Lupin Protein Isolate Structure Diversity in Frozen-Cast Foams: Effects of Transglutaminases and Edible Fats
Abstract
1. Introduction
2. Results and Discussion
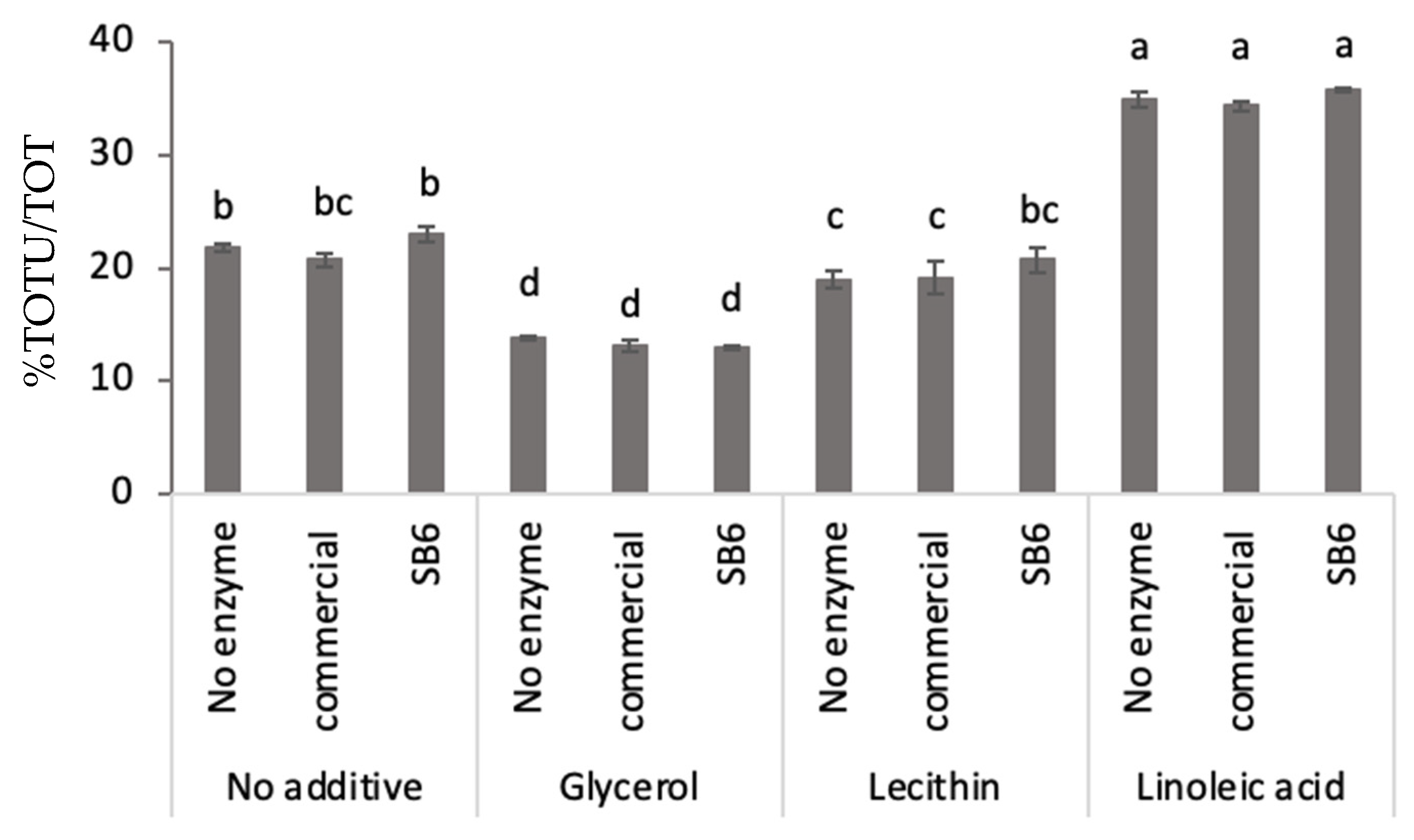
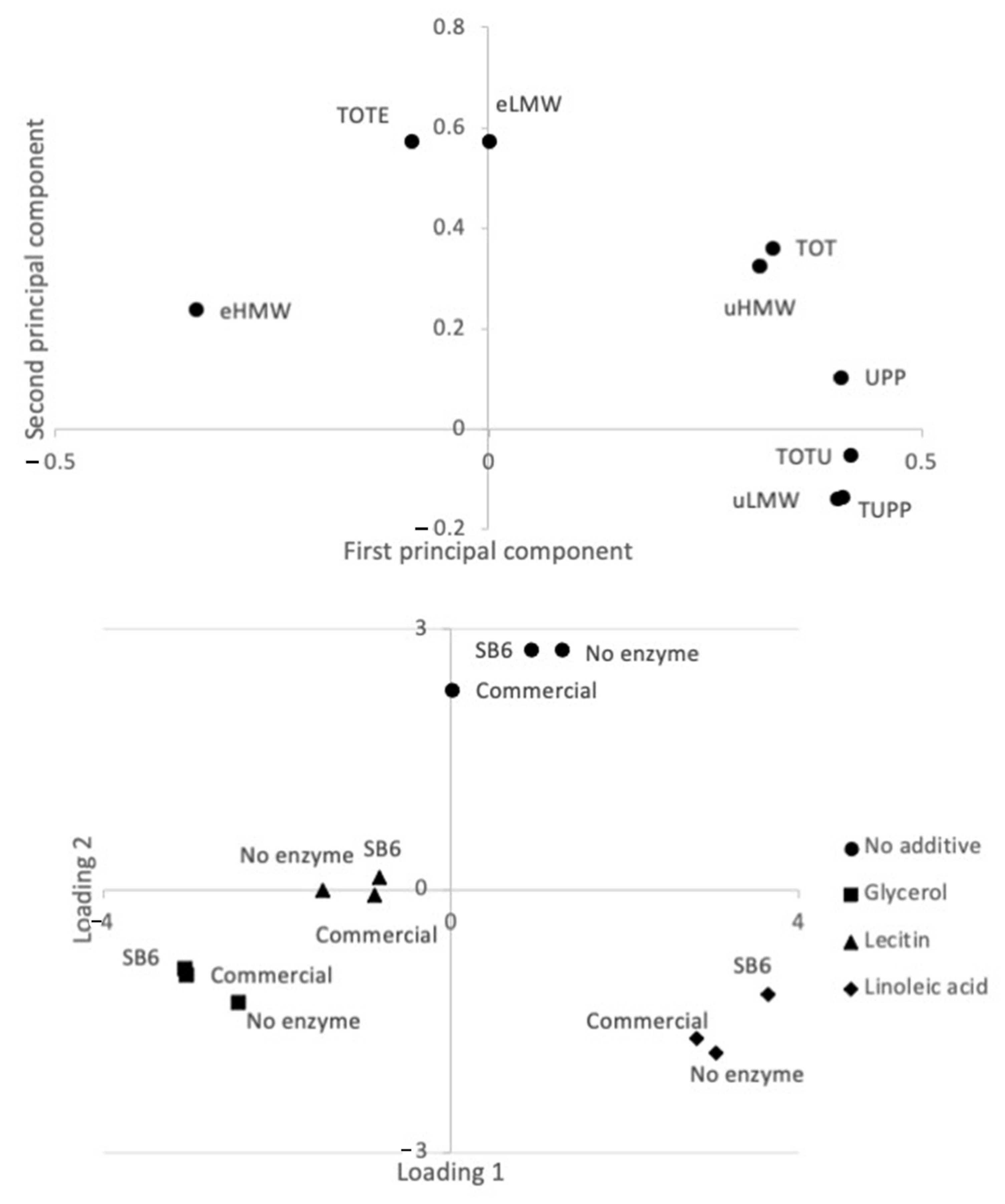
2.1. Effect of TGs and Additives on Lupin Protein Polymerization in Frozen-Cast Lupin Foams
2.2. Lupin Foam Morphology and Structure as Affected by Additives
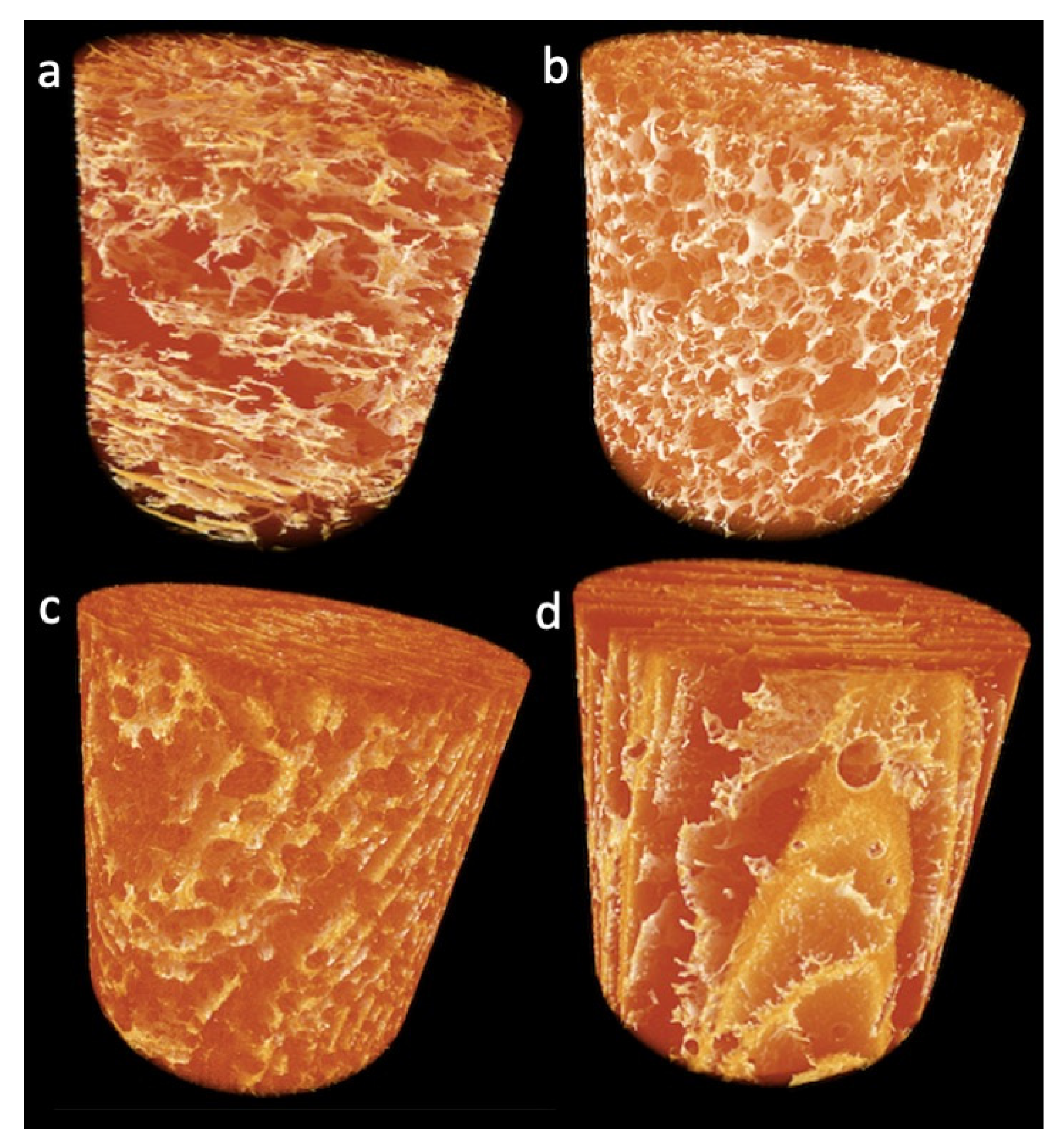
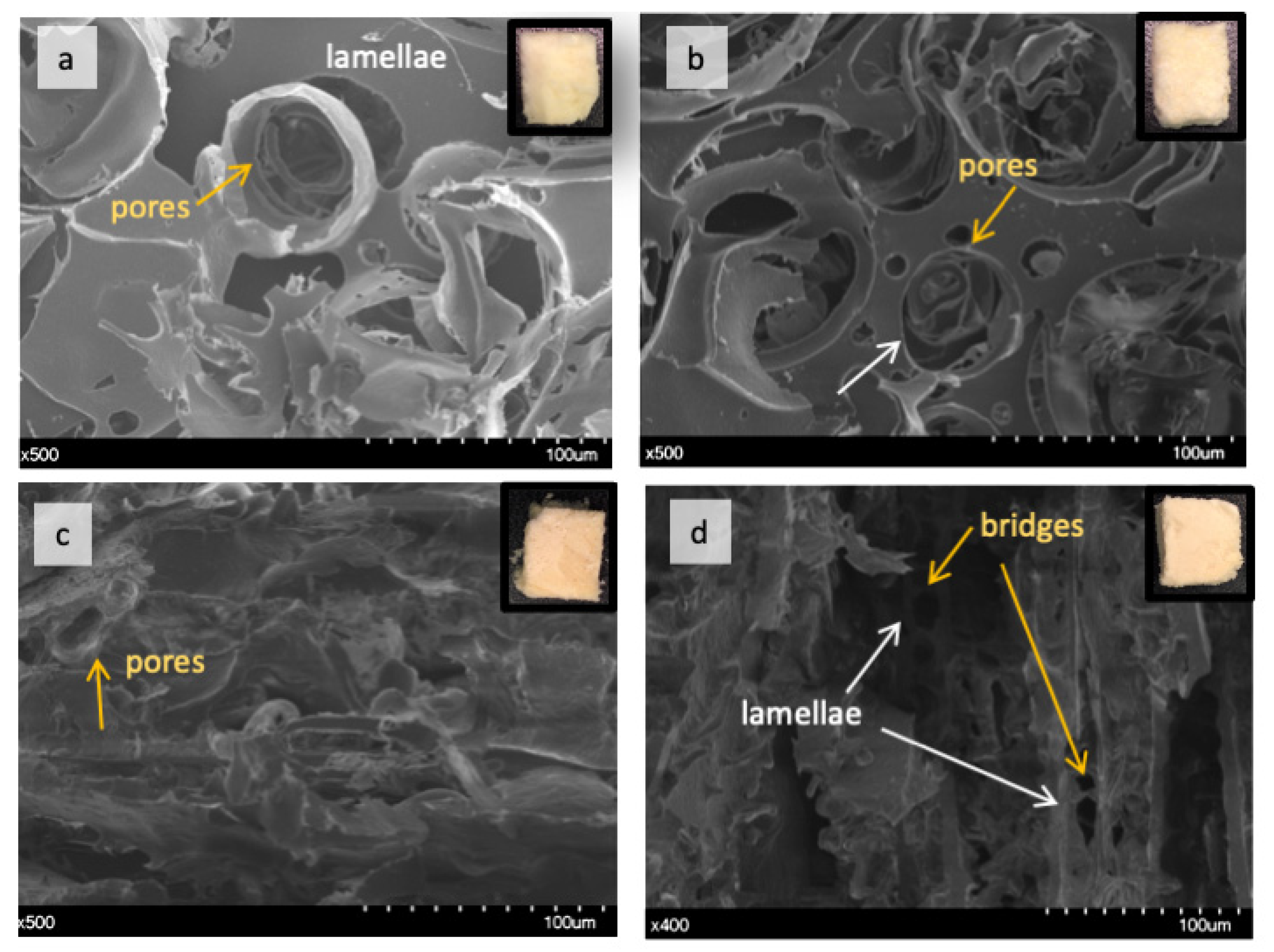
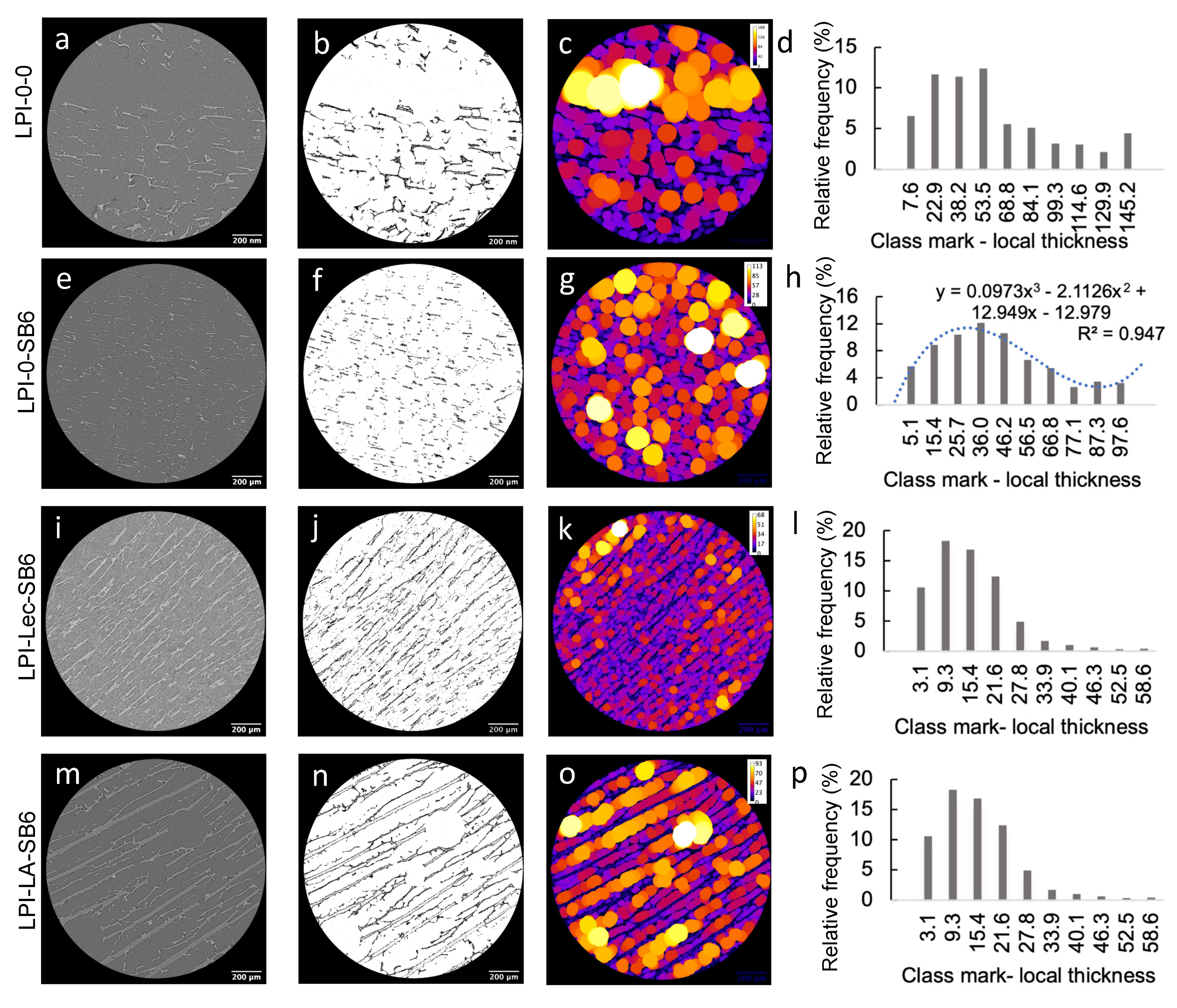
2.2.1. Effect of TGs on Lupin Foam Morphology and Structure
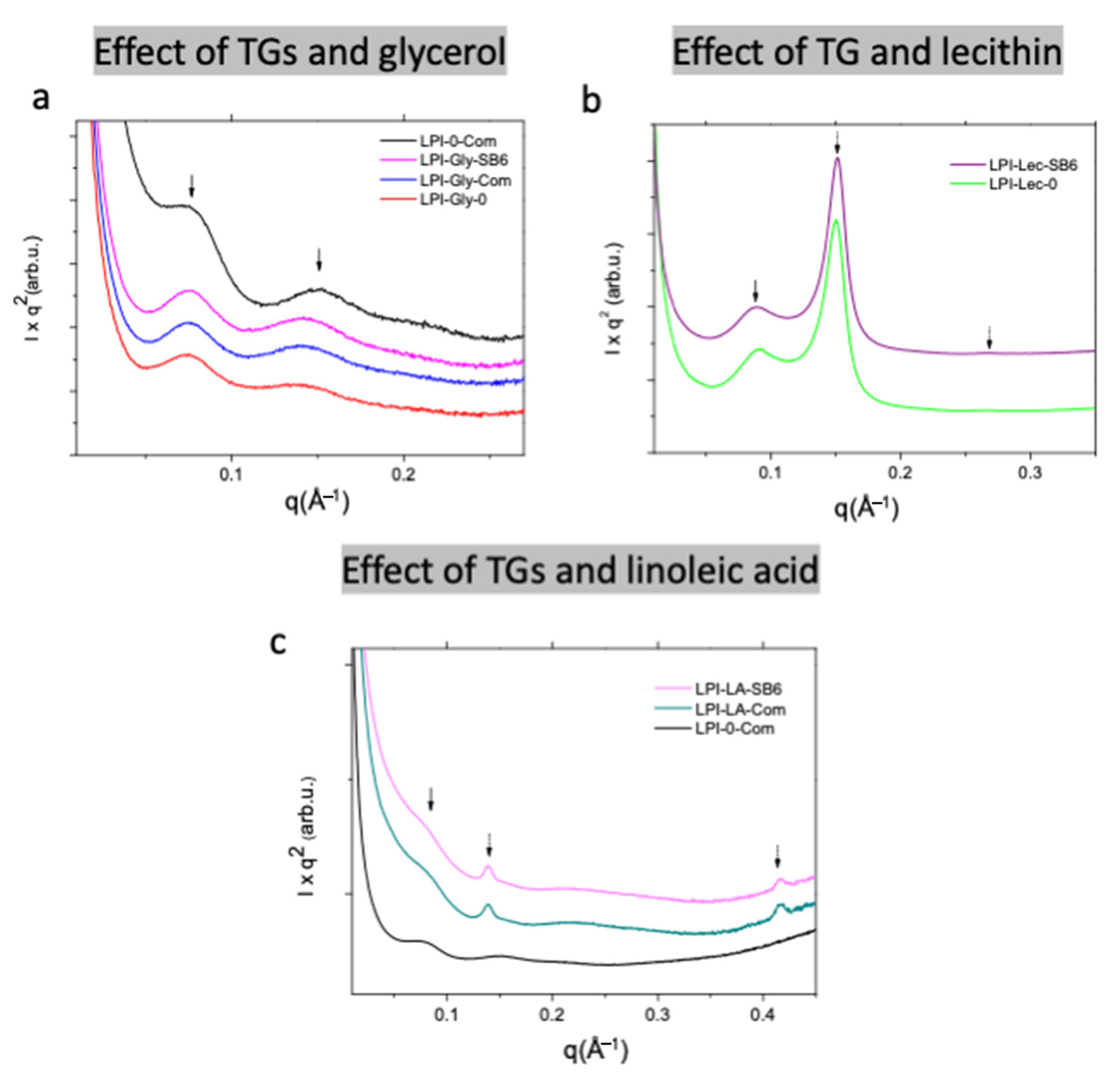
2.2.2. Effect of Lecithin and Glycerol on Lupin Functional Structuring Performance in Frozen-Cast Foams
2.2.3. Effect of Linoleic Acid on Lupin Structuring Performance in Frozen-Cast Foams
3. Materials and Methods
3.1. Lupin Protein Isolate
3.2. Additives
3.3. Foam Preparation
3.4. Chemicals
3.5. Size Exclusion-High Performance Liquid Chromatography (SE-HPLC)
3.6. Tomography
3.7. Scanning Electron Microscopy (SEM)
3.8. Small Angle X-ray Scattering (SAXS)
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Van de Noort, M. Lupin: An important protein and nutrient source. In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 165–183. [Google Scholar] [CrossRef]
- Muranyi, I.S.; Volke, D.; Hoffmann, R.; Eisner, P.; Herfellner, T.; Brunnbauer, M.; Koehler, P.; Schweiggert-Weisz, U. Protein distribution in lupin protein isolates from Lupinus angustifolius L. prepared by various isolation techniques. Food Chem. 2016, 207, 6–15. [Google Scholar] [CrossRef]
- D’Agostina, A.; Antonioni, C.; Resta, D.; Arnoldi, A.; Bez, J.; Knauf, U.; Wäsche, A. Optimization of a Pilot-Scale Process for producing lupin protein isolates with valuable technological properties and minimum thermal damage. J. Agric. Food Chem. 2006, 54, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Wäsche, A.; Müller, K.; Knauf, U. New processing of lupin protein isolates and functional properties. Food/Nahrung 2001, 45, 393–395. [Google Scholar] [CrossRef]
