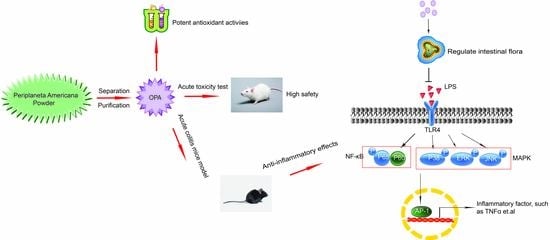
Periplaneta americana Oligosaccharides Exert Anti-Inflammatory Activity through Immunoregulation and Modulation of Gut Microbiota in Acute Colitis Mice Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Separation and Purification of OPA
2.3. Biochemical Characterization of OPA
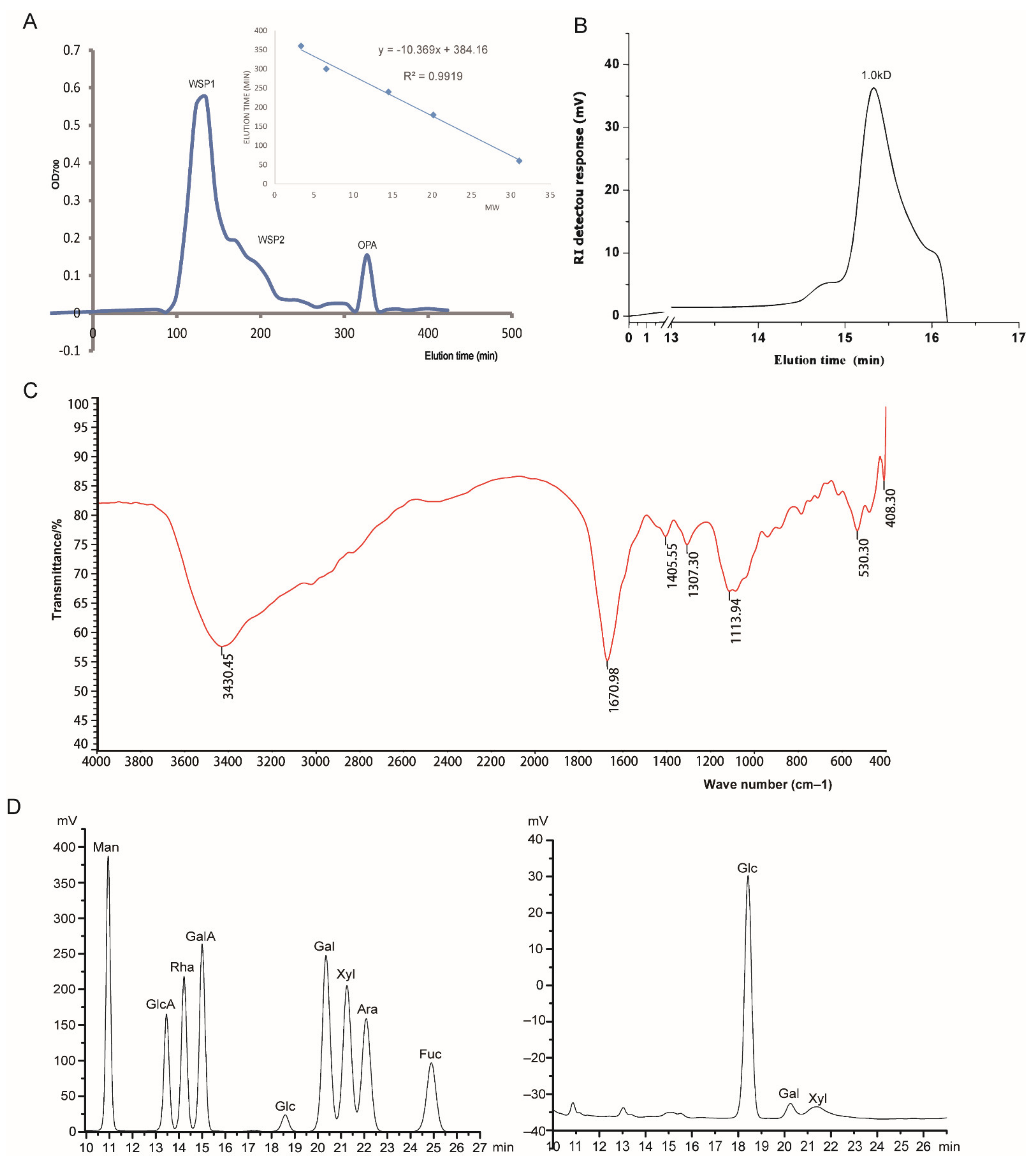
2.3.1. Molecular Weight and Homogeneity Determination
2.3.2. FTIR Analysis
2.3.3. Monosaccharide Composition Analysis
2.3.4. Methylation Analysis of OPA
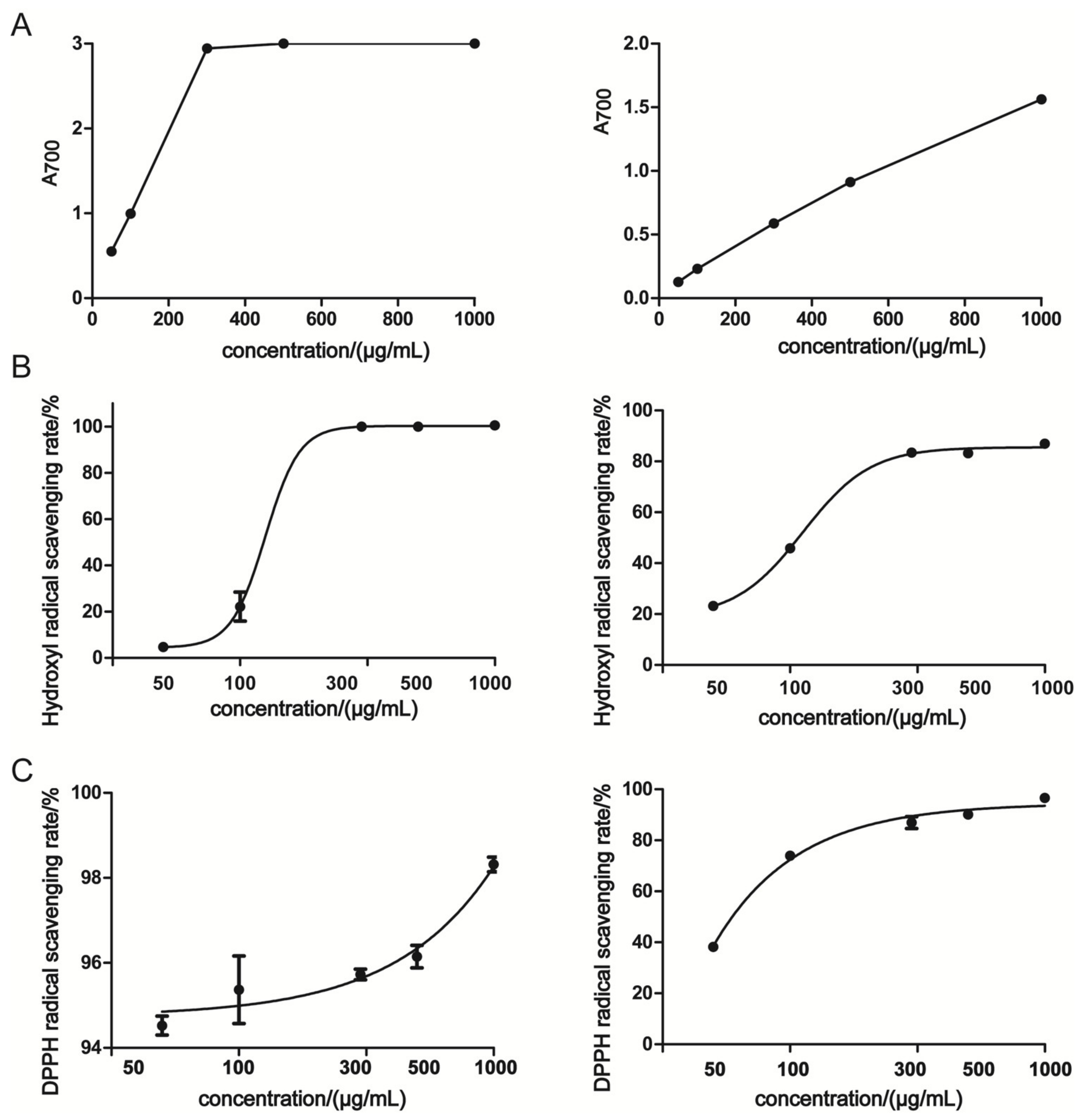
2.4. Determination of Antioxidant Activities
2.5. Anti-Inflammation Activity
2.5.1. Animals
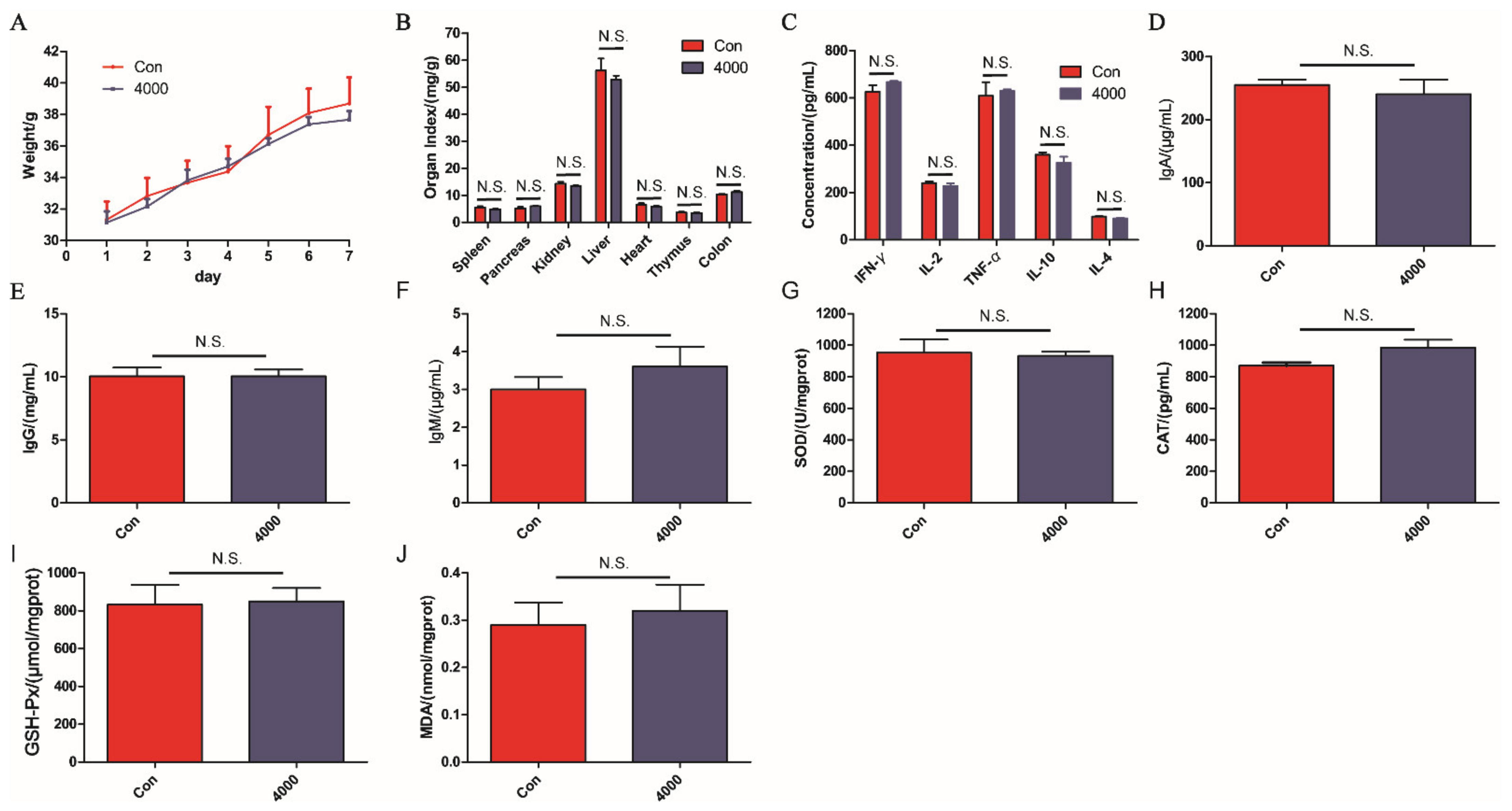
2.5.2. Acute Toxicity Test
2.5.3. Establishment of Acute Colitis Mice Model
2.5.4. Measurement of Cytokines
2.5.5. Histology Analysis
2.5.6. Immunohistochemistry Staining
2.5.7. Measurement of Oxidative Stress
2.5.8. Western Blot
2.5.9. Microbial Community Analysis
2.6. Statistical Analysis
3. Results
3.1. Purification and Biochemical Characterization of OPA
3.2. Antioxidant Activities In Vitro
3.3. Acute Toxicity
3.4. Intestinal Protective Activity
3.4.1. OPA Treatment Ameliorated DSS-Induced Colitis
3.4.2. OPA Regulated Immune in DSS-Induced Colitis
3.4.3. Histology Analysis
3.4.4. Immunohistochemistry Characterization
3.4.5. OPA Suppressed Oxidative Stress
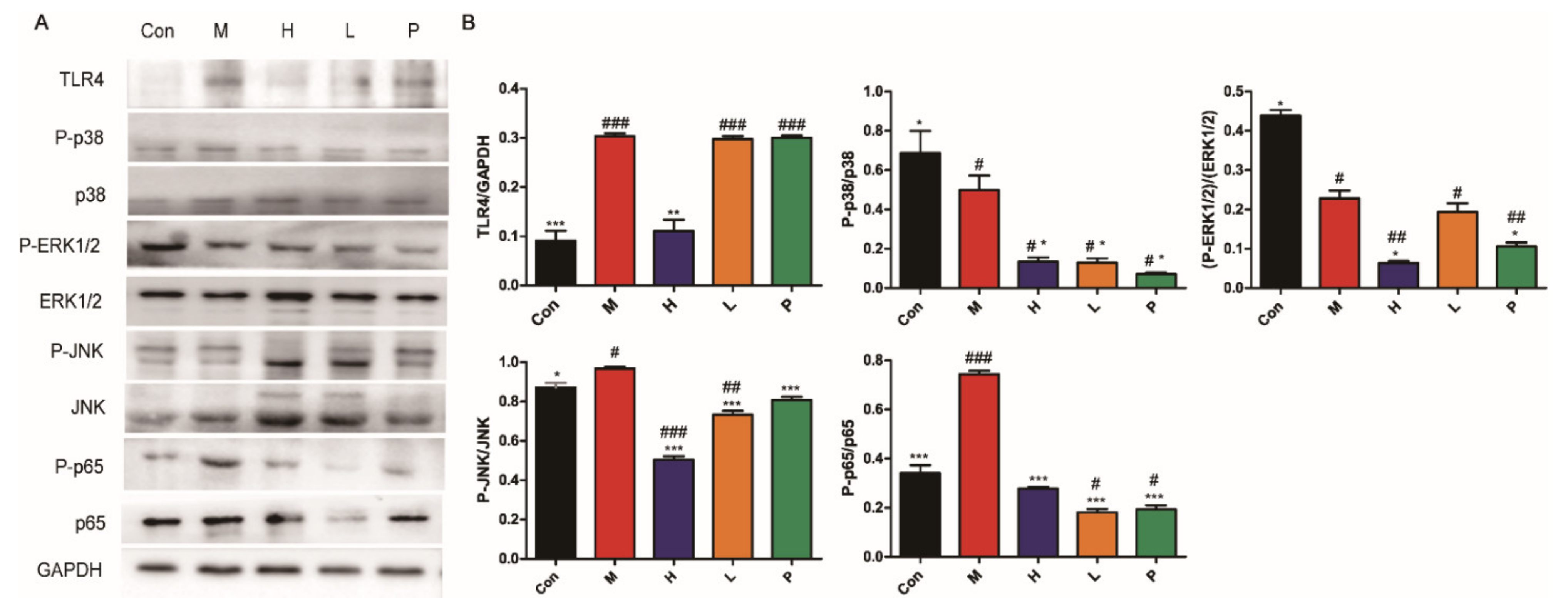
3.4.6. OPA Inhibited TLR4/MAPK/NF-κB Signaling Pathway
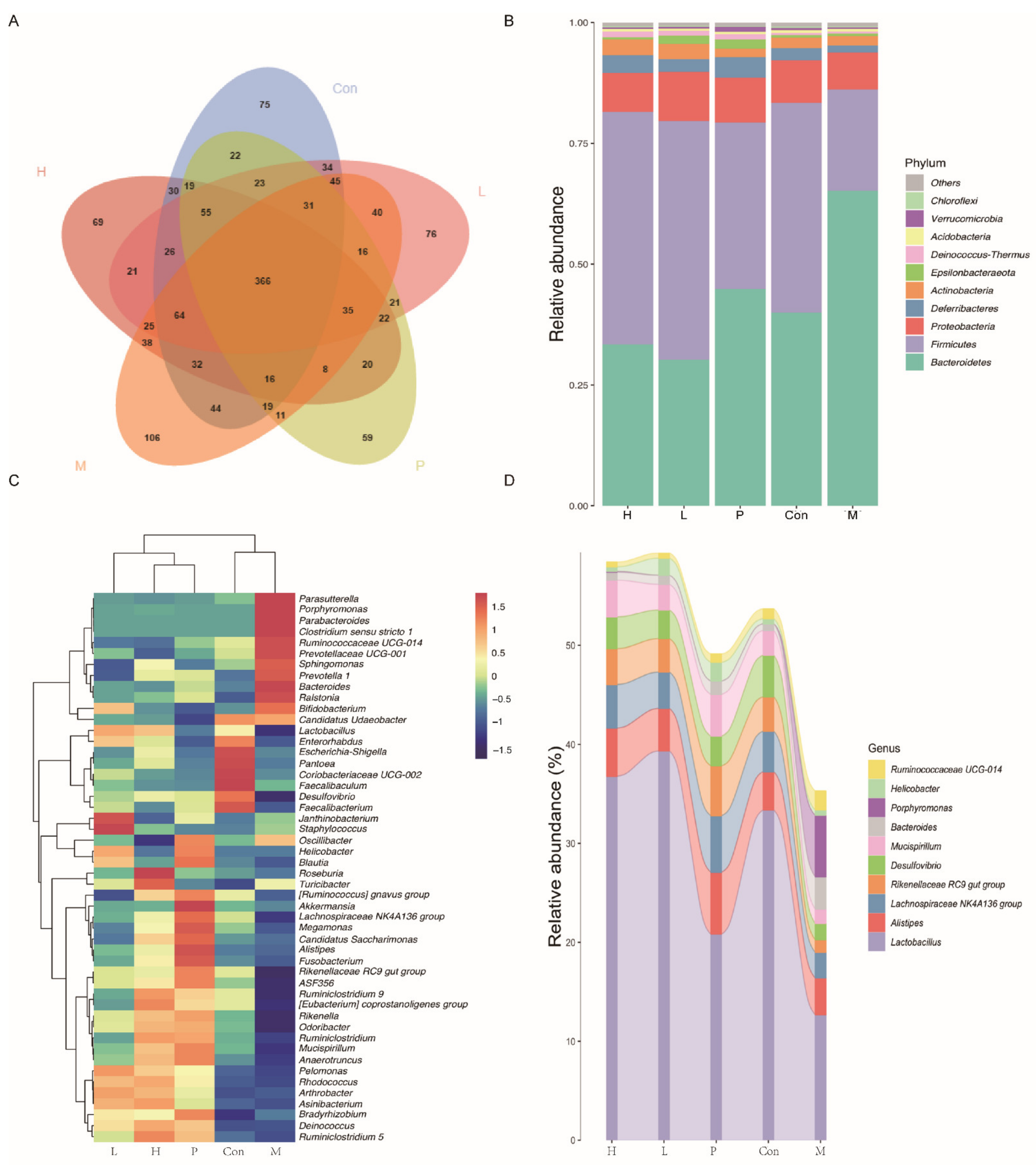
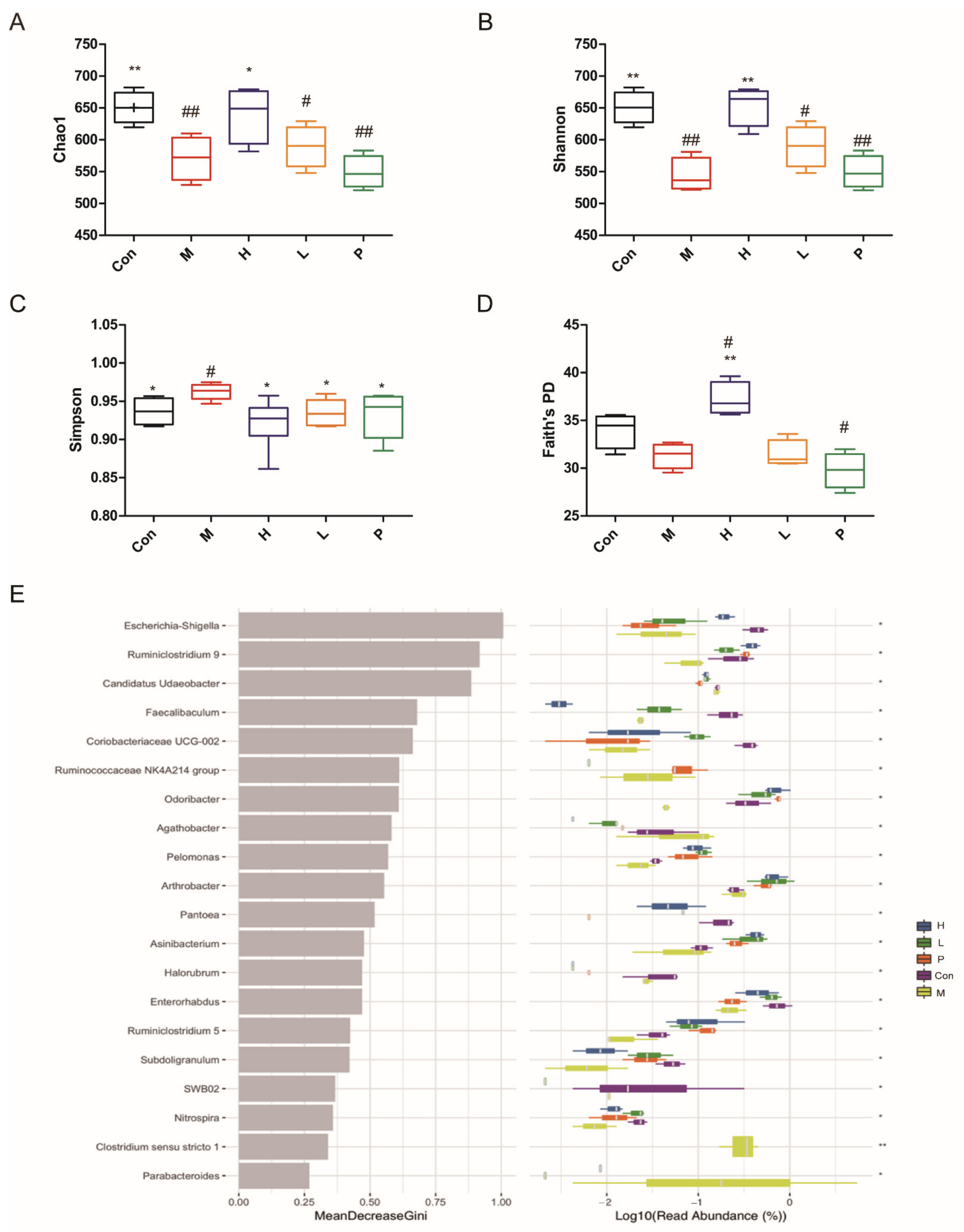
3.4.7. Effects of OPA on Gut Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| 5-ASA | 5-aminosalicylates |
| CAT | Catalase |
| COS | Chitosan oligosaccharides |
| DAI | Disease activity index |
| DSS | Dextran sulfate sodium |
| GSH-Px | Glutathione peroxidase |
| H&E | Hematoxylin and eosin |
| IBD | Inflammatory bowel disorder |
| IECs | Intestinal epithelial cells |
| IOD | Integrated optical density |
| LPS | Cellular endotoxin lipopolysaccharides |
| MDA | Malondialdehyde |
| MPO | Myeloperoxidase |
| OPA | Oligosaccharides from P. Americana |
| OTUs | Operational taxonomic units |
| P. Americana | Periplaneta Americana |
| PAE | P. americana extract |
| PMP | Phenyl-3-methyl-5-pyrazolone |
| ROS | Reactive oxygen species |
| SCFAs | Short-chain fatty acids |
| SOD | Superoxide dismutase |
| TFA | Trifluoroacetic acid |
| TJs | Tight junctions |
| TLR4 | Toll-like receptor 4 |
| VC | Ascorbic acid |
| ZO-1 | Zonula occludens-1 |
References
