Role of Zinc-Doped Bioactive Glass Encapsulated with Microspherical Gelatin in Localized Supplementation for Tissue Regeneration: A Contemporary Review
Abstract
1. Introduction
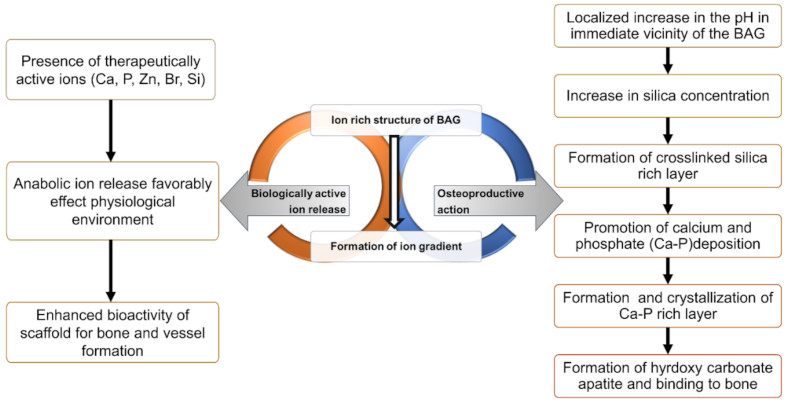
2. Mechanism of Action of Bioactive Glass
3. Use of Local Essential Mineral Supplements
4. Functional Activity of ZBG
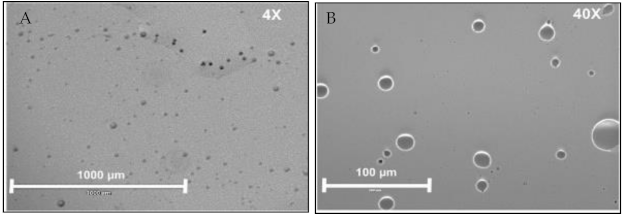
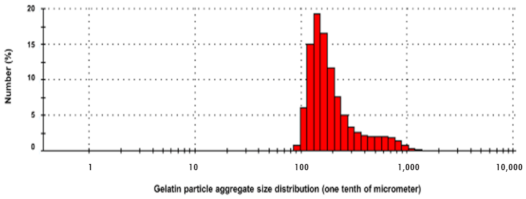
5. Role of Micro/Nanocarriers: Gelatin Microspheres
6. Synthesis of Gelatin-ZBG Microcapsules
7. BAG Gelatin Combination in Tissue Engineering
8. Summary and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kononen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A multifaceted disease of tooth-supporting tissues. J. Clin. Med. 2019, 8. [Google Scholar] [CrossRef]
- Gulati, M.; Anand, V.; Govila, V.; Jain, N. Host modulation therapy: An indispensable part of perioceutics. J. Indian Soc. Periodontol. 2014, 18, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Cafferata, E.A.; Alvarez, C.; Diaz, K.T.; Maureira, M.; Monasterio, G.; Gonzalez, F.E.; Covarrubias, C.; Vernal, R. Multifunctional nanocarriers for the treatment of periodontitis: Immunomodulatory, antimicrobial, and regenerative strategies. Oral Dis. 2019, 25, 1866–1878. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Boccardi, E.; Philippart, A.; Melli, V.; Altomare, L.; De Nardo, L.; Novajra, G.; Vitale-Brovarone, C.; Fey, T.; Boccaccini, A.R. Bioactivity and mechanical stability of 45S5 bioactive glass scaffolds based on natural marine sponges. Ann. Biomed. Eng. 2016, 44, 1881–1893. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Lin, Z.Y.W.; Wong, K.K.Y.; Lin, M.; Yildirimer, L.; Zhao, X. Electrospun polymeric micro/nanofibrous scaffolds for long-term drug release and their biomedical applications. Drug Discov. Today 2017, 22, 1351–1366. [Google Scholar] [CrossRef]
- Hum, J.; Boccaccini, A.R. Bioactive glasses as carriers for bioactive molecules and therapeutic drugs: A review. J. Mater. Sci. Mater. Med. 2012, 23, 2317–2333. [Google Scholar] [CrossRef]
- Hench, L.L.; Xynos, I.D.; Polak, J.M. Bioactive glasses for in situ tissue regeneration. J. Biomater. Sci. Polym. Ed. 2004, 15, 543–562. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, C.; Cadiz, M.; Maureira, M.; Celhay, I.; Cuadra, F.; von Marttens, A. Bionanocomposite scaffolds based on chitosan-gelatin and nanodimensional bioactive glass particles: In vitro properties and in vivo bone regeneration. J. Biomater. Appl. 2018, 32, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Schuhladen, K.; Stich, L.; Schmidt, J.; Steinkasserer, A.; Boccaccini, A.R.; Zinser, E. Cu, Zn doped borate bioactive glasses: Antibacterial efficacy and dose-dependent in vitro modulation of murine dendritic cells. Biomater. Sci. 2020, 8, 2143–2155. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Guldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Ehara, Y. Zinc decrease and bone metabolism in the femoral-metaphyseal tissues of rats with skeletal unloading. Calcif. Tissue Int. 1995, 57, 218–223. [Google Scholar] [CrossRef]
