New Helvolic Acid Derivatives with Antibacterial Activities from Sarocladium oryzae DX-THL3, an Endophytic Fungus from Dongxiang Wild Rice (Oryza rufipogon Griff.)
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification of Fungus Identification
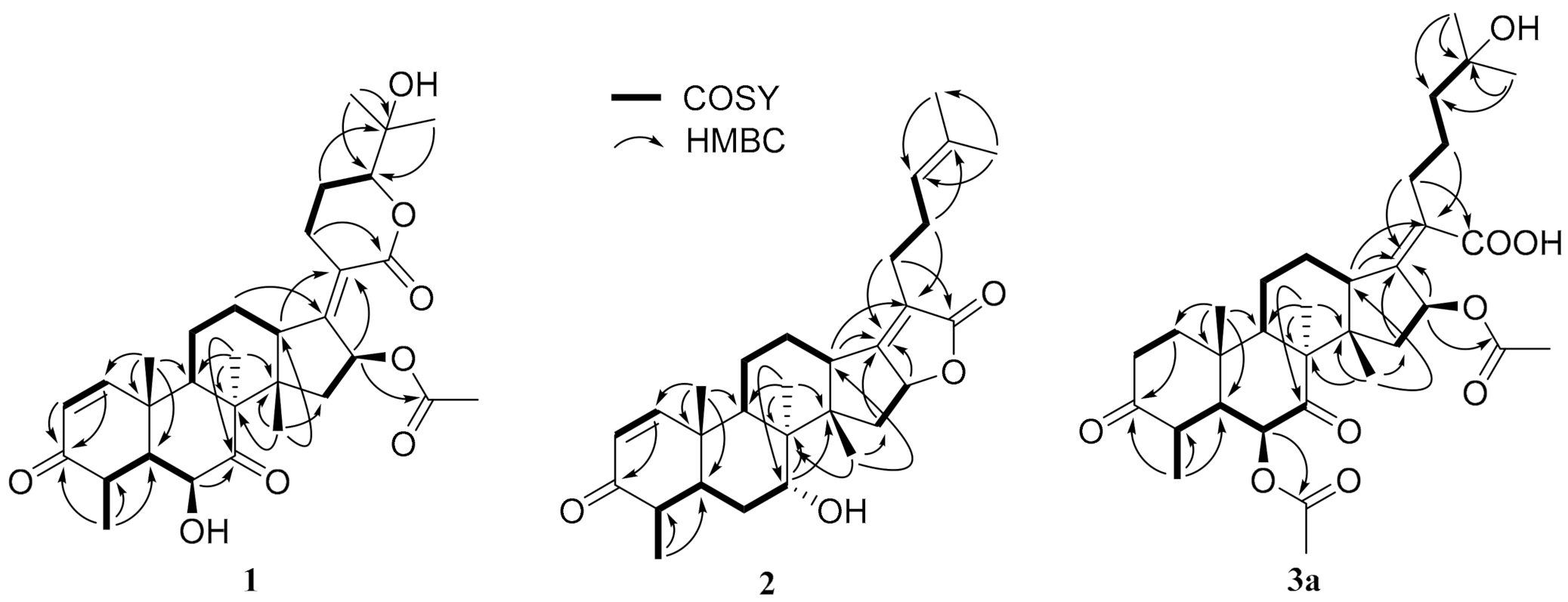
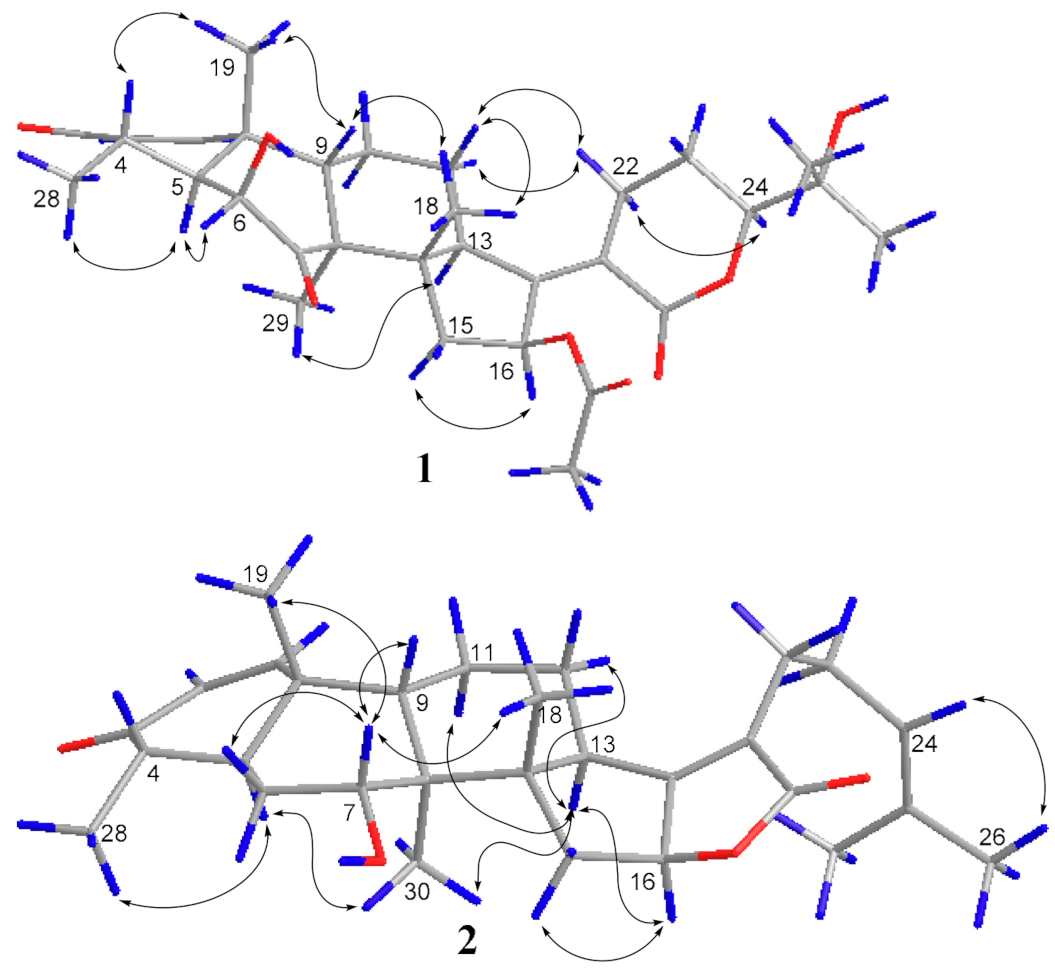
2.2. Structure Elucidation
2.3. Characterization of Compounds 1,2 and 3a
2.4. Biological Activities
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungus Material
3.3. Identification of Strain DX-THL3
3.4. Fermentation, Extraction and Metabolite Isolation
3.5. Antibacterial Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Daletos, G.; Proksch, P. Bioactive Secondary Metabolites from Endophytic Fungi. Curr. Med. Chem. 2020, 27, 1836–1854. [Google Scholar] [CrossRef]
- Venugopalan, A.; Srivastava, S. Endophytes as in vitro production platforms of high value plant secondary metabolites. Biotechnol. Adv. 2015, 33, 873–887. [Google Scholar] [CrossRef]
- Kaul, S.; Gupta, S.; Ahmed, M.; Dhar, M.K. Endophytic fungi from medicinal plants: A treasure hunt for bioactive metabolites. Phytochem. Rev. 2012, 11, 487–505. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, B.L.; Li, X.X.; Bin Zhang, Z.; Yan, R.M.; Yang, H.L.; Zhu, D. Phylogenetic diversity of culturable endophytic fungi in Dongxiang wild rice (Oryza rufipogon Griff), detection of polyketide synthase gene and their antagonistic activity analysis. Fungal Biol. 2015, 119, 1032–1045. [Google Scholar] [CrossRef]
- Ayyadurai, N.; Kirubakaran, S.I.; Srisha, S.; Sakthivel, N. Biological and Molecular Variability of Sarocladium oryzae, the Sheath Rot Pathogen of Rice (Oryza sativa L.). Curr. Microbiol. 2005, 50, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Amudha, R.; Jayachandran, S.; Sakthivel, N. Detection and quantification of phytotoxic metabolites of Sarocladium oryzae in sheath rot-infected grains of rice. Lett. Appl. Microbiol. 2002, 34, 398–401. [Google Scholar] [CrossRef]