- Chen, W.-X.; Ren, L.-H.; Shi, R.-H. Enteric microbiota leads to new therapeutic strategies for ulcerative colitis. World J. Gastroenterol. 2014, 20, 15657–15663. [Google Scholar] [CrossRef] [PubMed]
- Bouma, G.; Strober, W. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 2003, 3, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Basso, P.J.; Camara, N.O.S.; Sales-Campos, H. Microbial-Based Therapies in the Treatment of Inflammatory Bowel Disease—An Overview of Human Studies. Front. Pharmacol. 2019, 9, 1571. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Dai, Z.Q.; Feng, S.M.; Liu, A.N.; Wang, H.; Zeng, X.X.; Yang, C.S. Anti-inflammatory effects of newly synthesized a-galacto-oligosaccharides on dextran sulfate sodium-induced colitis in C57BL/6J mice. Food Res. Int. 2018, 109, 350–357. [Google Scholar] [CrossRef]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015, 149, 102–109. [Google Scholar] [CrossRef]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef]
- Lightner, A.L.; Shen, B. Perioperative use of immunosuppressive medications in patients with Crohn’s disease in the new “biological era”. Gastroenterol. Rep. 2017, 5, 165–177. [Google Scholar] [CrossRef]
- Ma, X.W.; Hu, Y.C.; Li, X.; Zheng, X.T.; Wang, Y.T.; Zhang, J.M.; Fu, C.M.; Geng, F.N. Periplaneta americana Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Rats by Keap1/Nrf-2 Activation, Intestinal Barrier Function, and Gut Microbiota Regulation. Front. Pharmacol. 2018, 9, 16. [Google Scholar] [CrossRef]
- Jin, M.Y.; Wang, Y.X.; Yang, X.B.; Yin, H.; Nie, S.P.; Wu, X.Y. Structure characterization of a polysaccharide extracted from noni (Morinda citrifolia L.) and its protective effect against DSS-induced bowel disease in mice. Food Hydrocoll. 2019, 90, 189–197. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, N.F.; Kan, J.; Zhang, X.; Wu, X.N.; Sun, R.; Tang, S.X.; Liu, J.; Qian, C.L.; Jin, C.H. Structural characterization of water-soluble polysaccharide from Arctium lappa and its effects on colitis mice. Carbohydr. Polym. 2019, 213, 89–99. [Google Scholar] [CrossRef]
- Liu, X.; Tang, Y.-P.; Liu, R.; Jin, Y.; Guo, J.-M.; Zhao, J.-L.; Ding, S.-X.; Lin, X.-Z.; Lin, R.-R.; Duan, J.-A. Antipyretic and anti-inflammatory activities of Thais luteostoma extracts and underlying mechanisms. Chin. J. Nat. Med. 2015, 13, 192–198. [Google Scholar] [CrossRef]