- Ceresino, E.B.; Kuktaite, R.; Hedenqvist, M.S.; Sato, H.H.; Johansson, E. Processing conditions and transglutaminase sources to “drive” the wheat gluten dough quality. Innov. Food Sci. Emerg. Technol. 2020, 65, 102439. [Google Scholar] [CrossRef]
- Duranti, M.; Consonni, A.; Magni, C.; Sessa, F.; Scarafoni, A. The major proteins of lupin seed: Characterisation and molecular properties for use as functional and nutraceutical ingredients. Trends Food Sci. Technol. 2008, 19, 624–633. [Google Scholar] [CrossRef]
- Kohajdová, Z.; Karovičová, J.; Schmidt, Š. Lupin composition and possible use in bakery—A review. Czech J. Food Sci. 2011, 29, 203–211. [Google Scholar] [CrossRef]
- Pollard, N.J.; Stoddard, F.L.; Popineau, Y.; Wrigley, C.W.; MacRitchie, F. Lupin flours as additives: Dough mixing, breadmaking, emulsifying, and foaming. Cereal Chem. 2002, 79, 662–669. [Google Scholar] [CrossRef]
- Maghaydah, S.; Abdul-hussain, S.; Ajo, R.; Tawalbeh, Y.; Elsahoryi, N. Effect of lupine flour on baking characteristics of gluten free cookies. Adv. J. Food Sci. Technol. 2013, 5, 600–605. [Google Scholar] [CrossRef]
- Banerjee, S.; Bhattacharya, S. Food gels: Gelling process and new applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 334–346. [Google Scholar] [CrossRef]
- Ceresino, E.B.; Kuktaite, R.; Sato, H.H.; Hedenqvist, M.S.; Johansson, E. Impact of gluten separation process and transglutaminase source on gluten based dough properties. Food Hydrocoll. 2019, 87, 661–669. [Google Scholar] [CrossRef]
- Dube, M.; Schäfer, C.; Neidhart, S.; Carle, R. Texturisation and modification of vegetable proteins for food applications using microbial transglutaminase. Eur. Food Res. Technol. 2006, 225, 287. [Google Scholar] [CrossRef]
- Mattice, K.D.; Marangoni, A.G. Physical properties of zein networks treated with microbial transglutaminase. Food Chem. 2021, 338, 128010. [Google Scholar] [CrossRef]
- Lerner, A.; Matthias, T. Processed food additive microbial transglutaminase and its cross-linked gliadin complexes are potential public health concerns in celiac disease. Int. J. Mol. Sci. 2020, 21, 1127. [Google Scholar] [CrossRef]
