On the Identification and Quantification of Ergothioneine and Lovastatin in Various Mushroom Species: Assets and Challenges of Different Analytical Approaches
Abstract
1. Introduction
2. Results and Discussion
2.1. Results of Validation of LC-MS Methods
2.2. Determination of Ergothioneine and Lovastatin Content of Mushrooms Produced in Conventional Substrates
2.3. Determination of Ergothioneine and Lovastatin Content of P. citrinopileatus Mushrooms from Different Substrates
2.4. Comparing Analytical Approaches for the Identification and Quantification of Ergothioneine and Lovastatin in Mushrooms
2.4.1. Ultraviolet-visibleSpectroscopy (UV–Vis)
2.4.2. Liquid Chromatography–Mass Spectrometry (LC–MS)
3. Materials and Methods
3.1. Reagents and Standards
3.2. Biological Material–Mushroom Cultivation
3.3. Sample Preparation
3.4. Extraction Procedure
3.5. Ergothioneine–Lovastatin Analysis
3.5.1. Ultraviolet–Visible Spectroscopy (UV–Vis)
3.5.2. Liquid Chromatography–Mass Spectrometry (LC–MS)
3.6. Liquid Chromatography–Mass Spectrometry Methods’ Validation
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Rathore, H.; Prasad, S.; Sharma, S. Mushroom Nutraceuticals for Improved Nutrition and Better Human Health: A Review. Pharma Nutr. 2017, 5, 35–46. [Google Scholar] [CrossRef]
- Gargano, M.L.; van Griensven, L.J.; Isikhuemhen, O.S.; Lindequist, U.; Venturella, G.; Wasser, S.P.; Zervakis, G.I. Meidicinal mushrooms: Valuable biological resources of high exploitation potential. Plant Biosyst. 2017, 151, 548–565. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Recent Developments in Mushrooms as Anti-Cancer Therapeutics: A Review. 3 Biotech 2012, 2, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Koutrotsios, G.; Patsou, M.; Mitsou, E.K.; Bekiaris, G.; Kotsou, M.; Tarantilis, P.; Pletsa, V.; Kyriakou, A.; Zervakis, G.I. Valorization of olive by-products as substrates for the cultivation of Ganoderma lucidum and Pleurotus ostreatus mushrooms with enhanced functional and prebiotic properties. Catalysts 2019, 9, 537. [Google Scholar] [CrossRef]
- El Enshasy, H.A.; Hatti-Kaul, R. Mushroom Immunomodulators: Unique Molecules with Unlimited Applications. Trends Biotechnol. 2013, 31, 668–677. [Google Scholar] [CrossRef]
- Boulaka, Α.; Christodoulou, P.; Vlassopoulou, M.; Koutrotsios, G.; Bekiaris, G.; Zervakis, G.I.; Kyriacou, A.; Zervou, M.; Georgiadis, P.; Pletsa, V. Genoprotective properties and metabolites of β-glucan-rich edible mushrooms following in vitro human faecal fermentation. Molecules 2020, 25, 3554. [Google Scholar] [CrossRef]
- Muszyńska, B.; Grzywacz-Kisielewska, A.; Kała, K.; Gdula-Argasińska, J. Anti-Inflammatory Properties of Edible Mushrooms: A Review. Food Chem. 2018, 243, 373–381. [Google Scholar] [CrossRef]
- Kała, K.; Kryczyk-Poprawa, A.; Rzewińska, A.; Muszyńska, B. Fruiting Bodies of Selected Edible Mushrooms as a Potential Source of Lovastatin. Eur. Food Res. Technol. 2020, 246, 246–722. [Google Scholar] [CrossRef]
- Reis, F.S.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Chemical Composition and Nutritional Value of the Most Widely Appreciated Cultivated Mushrooms: An Inter-Species Comparative Study. Food Chem. Toxicol. 2012, 50, 191–197. [Google Scholar] [CrossRef]
- Atila, F.; Owaid, M.N.; Shariati, M.A. The Nutritional and Medical Benefits of Agaricus bisporus: A Review. J. Microbiol. Biotechnol. Food Sci. 2017, 7, 281–286. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/TP/visualize (accessed on 25 November 2020).