- Song, Q.; Xie, Y.; Gou, Q.; Guo, X.; Yao, Q.; Gou, X. JAK/STAT3 and Smad3 activities are required for the wound healing properties of Periplaneta americana extracts. Int. J. Mol. Med. 2017, 40, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liao, Q.; Wu, Y.; Wang, X.; Fu, C.; Geng, F.; Qu, Y.; Zhang, J. A composite hydrogel loading natural polysaccharides derived from Periplaneta americana herbal residue for diabetic wound healing. Int. J. Biol. Macromol. 2020, 164, 3846–3857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-W.; Wei, L.-Y.; Zhao, G.; Yang, Y.-J.; Liu, S.-Z.; Zhang, Z.-Y.; Jing, Z.; Hu, Y.-L. Periplaneta americana extract used in patients with systemic inflammatory response syndrome. World J. Emerg. Med. 2016, 7, 50–54. [Google Scholar] [CrossRef]
- Yan, J.K.; Wang, Y.Y.; Ma, H.L.; Wang, Z.B. Ultrasonic effects on the degradation kinetics, preliminary characterization and antioxidant activities of polysaccharides from Phellinus linteus mycelia. Ultrason. Sonochem. 2016, 29, 251–257. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, Y.Y.; Ren, G.X. Immunoregulatory activities of polysaccharides from mung bean. Carbohydr. Polym. 2016, 139, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Thurl, S.; Munzert, M.; Boehm, G.; Matthews, C.; Stahl, B. Systematic review of the concentrations of oligosaccharides in human milk. Nutr. Rev. 2017, 75, 920–933. [Google Scholar] [CrossRef]
- Liu, Q.M.; Zhang, Y.F.; Shu, Z.D.; Liu, M.; Zeng, R.Y.; Wang, Y.B.; Liu, H.; Cao, M.J.; Su, W.J.; Liu, G.M. Sulfated oligosaccharide of Gracilaria lemaneiformis protect against food allergic response in mice by up-regulating immunosuppression. Carbohydr. Polym. 2020, 230, 12. [Google Scholar] [CrossRef] [PubMed]
- Ciucanu, I.; Kerek, F. A Simple and Rapid Method for the Permethylation of Carbohydrates. Carbohydr. Res. 1984, 131, 209–217. [Google Scholar] [CrossRef]
- Dhingra, N.; Sharma, R.; Kar, A. Towards further understanding on the antioxidative activities of Prunus persica fruit: A comparative study with four different fractions. Spectrochim. Acta Part Mol. Biomol. Spectrosc. 2014, 132, 582–587. [Google Scholar] [CrossRef]
- Zhao, H.T.; Wang, Z.Y.; Cheng, C.L.; Yao, L.; Wang, L.; Lu, W.H.; Yang, X.; Ma, F.M. In-vitro free radical scavenging activities of anthocyanins from three berries. J. Med. Plants Res. 2011, 5, 7036–7042. [Google Scholar] [CrossRef]
