Mid-Infrared Spectroscopy as a Rapid Tool to Qualitatively Predict the Effects of Species, Regions and Roasting on the Nutritional Composition of Australian Acacia Seed Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Spectra Collection
2.3. Data Analysis
3. Results and Discussion
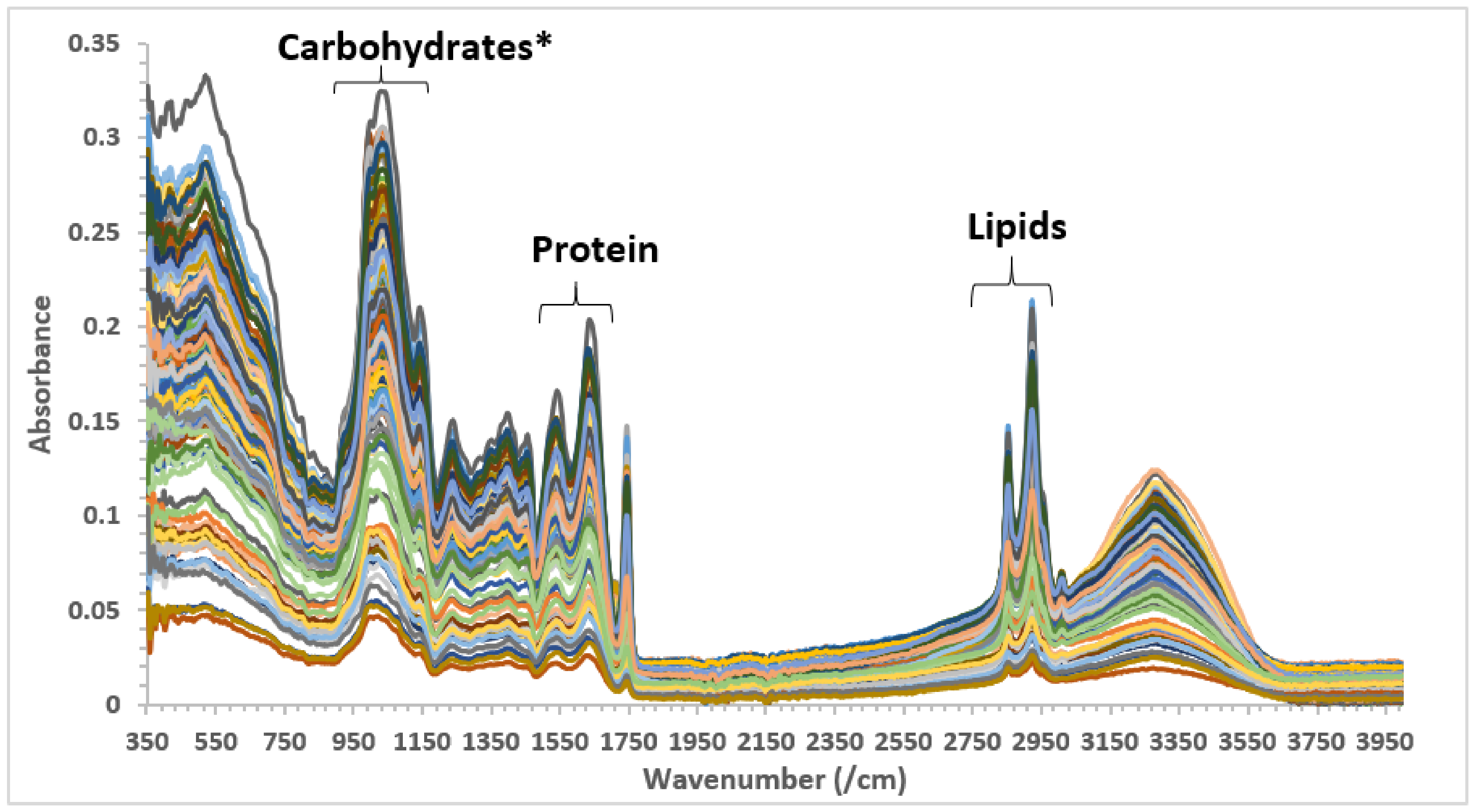
3.1. Essential Features in the MIR Spectra Related to the AS Samples
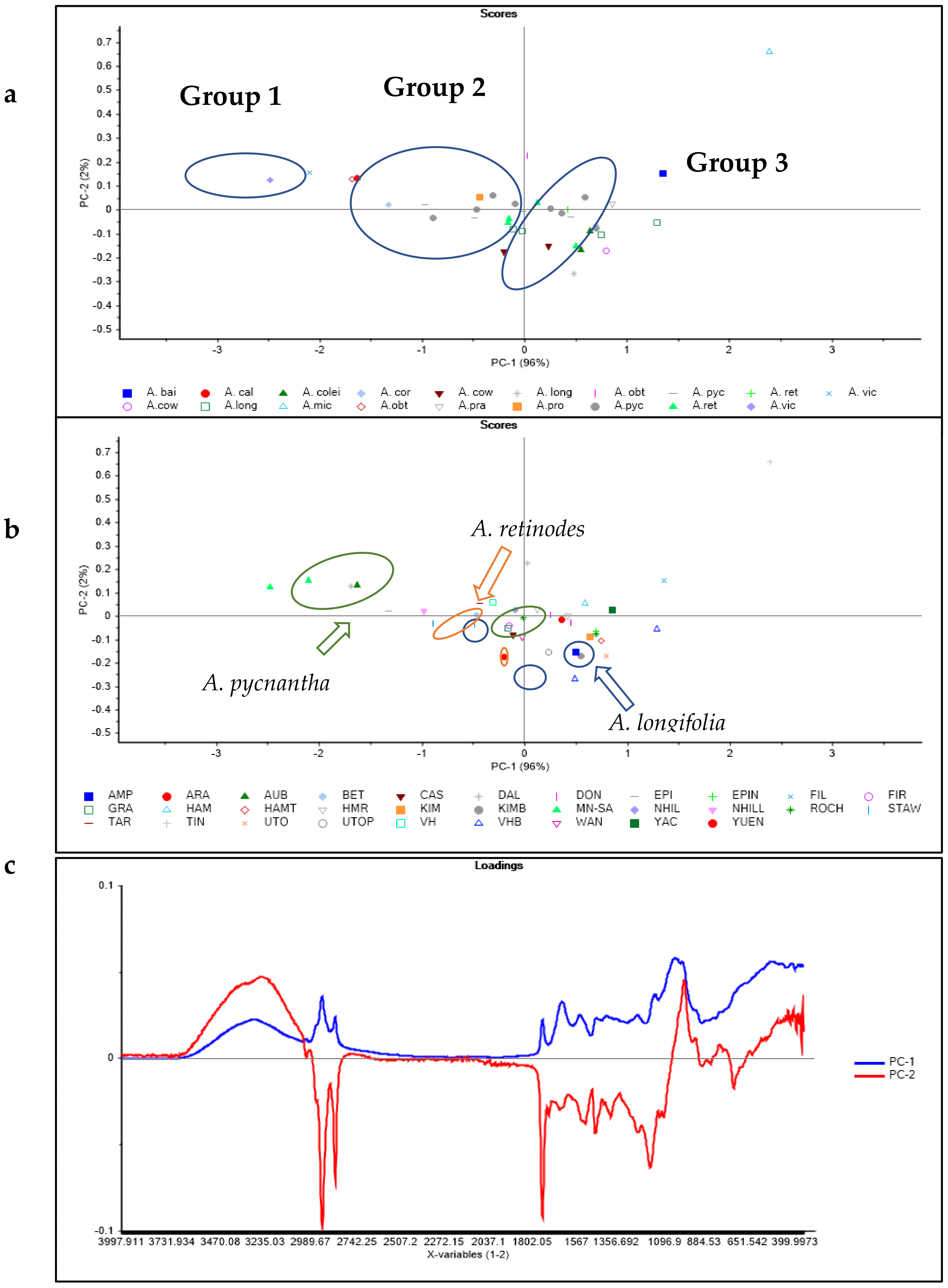
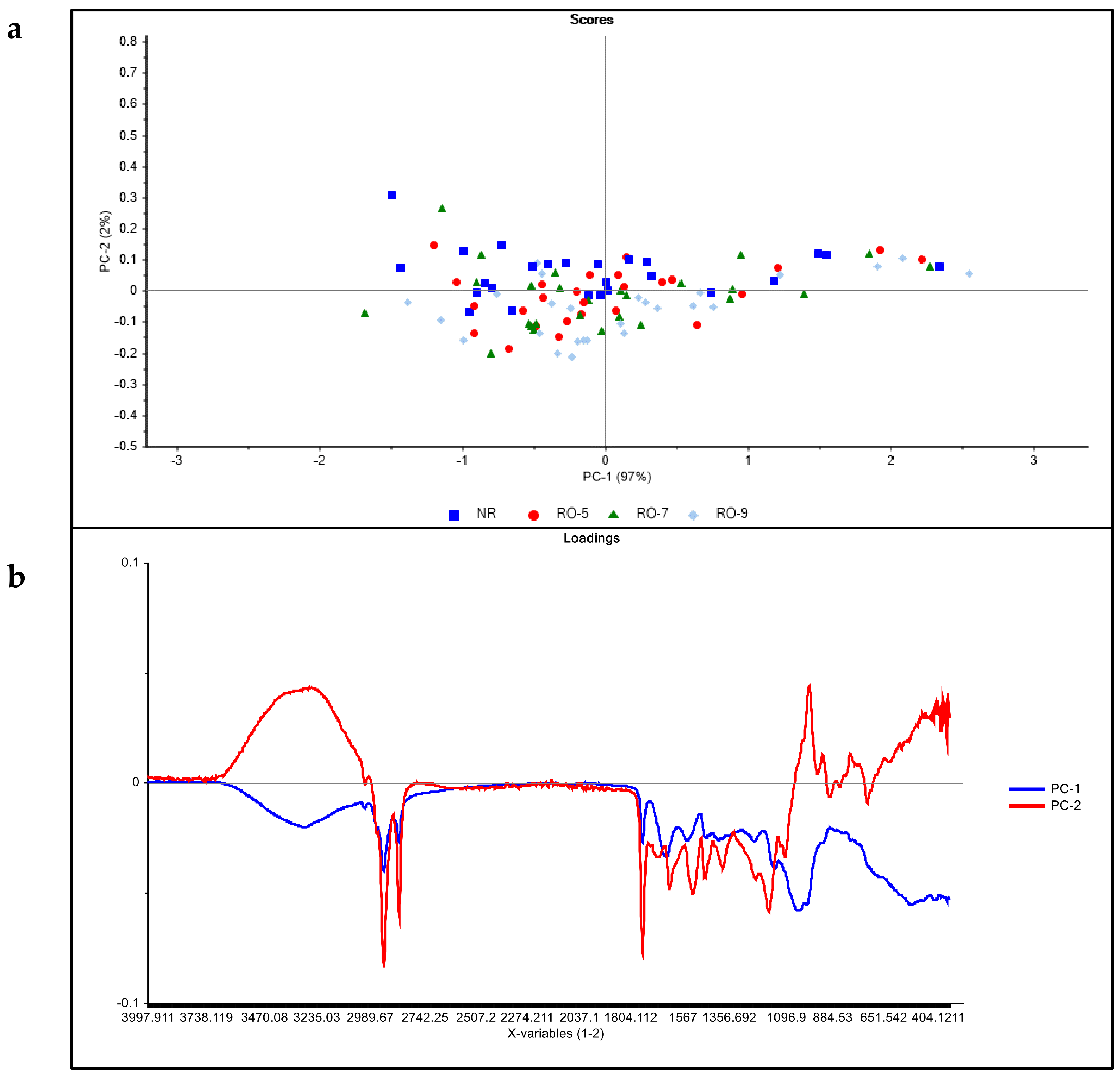
3.2. Principal Component Analysis
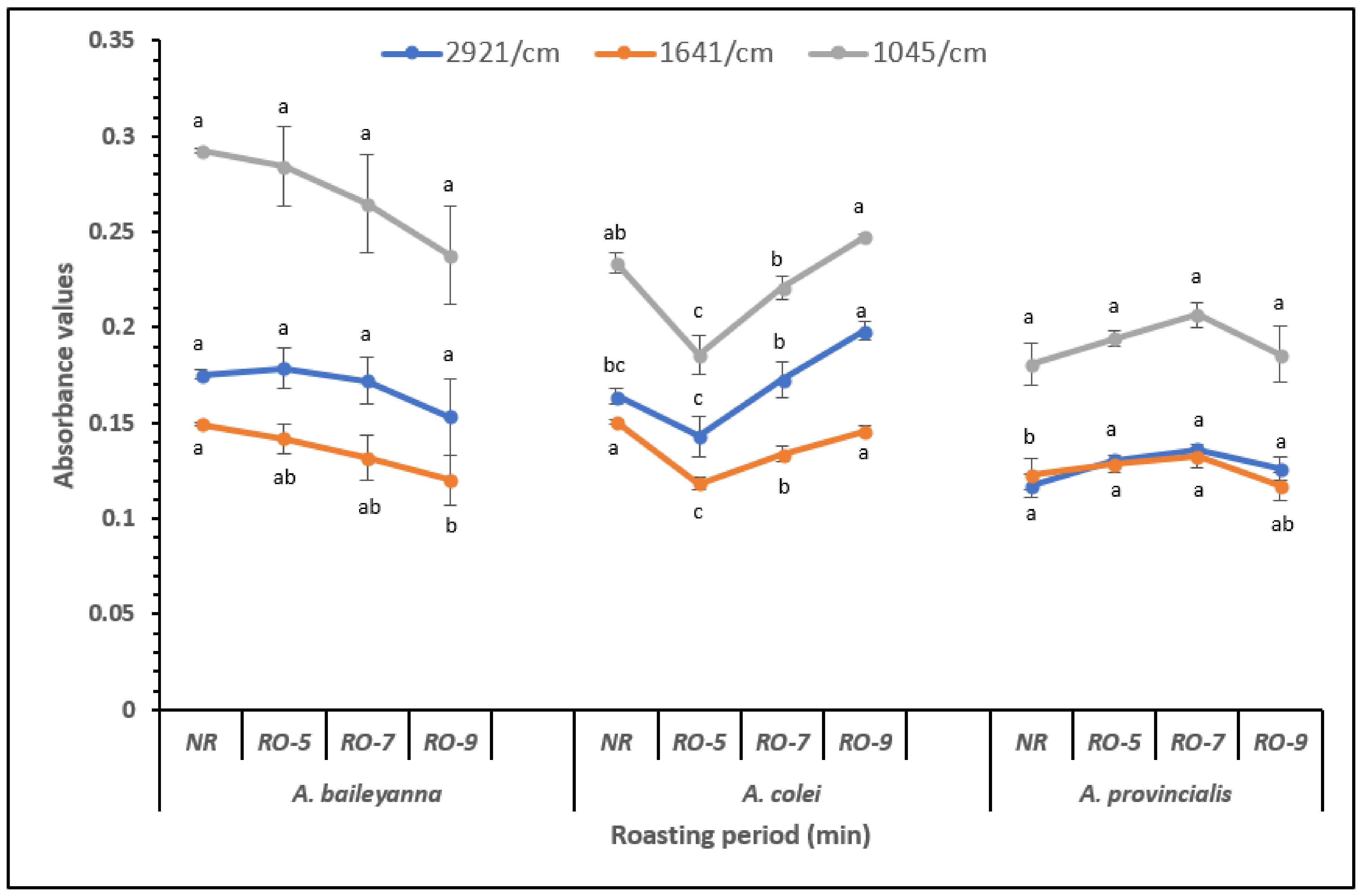
3.3. Analysis of Absorbance at Specific Wavenumbers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Maslin, B.R.; Thomson, L.; McDonald, M.; Hamilton-Brown, S. Edible Wattle Seeds of Southern Australia: A Review of Species for Use in Semi-Arid Regions; CSIRO: Canberra, Australia, 1998. [Google Scholar]
- Simpson, S.; Chudleigh, P. Wattle Seed Production in Low Rainfall Areas. In A Report for the Rural Industries Research and Development Corporation (RIRDC)/Land and Water Australia/Forest and Wood Products (FWPRDC), Joint Venture Agroforestry Program; RIRDC Publication No 01/08, RIRDC Project No AGT-7A; Rural Industries Research and Development Corporation: Canberra, Australia, 2001. [Google Scholar]
- Maslin, B.R.; Miller, J.T.; Seigler, D.S. Overview of the generic status of Acacia (Leguminosae: Mimosoideae). Aust. Syst. Bot. 2003, 16, 1–18. [Google Scholar] [CrossRef]
- Latz, P.K.; Green, J. Bushfires & Bushtucker: Aboriginal Plant Use in Central Australia; IAD Press: Alice Springs, Australia, 1995. [Google Scholar]
- Lister, P.R.; Holford, P.; Morrison, D.A. Acacia in Australia: Ethnobotany and potential food crop. In Progress in New Crops; ASHA Press: Alexandria, VA, USA, 1996. [Google Scholar]