- Royse, D.J.; Baars, J.; Tan, Q. Edible and Medicinal Mushrooms: Technology and Applications; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 5–13. [Google Scholar]
- Kim, M.Y.; Lee, S.J.; Ahn, J.K.; Kim, E.H.; Kim, M.J.; Kim, S.L.; Moon, H.I.; Ro, H.M.; Kang, E.Y.; Seo, S.H.; et al. Comparison of Free Amino Acid, Carbohydrates Concentrations in Korean Edible and Medicinal Mushrooms. Food Chem. 2009, 113, 386–393. [Google Scholar] [CrossRef]
- Stampfli, A.R.; Blankenfeldt, W.; Seebeck, F.P. Structural Basis of Ergothioneine Biosynthesis. Curr. Opin. Struct. Biol. 2020, 65, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Cheah, I.K.; Tang, R.M.Y. Ergothioneine–a Diet-Derived Antioxidant with Therapeutic Potential. FEBS Lett. 2018, 592, 3357–3366. [Google Scholar] [CrossRef]
- Ey, J.; Schömig, E.; Taubert, D. Dietary Sources and Antioxidant Effects of Ergothioneine. J. Agric. Food Chem. 2007, 55, 6466–6474. [Google Scholar] [CrossRef] [PubMed]
- Lingappa, K.; Babu, C.V.; Siddalingeshwar, K.G.; Pramod, T. Isolation, Screening and Rapid Confirmation of Lovastatin Producing Strains of Aspergillus Terreus. Indian J. Microbiol. 2004, 44, 133–136. [Google Scholar]
- Seenivasan, A.; Subhagar, S.; Aravindan, R.; Viruthagiri, T. Microbial Production and Biomedical Applications of Lovastatin. Indian J. Pharm. Sci. 2008, 70, 701–709. [Google Scholar] [PubMed]
- Patel, Y. Medicinal Properties of Pleurotus Species (Oyster Mushroom): A Review. World J. Fun. Plant. Biol. 2012, 3, 01–12. [Google Scholar]
- Goswami, S.; Vidyarthi, A.S.; Bhunia, B.; Mandal, T. A Review on Lovastatin and Its Production. J. Biochem. Technol. 2013, 4, 581–587. [Google Scholar]
- Mulder, K.C.L.; Mulinari, F.; Franco, O.L.; Soares, M.S.F.; Magalhães, B.S.; Parachin, N.S. Lovastatin Production: From Molecular Basis to Industrial Process Optimization. Biotechnol. Adv. 2015, 33, 648–665. [Google Scholar] [CrossRef]
- Lisec, B.; Radež, I.; Žilnik, L.F. Solvent Extraction of Lovastatin from a Fermentation Broth. Sep. Purif. Technol. 2012, 96, 187–193. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Ho, K.-J.; Hsieh, Y.-J.; Wang, L.-T.; Mau, J.-L. Contents of Lovastatin, γ-Aminobutyric Acid and Ergothioneine in Mushroom Fruiting Bodies and Mycelia. LWT 2012, 47, 274–278. [Google Scholar] [CrossRef]
- Lo, Y.-C.; Lin, S.-Y.; Ulziijargal, E.; Chen, S.-Y.; Chien, R.-C.; Tzou, Y.-J.; Mau, J.-L. Comparative Study of Contents of Several Bioactive Components in Fruiting Bodies and Mycelia of Culinary-Medicinal Mushrooms. Int. J. Med. Mushrooms 2012, 14, 357–363. [Google Scholar] [CrossRef]
- Lam, Y.S.; Okello, E.J. Determination of Lovastatin, β-Glucan, Total Polyphenols, and Antioxidant Activity in Raw and Processed Oyster Culinary-Medicinal Mushroom, Pleurotus ostreatus (Higher Basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Wang, F.; Tu, J.; Peng, W.; Li, H. Determination of Lovastatin in Human Plasma by Ultra-Performance Liquid Chromatography–Electrospray Ionization Tandem Mass Spectrometry and Its Application in a Pharmacokinetic Study. J. Pharm. Biomed. 2008, 46, 808–813. [Google Scholar] [CrossRef]
