Nordihydroguaiaretic Acid as a Novel Substrate and Inhibitor of Catechol O-Methyltransferase Modulates 4-Hydroxyestradiol-Induced Cyto- and Genotoxicity in MCF-7 Cells
Abstract
1. Introduction
2. Results
2.1. COMT-Mediated Metabolism of NDGA
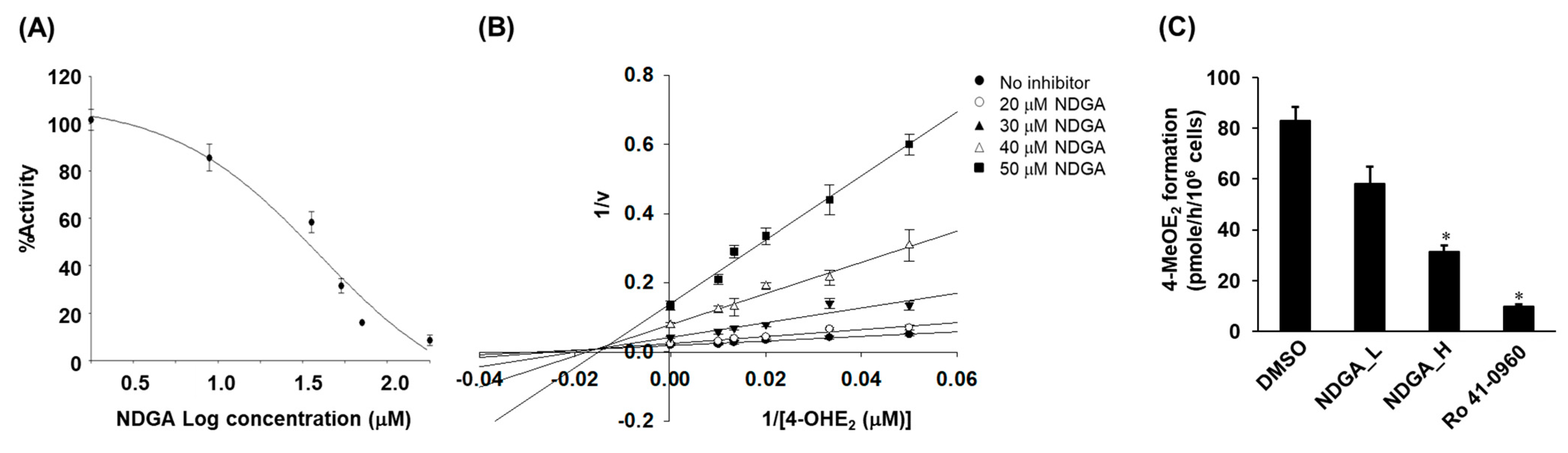
2.2. Inhibition of the COMT-Mediated O-Methylation of 4-OHE2 by NDGA
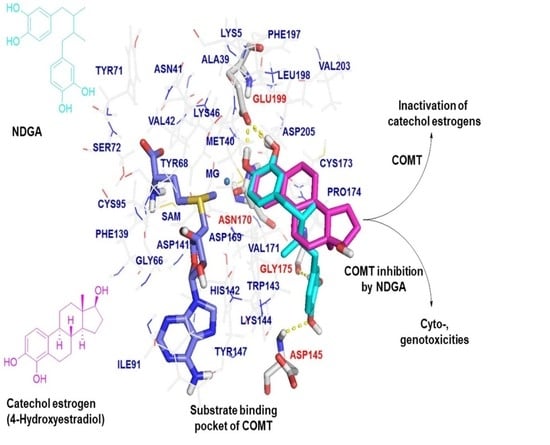
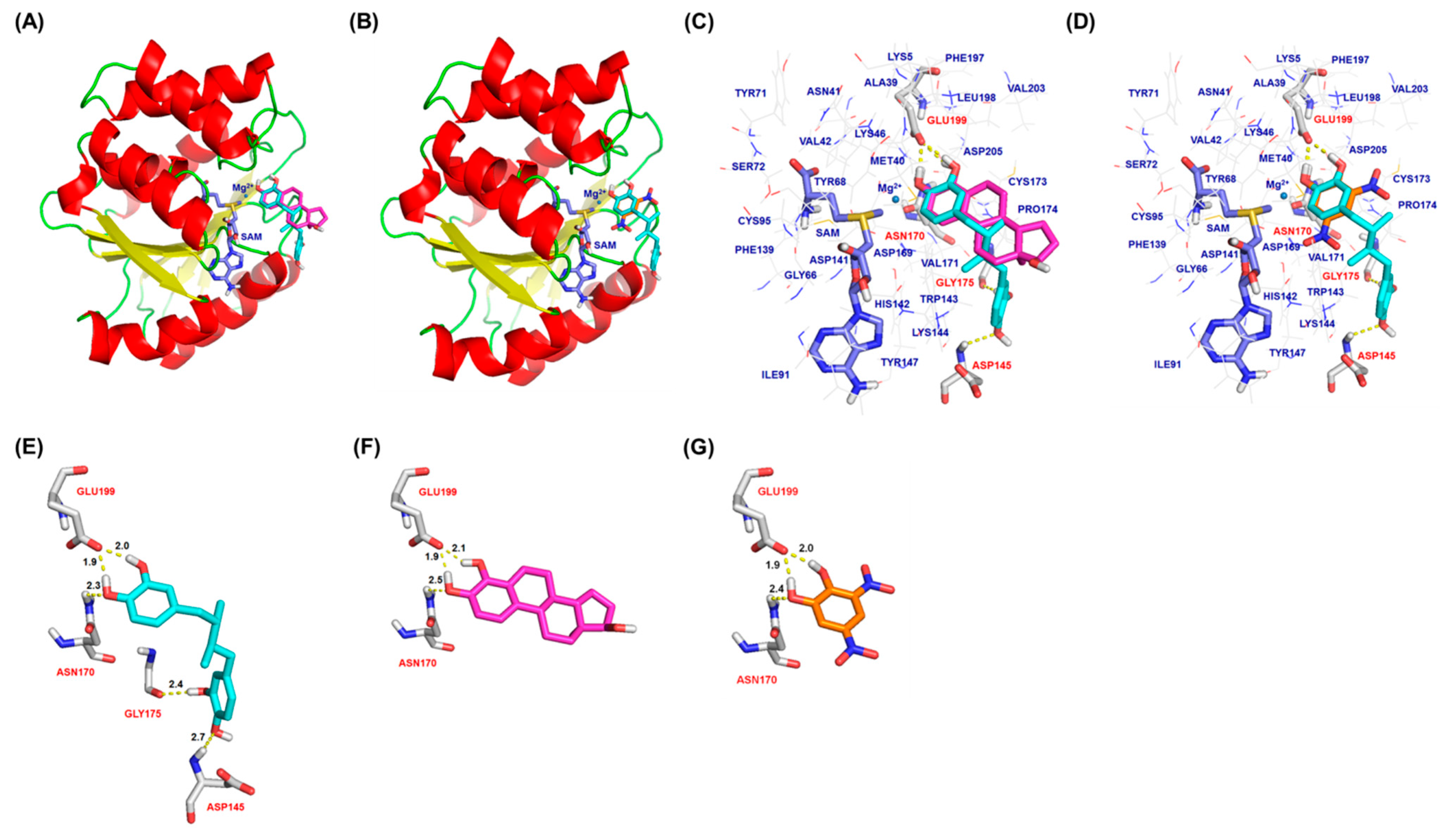
2.3. Molecular Docking Analysis of NDGA Bound to the Substrate Binding Pocket of COMT
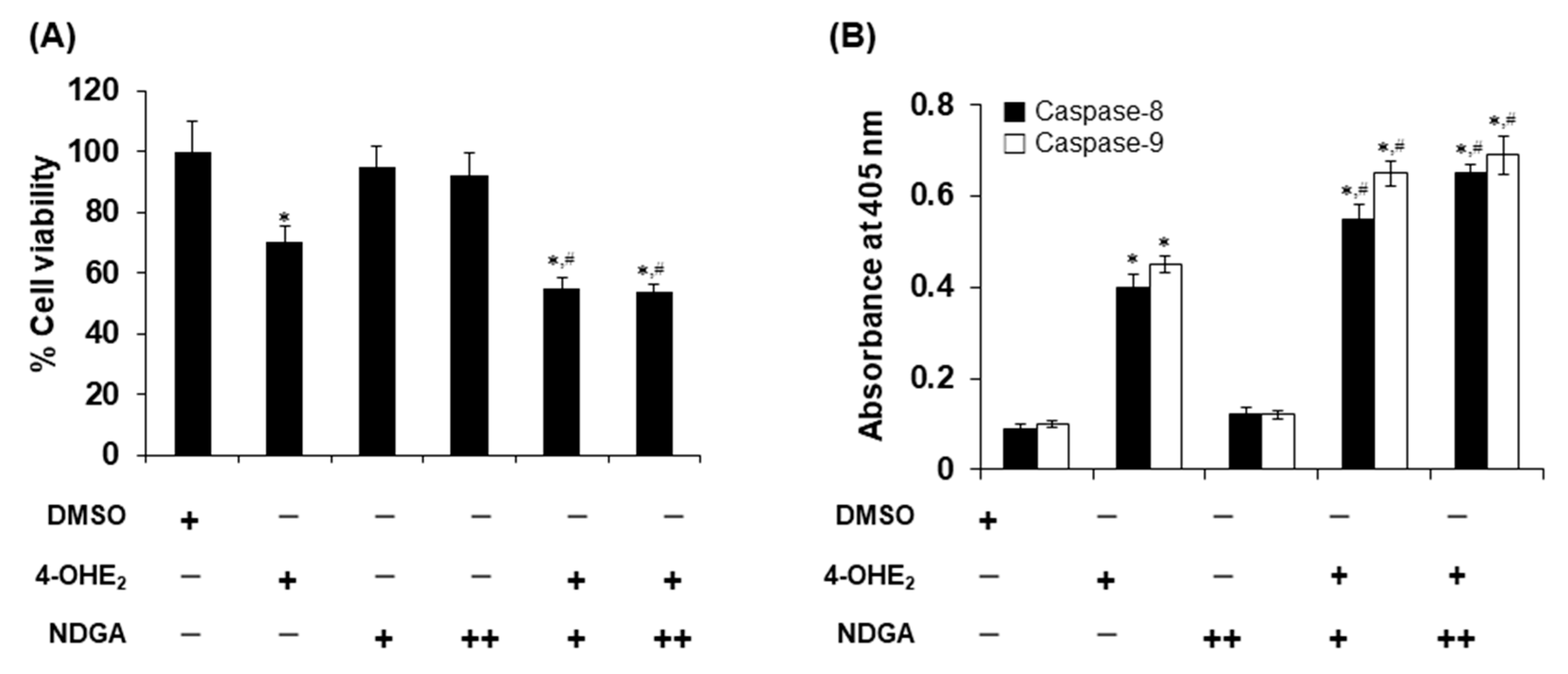
2.4. Effects of NDGA on 4-OHE2-Induced Cytotoxicity and Apoptosis in MCF-7 Cells
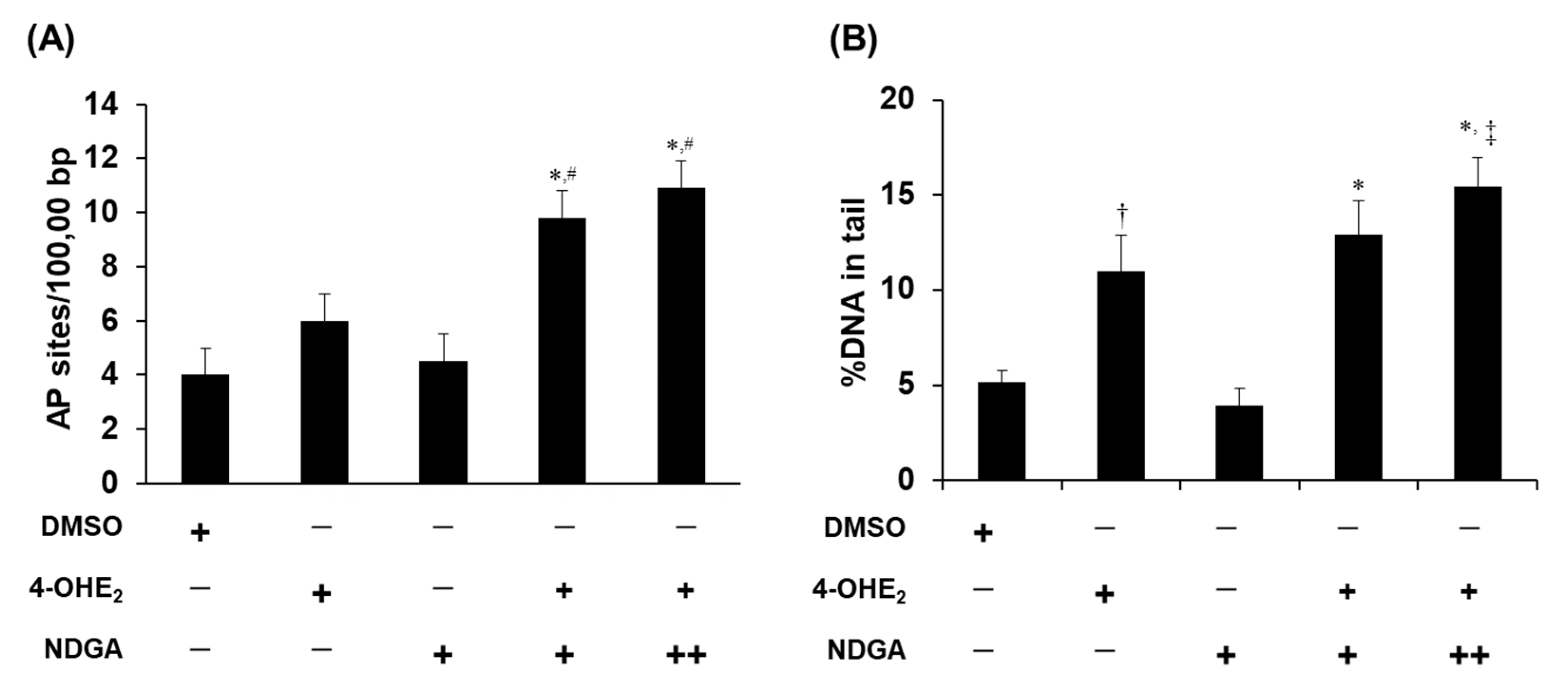
2.5. Effects of NDGA on 4-OHE2-Induced DNA Damage
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Enzyme Assays and Kinetic Measurements
4.3. Molecular Docking Study
4.4. Cell Culture
4.5. Cytotoxicity and Apoptosis Assays
4.6. Alkaline Single-Cell Gel Electrophoresis (Comet) Assay
4.7. Apurinic/apyrimidinic Site Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Arteaga, S.; Andrade-Cetto, A.; Cardenas, R. Larrea tridentata (Creosote bush), an abundant plant of Mexican and US-American deserts and its metabolite nordihydroguaiaretic acid. J. Ethnopharmacol. 