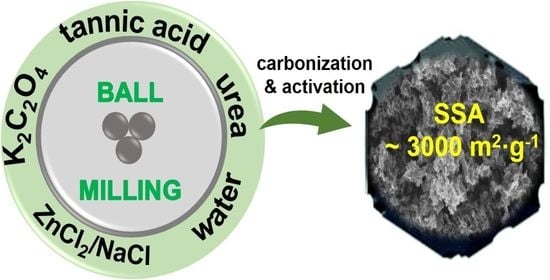
Highly Porous Carbons Synthesized from Tannic Acid via a Combined Mechanochemical Salt-Templating and Mild Activation Strategy
Abstract
1. Introduction
2. Results and Discussion
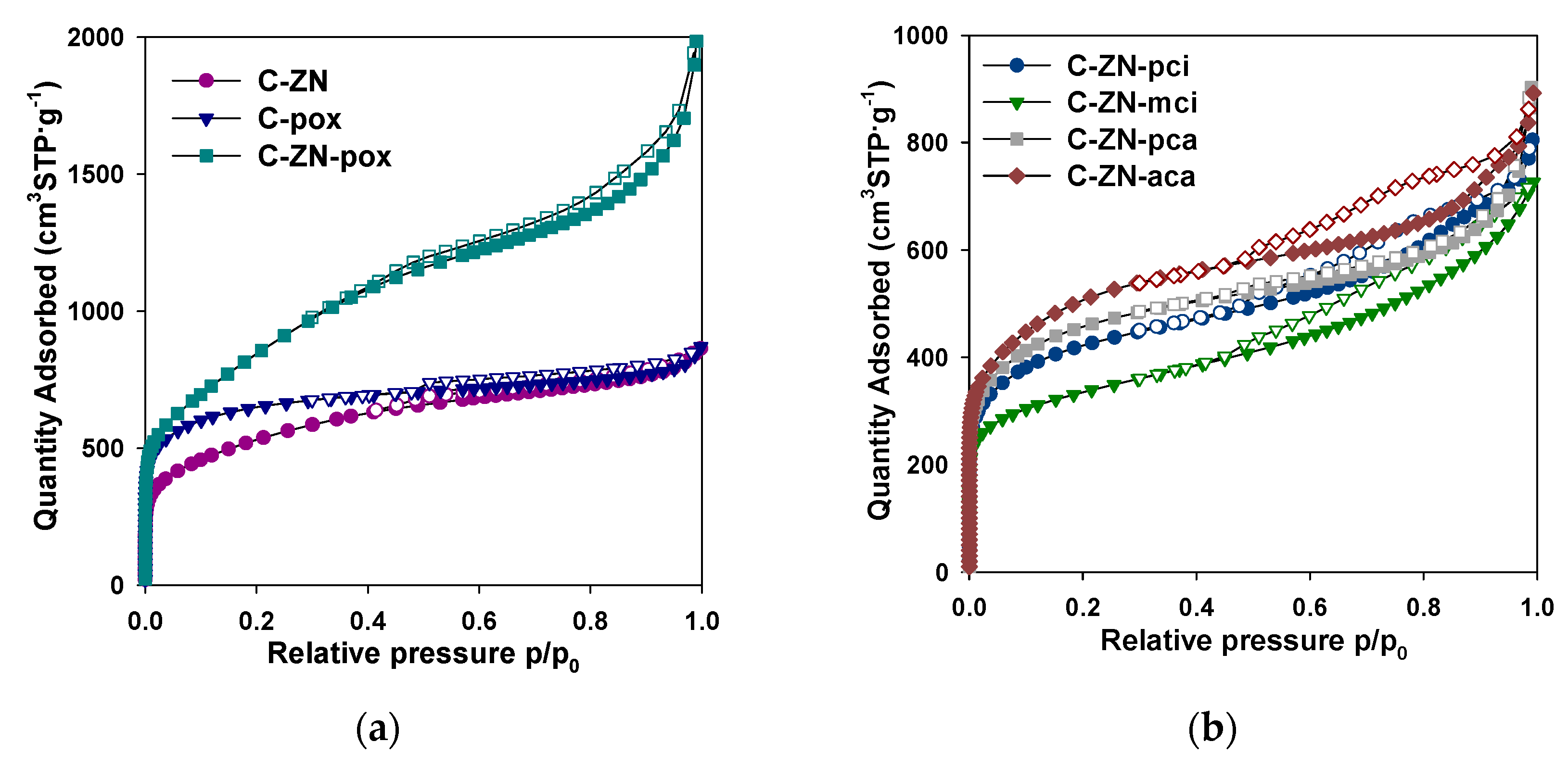
2.1. Morphological and Structural Characterization
2.2. H2 and CO2 Adsorption
3. Materials and Methods
3.1. Starting Materials
3.2. Synthesis Procedure
3.3. Measurements and Calculations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ali, I.; Asim, M.; Khan, T.A. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; You, S.; Igalavithana, A.D.; Xia, Y.; Bhatnagard, A.; Gupta, S.; Kua, H.W.; Kim, S.; Kwon, J.H.; Tsang, D.C.W.; et al. Biochar-based adsorbents for carbon dioxide capture: A critical review. Renew. Sustain. Energy Rev. 2020, 119, 109582. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Li, Z.; Ma, Y.; Ma, L. Recent progress of biomass-derived carbon materials for supercapacitors. J. Power Sources 2020, 451, 227794. [Google Scholar] [CrossRef]
- Pampel, J.; Denton, C.; Fellinger, T.P. Glucose derived ionothermal carbons with tailor-made porosity. Carbon 2016, 107, 288–296. [Google Scholar] [CrossRef]
- Díez, N.; Ferrero, G.A.; Sevilla, M.; Fuertes, A.B. A sustainable approach to hierarchically porous carbons from tannic acid and their utilization in supercapacitive energy storage systems. J. Mater. Chem. A 2019, 7, 14280–14290. [Google Scholar] [CrossRef]
- Tan, D.; García, F. Main group mechanochemistry: From curiosity to established protocols. Chem. Soc. Rev. 2019, 48, 2274–2292. [Google Scholar] [CrossRef] [PubMed]
- Rightmire, N.R.; Hanusa, T.P. Advances in organometallic synthesis with mechanochemical methods. Dalton Trans. 2016, 45, 2352–2362. [Google Scholar] [CrossRef] [PubMed]
- Horie, K.; Barón, M.; Fox, R.B.; He, J.; Hess, M.; Kahovec, J.; Kitayama, T.; Kubisa, P.; Maréchal, E.; Mormann, W.; et al. Definitions of terms relating to reactions of polymers and to functional polymeric materials (IUPAC Recommendations 2003). Pure Appl. Chem. 2004, 76, 889–906. [Google Scholar] [CrossRef]
- Takacs, L. Two important periods in the history of mechanochemistry. J. Mater. Sci. 2018, 53, 13324–13330. [Google Scholar] [CrossRef]
- Takacs, L. The historical development of mechanochemistry. Chem. Soc. Rev. 2013, 42, 7649–7659. [Google Scholar] [CrossRef]
- Boldyreva, E. Mechanochemistry of inorganic and organic systems: What is similar, what is different? Chem. Soc. Rev. 2013, 42, 7719–7738. [Google Scholar] [CrossRef]