- Cheah, I.K.; Tang, R.M.Y.; Yew, T.S.Z.; Lim, K.H.C.; Halliwell, B. Administration of Pure Ergothioneine to Healthy Human Subjects: Uptake, Metabolism, and Effects on Biomarkers of Oxidative Damage and Inflammation. Antioxid. Redox. Sign. 2017, 26, 193–206. [Google Scholar] [CrossRef] [PubMed]
- González, A.G.; Herrador, M.Á.; Asuero, A.G. Intra-Laboratory Assessment of Method Accuracy (Trueness and Precision) by Using Validation Standards. Talanta 2010, 82, 1995–1998. [Google Scholar] [CrossRef] [PubMed]
- Sapozhnikova, Y.; Brydwell, W.C.; Lobato, A.; Roming, B. Effect of UV-B radiation levels on concentrations of phytosterols, ergothioneine and polyphenolic compounds in mushroom powders used as dietary supplements. J. Agric. Food Chem. 2014, 62, 3034–3042. [Google Scholar] [CrossRef]
- Maftoun, P.; Johari, H.; Soltani, M.; Malik, R.; Othman, N.Z.; El Enshasy, H.A. The Edible Mushroom Pleurotus Spp.: I. Biodiversity and Nutritional Values. Int. J. Biotechnol. Well. Indus. 2015, 4, 67–83. [Google Scholar]
- Alarcón, J.; Águila, S. Lovastatin Production by Pleurotus Ostreatus: Effects of the C: N Ratio. Z. für Nat. C 2006, 61, 95–98. [Google Scholar] [CrossRef]
- Hajjaj, H.; Niederberger, P.; Duboc, P. Lovastatin Biosynthesis by Aspergillus Terreus in a Chemically Defined Medium. Appl. Environ. Microbiol. 2001, 67, 2596–2602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Z.; Wen, Q.; Xiong, Z.; Cao, X.; Zheng, Z.; Zhang, Y.; Huang, Z. An Overview on the Biosynthesis and Metabolic Regulation of Monacolin K/Lovastatin. Food Funct. 2020, 11, 5738–5748. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Kalogeropoulos, N.; Kaliora, A.; Zervakis, G.I. Toward an increased functionality in oyster (Pleurotus) mushrooms produced on grape marc or olive mill wastes serving as sources of bioactive compounds. J. Agric. Food Chem. 2018, 66, 5971–5983. [Google Scholar] [CrossRef]
- Porterfield, J.Z.; Zlotnick, A. A Simple and General Method for Determining the Protein and Nucleic Acid Content of Viruses by UV Absorbance. Virology 2010, 407, 281–288. [Google Scholar] [CrossRef]
- Hazra, C.; Samanta, T.; Mahalingam, V. A Resonance Energy Transfer Approach for the Selective Detection of Aromatic Amino Acids. J. Mater. Chem. C 2014, 2, 10157–10163. [Google Scholar] [CrossRef]
- Mattila, P.; Salo-Väänänen, P.; Könkö, K.; Aro, H.; Jalava, T. Basic Composition and Amino Acid Contents of Mushrooms Cultivated in Finland. J. Agric. Food Chem. 2002, 50, 6419–6422. [Google Scholar] [CrossRef] [PubMed]
- Chirinang, P.; Intarapichet, K.-O. Amino Acids and Antioxidant Properties of the Oyster Mushrooms, Pleurotus ostreatus and Pleurotus sajor-caju. Sci. Asia 2009, 35, 326–331. [Google Scholar] [CrossRef]
- Lafka, T.-I.; Sinanoglou, V.; Lazos, E.S. On the Extraction and Antioxidant Activity of Phenolic Compounds from Winery Wastes. Food Chem. 2007, 104, 1206–1214. [Google Scholar] [CrossRef]
- Leouifoudi, I.; Harnafi, H.; Zyad, A. Olive Mill Waste Extracts: Polyphenols Content, Antioxidant, and Antimicrobial Activities. Adv. Phar. Sci. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Rubilar, M.; Pinelo, M.; Shene, C.; Sineiro, J.; Nuñez, M.J. Separation and HPLC-MS Identification of Phenolic Antioxidants from Agricultural Residues: Almond Hulls and Grape Pomace. J. Agric. Food Chem. 2007, 55, 10101–10109. [Google Scholar] [CrossRef]
