Optimization of Enzyme-Assisted Extraction and Purification of Flavonoids from Pinus koraiensis Nut-Coated Film and Antioxidant Activity Evaluation
Abstract
1. Introduction
2. Results
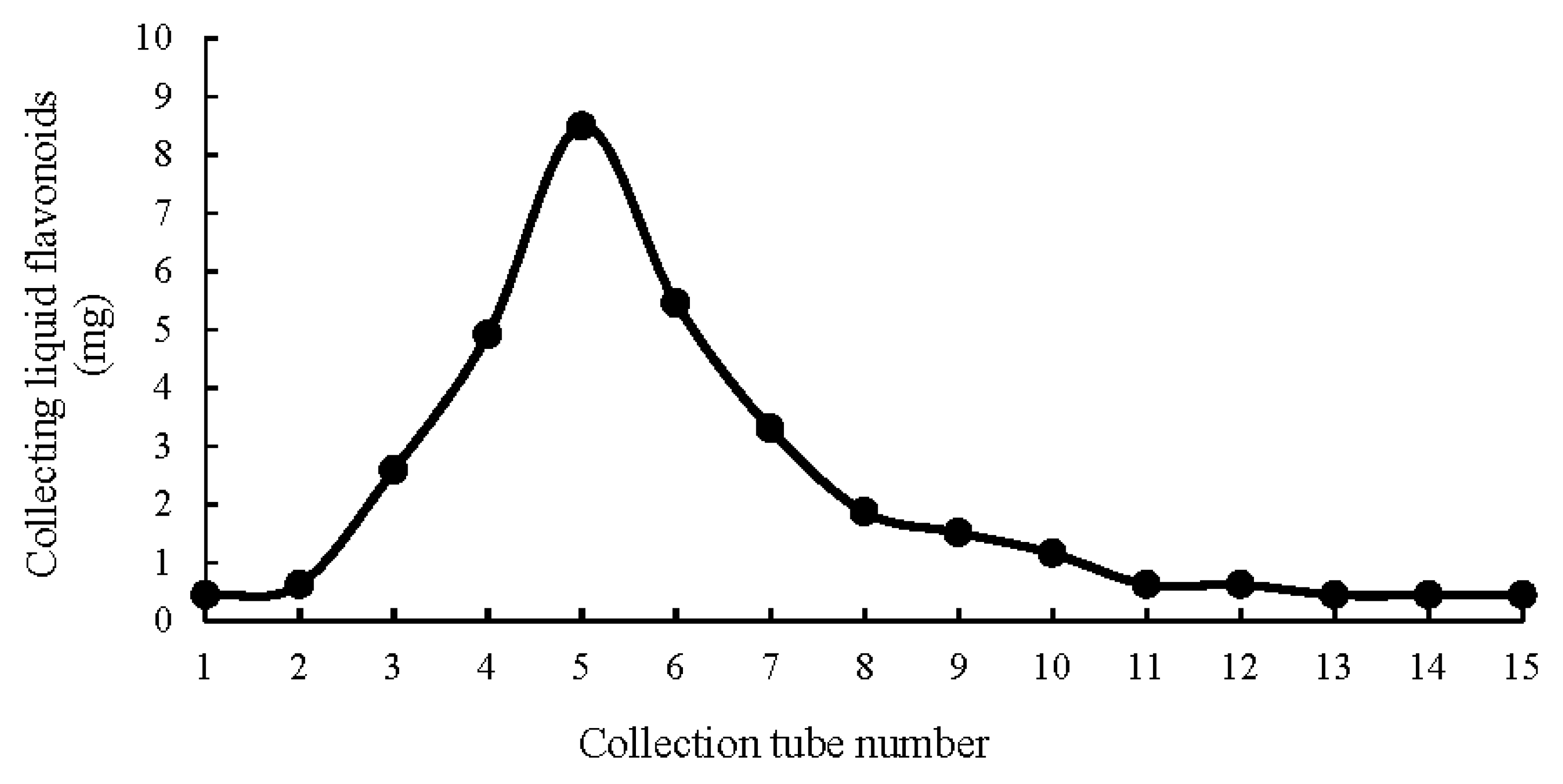
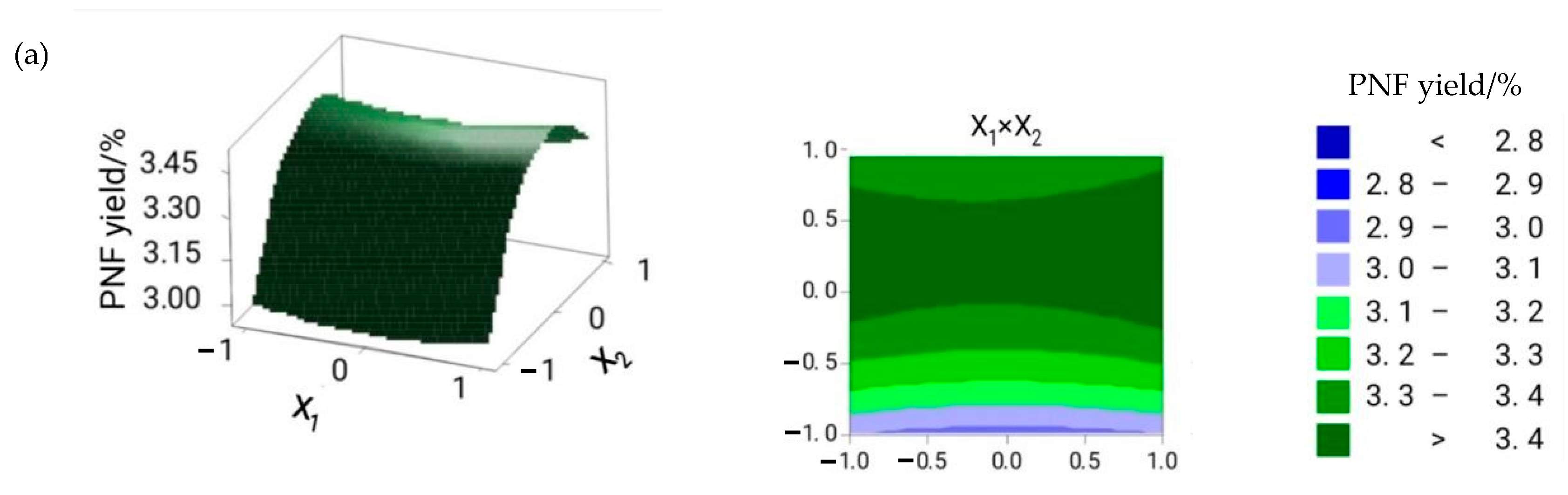
2.1. Optimization of the Extraction Conditions
0.18458X32 + 0.01000X1X2 − 0.00250X1X3 + 0.01500X2X3
0.3612X32 + 1.8225X1X2 − 0.2550X1X3 + 0.2200X2X3
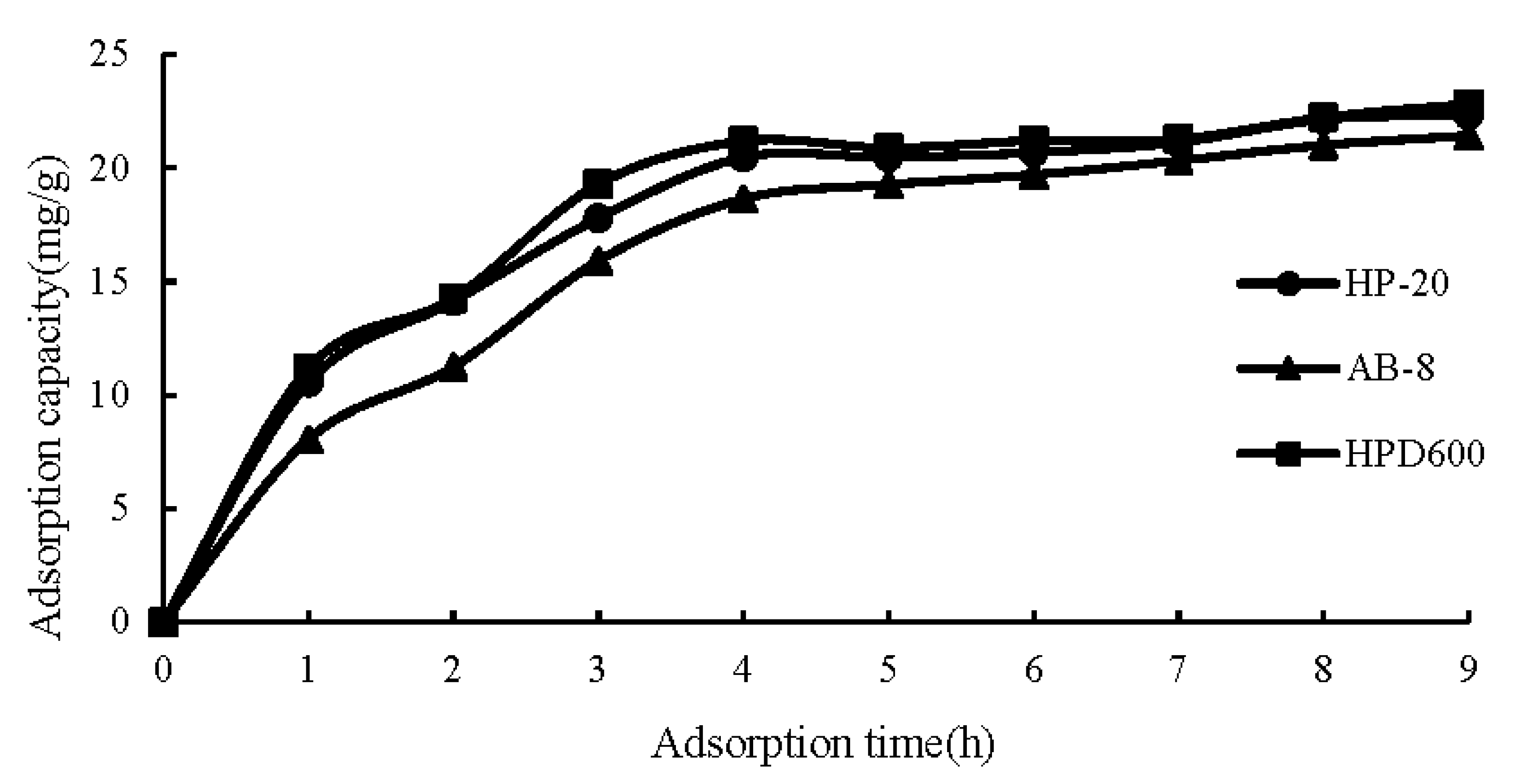
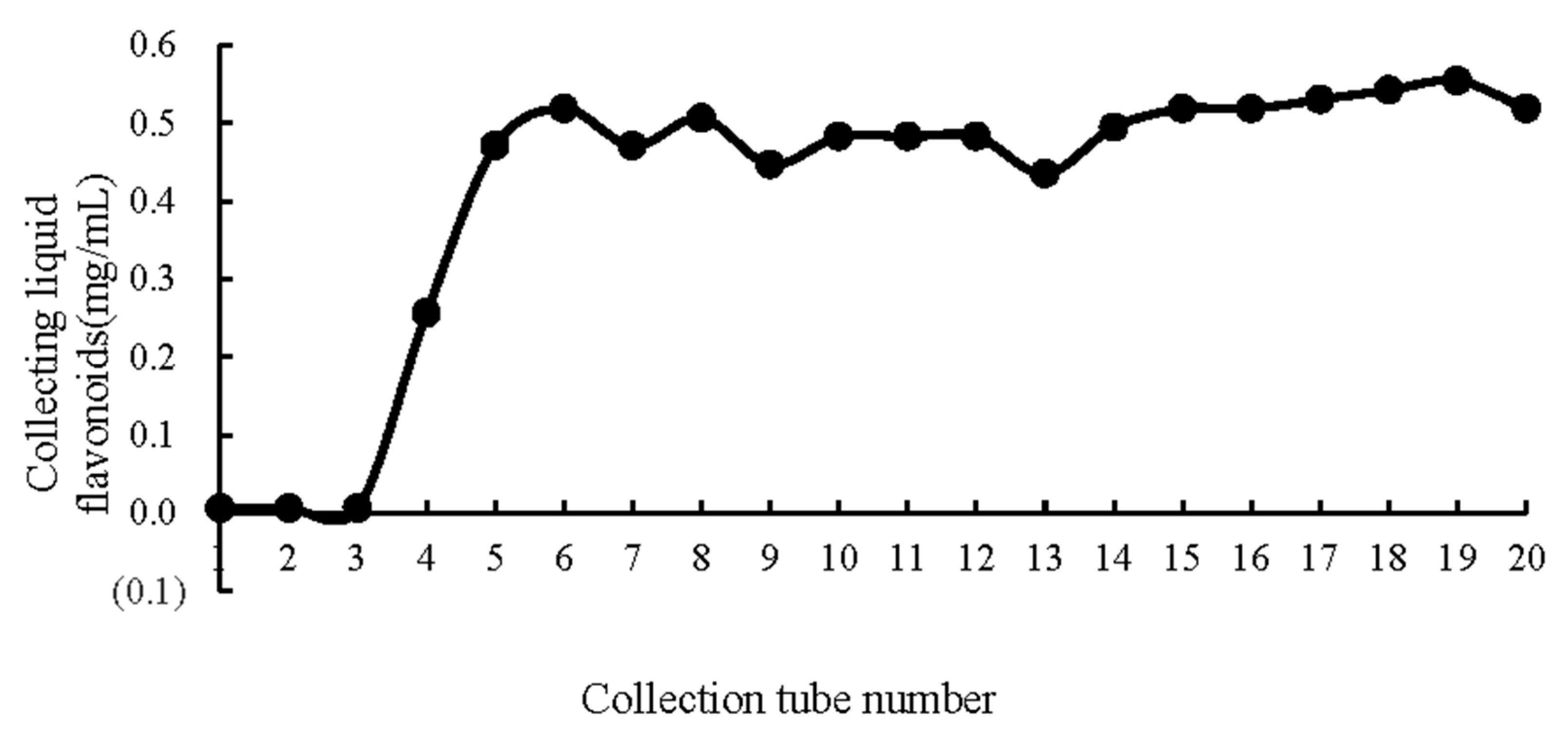
2.2. Optimization of the Purification Conditions
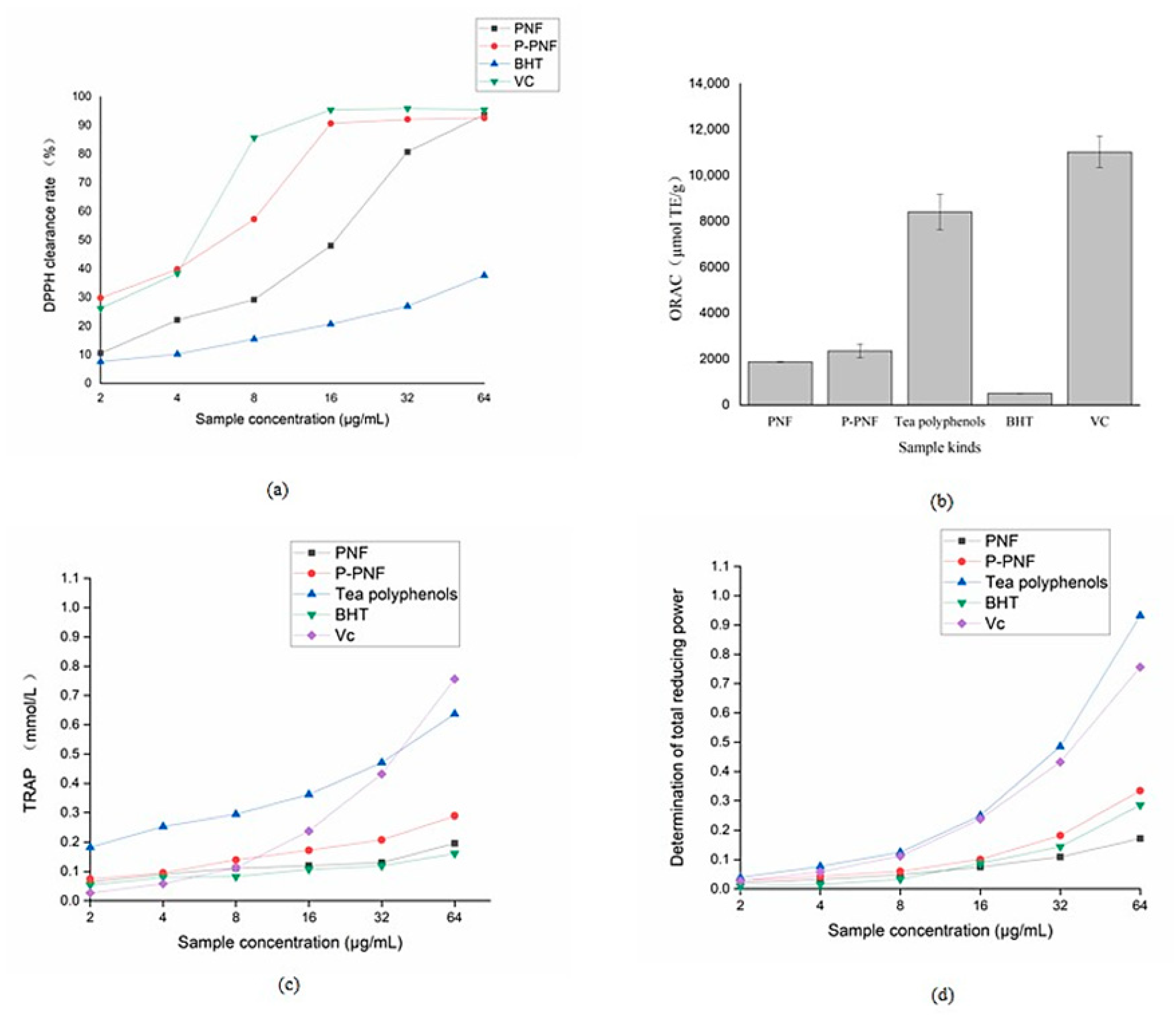
2.3. In Vitro Antioxidant Activity
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Extraction Conditions Optimization
4.2.1. Enzyme-Assisted Extraction
4.2.2. RSM Experimental Design
4.2.3. ABTS Radical Scavenging Assay
4.3. Purification Conditions Optimization
4.3.1. Selection and Preparation of Macroporous Resin
4.3.2. Purification of PNF
4.4. In Vitro Antioxidant Activity
4.4.1. DPPH Radical Scavenging Activity
4.4.2. Determination of ORAC
4.4.3. Determination of TRAP
4.4.4. Determination of Iron Ion Reduction Capacity
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Indicator | Content (%) |
|---|---|
| Protein | 4.63 |
| Axunge | 0.18 |
| Total sugar | 2.79 |
| Reducing sugar | 1.90 |
| Flavone | 2.83 |
| Polyphenol | 2.96 |
| VC | 0.03 |
| Source | DF | Seq SS | Adj SS | F | P |
|---|---|---|---|---|---|
| Regression | 9 | 0.644568 | 0.071619 | 4.80 | 0.049 |
| Linear | 3 | 0.209925 | 0.069975 | 4.69 | 0.065 |
| Square | 3 | 0.433318 | 0.144439 | 9.68 | 0.016 |
| Interaction | 3 | 0.001325 | 0.000442 | 0.03 | 0.992 |
| Residual Error | 5 | 0.074592 | 0.014918 | 14.56 | 0.065 |
| Lack-of-Fit | 3 | 0.071325 | 0.023775 | ||
| Pure Error | 2 | 0.003267 | 0.001633 | ||
| Total | 14 | ||||
| Regression | 9 | 198.433 | 22.0482 | 24.50 | 0.001 |
| Linear | 3 | 52.436 | 17.4787 | 19.42 | 0.003 |
| Square | 3 | 132.258 | 44.0859 | 48.98 | 0.000 |
| Interaction | 3 | 13.740 | 4.5799 | 5.09 | 0.056 |
| Interaction | 3 | 13.740 | 4.5799 | 5.09 | 0.056 |
| Residual Error | 5 | 4.500 | 0.9001 | 2.81 | 0.273 |
| Lack-of-Fit | 3 | 3.639 | 1.2129 | ||
| Pure Error | 2 | 0.862 | 0.4309 | ||
| Total | 14 |
| Serial Number | Resin Model | Polarity | Specific Surface Area (m2/g) | Balanced Concentration of Flavonoids in Solution (mg/mL) | Adsorption Amount (mg/g) | Adsorption Rate (%) | Eluent Mass Concentration (mg/mL) | Desorption Rate (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | AB-8 | Weak polarity | 450–500 | 0.06557 | 12.9957 | 95.1965 | 0.7958 | 61.2385 |