- Ceresino, E.B.; de Melo, R.R.; Kuktaite, R.; Hedenqvist, M.S.; Zucchi, T.D.; Johansson, E.; Sato, H.H. Transglutaminase from newly isolated Streptomyces sp. CBMAI 1617: Production optimization, characterization and evaluation in wheat protein and dough systems. Food Chem. 2018, 241, 403–410. [Google Scholar] [CrossRef]
- Ceresino, E.B.; Johansson, E.; Sato, H.H.; Plivelic, T.S.; Hall, S.A.; Kuktaite, R. Morphological and structural heterogeneity of solid gliadin food foams modified with transglutaminase and food grade dispersants. Food Hydrocoll. 2020, 108, 105995. [Google Scholar] [CrossRef]
- Johansson, E.; Prieto-Linde, M.L.; Jönsson, J.Ö. Effects of wheat cultivar and nitrogen application on storage protein composition and breadmaking quality. Cereal Chem. 2001, 78, 19–25. [Google Scholar] [CrossRef]
- Muneer, F.; Johansson, E.; Hedenqvist, M.S.; Plivelic, T.S.; Markedal, K.E.; Petersen, I.L.; Sørensen, J.C.; Kuktaite, R. The impact of newly produced protein and dietary fiber rich fractions of yellow pea (Pisum sativum L.) on the structure and mechanical properties of pasta-like sheets. Food Res. Int. 2018, 106, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Kuktaite, R.; Repo-Carrasco-Valencia, R.; Hurtado de Mendoza, C.J.; Plivelic, T.S.; Hall, S.; Johansson, E. Innovatively processed quinoa (Chenopodium quinoa Willd) food: Chemistry, structure and end-use characteristics. J. Sci. Food Agric. 2021. [Google Scholar] [CrossRef] [PubMed]
- Eibl, I.; von der Haar, D.; Jesdinszki, M.; Stäbler, A.; Schmid, M.; Langowski, H.-C. Adhesive based on micellar lupin protein isolate exhibiting oxygen barrier properties. J. Appl. Polym. Sci. 2018, 135, 46383. [Google Scholar] [CrossRef]
- Capezza, A.J.; Cui, Y.; Numata, K.; Lundman, M.; Newson, W.R.; Olsson, R.T.; Johansson, E.; Hedenqvist, M.S. High capacity functionalized protein superabsorbents from an agricultural co-product: A cradle-to-cradle approach. Adv. Sustain. Syst. 2020, 4, 2000110. [Google Scholar] [CrossRef]