- Konczak, I.; Zabaras, D.; Dunstan, M.; Aguas, P. Antioxidant capacity and phenolic compounds in commercially grown native Australian herbs and spices. Food Chem. 2010, 122, 260–266. [Google Scholar] [CrossRef]
- Adiamo, O.Q.; Netzel, M.E.; Hoffman, L.C.; Sultanbawa, Y. Acacia seed proteins: Low or high quality? A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 19, 21–43. [Google Scholar] [CrossRef]
- Shelat, K.J.; Adiamo, O.Q.; Mantilla, S.M.O.; Smyth, H.E.; Tinggi, U.; Hickey, S.; Rühmann, B.; Sieber, V.; Sultanbawa, Y. Overall Nutritional and Sensory Profile of Different Species of Australian Wattle Seeds (Acacia spp.): Potential Food Sources in the Arid Semi-Arid Regions. Foods 2019, 8, 482. [Google Scholar] [CrossRef] [PubMed]
- Ee, K.Y.; Zhao, J.; Rehman, A.-U.; Agboola, S. Effects of Roasting on the Characteristics of Australian Wattle (Acacia victoriaeBentham) Seed and Extracts. Int. J. Food Prop. 2013, 16, 1135–1147. [Google Scholar] [CrossRef]
- Forbes-Smith, M.; Paton, J. Innovative Products from Australian Native Foods; Rural Industries Research Development Corporation: Canberra, Australia, 2002. [Google Scholar]
- Hegarty, M.P.; Hegarty, E.E.; Wills, R.B.H. Food Safety of Australian Plant Bushfoods: RIRDC Publication No. 01; Union Offset Printing: Canberra, Australia, 2001. [Google Scholar]
- Baeten, V.; Dardenne, P. Spectroscopy: Developments in instrumentation and analysis. Grasas y Aceites 2002, 53, 45–63. [Google Scholar] [CrossRef]
- Barbin, D.F.; Felicio, A.L.D.S.M.; Sun, D.-W.; Nixdorf, S.L.; Hirooka, E.Y. Application of infrared spectral techniques on quality and compositional attributes of coffee: An overview. Food Res. Int. 2014, 61, 23–32. [Google Scholar] [CrossRef]