- Yamaguchi, M. Role of zinc in bone formation and bone resorption. J. Trace Elem. Exp. Med. 1998, 11, 119–135. [Google Scholar] [CrossRef]
- Ishikawa, K.; Miyamoto, Y.; Yuasa, T.; Ito, A.; Nagayama, M.; Suzuki, K. Fabrication of Zn containing apatite cement and its initial evaluation using human osteoblastic cells. Biomaterials 2002, 23, 423–428. [Google Scholar] [CrossRef]
- Wey, A.; Cunningham, C.; Hreha, J.; Breitbart, E.; Cottrell, J.; Ippolito, J.; Clark, D.; Lin, H.N.; Benevenia, J.; O’Connor, J.P.; et al. Local ZnCl2 accelerates fracture healing. J. Orthop. Res. 2014, 32, 834–841. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.P.; Kanjilal, D.; Teitelbaum, M.; Lin, S.S.; Cottrell, J.A. Zinc as a therapeutic agent in bone regeneration. Materials 2020, 13, 2211. [Google Scholar] [CrossRef] [PubMed]
- Baino, F. Bioactive glasses—When glass science and technology meet regenerative medicine. Ceram. Int. 2018, 44, 14953–14966. [Google Scholar] [CrossRef]
- Sanchez-Salcedo, S.; Shruti, S.; Salinas, A.J.; Malavasi, G.; Menabue, L.; Vallet-Regi, M. In vitro antibacterial capacity and cytocompatibility of SiO2-CaO-P2O5 meso-macroporous glass scaffolds enriched with ZnO. J. Mater. Chem. B 2014, 2, 4836–4847. [Google Scholar] [CrossRef]
- Du, R.L.; Chang, J.; Ni, S.Y.; Zhai, W.Y.; Wang, J.Y. Characterization and in vitro bioactivity of zinc-containing bioactive glass and glass-ceramics. J. Biomater. Appl. 2006, 20, 341–360. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Motelica-Heino, M.; Guegan, R.; Buton, N. Evaluation of antibacterial activity of zinc-doped hydroxyapatite colloids and dispersion stability using ultrasounds. Nanomaterials 2019, 9, 515. [Google Scholar] [CrossRef] [PubMed]
- Wajda, A.; Goldmann, W.H.; Detsch, R.; Boccaccini, A.R.; Sitarz, M. Influence of zinc ions on structure, bioactivity, biocompatibility and antibacterial potential of melt-derived and gel-derived glasses from CaO-SiO2 system. J. Non-Cryst. Solids 2019, 511, 86–99. [Google Scholar] [CrossRef]
- Ylänen, H. Bioactive Glasses, 2nd ed.; Ylänen, H., Ed.; Woodhead Publishing: Sawston, UK, 2018; 4p. [Google Scholar]
- Negrila, C.C.; Predoi, M.V.; Iconaru, S.L.; Predoi, D. Development of zinc-doped hydroxyapatite by sol-gel method for medical applications. Molecules 2018, 23, 2986. [Google Scholar] [CrossRef] [PubMed]
- Iconaru, S.L.; Prodan, A.M.; Buton, N.; Predoi, D. Structural characterization and antifungal studies of zinc-doped hydroxyapatite coatings. Molecules 2017, 22, 604. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, S.L.; Deniaud, A.; Chevallet, M.; Michaud-Soret, I.; Buton, N.; Prodan, A.M. Textural, structural and biological evaluation of hydroxyapatite doped with zinc at low concentrations. Materials 2017, 10, 229. [Google Scholar] [CrossRef]
- Vyas, S.P.; Khar, R.K. Targeted & Controlled Drug Delivery: Novel Carrier Systems; CBS Publishers & Distributors: New Delhi, India, 2004. [Google Scholar]
- Lien, S.-M.; Ko, L.-Y.; Huang, T.-J. Effect of crosslinking temperature on compression strength of gelatin scaffold for articular cartilage tissue engineering. Mater. Sci. Eng. C 2010, 30, 631–635. [Google Scholar] [CrossRef]