2005, 98, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Floriano-Sanchez, E.; Villanueva, C.; Medina-Campos, O.N.; Rocha, D.; Sanchez-Gonzalez, D.J.; Cardenas-Rodriguez, N.; Pedraza-Chaverri, J. Nordihydroguaiaretic acid is a potent in vitro scavenger of peroxynitrite, singlet oxygen, hydroxyl radical, superoxide anion and hypochlorous acid and prevents in vivo ozone-induced tyrosine nitration in lungs. Free Radic. Res. 2006, 40, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.N.; Jeong, S.Y.; Bae, G.U.; Chang, M.; Zhang, D.; Liu, X.; Pei, Y.; Chin, Y.W.; Lee, J.; Oh, S.R.; et al. Selective estrogen receptor modulation by Larrea nitida on MCF-7 cell proliferation and immature rat uterus. Biomol. Ther. 2014, 22, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Gregus, A.M.; Dumlao, D.S.; Wei, S.C.; Norris, P.C.; Catella, L.C.; Meyerstein, F.G.; Buczynski, M.W.; Steinauer, J.J.; Fitzsimmons, B.L.; Yaksh, T.L.; et al. Systematic analysis of rat 12/15-lipoxygenase enzymes reveals critical role for spinal eLOX3 hepoxilin synthase activity in inflammatory hyperalgesia. FASEB J. 2013, 27, 1939–1949. [Google Scholar] [CrossRef]
- Comoglio, A.; Gibbs, A.H.; White, I.N.; Gant, T.; Martin, E.A.; Smith, L.L.; Gamalero, S.R.; DeMatteis, F. Effect of tamoxifen feeding on metabolic activation of tamoxifen by the liver of the rhesus monkey: Does liver accumulation of inhibitory metabolites protect from tamoxifen-dependent genotoxicity and cancer? Carcinogenesis 1996, 17, 1687–1693. [Google Scholar] [CrossRef][Green Version]