- Schneidermann, C.; Kensy, C.; Otto, P.; Oswald, S.; Giebeler, L.; Leistenschneider, D.; Grätz, S.; Dörfler, S.; Kaskel, S.; Borchardt, L. Nitrogen-doped biomass-derived carbon formed by mechanochemical synthesis for lithium–sulfur batteries. ChemSusChem 2019, 12, 310–319. [Google Scholar] [CrossRef]
- Schneidermann, C.; Jäckel, N.; Oswald, S.; Giebeler, L.; Presser, V.; Borchardt, L. Solvent-free mechanochemical synthesis of nitrogen-doped nanoporous carbon for electrochemical energy storage. ChemSusChem 2017, 10, 2416–2424. [Google Scholar] [CrossRef] [PubMed]
- Tzvetkov, G.; Mihaylova, S.; Stoitchkova, K.; Tzvetkov, P.; Spassov, T. Mechanochemical and chemical activation of lignocellulosic material to prepare powdered activated carbons for adsorption applications. Powder Technol. 2016, 299, 41–50. [Google Scholar] [CrossRef]
- Cai, C.; Fu, N.; Zhou, Z.; Wu, M.; Yang, Z.; Liu, R. Mechanochemical synthesis of tannic acid-Fe coordination compound and its derived porous carbon for CO2 adsorption. Energy Fuels 2018, 32, 10779–10785. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, W.; Xu, L. Solvent-free mechanochemical preparation of hierarchically porous carbon for supercapacitor and oxygen reduction reaction. Chem. Eur. J. 2018, 24, 18097–18105. [Google Scholar] [CrossRef]
- Rajendiran, R.; Nallal, M.; Park, K.H.; Li, O.L.; Kim, H.J.; Prabakar, K. Mechanochemical assisted synthesis of heteroatoms inherited highly porous carbon from biomass for electrochemical capacitor and oxygen reduction reaction electrocatalysis. Electrochim. Acta 2019, 317, 1–9. [Google Scholar] [CrossRef]
- Singh, G.; Kim, I.Y.; Lakhi, K.S.; Joseph, S.; Srivastava, P.; Naidu, R.; Vinu, A. Heteroatom functionalized activated porous biocarbons and their excellent performance for CO2 capture at high pressure. J. Mater. Chem. A 2017, 5, 21196–21204. [Google Scholar] [CrossRef]
- Schneidermann, C.; Otto, P.; Leistenschneider, D.; Grätz, S.; Eßbach, C.; Borchardt, L. Upcycling of polyurethane waste by mechanochemistry: Synthesis of N-doped porous carbon materials for supercapacitor applications. Beilstein J. Nanotechnol. 2019, 10, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Leistenschneider, D.; Wegner, K.; Eßbach, C.; Sander, M.; Schneidermann, C.; Borchardt, L. Tailoring the porosity of a mesoporous carbon by a solvent-free mechanochemical approach. Carbon 2019, 147, 43–50. [Google Scholar] [CrossRef]