- Cozzolino, D. Infrared methods for high throughput screening of metabolites: Food and medical applications. Comb. Chem. High Throughput Screen. 2011, 14, 125–131. [Google Scholar] [CrossRef]
- Gordon, R.; Chapman, J.; Power, A.; Chandra, S.; Roberts, J.; Cozzolino, D. Mid-infrared spectroscopy coupled with chemometrics to identify spectral variability in Australian barley samples from different production regions. J. Cereal Sci. 2019, 85, 41–47. [Google Scholar] [CrossRef]
- Bauer, R.; Nieuwoudt, H.; Bauer, F.F.; Kossmann, J.; Koch, K.R.; Esbensen, K.H. FTIR Spectroscopy for Grape and Wine Analysis. Anal. Chem. 2008, 80, 1371–1379. [Google Scholar] [CrossRef]
- Liao, Y.; Fan, Y.; Cheng, F. On-line prediction of pH values in fresh pork using visible/near-infrared spectroscopy with wavelet de-noising and variable selection methods. J. Food Eng. 2012, 109, 668–675. [Google Scholar] [CrossRef]
- Plans, M.; Simó, J.; Casañas, F.; Sabaté, J.; Rodriguez-Saona, L. Characterization of common beans (Phaseolus vulgaris L.) by infrared spectroscopy: Comparison of MIR, FT-NIR and dispersive NIR using portable and benchtop instruments. Food Res. Int. 2013, 54, 1643–1651. [Google Scholar] [CrossRef]
- Subramanian, A.; Prabhakar, V.; Rodriguez-Saona, L. Infrared spectroscopy in dairy analysis. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J., Ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 115–123. [Google Scholar]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Cozzolino, D.; Power, A.; Chapman, J. Interpreting and Reporting Principal Component Analysis in Food Science Analysis and Beyond. Food Anal. Methods 2019, 12, 2469–2473. [Google Scholar] [CrossRef]
- Carbonaro, M.; Maselli, P.; Dore, P.; Nucara, A. Application of Fourier transform infrared spectroscopy to legume seed flour analysis. Food Chem. 2008, 108, 361–368. [Google Scholar] [CrossRef]
