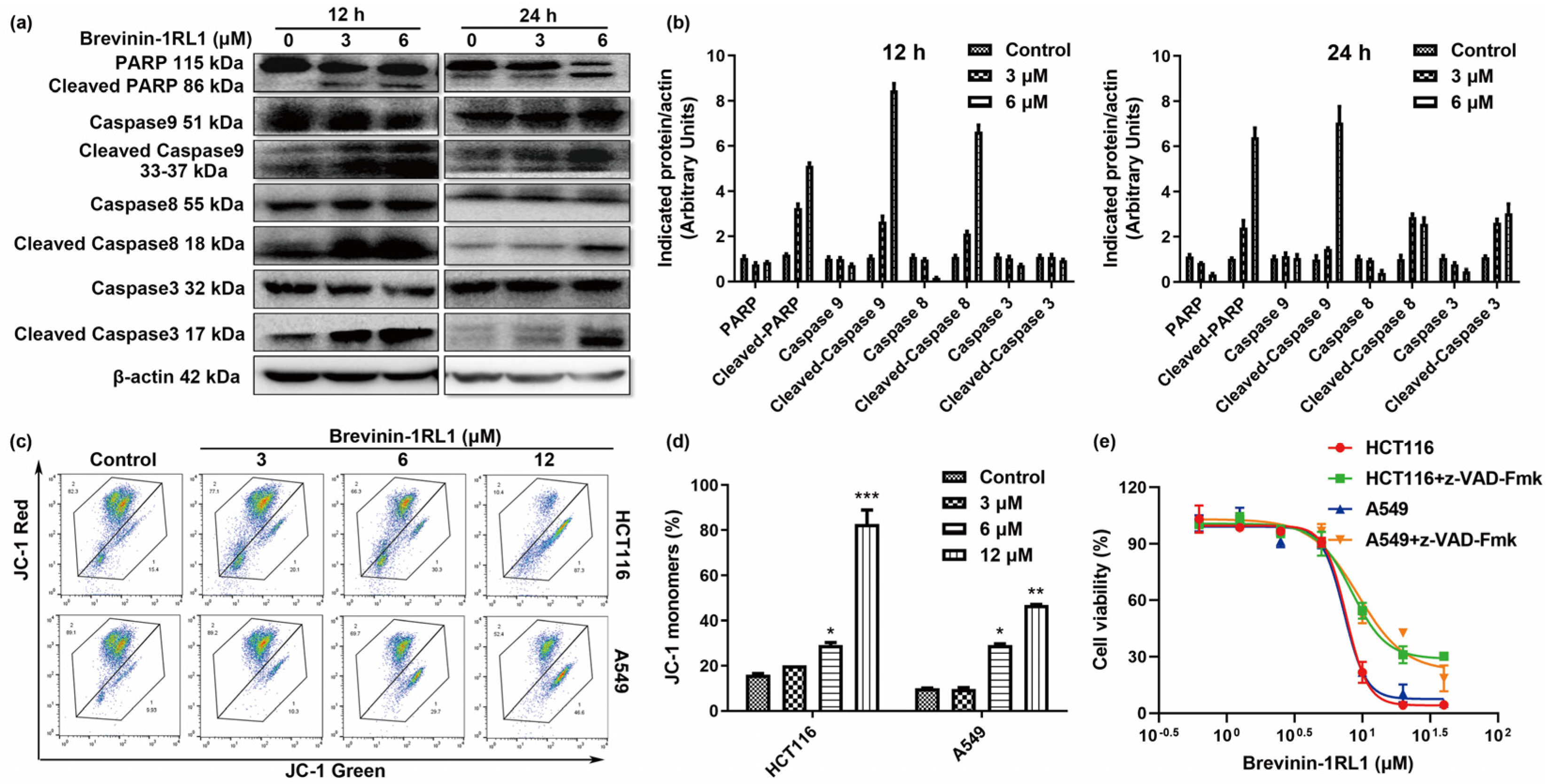
2.4. Brevinin-1RL1-Induced Cancer Cells Apoptosis Is Caspase-Dependent
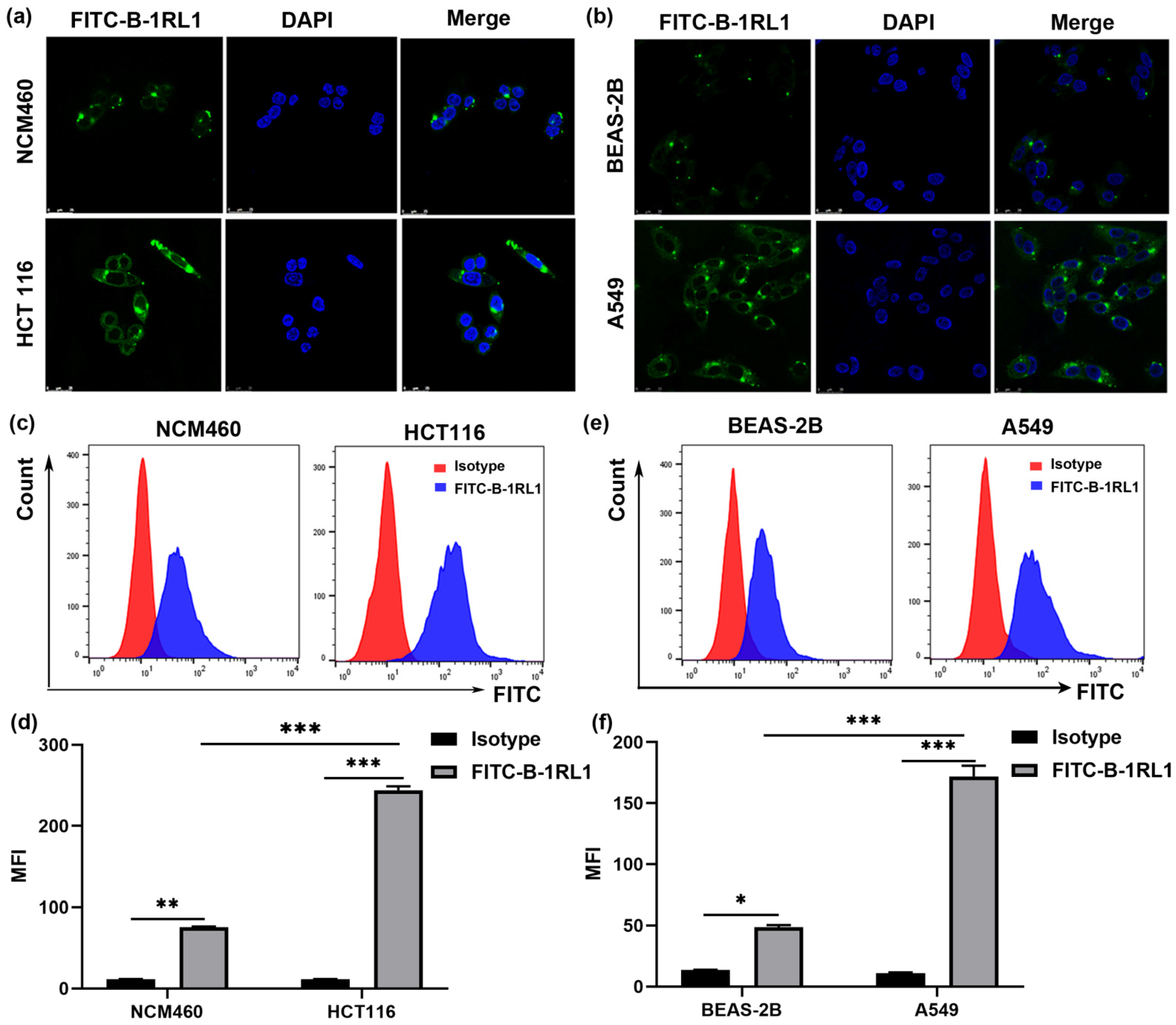
2.5. Brevinin-1RL1 Aggregates on the Surface of Tumor Cells
3. Discussion
4. Materials and Methods
4.1. Peptide Synthesis
4.2. Cell Culture
4.3. Cell Proliferation and Viability Assay
4.4. Hemolytic Activity
4.5. Cell Morphological Analysis
4.6. Lactate Dehydrogenase (LDH)-Release Assay
4.7. Analysis of Cell Cycle
4.8. Analysis of Cell Apoptosis
4.9. Mitochondrial Membrane Potential
4.10. Immunoblotting Analysis
4.11. Confocal Fluorescence Microscopy
4.12. TEM Assay
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Repetto, L.; Comandini, D.; Mammoliti, S. Life expectancy, comorbidity and quality of life: The treatment equation in the older cancer patients. Crit. Rev. Oncol. Hemat. 2001, 37, 147–152. [Google Scholar] [CrossRef]
- Stoletov, K.; Beatty, P.H.; Lewis, J.D. Novel therapeutic targets for cancer metastasis. Expert. Rev. Anticancer Ther. 2020, 20, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.; Beydoun, H.A.; Beydoun, M.A.; Barnes-Eley, M.; Davis, J.; Lance, R.; Schellhammer, P. Self-rated health as a tool for estimating health-adjusted life expectancy among patients newly diagnosed with localized prostate cancer: A preliminary study. Qual. Life Res. 2011, 20, 713–721. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luo, Y.C.; Ellis, L.Z.; Dallaglio, K.; Takeda, M.; Robinson, W.A.; Robinson, S.E.; Liu, W.M.; Lewis, K.D.; McCarter, M.D.; Gonzalez, R.; et al. Side Population Cells from Human Melanoma Tumors Reveal Diverse Mechanisms for Chemoresistance. J. Investig. Dermatol. 2012, 132, 2440–2450. [Google Scholar] [CrossRef]
- Vymetalkova, V.; Pardini, B.; Jiraskova, K.; Urbanova, M.; Levy, M.; Veskrnova, V.; Vodicka, P.; Naccarati, A. Repeated whole-exome sequencing for cell-free tumor DNA profiling of colon cancer patients: Searching for mechanisms of acquired chemoresistance. Cancer Res. 2018, 78, 1235. [Google Scholar]