- Schäfer, C.; Schott, M.; Brandl, F.; Neidhart, S.; Carle, R. Identification and quantification of ε-(γ-glutamyl)lysine in digests of enzymatically cross-linked leguminous proteins by high-performance liquid chromatography−electrospray ionization mass spectrometry (HPLC-ESI-MS). J. Agric. Food Chem. 2005, 53, 2830–2837. [Google Scholar] [CrossRef]
- Tang, C.-H. Nanostructured soy proteins: Fabrication and applications as delivery systems for bioactives (a review). Food Hydrocoll. 2019, 91, 92–116. [Google Scholar] [CrossRef]
- Alting, A.C.; van de Velde, F. 9—Proteins as clean label ingredients in foods and beverages. In Natural Food Additives, Ingredients and Flavourings; Baines, D., Seal, R., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 197–211. [Google Scholar] [CrossRef]
- Sousa, I.M.N.; Morgan, P.J.; Mitchell, J.R.; Harding, S.E.; Hill, S.E. Hydrodynamic characterization of lupin proteins: solubility, intrinsic viscosity, and molar mass. J. Agric. Food Chem. 1996, 44, 3018–3021. [Google Scholar] [CrossRef]
- Gekko, K.; Timasheff, S.N. Mechanism of protein stabilization by glycerol: Preferential hydration in glycerol-water mixtures. Biochemistry 1981, 20, 4667–4676. [Google Scholar] [CrossRef] [PubMed]
- Chanasattru, W.; Decker, E.A.; Julian McClements, D. Impact of cosolvents (polyols) on globular protein functionality: Ultrasonic velocity, density, surface tension and solubility study. Food Hydrocoll. 2008, 22, 1475–1484. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Bez, J.; Petersen, I.L.; Joehnke, M.S.; Detzel, A.; Busch, M.; Krueger, M.; Ispiryan, L.; O’Mahony, J.A.; Arendt, E.K.; et al. Techno-functional, nutritional and environmental performance of protein isolates from blue lupin and white lupin. Foods 2020, 9, 230. [Google Scholar] [CrossRef]
- Johnston, S.P.; Nickerson, M.T.; Low, N.H. The physicochemical properties of legume protein isolates and their ability to stabilize oil-in-water emulsions with and without genipin. J. Food Sci. Technol. 2015, 52, 4135–4145. [Google Scholar] [CrossRef]