- Fotakis, C.; Kokkotou, K.; Zoumpoulakis, P.; Zervou, M. NMR Metabolite Fingerprinting in Grape Derived Products: An Overview. Food. Res. Int. 2013, 54, 1184–1194. [Google Scholar] [CrossRef]
- Xie, X.; Watanabe, K.; Wojcicki, W.A.; Wang, C.C.C.; Tang, Y. Biosynthesis of Lovastatin Analogs with a Broadly Specific Acyltransferase. Chem. Biol. 2006, 13, 1161–1169. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, F.; Wang, Z.; Hu, Z. Identification and Chemical Profiling of Monacolins in Red Yeast Rice Using High-Performance Liquid Chromatography with Photodiode Array Detector and Mass Spectrometry. J. Pharm. Biomed. 2004, 35, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Kontogianni, V.G.; Gerothanassis, I.P. Phenolic Compounds and Antioxidant Activity of Olive Leaf Extracts. Nat. Prod. Res. 2012, 26, 186–189. [Google Scholar] [CrossRef]
- Seenivasan, A.; Gummadi, S.N.; Panda, T.; Théodore, T. Quantification of Lovastatin Produced by Monascus purpureus. Open Biotechnol. J. 2015, 9, 6–13. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Chen, Y.-K.; Yu, H.-T.; Barseghyan, G.S.; Asatiani, M.D.; Wasser, S.P.; Mau, J.-L. Comparative Study of Contents of Several Bioactive Components in Fruiting Bodies and Mycelia of Culinary-Medicinal Mushrooms. Int. J. Med. Mushrooms 2013, 15, 315–323. [Google Scholar] [CrossRef]
- Weigand-Heller, A.J.; Kris-Etherton, P.M.; Beelman, R.B. The Bioavailability of Ergothioneine from Mushrooms (Agaricus bisporus) and the Acute Effects on Antioxidant Capacity and Biomarkers of Inflammation. Prev. Med. 2012, 54, S75–S78. [Google Scholar] [CrossRef]
- Cohen, N.; Cohen, J.; Asatiani, M.D.; Varshney, V.K.; Yang, Y.-C.; Li, Y.-H.; Mau, J.-H.; Wasser, S.P. Chemical composition and nutritional and medicinal value of fruit bodies and submerged cultured mycelia of culinary-medicinal higher basidiomycetes mushrooms. Int. J. Med. Mushrooms 2014, 16, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Koutrotsios, G.; Mountzouris, K.C.; Chatzipavlidis, I.; Zervakis, G.I. Bioconversion of Lignocellulosic Residues by Agrocybe cylindracea and Pleurotus ostreatus Mushroom Fungi–Assessment of Their Effect on the Final Product and Spent Substrate Properties. Food Chem. 2014, 161, 127–135. [Google Scholar] [CrossRef]
- Ma, L.; Chen, H.; Zhu, W.; Wang, Z. Effect of Different Drying Methods on Physicochemical Properties and Antioxidant Activities of Polysaccharides Extracted from Mushroom Inonotus obliquus. Food. Res. Int. 2013, 50, 633–640. [Google Scholar] [CrossRef]
- Wujian, J.; Kuan-Wei, P.; Sihyung, Y.; Huijing, S.; Mario, S.; Zhuo, W.M. A Simple Protein Precipitation-Based Simultaneous Quantification of Lovastatin and Its Active Metabolite Lovastatin Acid in Human Plasma by Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry Using Polarity Switching. J. Chromatogr. Sep. Tech. 2015, 6, 268–287. [Google Scholar] [PubMed]
- Union, P.O. of the E. CELEX1, 2002/657/EC: Commission Decision of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results (Text with EEA relevance) (notified under document number C(2002) 3044). Available online: https://op.europa.eu/el/publication-detail/-/publication/ed928116-a955-4a84-b10a-cf7a82bad858/language-en (accessed on 15 November 2020).