- Liu, X.C.; Pang, H.; Gao, Z.; Zhao, H.J.; Zhang, J.J.; Jia, L. Antioxidant and hepatoprotective activities of residue polysaccharides by Check for Pleurotus citrinipileatus. Int. J. Biol. Macromol. 2019, 131, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.W.; Yue, Y.H.; Han, H.; Chen, X.L.; Lu, Y.G.; Zheng, J.M.; Hou, H.T.; Lang, X.M.; He, L.L.; Hu, Q.L.; et al. Effect of toll-like receptor 3 agonist poly I:C on intestinal mucosa and epithelial barrier function in mouse models of acute colitis. World J. Gastroenterol. 2017, 23, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Yuan, H.; Zhang, Y.; Wei, X.; Wang, D. Macromolecular glucocorticoid prodrug improves the treatment of dextran sulfate sodium-induced mice ulcerative colitis. Clin. Immunol. 2015, 160, 71–81. [Google Scholar] [CrossRef]
- Shi, L.; Dai, Y.; Jia, B.Y.; Han, Y.F.; Guo, Y.; Xie, T.H.; Liu, J.L.; Tan, X.; Ding, P.H.; Li, J.X. The inhibitory effects of Qingchang Wenzhong granule on the interactive network of inflammation, oxidative stress, and apoptosis in rats with dextran sulfate sodium-induced colitis. J. Cell. Biochem. 2019, 120, 9979–9991. [Google Scholar] [CrossRef]
- Wirtz, S.; Neufert, C.; Weigmann, B.; Neurath, M.F. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2007, 2, 541–546. [Google Scholar] [CrossRef]
- Li, L.J.; Xu, X.H.; Yuan, T.J.; Hou, J.; Yu, C.L.; Peng, L.H. Periplaneta Americana L. as a novel therapeutics accelerates wound repair and regeneration. Biomed. Pharmacother. 2019, 114, 108858. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, Y.; Li, J.; Zhang, C.; Gao, F.; Ma, X.; Zhang, J.; Fu, C.; Geng, F. A feasible biocompatible hydrogel film embedding Periplaneta americana extract for acute wound healing. Int. J. Pharm. 2019, 571, 118707. [Google Scholar] [CrossRef]
- Xue, N.N.; He, M. Periplaneta americana extract promotes intestinal mucosa repair of ulcerative colitis in rat. Acta Cir. Bras. 2020, 35, e202001002. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, M.; Zeng, D.; Ruan, Y.; Shen, L.; Mu, Z.; Zou, J.; Xie, C.; Yang, Z.; Qian, Z.; et al. Periplaneta americana Extracts Accelerate Liver Regeneration via a Complex Network of Pathways. Front. Pharmacol 2020, 11, 1174. [Google Scholar] [CrossRef]
- Hong, J.; Zhang, P.; Yoon, I.N.; Hwang, J.S.; Kang, J.K.; Kim, H. The American Cockroach Peptide Periplanetasin-2 Blocks Clostridium Difficile Toxin A-Induced Cell Damage and Inflammation in the Gut. J. Microbiol. Biotechnol. 2017, 27, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.W.; Lee, J.H.; Seo, M.; Lee, H.J.; Baek, M.; Kim, M.A.; Shin, Y.P.; Kim, S.H.; Kim, I.; Hwang, J.S. Anti-Inflammatory Activity of Antimicrobial Peptide Periplanetasin-5 Derived from the Cockroach Periplaneta americana. J. Microbiol. Biotechnol. 2020, 30, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B. Mechanisms of disease: Pathogenesis of Crohn’s disease and ulcerative colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 390–407. [Google Scholar] [CrossRef]