- Gnabre, J.N.; Brady, J.N.; Clanton, D.J.; Ito, Y.; Dittmer, J.; Bates, R.B.; Huang, R.C. Inhibition of human immunodeficiency virus type 1 transcription and replication by DNA sequence-selective plant lignans. Proc. Natl. Acad. Sci. USA 1995, 92, 11239–11243. [Google Scholar] [CrossRef]
- Youngren, J.F.; Gable, K.; Penaranda, C.; Maddux, B.A.; Zavodovskaya, M.; Lobo, M.; Campbell, M.; Kerner, J.; Goldfine, I.D. Nordihydroguaiaretic acid (NDGA) inhibits the IGF-1 and c-erbB2/HER2/neu receptors and suppresses growth in breast cancer cells. Breast Cancer Res. Treat. 2005, 94, 37–46. [Google Scholar] [CrossRef]
- Sadagurski, M.; Cady, G.; Miller, R.A. Anti-aging drugs reduce hypothalamic inflammation in a sex-specific manner. Aging Cell 2017, 16, 652–660. [Google Scholar] [CrossRef]
- Lambert, J.D.; Zhao, D.; Meyers, R.O.; Kuester, R.K.; Timmermann, B.N.; Dorr, R.T. Nordihydroguaiaretic acid: Hepatotoxicity and detoxification in the mouse. Toxicon 2002, 40, 1701–1708. [Google Scholar] [CrossRef]
- Chang, M. Dual roles of estrogen metabolism in mammary carcinogenesis. BMB Rep. 2011, 44, 423–434. [Google Scholar] [CrossRef]
- Cavalieri, E.L.; Rogan, E.G. Depurinating estrogen-DNA adducts in the etiology and prevention of breast and other human cancers. Future Oncol. 