- Bills, G.F.; Platas, G.; Gams, W. Conspecificity of the cerulenin and helvolic acid producing ‘Cephalosporium caerulens’, and the hypocrealean fungus Sarocladium oryzae. Mycol. Res. 2004, 108, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Chain, E.; Florey, H.W.; Jennings, M.A.; Williams, T.I. Helvolic Acid, an Antibiotic Produced by Aspergillus fumigatus, mut. helvola Yuill. Br. J. Exp. Pathol. 1943, 24, 108–119. [Google Scholar]
- Iwasaki, S.; Sair, M.I.; Igarashi, H.; Okuda, S. Revised structure of helvoic acid. J. Chem. Soc. D 1970, 17, 1119–1120. [Google Scholar] [CrossRef]
- Von Daehne, W.; Godtfredsen, W.; Rasmussen, P. Structure-Activity Relationships in Fusidic Acid-Type Antibiotics. Adv. Clin. Chem. 1979, 25, 95–146. [Google Scholar] [CrossRef]
- Fernandes, P. Fusidic Acid: A Bacterial Elongation Factor Inhibitor for the Oral Treatment of Acute and Chronic Staphylococcal Infections. Cold Spring Harb. Perspect. Med. 2016, 6, a025437. [Google Scholar] [CrossRef]
- Liang, X.-A.; Ma, Y.-M.; Zhang, H.-C.; Liu, R. A new helvolic acid derivative from an endophytic Fusarium sp. of Ficus carica. Nat. Prod. Res. 2016, 30, 2407–2412. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-H.; Li, T.-X.; Wang, Y.; Liu, R.-H.; Luo, J.; Kong, L.-Y. Antimicrobial metabolites from the plant endophytic fungus Penicillium sp. Fitoterapia 2017, 116, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, W.-L.; Fang, Y.-C.; Zhu, T.-J.; Gu, Q.-Q.; Zhu, W.-M. Cytotoxic alkaloids and antibiotic nordammarane triterpenoids from the marine-derived fungus Aspergillus sydowi. J. Nat. Prod. 2008, 71, 985–989. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Zhuravleva, O.I.; Antonov, A.S.; Kalinovsky, A.I.; Pivkin, M.V.; Menchinskaya, E.S.; Aminin, D.L. New Metabolites from the Marine-Derived Fungus Aspergillus Fumigatus. Nat. Prod. Commun. 2012, 7, 497–500. [Google Scholar] [CrossRef]