- Dressler, M.; Dombrowski, F.; Simon, U.; Börnstein, J.; Hodoroaba, V.D.; Feigl, M.; Grunow, S.; Gildenhaar, R.; Neumann, M. Influence of gelatin coatings on compressive strength of porous hydroxyapatite ceramics. J. Eur. Ceram. Soc. 2011, 31, 523–529. [Google Scholar] [CrossRef]
- Mozafari, M.; Moztarzadeh, F.; Rabiee, M.; Azami, M.; Maleknia, S.; Tahriri, M.; Moztarzadeh, Z.; Nezafati, N. Development of macroporous nanocomposite scaffolds of gelatin/bioactive glass prepared through layer solvent casting combined with lamination technique for bone tissue engineering. Ceram. Int. 2010, 36, 2431–2439. [Google Scholar] [CrossRef]
- Thomas, A.; Bera, J. Preparation and characterization of gelatin-bioactive glass ceramic scaffolds for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2019, 30, 561–579. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Ding, Y.; Scheithauer, E.C.; Goudouri, O.M.; Grunewald, A.; Detsch, R.; Agarwal, S.; Boccaccini, A.R. Antibacterial 45S5 bioglass(R)-based scaffolds reinforced with genipin cross-linked gelatin for bone tissue engineering. J. Mater. Chem. B 2015, 3, 3367–3378. [Google Scholar] [CrossRef]
- Saxena, A.; Sachin, K.; Bohidar, H.B.; Verma, A.K. Effect of molecular weight heterogeneity on drug encapsulation efficiency of gelatin nano-particles. Colloids Surf. B Biointerfaces 2005, 45, 42–48. [Google Scholar] [CrossRef]
- Nada, A.A.; El Aref, A.T.; Sharaf, S.S. The synthesis and characterization of zinc-containing electrospun chitosan/gelatin derivatives with antibacterial properties. Int. J. Biol. Macromol. 2019, 133, 538–544. [Google Scholar] [CrossRef]
- Yankova, I.; Shestakova, P.; Reis, R.L.; Pashkuleva, I.; Vassileva, E. Gelatin micro- and nanocapsules obtained via sonochemical method. J. Appl. Polym. Sci. 2020, 137. [Google Scholar] [CrossRef]
- Cascone, M.G.; Lazzeri, L.; Carmignani, C.; Zhu, Z. Gelatin nanoparticles produced by a simple W/O emulsion as delivery system for methotrexate. J. Mater. Sci. Mater. Med. 2002, 13, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Nasir, A.; Kausar, A.; Younus, A. A review on preparation, properties and applications of polymeric nanoparticle-based materials. Polym. Plast. Technol. Eng. 2014, 54, 325–341. [Google Scholar] [CrossRef]
- Esposito, E.; Cortesi, R.; Nastruzzi, C. Gelatin microspheres: Influence of preparation parameters and thermal treatment on chemico-physical and biopharmaceutical properties. Biomaterials 1996, 17, 2009–2020. [Google Scholar] [CrossRef]
- Jayan, S.C.; Sandeep, A.; Rifash, M.; Mareema, C.; Shamseera, S. Design and in-vitro evaluation of gelatin microspheres of salbutamol sulphate. J. Hygeia 2009, 1, 17–20. [Google Scholar]
- Habraken, W.J.; de Jonge, L.T.; Wolke, J.G.; Yubao, L.; Mikos, A.G.; Jansen, J.A. Introduction of gelatin microspheres into an injectable calcium phosphate cement. J. Biomed. Mater. Res. A 2008, 87, 643–655. [Google Scholar] [CrossRef]