2010, 6, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Dawling, S.; Roodi, N.; Mernaugh, R.L.; Wang, X.; Parl, F.F. Catechol-O-methyltransferase (COMT)-mediated metabolism of catechol estrogens: Comparison of wild-type and variant COMT isoforms. Cancer Res. 2001, 61, 6716–6722. [Google Scholar] [PubMed]
- Zhu, B.T.; Liehr, J.G. Quercetin increases the severity of estradiol-induced tumorigenesis in hamster kidney. Toxicol. Appl. Pharmacol. 1994, 125, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Sak, K. The Val158Met polymorphism in COMT gene and cancer risk: Role of endogenous and exogenous catechols. Drug Metab. Rev. 2017, 49, 56–83. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, Y.; Chen, A.; Tao, Y.; Song, H.; Li, W.; Tao, J.; Zuo, M. Association between the COMT Val158Met polymorphism and risk of cancer: Evidence from 99 case-control studies. Onco. Targets Ther. 2015, 8, 2791–2803. [Google Scholar] [CrossRef]
- van Duursen, M.B.; Sanderson, J.T.; de Jong, P.C.; Kraaij, M.; van den Berg, M. Phytochemicals inhibit catechol-O-methyltransferase activity in cytosolic fractions from healthy human mammary tissues: Implications for catechol estrogen-induced DNA damage. Toxicol. Sci. 2004, 81, 316–324. [Google Scholar] [CrossRef]
- Zhu, B.T.; Wang, P.; Nagai, M.; Wen, Y.; Bai, H.W. Inhibition of human catechol-O-methyltransferase (COMT)-mediated O-methylation of catechol estrogens by major polyphenolic components present in coffee. J Steroid. Biochem. Mol. Biol. 2009, 113, 65–74. [Google Scholar] [CrossRef]