- Kong, F.-D.; Huang, X.-L.; Ma, Q.-Y.; Xie, Q.-Y.; Wang, P.; Chen, P.-W.; Zhou, L.-M.; Yuan, J.-Z.; Dai, H.-F.; Luo, D.-Q.; et al. Helvolic Acid Derivatives with Antibacterial Activities against Streptococcus agalactiae from the Marine-Derived Fungus Aspergillus fumigatus HNMF0047. J. Nat. Prod. 2018, 81, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- Zaman, K.H.A.U.; Hu, Z.Q.; Wu, X.H.; Hou, S.B.; Saito, J.; Kondratyuk, T.P.; Pezzuto, J.M.; Cao, S.G. NF-κB Inhibitory and Antibacterial Helvolic and Fumagillin Derivatives from Aspergillus terreus. J. Nat. Prod. 2020, 83, 730–737. [Google Scholar] [CrossRef]
- Liu, D.; Gao, B.; Zhu, K.; Zou, Y.; Zhang, Z. Identification of Several Endophytic Fungi from Dongxiang Wild Rice (Oryza rufipogon Griff.) and its Activities to Rhizotonia solani. J. Yichun Univ. 2015, 37, 6–10. [Google Scholar]
- Okuda, S.; Nakayama, Y.; Tsuda, K. Studies on Microbial Products. I. 7-Desacetoxyhelvolic Acid and Helvolinic Acid. Chem. Pharm. Bull. 1966, 14, 436–441. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Kinoshita, H.; Ihara, F.; Igarashi, Y.; Nihira, T. Identification of novel derivative of helvolic acid from Metarhizium anisopliae grown in medium with insect component. J. Biosci. Bioeng. 2008, 105, 476–480. [Google Scholar] [CrossRef]
- Qin, L.; Li, B.; Guan, J.; Zhang, G. In vitro synergistic antibacterial activities of helvolic acid on multi-drug resistant Staphylococcus aureus. Nat. Prod. Res. 2009, 23, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.-M.; Chuan-Xi, W.; Gao, H.; Kushiro, T.; Awakawa, T.; Chen, G.-D.; Wang, C.-X.; Abe, I.; Yao, X.-S. Biosynthesis of helvolic acid and identification of an unusual C-4-demethylation process distinct from sterol biosynthesis. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sarıkahya, N.B.; Kırmızıgül, S. Antimicrobial Triterpenoid Glycosides from Cephalaria scoparia. J. Nat. Prod. 2010, 73, 825–830. [Google Scholar] [CrossRef] [PubMed]