- Mozafari, M.; Moztarzadeh, F.; Rabiee, M.; Azami, M.; Nezafati, N.; Moztarzadeh, Z.; Tahriri, M. Development of 3D bioactive nanocomposite scaffolds made from gelatin and nano bioactive glass for biomedical applications. Adv. Compos. Lett. 2010, 19. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Cannillo, V. A review of bioactive glass/natural polymer composites: State of the art. Materials 2020, 13, 5560. [Google Scholar] [CrossRef]
- Lacroix, J.; Jallot, E.; Lao, J. Gelatin-bioactive glass composites scaffolds with controlled macroporosity. Chem. Eng. J. 2014, 256, 9–13. [Google Scholar] [CrossRef]
- Johari, B.; Kadivar, M.; Lak, S.; Gholipourmalekabadi, M.; Urbanska, A.M.; Mozafari, M.; Ahmadzadehzarajabad, M.; Azarnezhad, A.; Afshari, S.; Zargan, J.; et al. Osteoblast-seeded bioglass/gelatin nanocomposite: A promising bone substitute in critical-size calvarial defect repair in rat. Int. J. Artif. Organs 2016, 39, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Wu, J.; Li, W.; Dippold, D.; Wan, Y.; Boccaccini, A.R. Incorporation of Cu-containing bioactive glass nanoparticles in gelatin-coated scaffolds enhances bioactivity and osteogenic activity. ACS Biomater. Sci. Eng. 2018, 4, 1546–1557. [Google Scholar] [CrossRef]
- Guo, W.; Zhao, F.; Wang, Y.; Tang, J.; Chen, X. Characterization of the mechanical behaviors and bioactivity of tetrapod ZnO whiskers reinforced bioactive glass/gelatin composite scaffolds. J. Mech. Behav. Biomed. Mater. 2017, 68, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Raz, M.; Moztarzadeh, F.; Kordestani, S.S. Sol-gel based fabrication and properties of Mg-Zn doped bioactive glass/gelatin composite scaffold for bone tissue engineering. Silicon 2017, 10, 667–674. [Google Scholar] [CrossRef]
- Moreira, C.D.F.; Carvalho, S.M.; Florentino, R.M.; Franca, A.; Okano, B.S.; Rezende, C.M.F.; Mansur, H.S.; Pereira, M.M. Injectable chitosan/gelatin/bioactive glass nanocomposite hydrogels for potential bone regeneration: In vitro and in vivo analyses. Int. J. Biol. Macromol. 2019, 132, 811–821. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Shim, Y.-S.; An, S.-Y.; Lee, M.-J. Role of Zinc-Doped Bioactive Glass Encapsulated with Microspherical Gelatin in Localized Supplementation for Tissue Regeneration: A Contemporary Review. Molecules 2021, 26, 1823. https://doi.org/10.3390/molecules26071823
Kim D, Shim Y-S, An S-Y, Lee M-J. Role of Zinc-Doped Bioactive Glass Encapsulated with Microspherical Gelatin in Localized Supplementation for Tissue Regeneration: A Contemporary Review. Molecules. 2021; 26(7):1823. https://doi.org/10.3390/molecules26071823
Chicago/Turabian StyleKim, Dokyeong, Youn-Soo Shim, So-Youn An, and Myung-Jin Lee. 2021. "Role of Zinc-Doped Bioactive Glass Encapsulated with Microspherical Gelatin in Localized Supplementation for Tissue Regeneration: A Contemporary Review" Molecules 26, no. 7: 1823. https://doi.org/10.3390/molecules26071823
APA StyleKim, D., Shim, Y.-S., An, S.-Y., & Lee, M.-J. (2021). Role of Zinc-Doped Bioactive Glass Encapsulated with Microspherical Gelatin in Localized Supplementation for Tissue Regeneration: A Contemporary Review. Molecules, 26(7), 1823. https://doi.org/10.3390/molecules26071823