- Tiruye, G.A.; Muñoz-Torrero, D.; Berthold, T.; Palma, J.; Antonietti, M.; Fechler, N.; Marcilla, R. Functional porous carbon nanospheres from sustainable precursors for high performance supercapacitors. J. Mater. Chem. A 2017, 5, 16263–16272. [Google Scholar] [CrossRef]
- Zhang, W.; Qi, J.; Bai, P.; Wang, H.; Xu, L. High-level nitrogen-doped, micro/mesoporous carbon as an efficient metal-free electrocatalyst for the oxygen reduction reaction: Optimizing the reaction surface area by a solvent-free mechanochemical method. New J. Chem. 2019, 43, 10878–10886. [Google Scholar] [CrossRef]
- Casco, M.E.; Kirchhoff, S.; Leistenschneider, D.; Rauche, M.; Brunner, E.; Borchardt, L. Mechanochemical synthesis of N-doped porous carbon at room temperature. Nanoscale 2019, 11, 4712–4718. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.-F.; Liu, S.-B.; Liu, Y.-G.; Gu, Y.-L.; Zeng, G.-M.; Hu, X.-J.; Wang, X.; Liu, S.-H.; Jiang, L.-H. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T. A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application. Renew. Sustain. Energ. Rev. 2018, 87, 1–21. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, D.; Wu, C.; Gu, S. State-of-the-art on the production and application of carbon nanomaterials from biomass. Green Chem. 2018, 20, 5031–5057. [Google Scholar] [CrossRef]
- Deng, J.; Li, M.; Wang, Y. Biomass-derived carbon: Synthesis and applications in energy storage and conversion. Green Chem. 2016, 18, 4824–4854. [Google Scholar] [CrossRef]
- Nowicki, P.; Kazmierczak, J.; Pietrzak, R. Comparison of physicochemical and sorption properties of activated carbons prepared by physical and chemical activation of cherry stones. Powder Technol. 2015, 269, 312–319. [Google Scholar] [CrossRef]
- Półrolniczak, P.; Nowicki, P.; Wasiński, K.; Pietrzak, R.; Walkowiak, M. Biomass-derived hierarchical carbon as sulfur cathode stabilizing agent for lithium-sulfur batteries. Solid State Ion. 2016, 297, 59–63. [Google Scholar] [CrossRef]
- Nowicki, P.; Pietrzak, R. Carbonaceous adsorbents prepared by physical activation of pine sawdust and their application for removal of NO2 in dry and wet conditions. Bioresour. Technol. 2010, 101, 5802–5807. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, P.; Kazmierczak-Razna, J.; Pietrzak, R. Physicochemical and adsorption properties of carbonaceous sorbents prepared by activation of tropical fruit skins with potassium carbonate. Mater. Des. 2016, 90, 579–585. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Technological application of tannin-based extracts. Molecules 2020, 25, 614. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins: Prospectives and actual industrial applications. Biomolecules 2019, 9, 344. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Fierro, V.; Izquierdo, M.T.; Parmentier, J.; Pizzi, A.; Celzard, A. Nitrogen-doped carbon materials produced from hydrothermally treated tannin. Carbon 2012, 50, 5411–5420. [Google Scholar] [CrossRef]