| Position | 1 a | 2 b | 3a b | 3b b | ||||
|---|---|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 157.8, CH | 7.30 d (10.0) | 161.3, CH | 7.42 d (10.2) | 33.8, CH2 | 2.02 m, 1.66 m | 160.2, CH | 7.50 (d 10.0) |
| 2 | 127.7, CH | 5.85 d (10.0) | 127.9, CH | 5.80 d (10.2) | 37.4, CH2 | 2.50 m | 128.2, CH | 5.83 (d 10.0) |
| 3 | 202.1, C | 204.3, C | 215.7, C | 203.7, C | ||||
| 4 | 40.0, CH | 3.02 dq (12.5, 6.6) | 44.0, CH | 2.38 m | 43.2, CH | 2.59 m | 41.5, CH | 2.83 m |
| 5 | 47.2, CH | 2.22 br d (12.5) | 43.4, CH | 2.41 dd (11.4, 5.7) | 46.4, CH | 2.22 m | 48.3, CH | 2.41 m |
| 6 | 73.5, CH | 4.03 br s | 34.4, CH2 | 1.92 m | 75.0, CHc | 5.19 br s | 74.8, CHc | 5.18 br s |
| 1.65 m | ||||||||
| 7 | 213.2, C | 70.6, CH | 4.04 dd (8.4, 4.1) | 211.3, C | 211.9, C | |||
| 8 | 52.3, C | 46.2, C | 54.2, C | 54.0, C | ||||
| 9 | 41.6, CH | 2.67 dd (13.2, 2.5) | 47.1, CH | 1.66 m | 43.1, CH | 2.65 m | 42.6, CH | 2.65 m |
| 10 | 38.2, C | 40.2, C | 36.2, C | 39.5, C | ||||
| 11 | 24.3, CH2 | 1.96 m | 26.3, CH2 | 2.03 m | 23.7, CH2 | 24.8, CH2 | ||
| 1.57 m | 1.54 m | |||||||
| 12 | 26.6, CH2 | 2.47 m | 22.9, CH2 | 2.14 m | 27.3, CH2 | 2.39 m | 27.3, CH2 | 2.39 m |
| 1.82 m | 1.76 m | 1.74 m | 1.74 m | |||||
| 13 | 51.6, CH | 2.62 br d (12.3) | 44.5, CH | 2.99 dd (12.5, 2.5) | 49.8, CH | 2.70 m | 49.6, CH | 2.70 m |
| 14 | 46.8, C | 56.3, C | 48.1, C | 47.9, C | ||||
| 15 | 41.1, CH2 | 2.12 m | 38.2, CH2 | 2.48 dd (15.2, 11.1) | 41.9, CH2 | 2.33 m | 41.7, CH2 | 2.33 m |
| 2.12 m | 1.32 dd (15.2, 4.7) | 1.75 m | 1.75 m | |||||
| 16 | 73.8, CH | 5.98 br d (6.8) | 84.1, CH | 4.99 dd (11.1, 4.7) | 75.1, CHc | 5.79 (d 8.4) | 75.1, CHc | 5.79 (d 8.4) |
| 17 | 155.0, C | 172.3, C | 145.7, C | 145.7, C | ||||
| 18 | 18.9, CH3 | 1.05 s | 20.7, CH3 | 0.93 s | 18.5, CH3 | 0.91 s | 18.4, CH3 | 0.93 s |
| 19 | 28.3, CH3 | 1.52 s | 25.8, CH3 | 1.14 s | 24.1, CH3 | 1.31 s | 27.8, CH3 | 1.49 s |
| 20 | 125.1, C | 124.4, C | 133.3, C | 133.2, C | ||||