- Nagai, M.; Conney, A.H.; Zhu, B.T. Strong inhibitory effects of common tea catechins and bioflavonoids on the O-methylation of catechol estrogens catalyzed by human liver cytosolic catechol-O-methyltransferase. Drug Metab. Dispos. 2004, 32, 497–504. [Google Scholar] [CrossRef]
- Yalcin, D.; Bayraktar, O. Inhibition of catechol-O-methyltransferase (COMT) by some plant-derived alkaloids and phenolics. J. Mol. Catal. B-Enzym. 2010, 64, 162–166. [Google Scholar] [CrossRef][Green Version]
- Jeong, H.; Kim, S.; Lee, J.; Park, J.Y.; Zhou, W.; Liu, X.; Kim, S.D.; Song, Y.S.; Jang, C.Y.; Oh, S.R.; et al. Characterization of phase I and phase II hepatic metabolism and reactive intermediates of Larrea nitida Cav. and its lignan compounds. Phytother. Res. 2017, 31, 140–151. [Google Scholar] [CrossRef]
- Rutherford, K.; Le Trong, I.; Stenkamp, R.E.; Parson, W.W. Crystal structures of human 108V and 108M catechol O-methyltransferase. J. Mol. Biol. 2008, 380, 120–130. [Google Scholar] [CrossRef]
- Barker, D. Lignans. Molecules 2019, 24, 1424. [Google Scholar] [CrossRef] [PubMed]
- Manda, G.; Rojo, A.I.; Martinez-Klimova, E.; Pedraza-Chaverri, J.; Cuadrado, A. Nordihydroguaiaretic acid: From herbal medicine to clinical development for cancer and chronic diseases. Front. Pharmacol. 2020, 11, 151. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, L.; Jiang, L.; Wagner, J. Soy isoflavones decrease the catechol-O-methyltransferase-mediated inactivation of 4-hydroxyestradiol in cultured MCF-7 cells. Carcinogenesis 2008, 29, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, C.Y.; Lambert, J.D.; Ai, N.; Welsh, W.J.; Yang, C.S. Inhibition of human liver catechol-O-methyltransferase by tea catechins and their metabolites: Structure-activity relationship and molecular-modeling studies. Biochem. Pharmacol. 2005, 69, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Passos, C.; Klein-Júnior, L.C.; de Mello Andrade, J.M.; Matté, C.; Henriques, A.T. The catechol-O-methyltransferase inhibitory potential of Z-vallesiachotamine by in silico and in vitro approaches. Rev. Bras. Farmacogn. 2015, 25, 382–386. [Google Scholar] [CrossRef]
- Lavigne, J.A.; Goodman, J.E.; Fonong, T.; Odwin, S.; He, P.; Roberts, D.W.; Yager, J.D. The effects of catechol-O-methyltransferase inhibition on estrogen metabolite and oxidative DNA damage levels in estradiol-treated MCF-7 cells. Cancer Res. 2001, 61, 7488–7494. [Google Scholar]
- Zavodovskaya, M.; Campbell, M.J.; Maddux, B.A.; Shiry, L.; Allan, G.; Hodges, L.; Kushner, P.; Kerner, J.A.; Youngren, J.F.; Goldfine, I.D. Nordihydroguaiaretic acid (NDGA), an inhibitor of the HER2 and IGF-1 receptor tyrosine kinases, blocks the growth of HER2-overexpressing human breast cancer cells. J. Cell Biochem. 2008, 103, 624–635. [Google Scholar] [CrossRef]