| 2 | HP-20 | Non-level | 550–600 | 0.10134 | 12.6381 | 92.5764 | 0.7661 | 60.6132 |
| 3 | HPD600 | Polarity | 550–600 | 0.08942 | 12.7572 | 93.4498 | 0.8376 | 65.6542 |
| 4 | HPD826 | Hydrogen bond | 500–600 | 0.12519 | 12.3996 | 90.8297 | 0.6528 | 52.6442 |
| 5 | D101 | Group polarity | 480–520 | 0.11327 | 12.5188 | 91.7031 | 0.7279 | 58.1429 |
References
- Carino, C.; Paolis, A.D.; Coïsson, J.D.; Bianchi, D.M.; Decastelli, L.; Arlorio, M. Sensitive and specific detection of pine nut (Pinus spp.) by real-time PCR in complex food products. Food Chem. 2016, 194, 980–985. [Google Scholar]
- Nergiz, C.; Dönmez, İ. Chemical composition and nutritive value of Pinus pinea L. seeds. Food Chem. 2004, 86, 365–368. [Google Scholar]
- Hou, L.X.; Li, C.C.; Qiu, J.H. Comparison of the Physicochemical Characteristics of Pinus koraiensis L. Nut Oils from Different Extraction Technologies. Grain Oil Sci. Technol. 2018, 3, 113–118. [Google Scholar]
- Xie, K.Y.; Miles, E.A.; Calder, P.C. A review of the potential health benefits of pine nut oil and its characteristic fatty acid pinolenic acid. J. Funct. Foods 2016, 23, 464–473. [Google Scholar]
- Yang, R.W.; Li, X.F.; Lin, S.Y.; Zhang, Z.M.; Chen, F. Identification of novel peptides from 3 to 10 kDa pine nut (Pinus koraiensis) meal protein, with an exploration of the relationship between their antioxidant activities and secondary structure. Food Chem. 2017, 219, 311–320. [Google Scholar]
- Zhang, H.; Zou, P.; Zhao, H.T.; Qiu, J.M.; Regenstein, J.M.; Yang, X. Isolation, purification, structure and antioxidant activity of polysaccharide from pinecones of Pinus koraiensis. Carbohydr. Polym. 2021, 251, 117078. [Google Scholar]
- Raffa, D.; Maggio, B.; Raimondi, M.V.; Plescia, F.; Daidone, G. Recent discoveries of anticancer flavonoids. Eur. J. Med. Chem. 2017, 142, 213–228. [Google Scholar]
- Perez-Vizcaino, F.; Fraga, C.G. Research trends in flavonoids and health. Arch. Biochem. Biophys. 2018, 646, 107–112. [Google Scholar]
- Zhao, J.J.; Yang, J.; Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 270, 118642. [Google Scholar]
- Owona, B.A.; Abia, W.A.; Moundipa, P.F. Natural compounds flavonoids as modulators of inflammasomes in chronic diseases. Int. Immunopharmacol. 2020, 84, 106498. [Google Scholar]