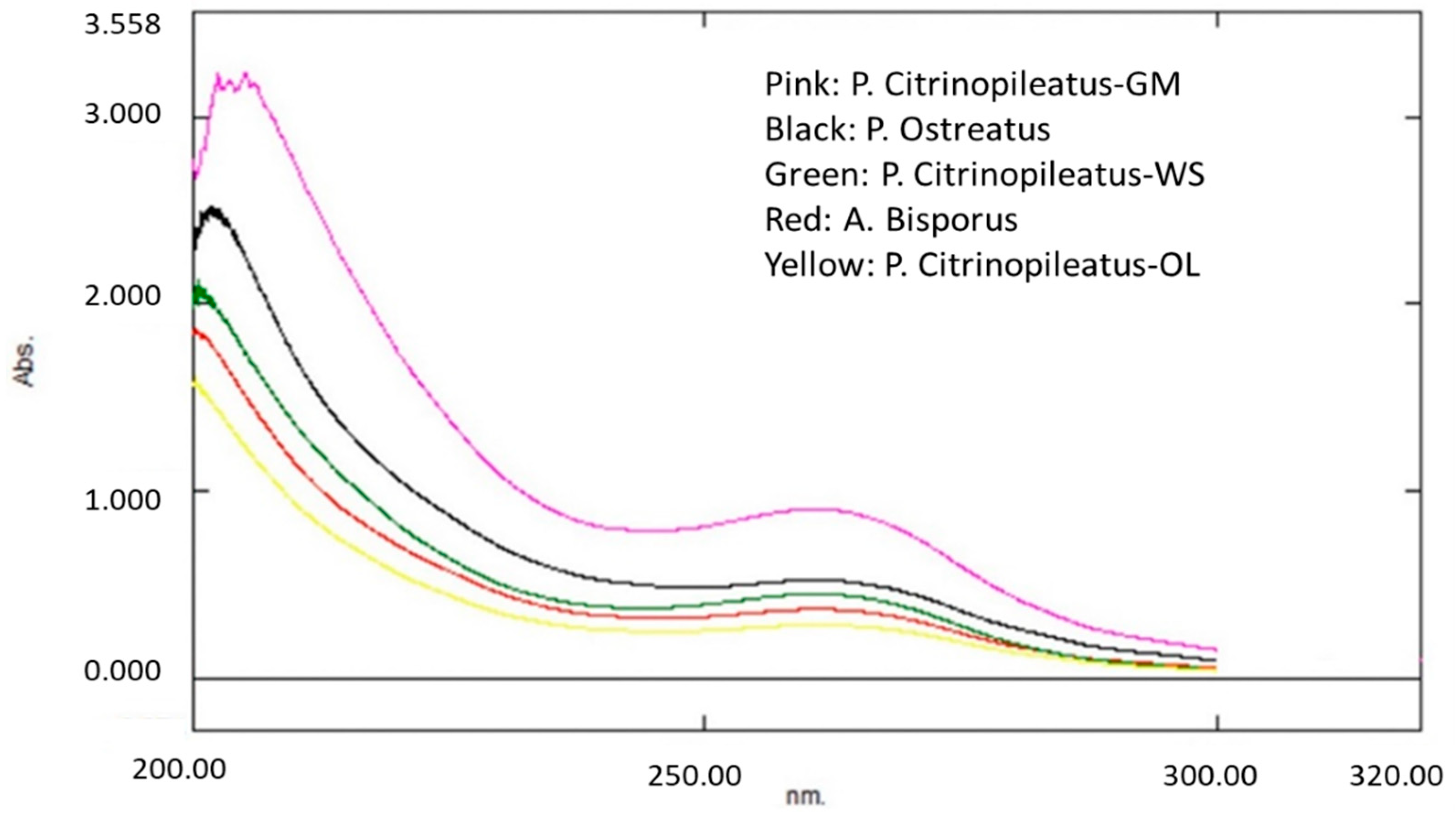
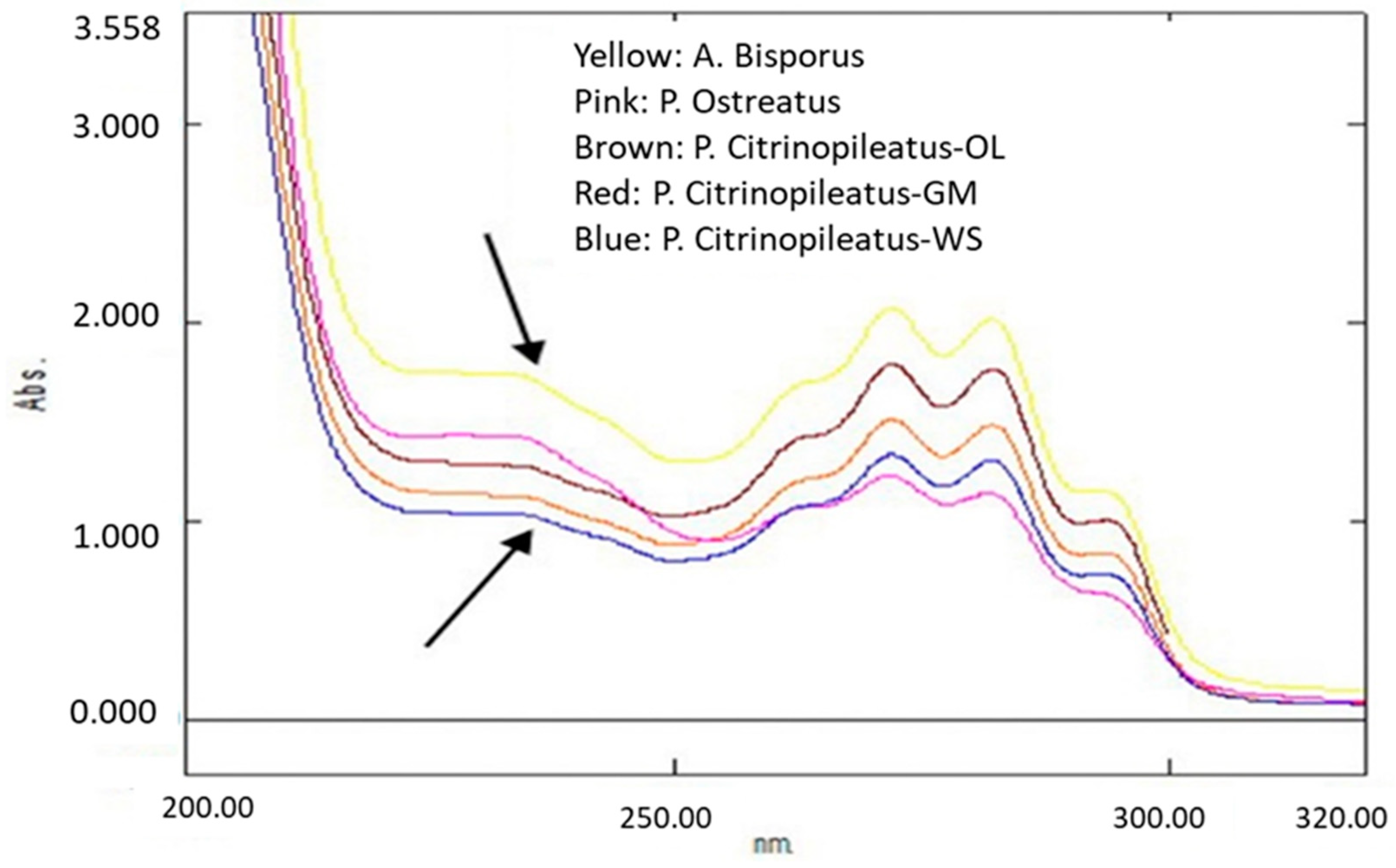


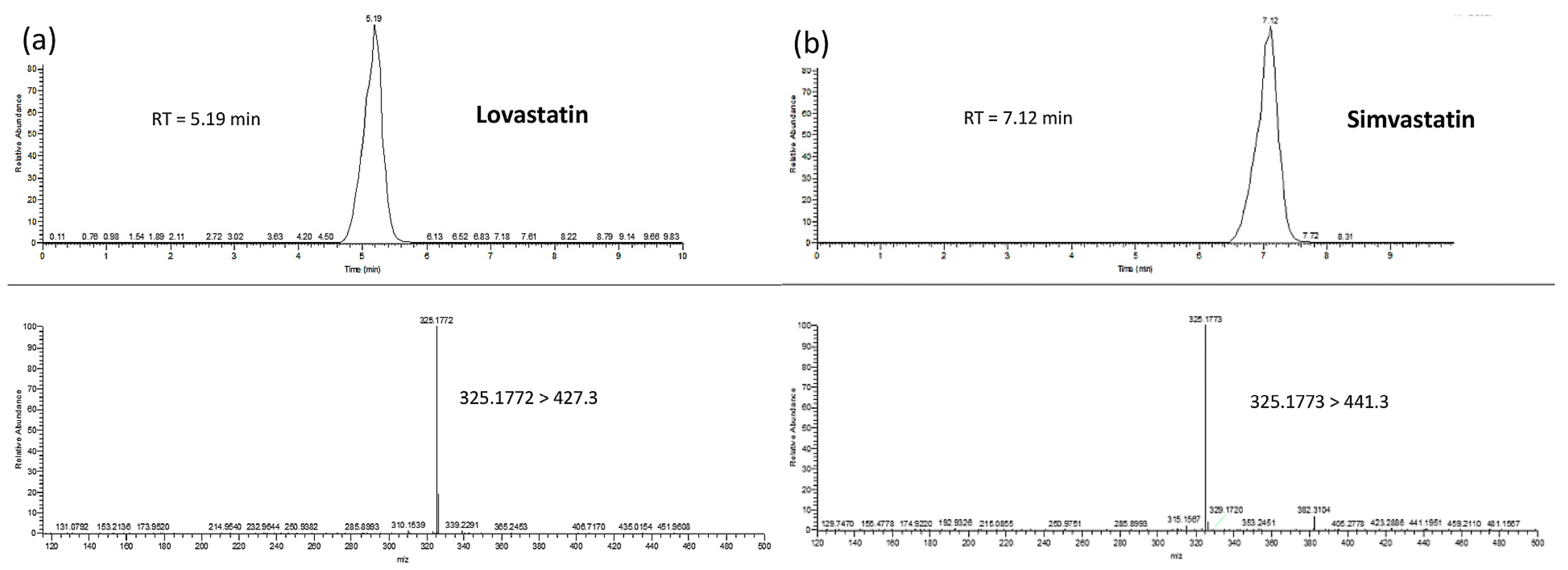
| Analytical Figures of Merit | ESH | LOV |
|---|---|---|
| Concentration range (μg mL−1) | 0.05–45 (n = 14) 1 | 0.001–1 (n = 10) 1 |
| Slope (a) (± standard error-sa) | 0.0307 (±0.00023) | 35.47 (±0.18) |
| Intercept (b) (± standard error-sb) | 0.0012 (±0.0051) | 0.090 (±0.065) |
| R2 (Correlation coefficient) | 0.9993 | 0.9998 |
| Limit of Detection-LoD (μg mL−1) | 0.02 | 0.00039 |
| Limit of Quantification LoQ (μg mL−1) | 0.06 | 0.0012 |
| Analyte | Quality Control Levels | ||
|---|---|---|---|
| Ergothioneine | 5.0 μg mL−1 (n = 3) 2 | 25.0 μg mL−1 (n = 3) 2 | 40 μg mL−1 (n = 3) 2 |
| Intra–Day Precision (%RSD) | 4.0 | 2.0 | 0.2 |
| Inter–Day Precision (%RSD) N = 3 1 | 7.5 | 1.9 | 2.5 |
| Accuracy | 102.95 | 100.95 | 99.67 |
| Matrix Effect (%) | 68.4 | 83.5 | 75.0 |
| Lovastatin | 0.005 μg mL−1 (n = 3) 2 | 0.05 μg mL−1 (n = 3) 2 | 0.5 μg mL−1 (n = 3) 2 |