- Day, L.; Zhai, J.; Xu, M.; Jones, N.C.; Hoffmann, S.V.; Wooster, T.J. Conformational changes of globular proteins adsorbed at oil-in-water emulsion interfaces examined by Synchrotron Radiation Circular Dichroism. Food Hydrocoll. 2014, 34, 78–87. [Google Scholar] [CrossRef]
- Rasheed, F.; Newson, W.R.; Plivelic, T.S.; Kuktaite, R.; Hedenqvist, M.S.; Gällstedt, M.; Johansson, E. Structural architecture and solubility of native and modified gliadin and glutenin proteins: Non-crystalline molecular and atomic organization. RSC Adv. 2014, 4, 2051–2060. [Google Scholar] [CrossRef]
- Pozani, S.; Doxastakis, G.; Kiosseoglou, V. Functionality of lupin seed protein isolate in relation to its interfacial behaviour. Food Hydrocoll. 2002, 16, 241–247. [Google Scholar] [CrossRef]
- Berghout, J.A.M.; Boom, R.M.; van der Goot, A.J. Understanding the differences in gelling properties between lupin protein isolate and soy protein isolate. Food Hydrocoll. 2015, 43, 465–472. [Google Scholar] [CrossRef]
- Lin, M.; Tay, S.H.; Yang, H.; Yang, B.; Li, H. Development of eggless cakes suitable for lacto-vegetarians using isolated pea proteins. Food Hydrocoll. 2017, 69, 440–449. [Google Scholar] [CrossRef]
- Paramita, V.D.; Panyoyai, N.; Kasapis, S. Molecular functionality of plant proteins from low-to high-solid systems with ligand and co-solute. Int. J. Mol. Sci. 2020, 21, 2550. [Google Scholar] [CrossRef] [PubMed]
- Agyare, K.K.; Addo, K.; Xiong, Y.L. Emulsifying and foaming properties of transglutaminase-treated wheat gluten hydrolysate as influenced by pH, temperature and salt. Food Hydrocoll. 2009, 23, 72–81. [Google Scholar] [CrossRef]
- Larre, C.; Chiarello, M.; Blanloeil, Y.; Chenu, M.; Gueguen, J. Gliadin modifications catalyzed by guinea pig liver transglutaminase. J. Food Biochem. 1993, 17, 267–282. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll. 2003, 17, 25–39. [Google Scholar] [CrossRef]
- Dimitrijev-Dwyer, M.; He, L.; James, M.; Nelson, A.; Wang, L.; Middelberg, A.P.J. The effects of acid hydrolysis on protein biosurfactant molecular, interfacial, and foam properties: pH responsive protein hydrolysates. Soft Matter 2012, 8, 5131–5139. [Google Scholar] [CrossRef]