- Yao, Y.; Yu, M.; Yin, H.; Wei, F.; Zhang, J.; Hu, H.; Wang, S. Tannic acid-Fe coordination derived Fe/N-doped carbon hybrids for catalytic oxidation processes. Appl. Surf. Sci. 2019, 489, 44–54. [Google Scholar] [CrossRef]
- Ruiz, B.; Ruisánchez, E.; Gil, R.R.; Ferrera-Lorenzo, N.; Lozano, M.S.; Fuente, E. Sustainable porous carbons from lignocellulosic wastes obtained from the extraction of tannins. Microporous Mesoporous Mater. 2015, 209, 23–29. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, A.; Izquierdo, M.T.; Medjahdi, G.; Ghanbaja, J.; Celzard, A.; Fierro, V. Ordered mesoporous carbons obtained by soft-templating of tannin in mild conditions. Microporous Mesoporous Mater. 2018, 270, 127–139. [Google Scholar] [CrossRef]
- Nelson, K.M.; Mahurin, S.M.; Mayes, R.T.; Williamson, B.; Teague, C.M.; Binder, A.J.; Baggetto, L.; Veith, G.M.; Dai, S. Preparation and CO2 adsorption properties of soft-templated mesoporous carbons derived from chestnut tannin precursors. Microporous Mesoporous Mater. 2016, 222, 94–103. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, A.; Izquierdo, M.T.; Ghanbaja, J.; Medjahdi, G.; Mathieu, S.; Celzard, A.; Fierro, V. Excellent electrochemical performances of nanocast ordered mesoporous carbons based on tannin-related polyphenols as supercapacitor electrodes. J. Power Sources 2017, 344, 15–24. [Google Scholar] [CrossRef]
- Bazan, A.; Nowicki, P.; Półrolniczak, P.; Pietrzak, R. Thermal analysis of activated carbon obtained from residue after supercritical extraction of hops. J. Therm. Anal. Calorim. 2016, 125, 1199–1204. [Google Scholar] [CrossRef]
- Sevilla, M.; Ferrero, G.A.; Fuertes, A.B. One-pot synthesis of biomass-based hierarchical porous carbons with a large porosity development. Chem. Mater. 2017, 29, 6900–6907. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.; Olivier, J.