- Hou, M.Y.; Hu, W.Z.; Xiu, Z.L.; Shi, Y.S.; Hao, K.X.; Cao, D.; Guan, Y.G.; Yin, H.L. Efficient enrichment of total flavonoids from Pteris ensiformis Burm. extracts by macroporous adsorption resins and in vitro evaluation of antioxidant and antiproliferative activities. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1138, 121960. [Google Scholar]
- Mai, X.M.; Liu, Y.T.; Tang, X.Y.; Wang, L.P.; Lin, Y.Y.; Zeng, H.Y.; Luo, L.C.; Fan, H.J.; Li, P.F. Sequential extraction and enrichment of flavonoids from Euonymus alatus by ultrasonic-assisted polyethylene glycol-based extraction coupled to temperature-induced cloud point extraction. Ultrason. Sonochem. 2020, 66, 105073. [Google Scholar]
- Bruno, S.F.; Ekorong, F.J.A.A.; Karkal, S.S. Green and innovative techniques for recovery of valuable compounds from seafood by-products and discards: A review. Trends Food Sci. Technol. 2018, 85, 10–12. [Google Scholar]
- Jiang, J.P.; Yan, L.; Shi, Z.; Wang, L.X.; Shan, L.T.; Thomas, E. Hepatoprotective and anti-inflammatory effects of total flavonoids of Qu Zhi Ke (peel of Citrus changshan-huyou) on non-alcoholic fatty liver disease in rats via modulation of NF-κB and MAPKs. Phytomedicine 2019, 64, 153082. [Google Scholar]
- Liu, B.Q.; Liu, J.F.; Huang, D.D.; Wei, J.T.; Di, D.L. Boric acid modified macroporous adsorption resin and its adsorption properties for catechol compounds. Colloids Surf. A 2020, 595, 124674. [Google Scholar]
- Ghasemi-Varnamkhasti, M.; Mohammad-Razdari, A.; Yoosefian, S.H.; Izadia, Z.; Siadat, M. Aging discrimination of French cheese types based on the optimization of an electronic nose using multivariate computational approaches combined with response surface method (RSM). LWT Food Sci. Technol. 2019, 111, 85–98. [Google Scholar]
- Eriheum; Wang, M.R.; Zhang, F.; Wang, D.S.; Zhao, M.; Cui, N.; Gao, G.; Guo, J.B.; Zhang, Q. Optimization of the process parameters of ultrasound on inhibition of polyphenol oxidase activity in whole potato tuber by response surface methodology. LWT Food Sci. Technol. 2021, 144, 111232. [Google Scholar]
- Mansur, A.P.; Song, N.; Jang, H.W.; Lim, T.; Yao, M.Y. Optimizing the ultrasound-assisted deep eutectic solvent extraction of flavonoids in common buckwheat sprouts. Food Chem. 2019, 293, 438–445. [Google Scholar]
- Wang, L.; Wu, Y.N.; Liu, Y.; Wu, Z.Q. Complex Enzyme-Assisted Extraction Releases Antioxidative Phenolic Compositions from Guava Leaves. Molecules 2017, 22, 1648. [Google Scholar]