- Moyna, G.; Hernandez, G.; Williams, H.J.; Nachman, R.J.; Scott, A.I. Development of Weiner et al. force field parameters suitable for conformational studies of [1,4]-benzodiazepines and related compounds. J. Chem. Inf. Comput. Sci. 1997, 37, 951–956. [Google Scholar] [CrossRef]
- Spitzer, R.; Jain, A.N. Surflex-Dock: Docking benchmarks and real-world application. J. Comput. Aided Mol. Des. 2012, 26, 687–699. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, J.; Kim, S.; Park, S.; Yang, H.; Ahn, B.H.; Jang, C.Y.; Jeong, H.C.; Lee, S.J.; Kim, S.L.; et al. Characterization of soybean germinated embryo extract as an estrogen receptor subtype-selective and tissue-specific modulator. J. Med. Food 2019, 22, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Gyori, B.M.; Venkatachalam, G.; Thiagarajan, P.S.; Hsu, D.; Clement, M.V. OpenComet: An automated tool for comet assay image analysis. Redox Biol. 2014, 2, 457–465. [Google Scholar] [CrossRef] [PubMed]
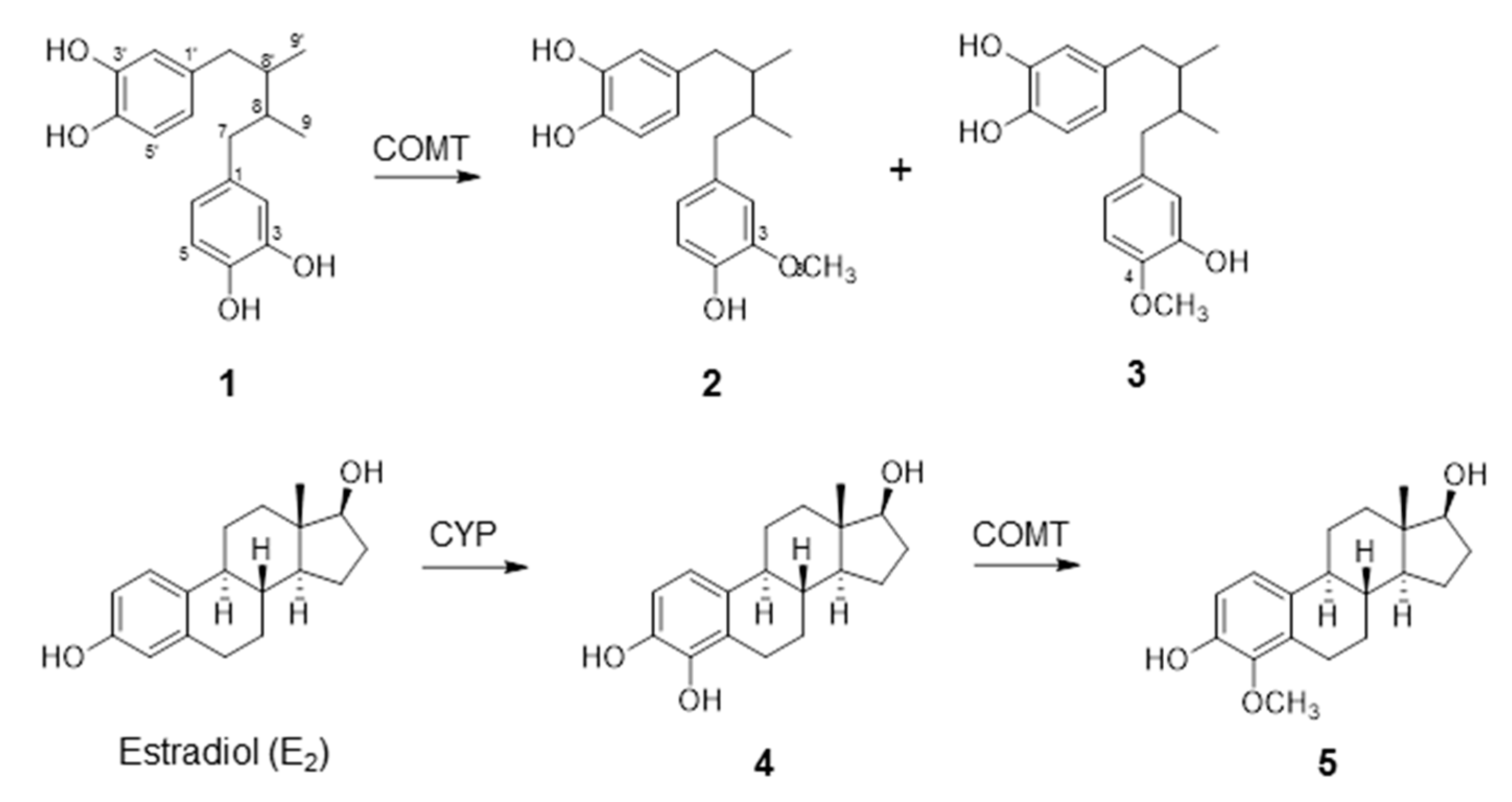
| Substrate | Product | Rate of O-Methylation (µmol/mg Protein/min) | Km (µM) | Vmax (µmol/mg Protein/min) | Vmax/Km |
|---|---|---|---|---|---|
| NDGA a | 3-O-methyl NDGA (3-MNDGA) | 10.2 | 2.57 | 15.7 | 6.12 |
| NDGA b | 4-O-methyl NDGA (4-MNDGA) | N.D c | 2.19 | N.D. | N.D |