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Phuriragpitikhon, J.; Ghimire, P.; Jaroniec, M. Tannin-derived micro-mesoporous carbons prepared by one-step activation with potassium oxalate and CO2. J. Colloid Interface Sci. 2020, 558, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Furmaniak, S.; Kowalczyk, P.; Terzyk, A.P.; Gauden, P.A.; Harris, P.J.F. Synergetic effect of carbon nanopore size and surface oxidation on CO2 capture from CO2/CH4 mixtures. J. Colloid Interface Sci. 2013, 397, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Babu, D.J.; Bruns, M.; Schneider, R.; Gerthsen, D.; Schneider, J.J. Understanding the influence of N-doping on the CO2 adsorption characteristics in carbon nanomaterials. J. Phys. Chem. C 2017, 121, 616–626. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Seredych, M.; Rodríguez-Castellon, E.; Cheng, Y.; Daemen, L.L.; Ramírez-Cuesta, A.J. Evidence for CO2 reactive adsorption on nanoporous S- and N-doped carbon at ambient conditions. Carbon 2016, 96, 856–863. [Google Scholar] [CrossRef]
- Fulvio, P.F.; Lee, J.S.; Mayes, R.T.; Wang, X.; Mahurin, S.M.; Dai, S. Boron and nitrogen-rich carbons from ionic liquid precursors with tailorable surface properties. Phys. Chem. Chem. Phys. 2011, 13, 13486–13491. [Google Scholar] [CrossRef]
- Balahmar, N.; Mitchell, A.C.; Mokaya, R. Generalized mechanochemical synthesis of biomass-derived sustainable carbons for high performance CO2 storage. Adv. Energy Mater. 2015, 5, 1500867. [Google Scholar] [CrossRef]
- Lin, X.; Liang, Y.; Lu, Z.; Lou, H.; Zhang, X.; Liu, S.; Zheng, B.; Liu, R.; Fu, R.; Wu, D. Mechanochemistry: A green, activation-free and top-down strategy to high-surface-area carbon materials. ACS Sustain. Chem. Eng. 2017, 5, 8535–8540. [Google Scholar] [CrossRef]
- Braunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Jagiello, J.; Olivier, J.P. 2D-NLDFT adsorption models for carbon slit-shaped pores with surface energetical heterogeneity and geometrical corrugation. Carbon 2013, 55, 70–80. [Google Scholar] [CrossRef]
- Jagiello, J.; Olivier, J.P. Carbon slit pore model incorporating surface energetical heterogeneity and geometrical corrugation. Adsorption 2013, 19, 777–783. [Google Scholar] [CrossRef]




| Samples | C (wt.%) | H (wt.%) | N (wt.%) |
|---|---|---|---|
| C-ZN | 67.52 | 2.84 | 5.60 |
| C-pox | 71.02 | 2.19 | 1.01 |
| C-ZN-pox | 74.20 | 1.83 | 5.94 |
| Sample | SSA 1 (m2·g−1) | Vt 2 (cm3·g−1) | Vultra 3 (cm3·g−1) | Vmicro 4 (cm3·g−1) | Vmeso 5 (cm3·g−1) |
|---|---|---|---|---|---|
| C-ZN | 1910 | 1.34 | 0.13 | 0.60 | 0.74 |
| C-pox | 2330 | 1.35 | 0.21 | 0.87 | 0.48 |
| C-ZN-pox | 3060 | 3.07 | 0.09 | 0.77 | 2.30 |
| C-ZN-aca | 1832 | 1.38 | 0.16 | 0.61 | 0.77 |
| C-ZN-pca | 1645 | 1.40 | 0.11 | 0.56 | 0.84 |
| C-ZN-pci | 1520 | 1.25 | 0.12 | 0.50 | 0.75 |