- Cozzolino, D.; Roumeliotis, S.; Eglinton, J. Feasibility study on the use of attenuated total reflectance infrared spectroscopy as high throughput screening tool to phenotype single barley seeds (Hordeum vulgare L.). Biosyst. Eng. 2013, 116, 379–384. [Google Scholar] [CrossRef]
- Cozzolino, D.; Degner, S.; Eglinton, J. Study of the role of sugar fatty esters in explaining differences in the malt composition of barley analysed using vibrational spectroscopy and chemometrics. Anal. Methods 2015, 7, 6152–6157. [Google Scholar] [CrossRef]
- Kacuráková, M. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Nakanishi, K.; Solomon, P.H. Infrared Absorption Spectroscopy, 2nd ed.; Holden-Day, Inc.: San Francisco, CA, USA, 1977; Chapter 2. [Google Scholar]
- Fernández, K.; Agosin, E. Quantitative Analysis of Red Wine Tannins Using Fourier-Transform Mid-Infrared Spectrometry. J. Agric. Food Chem. 2007, 55, 7294–7300. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Zhihua, L.; Jiyong, S.; Zhai, X.; Wang, S.; Mariod, A.A. Rapid prediction of phenolic compounds and antioxidant activity of Sudanese honey using Raman and Fourier transform infrared (FT-IR) spectroscopy. Food Chem. 2017, 226, 202–211. [Google Scholar] [CrossRef]
- Jackson, M.; Mantsch, H.H. The Use and Misuse of FTIR Spectroscopy in the Determination of Protein Structure. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Chukwumah, Y.; Walker, L.; Vogler, B.; Verghese, M. Changes in the Phytochemical Composition and Profile of Raw, Boiled, and Roasted Peanuts. J. Agric. Food Chem. 2007, 55, 9266–9273. [Google Scholar] [CrossRef] [PubMed]