| 21 | 164.8, C | 178.8, C | 174.2, C | 174.1, C | ||||
| 22 | 24.7, CH2 | 3.11 br d (15.2) | 24.9, CH2 | 2.36 m | 25.6, CH2 | 1.48 m | 25.6, CH2 | 1.48 m |
| 2.44 m | ||||||||
| 23 | 24.0, CH2 | 2.06 m | 28.3, CH2 | 2.27 m | 29.8, CH2 | 2.58 m | 29.8, CH2 | 2.58 m |
| 1.74 m | 2.34 m | 2.34 m | ||||||
| 24 | 85.3, CH | 4.09 dd (11.0, 3.6) | 124.4, CH | 5.14 m | 44.2, CH2 | 1.48 m | 44.2, CH2 | 1.48 m |
| 25 | 71.8, C | 133.8, C | 71.2, C | 71.2, C | ||||
| 26 | 25.6, CH3 | 1.27 s | 17.8, CH3 | 1.60 s | 29.3, CH3 d | 1.17 s | 29.2, CH3 d | 1.17 s |
| 27 | 24.2, CH3 | 1.22 s | 25.8, CH3 | 1.68 s | 29.1, CH3 d | 1.18 s | 29.1, CH3 d | 1.18 s |
| 28 | 12.5, CH3 | 1.23 d (7.1) | 12.8, CH3 | 1.10 d (6.1) | 13.4, CH3 | 1.13 (d 6.8) | 13.1, CH3 | 1.24 (d 6.8) |
| 30 | 17.5, CH3 | 1.13 s | 14.6, CH3 | 1.12 s | 17.7, CH3 | 1.36 s | 18.7, CH3 | 1.21 s |
| 6-OAC | 20.6, CH3 e | 1.97 s | 20.6, CH3e | 1.97 s | ||||
| 172.4, C | 172.4, C | |||||||
| 16-OAC | 169.9, C | 20.7, CH3 e | 2.10 s | 20.7, CH3e | 2.12 s | |||
| 20.8, CH3 | 2.01 s | 170.9, C | 170.9, C | |||||
| Position | 4 a | 5 a | 6 a | 7 b | ||||
|---|---|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 157.2, CH | 7.31, d (10.1) | 158.4, CH | 7.33, d (10.0) | 157.6, CH | 7.34, d (10.0) | 33.8, CH2 | |
| 2 | 127.8, CH | 5.87, d (10.1) | 127.5, CH | 5.85, d (10.0) | 128.4, CH | 5.85, d (10.0) | 37.4, CH2 | |
| 3 | 201.4, C | 202.4, C | 201.5, C | 215.7, C | ||||
| 4 | 40.4, CH | 2.78, m | 39.9, CH | 3.05, m | 41.9, CH | 2.56, m | 42.6, CH | |
| 5 | 47.2, CH | 2.27, d (11.3) | 47.1, CH | 2.15, d (12.4) | 43.7, CH | 2.06, overlapped | 46.4, CH | 2.22, d (12.3) |
| 6 | 73.8, CH | 5.24, s | 73.9, CH | 3.99, s | 39.8, CH2 | 2.37, m | 75.1, CH | 5.19, s |
| 2.24, m | ||||||||
| 7 | 208.8, C | 216.0, C | 216.1, C | 211.3, C | ||||
| 8 | 52.6, C | 52.4, C | 52.15, C | 2.06, overlapped | 54.2, C | |||
| 9 | 41.7, CH | 2.62, dd (13.3, 6.7) | 41.3, CH | 2.70, br d(12.8) | 43.3, CH | 43.2, CH | ||