| C-ZN-mci | 1190 | 1.12 | 0.14 | 0.38 | 0.74 |
| Samples | CO2 (mmol·g−1) 0 °C, 1 bar | H2 (mmol·g−1) −196 °C, 1 bar |
|---|---|---|
| C-ZN | 4.4 | 8.9 |
| C-pox | 6.4 | 12.9 |
| C-ZN-pox | 4.7 | 13.2 |
| Sample | Carbon Source/Activator | SSA [m2·g−1] | Application | Ref. |
|---|---|---|---|---|
| C-ZN | Tannic acid/ZnCl2 | 1910 | CO2 adsorption (4.4 mmol·g−1 at 0 °C and 1 bar) | This work |
| H2 adsorption (8.9 mmol·g−1 at −196 °C and 1 bar) | ||||
| C-pox | Tannic acid/K2C2O4 | 2330 | CO2 adsorption (6.4 mmol·g−1 at 0 °C and 1 bar) | This work |
| H2 adsorption (12.9 mmol·g−1 at −196 °C and 1 bar) | ||||
| C-ZN-pox | Tannic acid/ZnCl2, K2C2O4 | 3060 | CO2 adsorption (4.7 mmol·g−1 at 0 °C and 1 bar) | This work |
| H2 adsorption (13.2 mmol·g−1 at −196 °C and 1 bar) | ||||
| LUPC | Lignin/K2CO3 | 3199 | Li–S batteries | [12] |
| WWUPC | Wood waste/K2CO3 | 2988 | Li–S batteries | [12] |
| TUPC | Tannic acid/K2CO3 | 2873 | Li–S batteries | [12] |
| LD2600P | Lignin/KOH | 2224 | CO2 adsorption (4.5 mmol·g−1 at 25 °C and 1 bar) | [48] |
| NDAB3-500 | Arundo donax and chitosan/ZnCl2 | 1863 | CO2 adsorption (2.1 mmol·g−1 at 25 °C and 1 bar) | [18] |
| HSAC-MCS-900-9 | Coconut shel/- | 1771 | Li–S batteries and creatinine adsorption | [49] |
| TA_0 | Tannic acid/ZnCl2 | 1570 | Supercapacitors | [21] |
| SD2650P | Sawdust/KOH | 1313 | CO2 adsorption (5.8 mmol·g−1 at 25 °C and 1 bar) | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Głowniak, S.; Szczęśniak, B.; Choma, J.; Jaroniec, M. Highly Porous Carbons Synthesized from Tannic Acid via a Combined Mechanochemical Salt-Templating and Mild Activation Strategy. Molecules 2021, 26, 1826. https://doi.org/10.3390/molecules26071826
Głowniak S, Szczęśniak B, Choma J, Jaroniec M. Highly Porous Carbons Synthesized from Tannic Acid via a Combined Mechanochemical Salt-Templating and Mild Activation Strategy. Molecules. 2021; 26(7):1826. https://doi.org/10.3390/molecules26071826
Chicago/Turabian StyleGłowniak, Sylwia, Barbara Szczęśniak, Jerzy Choma, and Mietek Jaroniec. 2021. "Highly Porous Carbons Synthesized from Tannic Acid via a Combined Mechanochemical Salt-Templating and Mild Activation Strategy" Molecules 26, no. 7: 1826. https://doi.org/10.3390/molecules26071826
APA StyleGłowniak, S., Szczęśniak, B., Choma, J., & Jaroniec, M. (2021). Highly Porous Carbons Synthesized from Tannic Acid via a Combined Mechanochemical Salt-Templating and Mild Activation Strategy. Molecules, 26(7), 1826. https://doi.org/10.3390/molecules26071826