- Damodaran, S.; Parkin, K.L.; Fennema, O.R. Fennema’s Food Chemistry, 4th ed.; CRC Press: Boca Raton, FL, USA, 2007; p. 1144. [Google Scholar]
- Piornos, J.A.; Burgos-Díaz, C.; Ogura, T.; Morales, E.; Rubilar, M.; Maureira-Butler, I.; Salvo-Garrido, H. Functional and physicochemical properties of a protein isolate from AluProt-CGNA: A novel protein-rich lupin variety (Lupinus luteus). Food Res. Int. 2015, 76, 719–724. [Google Scholar] [CrossRef]
- Cabello-Hurtado, F.; Keller, J.; Ley, J.; Sanchez-Lucas, R.; Jorrín-Novo, J.V.; Aïnouche, A. Proteomics for exploiting diversity of lupin seed storage proteins and their use as nutraceuticals for health and welfare. J. Proteom. 2016, 143, 57–68. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Guo, S. The binding mechanism of lecithin to soybean 11S and 7S globulins using fluorescence spectroscopy. Food Sci. Biotechnol. 2014, 23, 1785–1791. [Google Scholar] [CrossRef]
- Sun, X.M.; Wang, C.N.; Guo, M.R. Interactions between whey protein or polymerized whey protein and soybean lecithin in model system. J. Dairy Sci. 2018, 101, 9680–9692. [Google Scholar] [CrossRef]
- Murthy, A.; Ravi, P.R.; Kathuria, H.; Vats, R. Self-assembled lecithin-chitosan nanoparticles improve the oral bioavailability and alter the pharmacokinetics of raloxifene. Int. J. Pharm. 2020, 588, 119731. [Google Scholar] [CrossRef]
- Kulkarni, C.V.; Wachter, W.; Iglesias-Salto, G.; Engelskirchen, S.; Ahualli, S. Monoolein: A magic lipid? Phys. Chem. Chem. Phys. 2011, 13, 3004–3021. [Google Scholar] [CrossRef]
- Kuktaite, R.; Plivelic, T.S.; Cerenius, Y.; Hedenqvist, M.S.; Gällstedt, M.; Marttila, S.; Ignell, R.; Popineau, Y.; Tranquet, O.; Shewry, P.R.; et al. Structure and morphology of wheat gluten films: From polymeric protein aggregates toward superstructure arrangements. Biomacromolecules 2011, 12, 1438–1448. [Google Scholar] [CrossRef]