| 10 | 38.1, C | 38.3, C | 38.5, C | 36.2, C | ||||
| 11 | 23.9, CH2 | 1.97, m | 24.0, CH2 | 1.97, m | 24.2, CH2 | 1.93, m | 23.7, CH2 | |
| 1.58, m | 1.55, m | 1.51 m | ||||||
| 12 | 25.9, CH2 | 2.43, m | 25.9, CH2 | 2.40, m | 25.9, CH2 | 2.39, m | 27.4, CH2 | |
| 1.83, m | 1.82, m | 1.76, m | ||||||
| 13 | 49.4, CH | 2.60, dd (13.3, 6.7) | 49.6, CH | 2.57, br d (11.7) | 49.3, CH | 2.53, overlapped | 49.7, CH | 2.67, br d (11.6) |
| 14 | 46.6, C | 46.5, C | 46.8, C | 48.1, C | ||||
| 15 | 40.6, CH2 | 2.25, dd (13.3, 6.7) | 40.8, CH | 2.24, m | 40.6, CH2 | 2.22, m | 41.7, CH2 | 2.32,dd(13.3,6.7) |
| 1.92, d (15.0) | 1.83, m | 1.91, m | 1.74, d (14.7) | |||||
| 16 | 73.4, CH | 5.88, d (8.3) | 73.8, CH | 5.85, d (10.0) | 73.6, CH | 5.87, br d (9.4) | 74.8,CH | 5.78, d (8.3) |
| 17 | 147.9, C | 148.5, C | 147.8, C | 146.2, C | ||||
| 18 | 17.9, CH3 | 0.93, s | 18.2, CH3 | 0.95, s | 17.4, CH3 | 0.90, s | 17.8, CH3 | 0.91, s |
| 19 | 27.5, CH3 | 1.45, s | 28.1, CH3 | 1.55, s | 25.5, CH3 | 1.30, s | 24.1, CH3 | 1.31, s |
| 20 | 130.2, C | 130.4, C | 130.3, C | 132.9, C | ||||
| 21 | 173.5, C | 174.2, C | 174.6, C | 174.0, C | ||||
| 22 | 28.6, CH2 | 2.48, m | 28.4, CH2 | 2.48, m | 28.5, CH2 | 2.48, m | 29.6, CH2 | |
| 23 | 28.3, CH2 | 2.10, m | 28.4, CH2 | 2.06, m | 28.3, CH2 | 2.08, m | 29.1, CH2 | |
| 1.62, m | ||||||||
| 24 | 122.7, CH | 5.11, m | 122.8, CH | 5.10, (t like) | 122.8, CH | 5.10, m | 124.3, CH | 5.14, t (7.0) |
| 25 | 133.0, C | 132.9, C | 132.9, C | 133.4, C | ||||
| 26 | 25.7, CH3 | 1.70, s | 25.7, CH3 | 1.69, s | 25.7, CH3 | 1.69, s | 25.9, CH3 | 1.68, s |
| 27 | 17.8, CH3 | 1.61, s | 17.7, CH3 | 1.60, s | 17.8, CH3 | 1.60, s | 17.7, CH3 | 1.62, s |
| 28 | 13.1, CH3 | 1.28, d (6.8) | 12.4, CH3 | 1.21, d (6.6) | 13.0, CH3 | 1.15, d (6.6) | 13.4, CH3 | 1.12, d (6.7) |
| 30 | 18.3, CH3 | 1.18, s | 17.9, CH3 | 1.13, s | 17.7, CH3 | 1.11, s | 18.5, CH3 | 1.35, s |