- Strober, W.; Fuss, I.; Mannon, P. The fundamental basis of inflammatory bowel disease. J. Clin. Investig. 2007, 117, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yan, Y.; Wan, P.; Chen, D.; Ding, Y.; Ran, L.; Mi, J.; Lu, L.; Zhang, Z.; Li, X.; et al. Gut microbiota modulation and anti-inflammatory properties of anthocyanins from the fruits of Lycium ruthenicum Murray in dextran sodium sulfate-induced colitis in mice. Free Radic. Biol. Med. 2019, 136, 96–108. [Google Scholar] [CrossRef]
- Xia, X.; Zhu, L.; Lei, Z.; Song, Y.; Tang, F.; Yin, Z.; Wang, J. Feruloylated Oligosaccharides Alleviate Dextran Sulfate Sodium-Induced Colitis in Vivo. J. Agr. Food Chem. 2019, 67, 9522–9531. [Google Scholar] [CrossRef]
- Valatas, V.; Bamias, G.; Kolios, G. Experimental colitis models: Insights into the pathogenesis of inflammatory bowel disease and translational issues. Eur. J. Pharmacol. 2015, 759, 253–264. [Google Scholar] [CrossRef]
- Maloy, K.J.; Powrie, F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 2011, 474, 298–306. [Google Scholar] [CrossRef]
- Yousef, M.; Pichyangkura, R.; Soodvilai, S.; Chatsudthipong, V.; Muanprasat, C. Chitosan oligosaccharide as potential therapy of inflammatory bowel disease: Therapeutic efficacy and possible mechanisms of action. Pharmacol. Res. 2012, 66, 66–79. [Google Scholar] [CrossRef]
- De Roock, S.; Van Elk, M.; Van Dijk, M.E.; Timmerman, H.M.; Rijkers, G.T.; Prakken, B.J.; Hoekstra, M.O.; De Kleer, I.M. Lactic acid bacteria differ in their ability to induce functional regulatory T cells in humans. Clin. Exp. Allergy 2010, 40, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Boirivant, M.; Strober, W. The mechanism of action of probiotics. Curr. Opin. Gastroenterol. 2007, 23, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.X.; Li, Y.C.; Zhang, B. The effects of konjac oligosaccharide on TNBS-induced colitis in rats. Int. Immunopharmacol. 2016, 40, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.; Talens-Visconti, R.; Rius-Perez, S.; Finamor, I.; Sastre, J. Redox signaling in the gastrointestinal tract. Free Radic. Biol. Med. 2017, 104, 75–103. [Google Scholar] [CrossRef]