- Ee, K.; Yates, P. Nutritional and antinutritional evaluation of raw and processed Australian wattle (Acacia saligna) seeds. Food Chem. 2013, 138, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, A.; Ayedun, H.; Sanni, L. Chemical composition and functional properties of raw and roasted Nigerian benniseed (Sesamum indicum) and bambara groundnut (Vigna subterranean). Food Chem. 2008, 111, 277–282. [Google Scholar] [CrossRef]
- Alessandrini, L.; Romani, S.; Pinnavaia, G.; Rosa, M.D. Near infrared spectroscopy: An analytical tool to predict coffee roasting degree. Anal. Chim. Acta 2008, 625, 95–102. [Google Scholar] [CrossRef]
- Oracz, J.; Nebesny, E. Effect of roasting parameters on the physicochemical characteristics of high-molecular-weight Maillard reaction products isolated from cocoa beans of different Theobroma cacao L. groups. Eur. Food Res. Technol. 2019, 245, 111–128. [Google Scholar] [CrossRef]
- Skåra, T.; Stormo, S.K.; Skipnes, D.; Kondjoyan, A.; Sivertsen, A.; Gins, G.; Van Derlinden, E.; Valdramidis, V.P.; Van Impe, J.F. Estimation of surface temperature and thermal load in short-time heat treatment of surimi through reflectance spectroscopy and heat transfer modeling. J. Food Eng. 2014, 120, 75–80. [Google Scholar] [CrossRef]
- Stormo, S.K.; Sivertsen, A.H.; Heia, K.; Skipnes, D. Endpoint temperature of heat-treated surimi can be measured by visible spectroscopy. Food Control. 2012, 26, 92–97. [Google Scholar] [CrossRef]
- Mubaiwa, J.; Fogliano, V.; Chidewe, C.; Linnemann, A.R. Bambara groundnut (Vigna subterranea (L.) Verdc.) flour: A functional ingredient to favour the use of an unexploited sustainable protein source. PLoS ONE 2018, 13, e0205776. [Google Scholar] [CrossRef]
- Agboola, S.; Ee, K.; Huhn, A. Functional properties of processed Australian wattle (Acacia victoriae Bentham) seed extracts. Food Chem. 2012, 133, 990–997. [Google Scholar] [CrossRef]