- Xiao, Q.; Weng, H.; Ni, H.; Hong, Q.; Lin, K.; Xiao, A. Physicochemical and gel properties of agar extracted by enzyme and enzyme-assisted methods. Food Hydrocoll. 2019, 87, 530–540. [Google Scholar]
- Yao, X.J.; Xu, J.C.; Adhikari, B.; Lv, W.Q. Microwave-Assisted Enzymatic Extraction of Flavonoids from Armeniaca mume Sieb. Blossom and Their Immunomodulating Effect in Mice with DSS-Induced Colitis. Molecules 2021, 26, 855. [Google Scholar]
- Moon, J.M.; Park, S.H.; Jhee, K.H.; Yang, S.A. Protection against UVB-Induced Wrinkle Formation in SKH-1 Hairless Mice: Efficacy of Tricin Isolated from Enzyme-Treated Zizania latifolia Extract. Molecules 2018, 23, 2254. [Google Scholar]
- Ng, K.P.; Lyu, X.M.; Mark, R.; Chen, W.N. Antimicrobial and antioxidant activities of phenolic metabolites from flavonoid-producing yeast: Potential as natural food preservatives. Food Chem. 2019, 270, 123–129. [Google Scholar]
- Bouras, M.; Grimi, N.; Bals, O.; Vorobiev, E. Impact of pulsed electric fields on polyphenols extraction from Norway spruce bark. Ind. Crop. Prod. 2016, 80, 50–58. [Google Scholar]
- Son, N.T.; Thanh, D.T.M.; Trang, V.N. Flavone norartocarpetin and isoflavone 2′-hydroxygenistein: A spectroscopic study for structure, electronic property and antioxidant potential using DFT (Density functional theory). J. Mol. Struct. 2019, 1193, 76–88. [Google Scholar]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar]
- Tsaia, C.E.; Lin, L.H. DPPH scavenging capacity of extracts from Camellia seed dregs using polyol compounds as solvents. Heliyon 2019, 5, e02315. [Google Scholar]
- Dong, Y.; Zhao, M.M.; Sun-Waterhouse, X.D.; Zhuang, M.Z.; Chen, H.P.; Feng, M.Y.; Lin, L.Z. Absorption and desorption behaviour of the flavonoids from Glycyrrhiza glabra L. leaf on macroporous adsorption resins. Food Chem. 2015, 168, 538–545. [Google Scholar]
- Hou, Z.Q.; Wang, Y.T.; Zhou, L.; Liao, X.J. Processing of chestnut rose juice using three-stage ultra-filtration combined with high pressure processing. LWT Food Sci. Technol. 2021, 143, 111127. [Google Scholar]
- Wan, W.J.; Xia, N.; Zhu, S.M.; Liu, Q.; Gao, Y.C. Synthesis and characterization of a novel soluble hesperetin monoglucosidecopper (II) complex using ion exchange column. Inorg. Chim. Acta 2020, 512, 119857. [Google Scholar] [CrossRef]
- Li, J.; Chase, H.A. Use of expanded bed adsorption to purify flavonoids from Ginkgo biloba L. J. Chromatogr. A 2009, 1216, 8759–8770. [Google Scholar] [CrossRef]