- Hancock, R.E.W.; Diamond, G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000, 8, 402–410. [Google Scholar] [CrossRef]
- Wiesner, J.; Vilcinskas, A. Antimicrobial peptides: The ancient arm of the human immune system. Virulence 2010, 1, 440–464. [Google Scholar] [CrossRef]
- Giuliani, A.; Pirri, G.; Nicoletto, S.F. Antimicrobial peptides: An overview of a promising class of therapeutics. Cent. Eur. J. Biol. 2007, 2, 1–33. [Google Scholar] [CrossRef]
- Boman, H.G. Peptide Antibiotics and Their Role in Innate Immunity. Annu. Rev. Immunol. 1995, 13, 61–92. [Google Scholar] [CrossRef]
- Simmaco, M.; Mignogna, G.; Barra, D. Antimicrobial peptides from amphibian skin: What do they tell us? Biopolymers 1998, 47, 435–450. [Google Scholar] [CrossRef]
- Lazzaro, B.P.; Zasloff, M.; Rolff, J. Antimicrobial peptides: Application informed by evolution. Science 2020, 368, 487–493. [Google Scholar] [CrossRef]
- Deslouches, B.; Di, Y.P. Antimicrobial peptides with selective antitumor mechanisms prospect for anticancer applications. Oncotarget 2017, 8, 46635–46651. [Google Scholar] [CrossRef]
- Iwasaki, T.; Ishibashi, J.; Tanaka, H.; Sato, M.; Asaoka, A.; Taylor, D.; Yamakawa, M. Selective cancer cell cytotoxicity of enantiomeric 9-mer peptides derived from beetle defensins depends on negatively charged phosphatidylserine on the cell surface. Peptides 2009, 30, 660–668. [Google Scholar] [CrossRef]
- Schroder-Borm, H.; Bakalova, R.; Andra, J. The NK-lysin derived peptide NK-2 preferentially kills cancer cells with increased surface levels of negatively charged phosphatidylserine. FEBS Lett. 2005, 579, 6128–6134. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Liu, L.W.; Wang, K.R.; Song, J.J.; Yan, J.X.; Li, Z.Y.; Zhang, B.Z.; Wang, R. A novel analog of antimicrobial peptide Polybia-MPI, with thioamide bond substitution, exhibits increased therapeutic efficacy against cancer and diminished toxicity in mice. Peptides 2010, 31, 1832–1838. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, T.Y.; Zhang, N.; Yang, M.J.; Li, B.; Lu, X.; Cao, X.T.; Ling, C.Q. Melittin, a Major Component of Bee Venom, Sensitizes Human Hepatocellular Carcinoma Cells to Tumor Necrosis Factor-related Apoptosis-inducing Ligand (TRAIL)-induced Apoptosis by Activating CaMKII-TAK1-JNK/p38 and Inhibiting I kappa B alpha Kinase-NF kappa B. J. Biol. Chem. 2009, 284, 3804–3813. [Google Scholar] [PubMed]
- Han, Y.F.; Cui, Z.B.; Li, Y.H.; Hsu, W.H.; Lee, B.H. In Vitro and in Vivo Anticancer Activity of Pardaxin against Proliferation and Growth of Oral Squamous Cell Carcinoma. Mar. Drugs 2016, 14, 2. [Google Scholar] [CrossRef]
- Lehmann, J.; Retz, M.; Sidhu, S.S.; Suttmann, H.; Sell, M.; Paulsen, F.; Harder, J.; Unteregger, G.; Stockle, M. Antitumor activity of the antimicrobial peptide Magainin II against bladder cancer cell lines. Eur. Urol. 2006, 50, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Hsieh, C.L.; Bhansali, M.; Kannan, A.; Shemshedini, L. A Peptide against Soluble Guanylyl Cyclase alpha 1: A New Approach to Treating Prostate Cancer. PLoS ONE 2013, 8, e64189. [Google Scholar]
- Wang, Y.S.; Li, D.; Shi, H.S.; Wen, Y.J.; Yang, L.; Xu, N.; Chen, X.C.; Chen, X.; Chen, P.; Li, J.; et al. Intratumoral Expression of Mature Human Neutrophil Peptide-1 Mediates Antitumor Immunity in Mice. Clin Cancer Res 2009, 15, 6901–6911. [Google Scholar] [CrossRef]
- Chen, J.G.; Xu, X.N.; Underhfll, C.B.; Yang, S.M.; Wang, L.P.; Chen, Y.X.; Hong, S.G.; Creswell, K.; Zhang, L.R. Tachyplesin activates the classic complement pathway to kill tumor cells. Cancer Res. 2005, 65, 4614–4622. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.X.; Shen, J.; Cheng, A.S.L.; Lu, L.; Chan, R.L.Y.; Li, Z.J.; Wang, X.J.; Wong, C.C.M.; Zhang, L.; Ng, S.S.M.; et al. FK-16 Derived from the Anticancer Peptide LL-37 Induces Caspase-Independent Apoptosis and Autophagic Cell Death in Colon Cancer Cells. PLoS ONE 2013, 8, e63641. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Y.; Tu, Q.; Zhang, Z.; Chen, M.; Mwangi, J.; Li, Y.; Jin, Y.; Zhao, X.; Lai, R. Targeting surface nucleolin induces autophagy-dependent cell death in pancreatic cancer via AMPK activation. Oncogene 2019, 38, 1832–1844. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Lee, W.H.; Yang, X.W.; Zhang, Y. Novel Peptides from Skins of Amphibians Showed Broad-Spectrum Antimicrobial Activities. Chem. Biol. Drug Des. 2016, 87, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wu, Y.; Wang, T.; Chen, X.; Zhou, M.; Ma, C.; Xi, X.; Zhang, Y.; Chen, T.; Shaw, C.; et al. Brevinin-1GHd: A novel Hylarana guentheri skin secretion-derived Brevinin-1 type peptide with antimicrobial and anticancer therapeutic potential. Biosci. Rep. 2020, 40, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wodlej, C.; Riedl, S.; Rinner, B.; Leber, R.; Drechsler, C.; Voelker, D.R.; Choi, J.Y.; Lohner, K.; Zweytick, D. Interaction of two antitumor peptides with membrane lipids - Influence of phosphatidylserine and cholesterol on specificity for melanoma cells. PLoS ONE 2019, 14, e0211187. [Google Scholar] [CrossRef] [PubMed]
- Los, M.; Mozoluk, M.; Ferrari, D.; Stepczynska, A.; Stroh, C.; Renz, A.; Herceg, Z.; Wang, Z.Q.; Schulze-Osthoff, K. Activation and caspase-mediated inhibition of PARP: A molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol. Biol. Cell 2002, 13, 978–988. [Google Scholar] [CrossRef]
- Riedl, S.; Rinner, B.; Schaider, H.; Lohner, K.; Zweytick, D. Killing of melanoma cells and their metastases by human lactoferricin derivatives requires interaction with the cancer marker phosphatidylserine. Biometals 2014, 27, 981–997. [Google Scholar] [CrossRef]
- Green, D.; Kroemer, G. The central executioners of apoptosis: Caspases or mitochondria? Trends Cell Biol. 1998, 8, 267–271. [Google Scholar] [CrossRef]
- Conlon, J.M.; Mechkarska, M.; Lukic, M.L.; Flatt, P.R. Potential therapeutic applications of multifunctional host-defense peptides from frog skin as anti-cancer, anti-viral, immunomodulatory, and anti-diabetic agents. Peptides 2014, 57, 67–77. [Google Scholar] [CrossRef]