| Species (spp. Abbreviation *) | Common Name | Regions | Abbreviation ** | Harvest Date |
|---|---|---|---|---|
| Acacia baileyana (A.bai) | Cootamundra wattle | Firgrove bulk, South West (SW) Victoria | FIL | December 2019 |
| Acacia calamifolia (A. cal) | Wallowa wattle | Auburn, South Australia (SA) | AUB | December 2019 |
| Acacia colei (A. col) | Cole’s wattle | Kimberly, Western Australia (WA) | KIM | December 2019 |
| Acacia coriacea (A. cor) | Wiry wattle | Epinara station, North East (NE) Alice Springs | EPI | November 2018 |
| Acacia cowleana (A. cow) | Halls creek wattle | Utopia, NE Alice Springs | UTO | November 2018 |
| Yuendumu, North West (NW) Alice Springs | YUEN | November 2018 | ||
| Acacia longifoliaspp. longifolia (A. lon) | Coastal wattle | Hamilton/Firgrove | HAMT | December 2019 |
| Wannon, SW Victoria | WAN | December 2019 | ||
| Casterton (Schurmanns), SW Victoria | CAS | December 2019 | ||
| Acacia longifolia spp. sophorae (A. long) | Coastal wattle | Victor Harbour, SA coast | VHB | December 2019 |
| Acacia microbotrya (A. mic) | Manna wattle | Tincurren, WA | TIN | December 2019 |
| Acacia retinodes (A. ret) | Swamp wattle | Harmans road, SW, Victoria | HMR | January 2020 |
| Firgrove sein Bulk, SW Victoria | FIR | December 2019 | ||
| Amphitheatre, SW Victoria | AMP | December 2019 | ||
| Grampians, SW Victoria | GRA | December 2019 | ||
| Acacia pravissima (A. pra) | Ovens wattle | Yackandandah, NE Victoria | YAC | December 2019 |
| Acacia provincialis (A. pro) | Water/swamp wattle | Tarrington, SW Victoria | TAR | December 2018 |
| Acacia pycnantha (A. pyc) | Golden wattle | Rochester, NE Victoria | ROCH | December 2019 |
| Nhill, NW Victoria | NHILL | December 2019 | ||