| 4-OHE2 | 4-O-methylestradiol (4-MeOE2) | 2.62 | 37.9 | 3.26 | 0.086 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Lee, J.; Jeong, H.; Bang, M.S.; Jeong, J.-H.; Chang, M. Nordihydroguaiaretic Acid as a Novel Substrate and Inhibitor of Catechol O-Methyltransferase Modulates 4-Hydroxyestradiol-Induced Cyto- and Genotoxicity in MCF-7 Cells. Molecules 2021, 26, 2060. https://doi.org/10.3390/molecules26072060
Kim J-H, Lee J, Jeong H, Bang MS, Jeong J-H, Chang M. Nordihydroguaiaretic Acid as a Novel Substrate and Inhibitor of Catechol O-Methyltransferase Modulates 4-Hydroxyestradiol-Induced Cyto- and Genotoxicity in MCF-7 Cells. Molecules. 2021; 26(7):2060. https://doi.org/10.3390/molecules26072060
Chicago/Turabian StyleKim, Jin-Hee, Jimin Lee, Hyesoo Jeong, Mi Seo Bang, Jin-Hyun Jeong, and Minsun Chang. 2021. "Nordihydroguaiaretic Acid as a Novel Substrate and Inhibitor of Catechol O-Methyltransferase Modulates 4-Hydroxyestradiol-Induced Cyto- and Genotoxicity in MCF-7 Cells" Molecules 26, no. 7: 2060. https://doi.org/10.3390/molecules26072060
APA StyleKim, J.-H., Lee, J., Jeong, H., Bang, M. S., Jeong, J.-H., & Chang, M. (2021). Nordihydroguaiaretic Acid as a Novel Substrate and Inhibitor of Catechol O-Methyltransferase Modulates 4-Hydroxyestradiol-Induced Cyto- and Genotoxicity in MCF-7 Cells. Molecules, 26(7), 2060. https://doi.org/10.3390/molecules26072060