- Van Zoggel, H.; Hamma-Kourbali, Y.; Galanth, C.; Ladram, A.; Nicolas, P.; Courty, J.; Amiche, M.; Delbe, J. Antitumor and angiostatic peptides from frog skin secretions. Amino Acids 2012, 42, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Haney, E.F.; Hunter, H.N.; Matsuzaki, K.; Vogel, H.J. Solution NMR studies of amphibian antimicrobial peptides: Linking structure to function? Biochim. Biophys. Acta 2009, 1788, 1639–1655. [Google Scholar] [CrossRef]
- Morikawa, N.; Hagiwara, K.; Nakajima, T. Brevinin-1 and -2, unique antimicrobial peptides from the skin of the frog, Rana brevipoda porsa. Biochem. Biophys. Res. Commun. 1992, 189, 184–190. [Google Scholar] [CrossRef]
- Maurizio Simmaco, G.M. Donatella Barra and Francesco Bossa Novel antimicrobial peptides from skin secretion of the European frog Rana esculenta. FEBS Lett. 1993, 324, 159–161. [Google Scholar] [CrossRef]
- Goraya, J.; Knoop, F.C.; Conlon, J.M. Ranatuerins antimicrobial peptides isolated from the skin of the American bullfrog, Rana catesbeiana. Biochem. Biophys. Res. Commun. 1998, 250, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.P.; Durell, S.; Maloy, W.L.; Zasloff, M. Ranalexin. A novel antimicrobial peptide from bullfrog (Rana catesbeiana) skin, structurally related to the bacterial antibiotic, polymyxin. J. Biol. Chem. 1994, 269, 10849–10855. [Google Scholar] [CrossRef]
- Park, J.M.; Jung, J.E.; Lee, B.J. Antimicrobial peptides from the skin of a korean frog, rana rugosa. Biochem. Biophys. Res. Commun. 1994, 205, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Ahn, H.C.; Kim, S.S.; Lee, B.J. Structural study of novel antimicrobial peptides, nigrocins, isolated from rana nigromaculata. FEBS Lett. 2001, 507, 95–100. [Google Scholar] [CrossRef]
- Mangoni, M.L.; Papo, N.; Mignogna, G.; Andreu, D.; Shai, Y.; Barra, D. Ranacyclins, a new family of short cyclic antimicrobial peptides: Biological function, mode of action, and parameters involved in target specificity. Biochemistry 2003, 42, 14023–14035. [Google Scholar] [CrossRef]