- Limwachiranon, J.; Huang, H.; Li, L.; Duan, Z.H.; Luo, Z.S. Recovery of lotus (Nelumbo nucifera Gaertn.) seedpod flavonoids using polar macroporous resins: The updated understanding on adsorption/desorption mechanisms and the involved intermolecular attractions and bonding. Food Chem. 2019, 299, 125108. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chase, H.A. Development of adsorptive (non-ionic) macroporous resins and their uses in the purification of pharmacologically-active natural products from plant sources. Nat. Prod. Rep. 2010, 27, 1493–1510. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zhang, Q.; Pan, H.; Luan, L.; Liu, X.; Wu, Y. Optimization of integrated extraction-adsorption process for the extraction and purification of total flavonoids from Scutellariae barbatae herba. Sep. Purif. Technol. 2017, 175, 203–212. [Google Scholar] [CrossRef]
- Yin, P.P.; Yang, L.G.; Li, K.; Fan, H.; Xue, Q.; Li, X.; Sun, L.W.; Liu, Y.J. Bioactive components and antioxidant activities of oak cup crude extract and its four partially purified fractions by HPD-100 macroporous resin chromatography. Arab. J. Chem. 2019, 12, 249–261. [Google Scholar] [CrossRef]
- Xu, X.Q.; Liu, A.M.; Hu, S.Y.; Ares, I.; Martínez-Larrañaga, M.R.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M.A. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef]
- Vrchovska, V.; Sousa, C.; Valentao, P.; Ferreres, F.; Pereira, J.A.; Seabra, R.M.; Andrade, P.B. Antioxidative properties of tronchuda cabbage (Brassica oleracea L. var. costata DC) external leaves against DPPH, superoxide radical, hydroxyl radical and hypochlorous acid. Food Chem. 2006, 98, 416–425. [Google Scholar] [CrossRef]
- Grazul, M.; Budzisz, E. Biological activity of metal ions complexes of chromones, coumarins and flavones. Coord. Chem. Rev. 2009, 253, 2588–2598. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Wianowska, D.; Olszowy, M. On practical problems in estimation of antioxidant activity of compounds by DPPH method (Problems in estimation of antioxidant activity). Food Chem. 2012, 131, 1037–1043. [Google Scholar] [CrossRef]
- Gillespie, K.M.; Chae, J.M.; Ainsworth, E.A. Rapid measurement of total antioxidant capacity in plants. Nat. Protoc. 2007, 2, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, I.; Bajkacz, S. A new UHPLC-MS/MS method for the determination of flavonoids in supplements and DPPH%-UHPLC-UV method for the evaluation of the radical scavenging activity of flavonoids. Food Chem. 2018, 256, 333–341. [Google Scholar] [CrossRef]