| Intra–Day Precision (%RSD) | 13.6 | 4.91 | 4.04 |
| Inter–Day Precision (%RSD) N = 3 1 | 0.7 | 3.21 | 1.73 |
| Accuracy | 81.56 | 105.17 | 96.8 |
| Matrix Effect (%) | 42.08 | 15.9 | 8.3 |
| Method | Ergothioneine Content (mg kg−1 Dry Sample) a | ||
|---|---|---|---|
| A. bisporus | P. ostreatus | P. citrinopileatus | |
| UV–Vis | 7100 (±300) c | 9200 (± 800) b | 8300 (±1100) b |
| LC–MS | 521.2 (±14.7) d | 607.3 (±11.2) c | 822.1 (±20.6) b |
| Method | Lovastatin Content (mg kg−1 Dry Sample) a | ||
| UV–Vis | 1050 (±80) b | 930 (±100) b | 840 (±250) b |
| LC-MS | 1.39 (±0.014) b | 1.11 (±0.042) c | 0.158 (±0.005) d |
| Method | Ergothioneine Content (mg kg−1 Dry Sample) a | ||
|---|---|---|---|
| WS | GM | OL | |
| UV–Vis | 8300 (±1100) b | 11800 (±1400) b | 6700 (±1100) b |
| LC–MS | 822.1 (±20.6) b | 637.2 (±24.5) c | 884.5 (±20.0) b |
| Method | Lovastatin Content (mg kg−1 Dry Sample) a | ||
| UV–Vis | 840 (±250) b | 860 (±180) b | 904 (±0.241) b |
| LC-MS | 0.158 (±0.005) c | 0.218 (±0.014) b | 0.161 (±0.009) c |