- Kozic, M.; Vukicevic, D.; Simunic, J.; Roncevic, T.; Antcheva, N.; Tossi, A.; Juretic, D. Predicting the Minimal Inhibitory Concentration for Antimicrobial Peptides with Rana-Box Domain. J. Chem. Inf. Model. 2015, 55, 2275–2287. [Google Scholar] [CrossRef]
- Abraham, P.; Sundaram, A.; R, A.; V, R.; George, S.; Kumar, K.S. Structure-Activity Relationship and Mode of Action of a Frog Secreted Antibacterial Peptide B1CTcu5 Using Synthetically and Modularly Modified or Deleted (SMMD) Peptides. PLoS ONE 2015, 10, e0124210. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Cheng, P.; Ma, C.; Xi, X.; Wang, L.; Zhou, M.; Bian, H.; Chen, T. Evaluating the Bioactivity of a Novel Broad-Spectrum Antimicrobial Peptide Brevinin-1GHa from the Frog Skin Secretion of Hylarana guentheri and Its Analogues. Toxins 2018, 10, 413. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Ma, C.; Zhang, Y.; Xi, X.; Wang, L.; Zhou, M.; Burrows, J.F.; Chen, T. A novel antimicrobial peptide, Ranatuerin-2PLx, showing therapeutic potential in inhibiting proliferation of cancer cells. Biosci. Rep. 2018, 38, BSR20180710. [Google Scholar] [CrossRef]
- Marenah, L.; Flatt, P.R.; Orr, D.F.; Shaw, C.; Abdel-Wahab, Y.H. Skin secretions of Rana saharica frogs reveal antimicrobial peptides esculentins-1 and -1B and brevinins-1E and -2EC with novel insulin releasing activity. J. Endocrinol. 2006, 188, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Won, H.S.; Kang, S.J.; Lee, B.J. Action mechanism and structural requirements of the antimicrobial peptides, gaegurins. Biochim. Biophys. Acta 2009, 1788, 1620–1629. [Google Scholar] [CrossRef]
- Islas-Rodriguez, A.E.; Marcellini, L.; Orioni, B.; Barra, D.; Stella, L.; Mangoni, M.L. Esculentin 1-21: A linear antimicrobial peptide from frog skin with inhibitory effect on bovine mastitis-causing bacteria. J. Pept. Sci. 2009, 15, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Anticancer peptide: Physicochemical property, functional aspect and trend in clinical application (Review). Int. J. Oncol. 2020, 57, 678–696. [Google Scholar] [CrossRef] [PubMed]