- Fahimeh, N.; Naser, S.; Bahram, M.S.; Shams-Bakhsh, M.; Sharif, M. Bacillus subtilis affects miRNAs and flavanoids production in Agrobacterium-Tobacco interaction. Plant Physiol. Biochem. 2017, 118, 98–106. [Google Scholar]
- Pan, L.C.; Zhu, Y.M.; Zhu, Z.Y.; Xue, W.; Liu, C.Y.; Sun, H.Q.; Yin, Y. Chemical structure and effects of antioxidation and against α-glucosidaseof natural polysaccharide from Glycyrrhiza inflata Batalin. Int. J. Biol. Macromol. 2020, 155, 560–571. [Google Scholar] [CrossRef]
- Tang, D.; Zhu, J.X.; Nie, H.; He, B.; Xu, Y.H.; Zhu, Q. Simple and efficient approach for enrichment of major isoflavonoids from Astragalus membranaceus with macroporous resins and their nephroprotective activities. Ind. Crop. Prod. 2020, 125, 276–283. [Google Scholar] [CrossRef]
- Jiang, H.; Li, J.; Chen, L.; Wang, Z. Adsorption and desorption of chlorogenic acid by macroporous adsorbent resins during extraction of Eucommia ulmoides leaves. Ind. Crop. Prod. 2020, 149, 112336. [Google Scholar] [CrossRef]
- Kong, F.S.; Ding, Z.D.; Zhang, K.; Duan, W.J.; Qin, Y.R.; Su, Z.P. Optimization of extraction flavonoids from Exocarpium Citri Grandis and evaluation its hypoglycemic and hypolipidemic activities. J. Ethnopharmacol. 2020, 262, 113178. [Google Scholar] [CrossRef] [PubMed]
- Bier, M.C.J.; Medeiros, A.B.P.; Kimpe, N.D.; Soccol, C.R. Evaluation of antioxidant activity of the fermented product from the biotransformation of R-(+)-limonene in solid-state fermentation of orange waste by Diaporthe sp. Biotechnol. Res. Innov. 2019, 3, 168–176. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-woodll, M.; Prior, R. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Atki, Y.E.; Aouam, I.; Kamari, F.E.; Taroq, A.; Lyoussi, B.; Taleb, M.; Abdellaou, A. Total phenolic and flavonoid contents and antioxidant activities of extracts from Teucrium polium growing wild in Morocco. Mater. Today 2019, 13, 777–783. [Google Scholar]

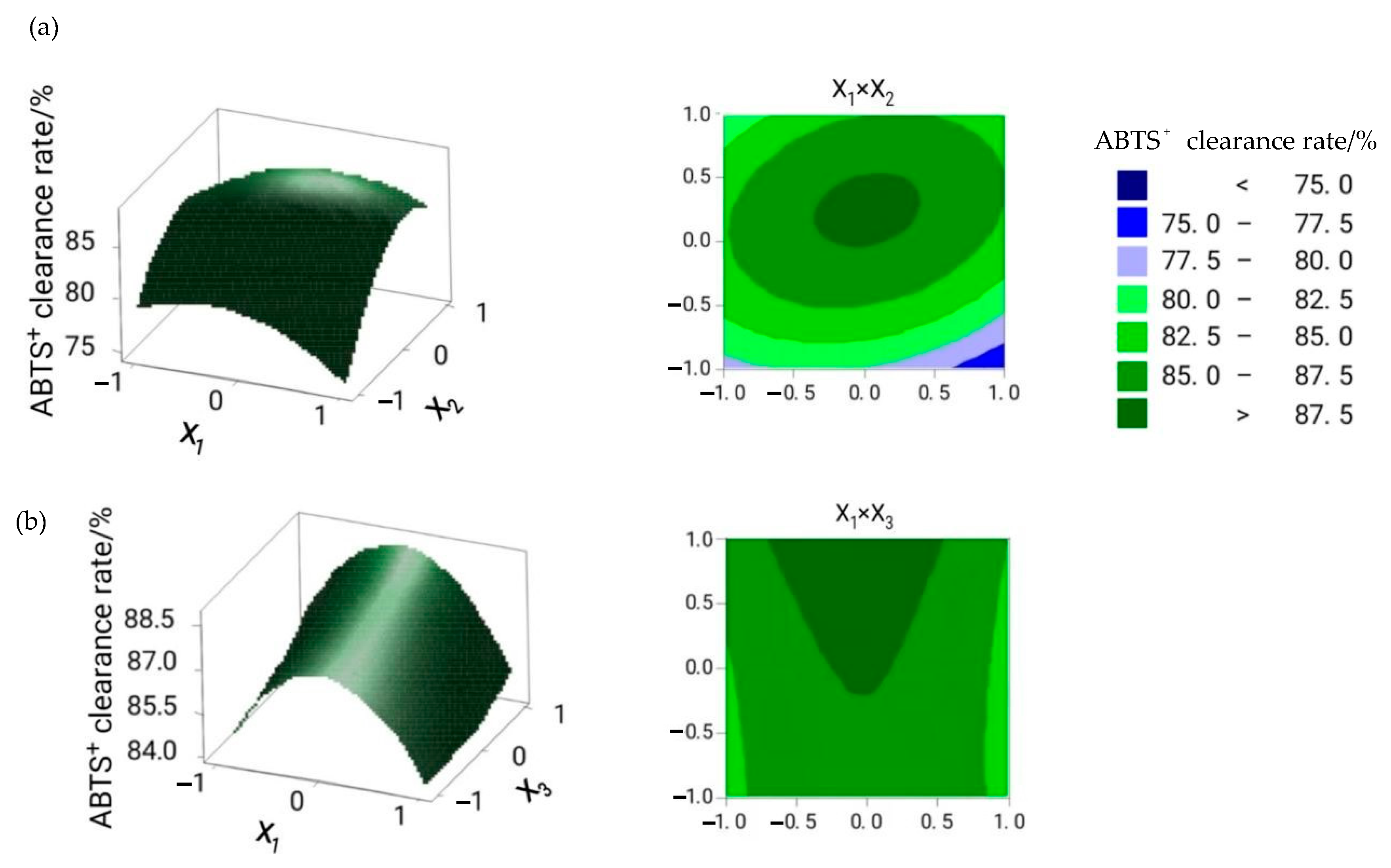
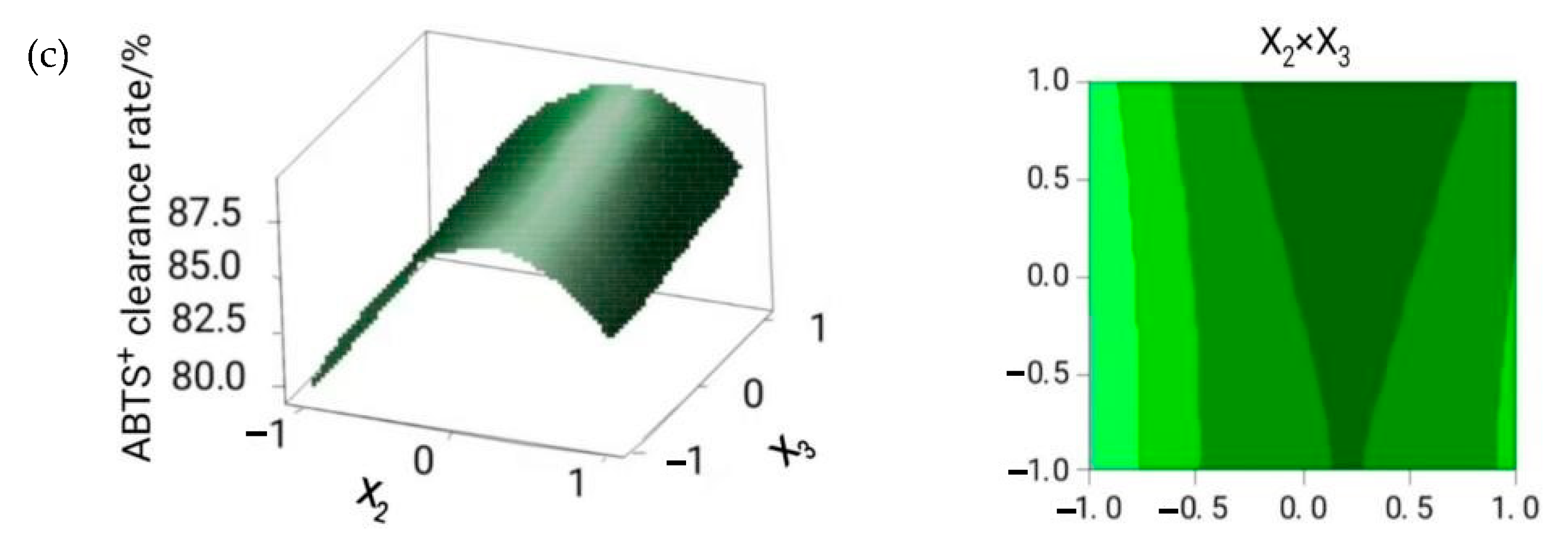
| Test Number | X1 Cellulase Dosage/(U/g) | X2 Material/liquid Ratio/(g/mL) | X3 Extraction Time/h | Flavonoid Yield/% | ABTS + Clearance Rate/% |
|---|---|---|---|---|---|
| 1 | 75 (−1) | 1:10 (−1) | 2 (0) | 3.07 | 78.10 |
| 2 | 105 (+1) | 1:10 (−1) | 2 (0) | 3.12 | 75.18 |