- Martiel, I.; Sagalowicz, L.; Mezzenga, R. Phospholipid-based nonlamellar mesophases for delivery systems: Bridging the gap between empirical and rational design. Adv. Colloid Interface Sci. 2014, 209, 127–143. [Google Scholar] [CrossRef]
- Bollom, M.A.; Clark, S.; Acevedo, N.C. Development and characterization of a novel soy lecithin-stearic acid and whey protein concentrate bigel system for potential edible applications. Food Hydrocoll. 2020, 101, 105570. [Google Scholar] [CrossRef]
- Le, T.T.; El-Bakry, M.; Neirynck, N.; Bogus, M.; Hoa, H.D.; Van der Meeren, P. Hydrophilic lecithins protect milk proteins against heat-induced aggregation. Colloids Surf. B Biointerfaces 2007, 60, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Kuktaite, R.; Newson, W.R.; Rasheed, F.; Plivelic, T.S.; Hedenqvist, M.S.; Gällstedt, M.; Johansson, E. Monitoring nanostructure dynamics and polymerization in glycerol plasticized wheat gliadin and glutenin films: Relation to mechanical properties. ACS Sustain. Chem. Eng. 2016, 4, 2998–3007. [Google Scholar] [CrossRef]
- Janssen, F.; Wouters, A.G.B.; Pauly, A.; Delcour, J.A. Relating the composition and air/water interfacial properties of wheat, rye, barley, and oat dough liquor. Food Chem. 2018, 264, 126–134. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, M.; Ma, X.; Ding, T.; Liu, D. Microstructure and permeability of hollow microcapsules produced from faba bean 11s protein. Food Hydrocoll. 2021, 112, 106292. [Google Scholar] [CrossRef]
- El-Fakharany, E.M.; Redwan, E.M. Protein-lipid complexes: Molecular structure, current scenarios and mechanisms of cytotoxicity. RSC Adv. 2019, 9, 36890–36906. [Google Scholar] [CrossRef]
- Angelova, A.; Garamus, V.M.; Angelov, B.; Tian, Z.; Li, Y.; Zou, A. Advances in structural design of lipid-based nanoparticle carriers for delivery of macromolecular drugs, phytochemicals and anti-tumor agents. Adv. Colloid Interface Sci. 2017, 249, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Foundation, P. The Keto Diet Market Growth and Industry Trends. 2020. Available online: https://paleofoundation.com/the-keto-diet-market-growth-and-industry-trends-2020/ (accessed on 24 January 2020).