- Ran, S.; Downes, A.; Thorpe, P.E. Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer Res. 2002, 62, 6132–6140. [Google Scholar]
- Connor, J.; Bucana, C.; Fidler, I.J.; Schroit, A.J. Differentiation-Dependent Expression of Phosphatidylserine in Mammalian Plasma-Membranes - Quantitative Assessment of Outer-Leaflet Lipid by Prothrombinase Complex-Formation. Prac. Natl. Acad. Sci. USA 1989, 86, 3184–3188. [Google Scholar] [CrossRef]
- Yavari, B.; Mahjub, R.; Saidijam, M.; Raigani, M.; Soleimani, M. The Potential Use of Peptides in Cancer Treatment. Curr. Protein Pept. Sci. 2018, 19, 759–770. [Google Scholar] [CrossRef]
- Kim, R.; Emi, M.; Tanabe, K. Role of mitochondria as the gardens of cell death. Cancer Chemoth. Pharm. 2006, 57, 545–553. [Google Scholar] [CrossRef] [PubMed]
- An, T.; Gong, Y.; Li, X.; Kong, L.; Ma, P.; Gong, L.; Zhu, H.; Yu, C.; Liu, J.; Zhou, H.; et al. USP7 inhibitor P5091 inhibits Wnt signaling and colorectal tumor growth. Biochem. Pharmacol. 2017, 131, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Minn, I.; Kim, H.S.; Kim, S.C. Antimicrobial peptides derived from pepsinogens in the stomach of the bullfrog, Rana catesbeiana. Bba-Mol. Basis Dis. 1998, 1407, 31–39. [Google Scholar] [CrossRef]
- Liang, X.; Wang, R.; Dou, W.; Zhao, L.; Zhou, L.; Zhu, J.; Wang, K.; Yan, J. Arminin 1a-C, a novel antimicrobial peptide from ancient metazoan Hydra, shows potent antileukemia activity against drug-sensitive and drug-resistant leukemia cells. Drug Des. Dev. Ther. 2018, 12, 3691–3703. [Google Scholar] [CrossRef]
- Zhu, H.F.; Wang, B.; Kong, L.M.; An, T.; Li, G.T.; Zhou, H.Y.; Gong, L.; Zhao, Z.X.; Gong, Y.X.; Sun, H.D.; et al. Parvifoline AA Promotes Susceptibility of Hepatocarcinoma to Natural Killer Cell-Mediated Cytolysis by Targeting Peroxiredoxin. Cell Chem. Biol. 2019, 26, 1122. [Google Scholar] [CrossRef] [PubMed]