| 6-OAC | 168.9, C | 172.4, C | ||||||
| 20.8, CH3 | 2.12, s | 20.6, CH3 | 1.96, s | |||||
| 16-OAC | 170.1, C | 170.9, C | 170.5, C | 170.9, C | ||||
| 20.5, CH3 | 1.95, s | 20.4, CH3 | 1.97, s | 20.5, CH3 | 1.97, s | 20.7, CH3 | 2.10, s | |
| Compounds | MIC (μg/mL) | |||
|---|---|---|---|---|
| S. aureus (ATCC 29213) | B. subtilis | E. coli (ATCC 25922) | X. oryzae pv.oryzicola | |
| 1 | 64 | >128 | >128 | >128 |
| 2 | 4 | >128 | 64 | >128 |
| 4 | 8 | >128 | >128 | >128 |
| 5 | 1 | 64 | 64 | >128 |
| 6 | 4 | >128 | >128 | >128 |
| 7 | 16 | >128 | 64 | >128 |
| Streptomycin sulphate | NT | NT | 8 | 64 |
| tobramycin | 1 | 64 | NT | NT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.-B.; Du, S.-Y.; Ji, B.; Ji, C.-J.; Xiao, Y.-W.; Yan, R.-M.; Zhu, D. New Helvolic Acid Derivatives with Antibacterial Activities from Sarocladium oryzae DX-THL3, an Endophytic Fungus from Dongxiang Wild Rice (Oryza rufipogon Griff.). Molecules 2021, 26, 1828. https://doi.org/10.3390/molecules26071828
Zhang Z-B, Du S-Y, Ji B, Ji C-J, Xiao Y-W, Yan R-M, Zhu D. New Helvolic Acid Derivatives with Antibacterial Activities from Sarocladium oryzae DX-THL3, an Endophytic Fungus from Dongxiang Wild Rice (Oryza rufipogon Griff.). Molecules. 2021; 26(7):1828. https://doi.org/10.3390/molecules26071828
Chicago/Turabian StyleZhang, Zhi-Bin, Si-Yao Du, Bo Ji, Chang-Jiu Ji, Yi-Wen Xiao, Ri-Ming Yan, and Du Zhu. 2021. "New Helvolic Acid Derivatives with Antibacterial Activities from Sarocladium oryzae DX-THL3, an Endophytic Fungus from Dongxiang Wild Rice (Oryza rufipogon Griff.)" Molecules 26, no. 7: 1828. https://doi.org/10.3390/molecules26071828
APA StyleZhang, Z.-B., Du, S.-Y., Ji, B., Ji, C.-J., Xiao, Y.-W., Yan, R.-M., & Zhu, D. (2021). New Helvolic Acid Derivatives with Antibacterial Activities from Sarocladium oryzae DX-THL3, an Endophytic Fungus from Dongxiang Wild Rice (Oryza rufipogon Griff.). Molecules, 26(7), 1828. https://doi.org/10.3390/molecules26071828