| 3 | 75 (−1) | 1:30 (+1) | 2 (0) | 3.19 | 79.71 |
| 4 | 105 (+1) | 1:30 (+1) | 2 (0) | 3.28 | 84.08 |
| 5 | 75 (−1) | 1:20 (0) | 1 (−1) | 3.30 | 84.67 |
| 6 | 105 (+1) | 1:20 (0) | 1 (−1) | 3.27 | 83.36 |
| 7 | 75 (−1) | 1:20 (0) | 3 (+1) | 3.28 | 86.86 |
| 8 | 105 (+1) | 1:20 (0) | 3 (+1) | 3.24 | 84.53 |
| 9 | 90 (0) | 1:10 (−1) | 1 (−1) | 2.74 | 80.15 |
| 10 | 90 (0) | 1:30 (+1) | 1 (−1) | 3.21 | 84.23 |
| 11 | 90 (0) | 1:10 (−1) | 3 (+1) | 2.64 | 80.88 |
| 12 | 90 (0) | 1:30 (+1) | 3 (+1) | 3.17 | 85.84 |
| 13 | 90 (0) | 1:20 (0) | 2 (0) | 3.41 | 87.59 |
| 14 | 90 (0) | 1:20 (0) | 2 (0) | 3.38 | 88.32 |
| 15 | 90 (0) | 1:20 (0) | 2 (0) | 3.46 | 87.01 |
| Source | DF | Seq SS | Adj SS | F-Value | p-Value |
|---|---|---|---|---|---|
| Regression | 9 | 0.644568 | 0.071619 | 4.80 | 0.049 * |
| Linear | 3 | 0.209925 | 0.069975 | 4.69 | 0.065 |
| Square | 3 | 0.433318 | 0.144439 | 9.68 | 0.016 * |
| Interaction | 3 | 0.001325 | 0.000442 | 0.03 | 0.992 |
| Residual Error | 5 | 0.074592 | 0.014918 | 14.56 | 0.065 |
| Lack-of-Fit | 3 | 0.071325 | 0.023775 | ||
| Pure Error | 2 | 0.003267 | 0.001633 | ||
| Total | 14 | ||||
| Regression | 9 | 198.433 | 22.0482 | 24.50 | 0.001 * |
| Linear | 3 | 52.436 | 17.4787 | 19.42 | 0.003 * |
| Square | 3 | 132.258 | 44.0859 | 48.98 | 0.000 * |
| Interaction | 3 | 13.740 | 4.5799 | 5.09 | 0.056 |
| Interaction | 3 | 13.740 | 4.5799 | 5.09 | 0.056 |
| Residual Error | 5 | 4.500 | 0.9001 | 2.81 | 0.273 |
| Lack-of-Fit | 3 | 3.639 | 1.2129 | ||
| Pure Error | 2 | 0.862 | 0.4309 | ||
| Total | 14 |
| Item | Coefficient | St Dev | T | p-Value |
|---|---|---|---|---|
| Constant | 3.41667 | 0.07052 | 48.451 | 0.000 |
| X1 | 0.00875 | 0.04318 | 0.203 | 0.847 |
| X2 | 0.16000 | 0.04318 | 3.705 | 0.014 * |
| X3 | −0.02375 | 0.04318 | −0.550 | 0.606 |
| X12 | 0.04042 | 0.06356 | 0.636 | 0.553 |
| X22 | −0.29208 | 0.06356 | −4.595 | 0.006 * |
| X32 | −0.18458 | 0.06356 | −2.904 | 0.034 * |
| X1×X2 | 0.01000 | 0.06107 | 0.164 | 0.876 |
| X1×X3 | −0.00250 | 0.06107 | −0.041 | 0.969 |
| X2×X3 | 0.01500 | 0.06107 | 0.246 | 0.816 |
| X1 | −0.2737 | 0.3354 | −0.816 | 0.452 |
| X2 | 2.4438 | 0.3354 | 7.285 | 0.001 * |
| X3 | 0.7125 | 0.3354 | 2.124 | 0.087 |
| X12 | −3.1463 | 0.4937 | −6.372 | 0.001 * |
| X22 | −5.2263 | 0.4937 | −10.585 | 0.000 * |
| X32 | 0.3612 | 0.4937 | 0.732 | 0.497 |
| X1×X2 | 1.8225 | 0.4744 | 3.842 | 0.012 * |
| X1×X3 | −0.2550 | 0.4744 | −0.538 | 0.614 |