- Beltrán, B.; Nos, P.; Dasí, F.; Iborra, M.; Bastida, G.; Martínez, M.; O’Connor, J.E.; Sáez, G.; Moret, I.; Ponce, J. Mitochondrial dysfunction, persistent oxidative damage, and catalase inhibition in immune cells of naïve and treated Crohn’s disease. Inflamm. Bowel Dis. 2010, 16, 76–86. [Google Scholar] [CrossRef]
- Alzoghaibi, M.A.; Al Mofleh, I.A.; Al-Jebreen, A.M. Lipid peroxides in patients with inflammatory bowel disease. Saudi J. Gastroentero. 2007, 13, 187–190. [Google Scholar] [CrossRef]
- Ghia, J.-E.; Li, N.; Wang, H.; Collins, M.; Deng, Y.; El-Sharkawy, R.T.; Cote, F.; Mallet, J.; Khan, W.I. Serotonin Has a Key Role in Pathogenesis of Experimental Colitis. Gastroenterology 2009, 137, 1649–1660. [Google Scholar] [CrossRef]
- Lavi, I.; Levinson, D.; Peri, I.; Nimri, L.; Hadar, Y.; Schwartz, B. Orally administered glucans from the edible mushroom Pleurotus pulmonarius reduce acute inflammation in dextran sulfate sodium-induced experimental colitis. Br. J. Nutr. 2010, 103, 393–402. [Google Scholar] [CrossRef]
- Mancini, S.; Mariani, F.; Sena, P.; Benincasa, M.; Roncucci, L. Myeloperoxidase expression in human colonic mucosa is related to systemic oxidative balance in healthy subjects. Redox Rep. 2017, 22, 399–407. [Google Scholar] [CrossRef]
- Chen, L.; You, Q.; Hu, L.; Gao, J.; Meng, Q.Q.; Liu, W.T.; Wu, X.F.; Xu, Q. The antioxidant Procyanidin reduces Reactive Oxygen Species Signaling in Macrophages and Ameliorates Experimental Colitis in Mice. Front. Immunol. 2018, 8, 13. [Google Scholar] [CrossRef]
- Xia, F.; Wang, C.; Jin, Y.; Liu, Q.; Meng, Q.; Liu, K.; Sun, H. Luteolin protects HUVECs from TNF-α-induced oxidative stress and inflammation via its effects on the Nox4/ROS-NF-κB and MAPK pathways. J. Atheroscler. Thromb. 2014, 21, 768–783. [Google Scholar] [CrossRef] [PubMed]
- Ortega-González, M.; Ocón, B.; Romero-Calvo, I.; Anzola, A.; Guadix, E.; Zarzuelo, A.; Suárez, M.D.; Sánchez de Medina, F.; Martínez-Augustin, O. Nondigestible oligosaccharides exert nonprebiotic effects on intestinal epithelial cells enhancing the immune response via activation of TLR4-NFκB. Mol. Nutr. Food Res. 2014, 58, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Hatanaka, E.; Sato, F.T.; Sampaio, S.C.; Curi, R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 2011, 22, 849–855. [Google Scholar] [CrossRef]
- Andoh, A.; Zhang, Z.; Inatomi, O.; Fujino, S.; Deguchi, Y.; Araki, Y.; Tsujikawa, T.; Kitoh, K.; Kim-Mitsuyama, S.; Takayanagi, A.; et al. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology 2005, 129, 969–984. [Google Scholar] [CrossRef]