| Source Parameters | Lovastatin-Simvastatin | Ergothioneine-Methimidazole |
|---|---|---|
| S-LENS RF Amplitude (V) | 60 | 120 |
| Sheath gas flow rate (arbitrary units, a.u) | 8 | 7 |
| Auxiliary gas flow rate (arbitrary units, a.u) | 0 | 0 |
| Sweep gas flow rate (arbitrary units, a.u) | 0 | 0 |
| Vaporizer temperature (°C) | 320 | 300 |
| Capillary temperature (°C) | 220 | 200 |
| Cone voltage (kV) | 4 | 4.5 |
| Isolation mass width | 2 | 1.5 |
| Collision energy (eV) | 33 (lovastatin) 35 (simvastatin) | 15 for ESH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsiantas, K.; Tsiaka, T.; Koutrotsios, G.; Siapi, E.; Zervakis, G.I.; Kalogeropoulos, N.; Zoumpoulakis, P. On the Identification and Quantification of Ergothioneine and Lovastatin in Various Mushroom Species: Assets and Challenges of Different Analytical Approaches. Molecules 2021, 26, 1832. https://doi.org/10.3390/molecules26071832
Tsiantas K, Tsiaka T, Koutrotsios G, Siapi E, Zervakis GI, Kalogeropoulos N, Zoumpoulakis P. On the Identification and Quantification of Ergothioneine and Lovastatin in Various Mushroom Species: Assets and Challenges of Different Analytical Approaches. Molecules. 2021; 26(7):1832. https://doi.org/10.3390/molecules26071832
Chicago/Turabian StyleTsiantas, Konstantinos, Thalia Tsiaka, Georgios Koutrotsios, Eleni Siapi, Georgios I. Zervakis, Nick Kalogeropoulos, and Panagiotis Zoumpoulakis. 2021. "On the Identification and Quantification of Ergothioneine and Lovastatin in Various Mushroom Species: Assets and Challenges of Different Analytical Approaches" Molecules 26, no. 7: 1832. https://doi.org/10.3390/molecules26071832
APA StyleTsiantas, K., Tsiaka, T., Koutrotsios, G., Siapi, E., Zervakis, G. I., Kalogeropoulos, N., & Zoumpoulakis, P. (2021). On the Identification and Quantification of Ergothioneine and Lovastatin in Various Mushroom Species: Assets and Challenges of Different Analytical Approaches. Molecules, 26(7), 1832. https://doi.org/10.3390/molecules26071832