| X2×X3 | 0.2200 | 0.4744 | 0.464 | 0.662 |
| Test Number | A | B | C | D | Flavonoid Recovery (%) |
|---|---|---|---|---|---|
| Sample Concentration (mg/mL) | pH | Eluent Concentration (%) | Water Washing Volume (BV) | ||
| 1 | 1.0 | 5 | 30 | 3 | 43.54 |
| 2 | 1.0 | 6 | 50 | 4 | 64.07 |
| 3 | 1.0 | 7 | 70 | 5 | 66.91 |
| 4 | 1.5 | 5 | 50 | 5 | 76.07 |
| 5 | 1.5 | 6 | 70 | 3 | 64.05 |
| 6 | 1.5 | 7 | 30 | 4 | 37.69 |
| 7 | 2.0 | 5 | 70 | 4 | 75.67 |
| 8 | 2.0 | 6 | 30 | 5 | 32.73 |
| 9 | 2.0 | 7 | 50 | 3 | 71.17 |
| K1j | 174.52 | 195.28 | 113.95 | 178.76 | 531.9(T) |
| K2j | 177.81 | 160.84 | 211.30 | 177.43 | |
| K3j | 179.57 | 175.76 | 206.63 | 175.71 | |
| k1j | 58.17 | 65.09 | 37.99 | 59.59 | 59.1 |
| k2j | 59.27 | 53.62 | 70.43 | 59.14 | |
| k3j | 59.86 | 58.59 | 68.88 | 58.57 | |
| Rj | 1.86 | 11.48 | 32.45 | 1.02 |
| Source of Variation | SS | df | MS | F | Fa | Significant |
|---|---|---|---|---|---|---|
| A (Sample Concentration) | 4.3805 | 2 | 2.1902 | 2.8100 | F0.05 (2,8) = 4.46 | |
| B (pH) | 198.3023 | 2 | 99.1511 | 127.2093 | ** | |
| C (Eluent Concentration) | 2009.2594 | 2 | 1004.6297 | 1288.9232 | F0.01 (2,8) = 8.65 | ** |
| Error | 1.5589 | 2 | 0.7794 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Ma, W.; Wang, C.; Yang, X.; Lou, Y.; Xia, X.; Xu, H. Optimization of Enzyme-Assisted Extraction and Purification of Flavonoids from Pinus koraiensis Nut-Coated Film and Antioxidant Activity Evaluation. Molecules 2021, 26, 1950. https://doi.org/10.3390/molecules26071950
Zhang M, Ma W, Wang C, Yang X, Lou Y, Xia X, Xu H. Optimization of Enzyme-Assisted Extraction and Purification of Flavonoids from Pinus koraiensis Nut-Coated Film and Antioxidant Activity Evaluation. Molecules. 2021; 26(7):1950. https://doi.org/10.3390/molecules26071950
Chicago/Turabian StyleZhang, Mingyan, Wuchao Ma, Chao Wang, Ximing Yang, Yuhang Lou, Xinxiu Xia, and Hongyan Xu. 2021. "Optimization of Enzyme-Assisted Extraction and Purification of Flavonoids from Pinus koraiensis Nut-Coated Film and Antioxidant Activity Evaluation" Molecules 26, no. 7: 1950. https://doi.org/10.3390/molecules26071950
APA StyleZhang, M., Ma, W., Wang, C., Yang, X., Lou, Y., Xia, X., & Xu, H. (2021). Optimization of Enzyme-Assisted Extraction and Purification of Flavonoids from Pinus koraiensis Nut-Coated Film and Antioxidant Activity Evaluation. Molecules, 26(7), 1950. https://doi.org/10.3390/molecules26071950