- Yin, Y.; Ye, L.; Niu, Z.; Fang, W. Anti-inflammatory effects of Vicenin-2 on dextran sulfate sodium-induced colitis in mice. Drug Dev. Res. 2019, 80, 546–555. [Google Scholar] [CrossRef]
- Yeom, Y.; Kim, B.-S.; Kim, S.-J.; Kim, Y. Sasa quelpaertensis leaf extract regulates microbial dysbiosis by modulating the composition and diversity of the microbiota in dextran sulfate sodium-induced colitis mice. BMC Complement. Altern. Med. 2016, 16. [Google Scholar] [CrossRef]
- Hakansson, A.; Tormo-Badia, N.; Baridi, A.; Xu, J.; Molin, G.; Hagslatt, M.L.; Karlsson, C.; Jeppsson, B.; Cilio, C.M.; Ahrne, S. Immunological alteration and changes of gut microbiota after dextran sulfate sodium (DSS) administration in mice. Clin. Exper. Med. 2015, 15, 107–120. [Google Scholar] [CrossRef]
- Long, T.; Yu, Z.J.; Wang, J.; Liu, J.; He, B.S. Orally Administered Chitooligosaccharides Modulate Colon Microbiota in Normal and Colitis Mice. Int. J. Pharmacol. 2018, 14, 291–300. [Google Scholar] [CrossRef]
- Muñoz-Garach, A.; Diaz-Perdigones, C.; Tinahones, F.J. Gut microbiota and type 2 diabetes mellitus. Endocrinol. Nutr. 2016, 63, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Le Blay, G.; Michel, C.; Blottière, H.M.; Cherbut, C. Prolonged intake of fructo-oligosaccharides induces a short-term elevation of lactic acid-producing bacteria and a persistent increase in cecal butyrate in rats. J. Nutr. 1999, 129, 2231–2235. [Google Scholar] [CrossRef]
- Ferenczi, S.; Szegi, K.; Winkler, Z.; Barna, T.; Kovacs, K.J. Oligomannan Prebiotic Attenuates Immunological, Clinical and Behavioral Symptoms in Mouse Model of Inflammatory Bowel Disease. Sci. Rep. 2016, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Cherbut, C.; Michel, C.; Lecannu, G. The prebiotic characteristics of fructooligosaccharides are necessary for reduction of TNBS-induced colitis in rats. J. Nutr. 2003, 133, 21–27. [Google Scholar] [CrossRef]
- Islam, J.; Koseki, T.; Watanabe, K.; Ardiansyah; Budijanto, S.; Oikawa, A.; Alauddin, M.; Goto, T.; Aso, H.; Komai, M.; et al. Dietary Supplementation of Fermented Rice Bran Effectively Alleviates Dextran Sodium Sulfate-Induced Colitis in Mice. Nutrients 2017, 9, 747. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, S.; Calder, P.C.; Childs, C.E. Microbiota-independent immunological effects of non-digestible oligosaccharides in the context of inflammatory bowel diseases. Proc. Nutr. Soc. 2020, 1–11. [Google Scholar] [CrossRef]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef] [PubMed]