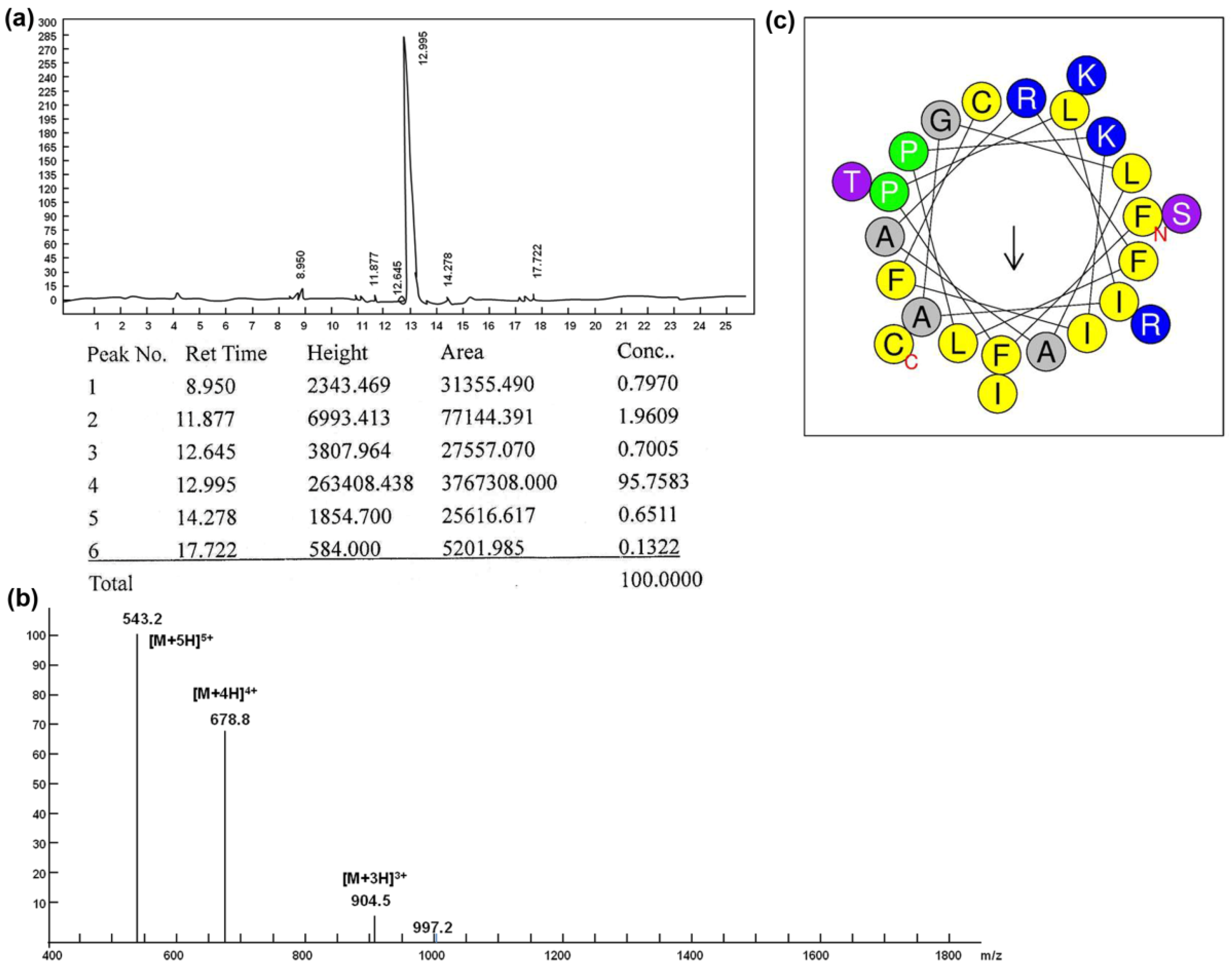
| Peptide | Sequence | Length (a.a) | MW (Da) | Net Charge | pI | Hydropho-bicity (H) | Freq Polar |
|---|---|---|---|---|---|---|---|
| Brevinin-1RL1 | FFPLIAGLAARFLPKIFCSITKRC | 24 | 2711.3 | 4 | 10.11 | 0.805 | 0.292 |
| Cell Type Caption | Cell Lines | Tumor Type | IC50 (Mean ± SD, μM) |
|---|---|---|---|
| Tumor cells | HCT116 | colorectal adenocarcinoma | 5.87 ± 0.15 |
| MDA-MB-231 | breast adenocarcinoma | 5.44 ± 0.33 | |
| SW480 | colorectal adenocarcinoma | 10.37 ± 0.40 | |
| A549 | lung adenocarcinoma | 5.81 ± 0.23 | |
| SMMC-7721 | hepatocellular carcinoma | 6.87 ± 0.51 | |
| B16-F10 | melanomas | 6.65 ± 0.33 | |
| Immortalized noncancer cells | NCM460 | colon mucosal epithelial | 16.84 ± 0.56 |
| BEAS-2B | bronchial epithelial | 16.57 ± 0.29 | |
| HaCaT | keratinocyte cell | 28.67 ± 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, X.; Fan, D.; Kong, L.; Yang, Q.; Zhu, Y.; Zhang, S.; Su, G.; Li, Y. Antimicrobial Peptide Brevinin-1RL1 from Frog Skin Secretion Induces Apoptosis and Necrosis of Tumor Cells. Molecules 2021, 26, 2059. https://doi.org/10.3390/molecules26072059
Ju X, Fan D, Kong L, Yang Q, Zhu Y, Zhang S, Su G, Li Y. Antimicrobial Peptide Brevinin-1RL1 from Frog Skin Secretion Induces Apoptosis and Necrosis of Tumor Cells. Molecules. 2021; 26(7):2059. https://doi.org/10.3390/molecules26072059
Chicago/Turabian StyleJu, Xiaoman, Dongmei Fan, Lingmei Kong, Qihong Yang, Yiying Zhu, Shaohua Zhang, Guifeng Su, and Yan Li. 2021. "Antimicrobial Peptide Brevinin-1RL1 from Frog Skin Secretion Induces Apoptosis and Necrosis of Tumor Cells" Molecules 26, no. 7: 2059. https://doi.org/10.3390/molecules26072059
APA StyleJu, X., Fan, D., Kong, L., Yang, Q., Zhu, Y., Zhang, S., Su, G., & Li, Y. (2021). Antimicrobial Peptide Brevinin-1RL1 from Frog Skin Secretion Induces Apoptosis and Necrosis of Tumor Cells. Molecules, 26(7), 2059. https://doi.org/10.3390/molecules26072059