| Donald, NW Victoria | DON | December 2019 | ||
| Stawell, NW Victoria | STAW | December 2019 | ||
| Ararat, SW Victoria | ARA | December 2019 | ||
| Betley, Central Victoria | BET | December 2019 | ||
| Victor Harbour, SA coast | VH | December 2019 | ||
| Hamilton/Wycliffe Seins bulk, SW Victoria | HAM | December 2019 | ||
| Acacia obteca (A. obt) | Dalwallinu, WA | DAL | December 2019 | |
| Acacia victoriae (A. vic) | Elegant wattle | Mid-north, SA | MN-SA | December 2019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adiamo, O.Q.; Sultanbawa, Y.; Cozzolino, D. Mid-Infrared Spectroscopy as a Rapid Tool to Qualitatively Predict the Effects of Species, Regions and Roasting on the Nutritional Composition of Australian Acacia Seed Species. Molecules 2021, 26, 1879. https://doi.org/10.3390/molecules26071879
Adiamo OQ, Sultanbawa Y, Cozzolino D. Mid-Infrared Spectroscopy as a Rapid Tool to Qualitatively Predict the Effects of Species, Regions and Roasting on the Nutritional Composition of Australian Acacia Seed Species. Molecules. 2021; 26(7):1879. https://doi.org/10.3390/molecules26071879
Chicago/Turabian StyleAdiamo, Oladipupo Q., Yasmina Sultanbawa, and Daniel Cozzolino. 2021. "Mid-Infrared Spectroscopy as a Rapid Tool to Qualitatively Predict the Effects of Species, Regions and Roasting on the Nutritional Composition of Australian Acacia Seed Species" Molecules 26, no. 7: 1879. https://doi.org/10.3390/molecules26071879
APA StyleAdiamo, O. Q., Sultanbawa, Y., & Cozzolino, D. (2021). Mid-Infrared Spectroscopy as a Rapid Tool to Qualitatively Predict the Effects of Species, Regions and Roasting on the Nutritional Composition of Australian Acacia Seed Species. Molecules, 26(7), 1879. https://doi.org/10.3390/molecules26071879