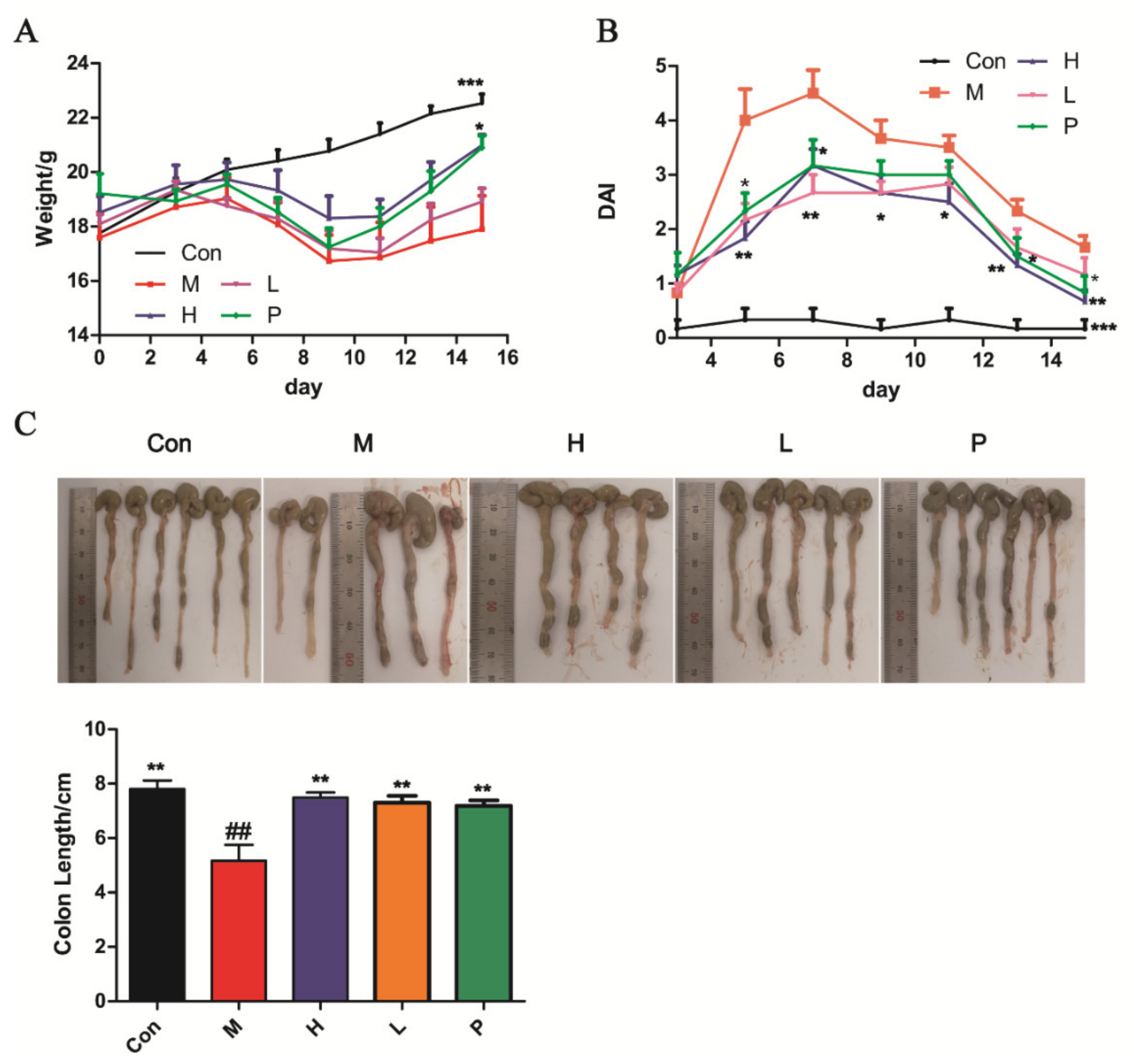
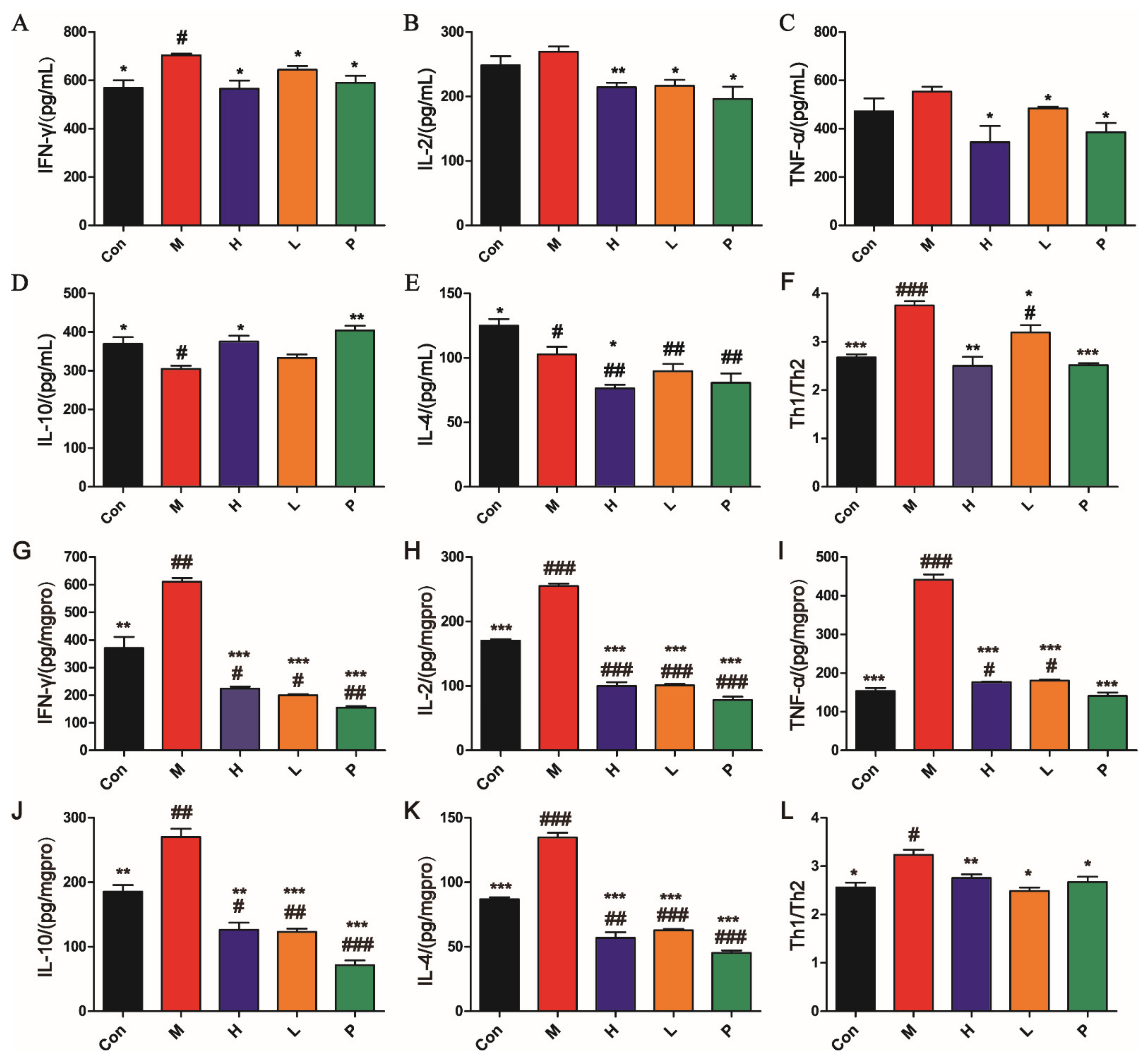
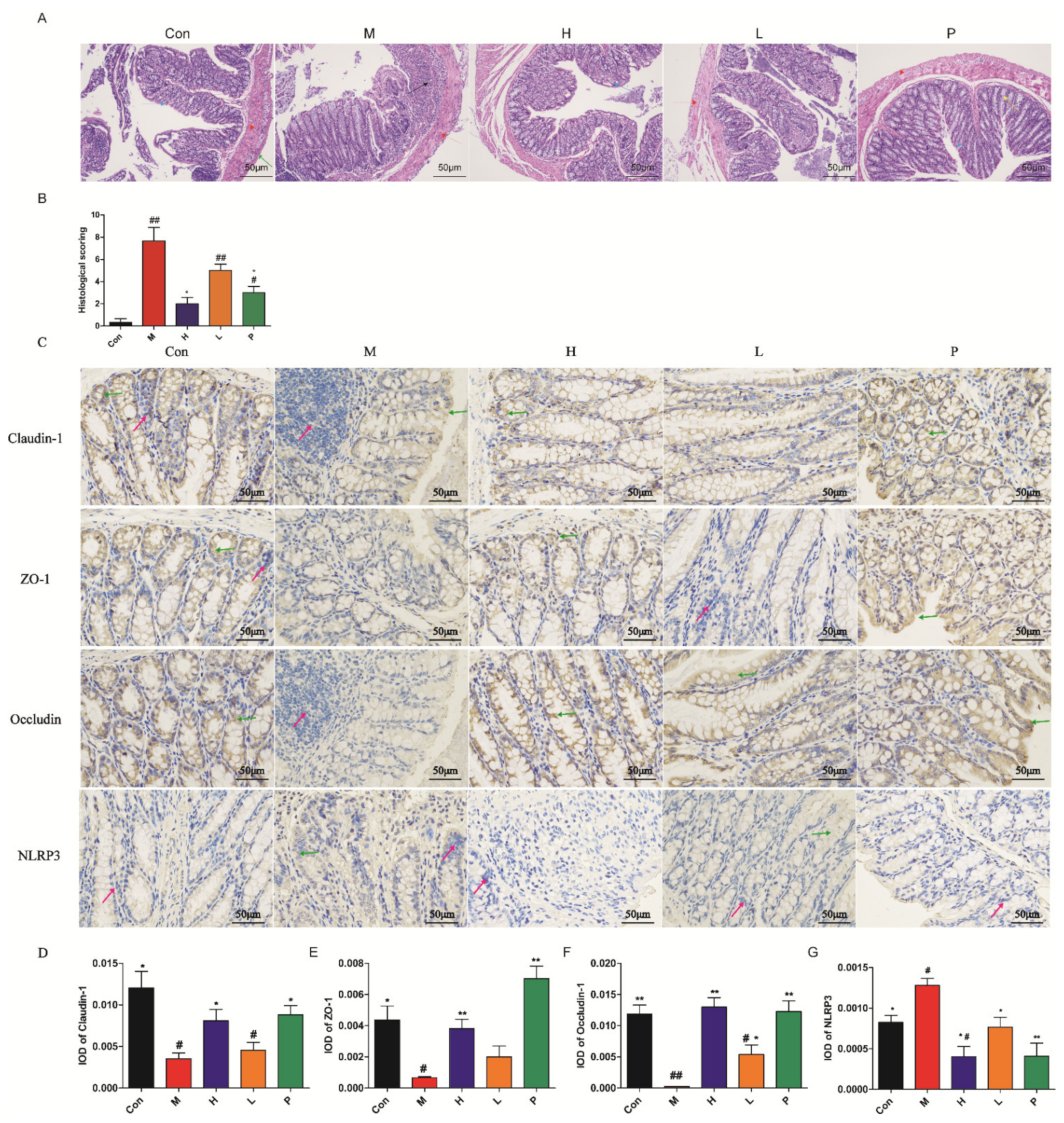
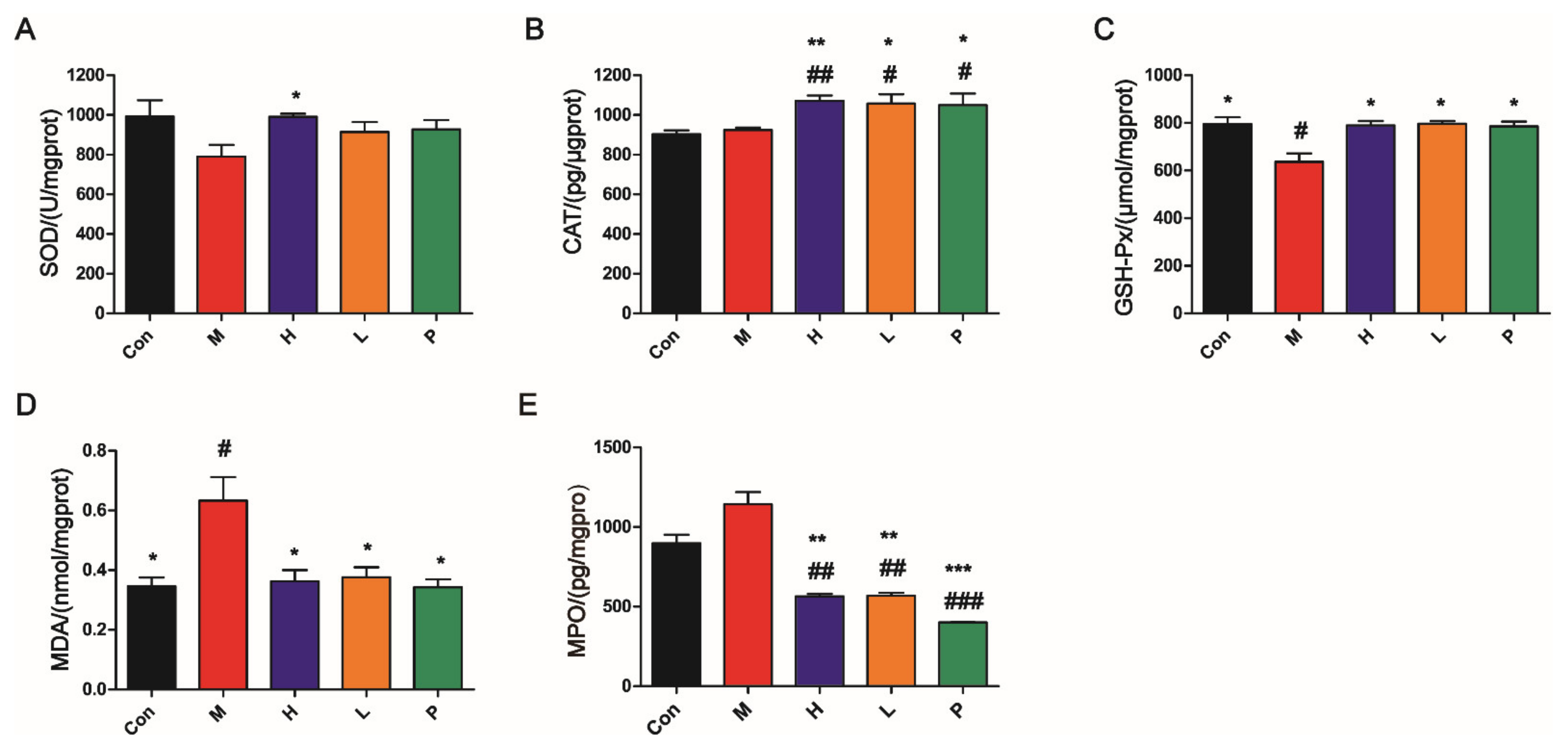
| Score | Weight Loss (%) | Diarrheal Stool Score | Bloody Stool Score |
|---|---|---|---|
| 0 | <1 | Normal | Negative |
| 1 | 1–5 | -- | -- |
| 2 | 5–10 | Loose | Positive |
| 3 | 10–15 | -- | -- |
| 4 | >15 | Diarrhea | Gross bleeding |
| Score | Inflammation | Mucosal Injury | Crypt Damage | Percent Involvement (%) |
|---|---|---|---|---|
| 0 | None | None | None | 0 |
| 1 | Slight | Mucous layer | 1/3 | 1–25 |
| 2 | Moderate | Submucosal | 2/3 | 26–50 |
| 3 | Serious | Muscular layer and serosa | 1 | 51–75 |
| 4 | -- | -- | 1 + epithelial loss | 76–100 |
| O-Me-Alditol Acetate | Linkages | Percent (%) |
|---|---|---|
| 2,3,6-Me3-Glcp | 1,4-Glcp | 74 |
| 2,3,4-Me3-Glcp | 1,6-Glcp | 2.9 |
| 2,3-Me2-Glcp | 1,4,6-Glcp | 5.6 |
| 2,3,4,6-Me4-Glcp | 1-Glcp | 7.8 |
| 2,3,4,6-Me4-Xylp | 1-Xyl | 9.7 |
| IC50 | VC | OPA |
|---|---|---|
| IC50 of hydroxyl radical scavenging (µg/mL) | 125.6 ± 1.445 | 112.3 ± 1.292 |
| IC50 of DPPH radical scavenging (µg/mL) | <<50 | 61.56 ± 0.893 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, K.; Zhou, J.; Deng, J.; Li, Y.; Wu, C.; Bao, J. Periplaneta americana Oligosaccharides Exert Anti-Inflammatory Activity through Immunoregulation and Modulation of Gut Microbiota in Acute Colitis Mice Model. Molecules 2021, 26, 1718. https://doi.org/10.3390/molecules26061718
Lu K, Zhou J, Deng J, Li Y, Wu C, Bao J. Periplaneta americana Oligosaccharides Exert Anti-Inflammatory Activity through Immunoregulation and Modulation of Gut Microbiota in Acute Colitis Mice Model. Molecules. 2021; 26(6):1718. https://doi.org/10.3390/molecules26061718
Chicago/Turabian StyleLu, Kaimin, Jing Zhou, Jie Deng, Yangjun Li, Chuanfang Wu, and Jinku Bao. 2021. "Periplaneta americana Oligosaccharides Exert Anti-Inflammatory Activity through Immunoregulation and Modulation of Gut Microbiota in Acute Colitis Mice Model" Molecules 26, no. 6: 1718. https://doi.org/10.3390/molecules26061718
APA StyleLu, K., Zhou, J., Deng, J., Li, Y., Wu, C., & Bao, J. (2021). Periplaneta americana Oligosaccharides Exert Anti-Inflammatory Activity through Immunoregulation and Modulation of Gut Microbiota in Acute Colitis Mice Model. Molecules, 26(6), 1718. https://doi.org/10.3390/molecules26061718