- Hildebrand, T.; Rüegsegger, P. A new method for the model-independent assessment of thickness in three-dimensional images. J. Microsc. 1997, 185, 67–75. [Google Scholar] [CrossRef]
- Labrador, A.; Cerenius, Y.; Svensson, C.; Theodor, K.; Plivelic, T. The yellow mini-hutch for SAXS experiments at MAX IV Laboratory. J. Phys. Conf. Ser. 2013, 425, 072019. [Google Scholar] [CrossRef]
| Samples | Distances (Å) * | |||||
|---|---|---|---|---|---|---|
| d1 | d2 | d1,H | d2,H | d1,L | d3,L | |
| LPI-0-commercial | 81.6 | 41.2 | - | - | - | - |
| LPI-Gly-0 | 84.2 | 44.2 | - | - | - | - |
| LPI-Gly-commercial | 82.3 | 43.6 | - | - | - | - |
| LPI-Gly-SB6 | 82.7 | 43.5 | - | - | - | - |
| LPI-Lec-0 | 68.5 | - | 41.8 | 23.6 | - | - |
| LPI-Lec-SB6 | 70.0 | - | 41.4 | 23.5 | - | - |
| LPI-LA-commercial | 83.4 | - | - | - | 45.1 | 15.1 |
| LPI-LA-SB6 | 83.4 | - | - | - | 45.0 | 15.0 |
| Samples | Lupin Content a | TG b | Gly Content c | Lec Content d | Linoleic Acid Content e | Water | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| g | wt% | mg | U/g | g | wt% | g | wt% | g | wt% | mL | |
| LPI-0-0 | 2.7 | 18 | 0 | 0 | 15 | ||||||
| LPI-0-commercial | 2.7 | 18 | 40 | 4.68 | 0 | 15 | |||||
| LPI-0-SB6 | 2.7 | 18 | 10 | 4.68 | 0 | 15 | |||||
| LPI-Gly-0 | 2.7 | 18 | 0 | 0.81 | 30 | 15 | |||||
| LPI-Gly-commercial | 2.7 | 18 | 40 | 4.68 | 0.81 | 30 | 15 | ||||
| LPI-Gly-SB6 | 2.7 | 18 | 10 | 4.68 | 0.81 | 30 | 15 | ||||
| LPI-Lec-0 | 2.7 | 18 | 0 | 0.81 | 30 | 15 | |||||
| LPI-Lec-commercial | 2.7 | 18 | 40 | 4.68 | 0.81 | 30 | 15 | ||||
| LPI-Lec-SB6 | 2.7 | 18 | 10 | 4.68 | 0.81 | 30 | 15 | ||||
| LPI-LA-0 | 2.7 | 18 | 0 | 0.81 | 30 | 15 | |||||
| LPI-LA-commercial | 2.7 | 18 | 40 | 4.68 | 0.81 | 30 | 15 | ||||
| LPI-LA-SB6 | 2.7 | 18 | 10 | 4.68 | 0.81 | 30 | 15 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceresino, E.B.; Johansson, E.; Sato, H.H.; Plivelic, T.S.; Hall, S.A.; Bez, J.; Kuktaite, R. Lupin Protein Isolate Structure Diversity in Frozen-Cast Foams: Effects of Transglutaminases and Edible Fats. Molecules 2021, 26, 1717. https://doi.org/10.3390/molecules26061717
Ceresino EB, Johansson E, Sato HH, Plivelic TS, Hall SA, Bez J, Kuktaite R. Lupin Protein Isolate Structure Diversity in Frozen-Cast Foams: Effects of Transglutaminases and Edible Fats. Molecules. 2021; 26(6):1717. https://doi.org/10.3390/molecules26061717
Chicago/Turabian StyleCeresino, Elaine Berger, Eva Johansson, Hélia Harumi Sato, Tomás S. Plivelic, Stephen A. Hall, Jürgen Bez, and Ramune Kuktaite. 2021. "Lupin Protein Isolate Structure Diversity in Frozen-Cast Foams: Effects of Transglutaminases and Edible Fats" Molecules 26, no. 6: 1717. https://doi.org/10.3390/molecules26061717
APA StyleCeresino, E. B., Johansson, E., Sato, H. H., Plivelic, T. S., Hall, S. A., Bez, J., & Kuktaite, R. (2021). Lupin Protein Isolate Structure Diversity in Frozen-Cast Foams: Effects of Transglutaminases and Edible Fats. Molecules, 26(6), 1717. https://doi.org/10.3390/molecules26061717








