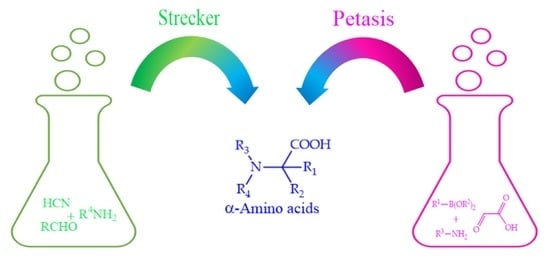
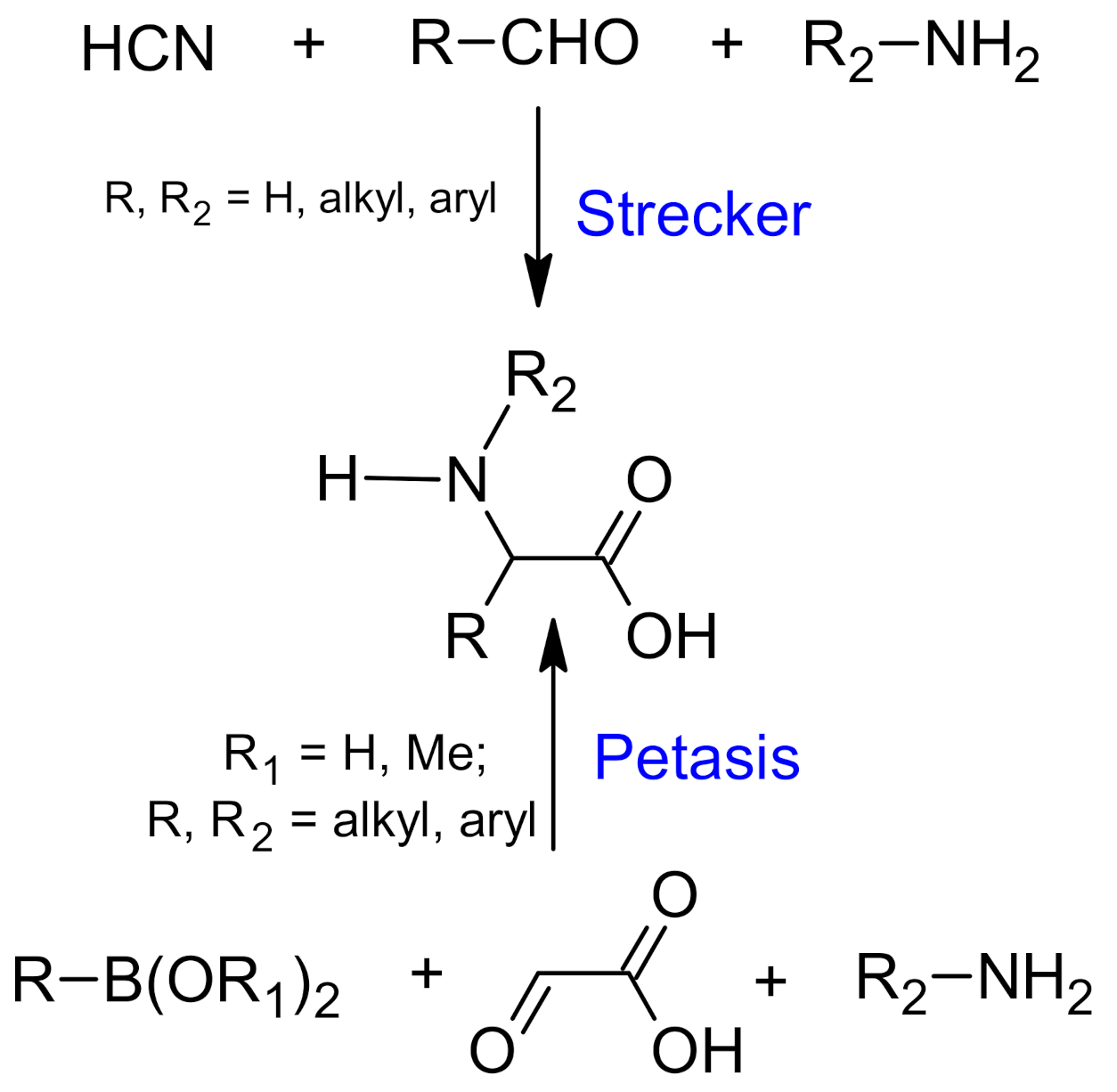
Petasis vs. Strecker Amino Acid Synthesis: Convergence, Divergence and Opportunities in Organic Synthesis
Abstract
1. Introduction
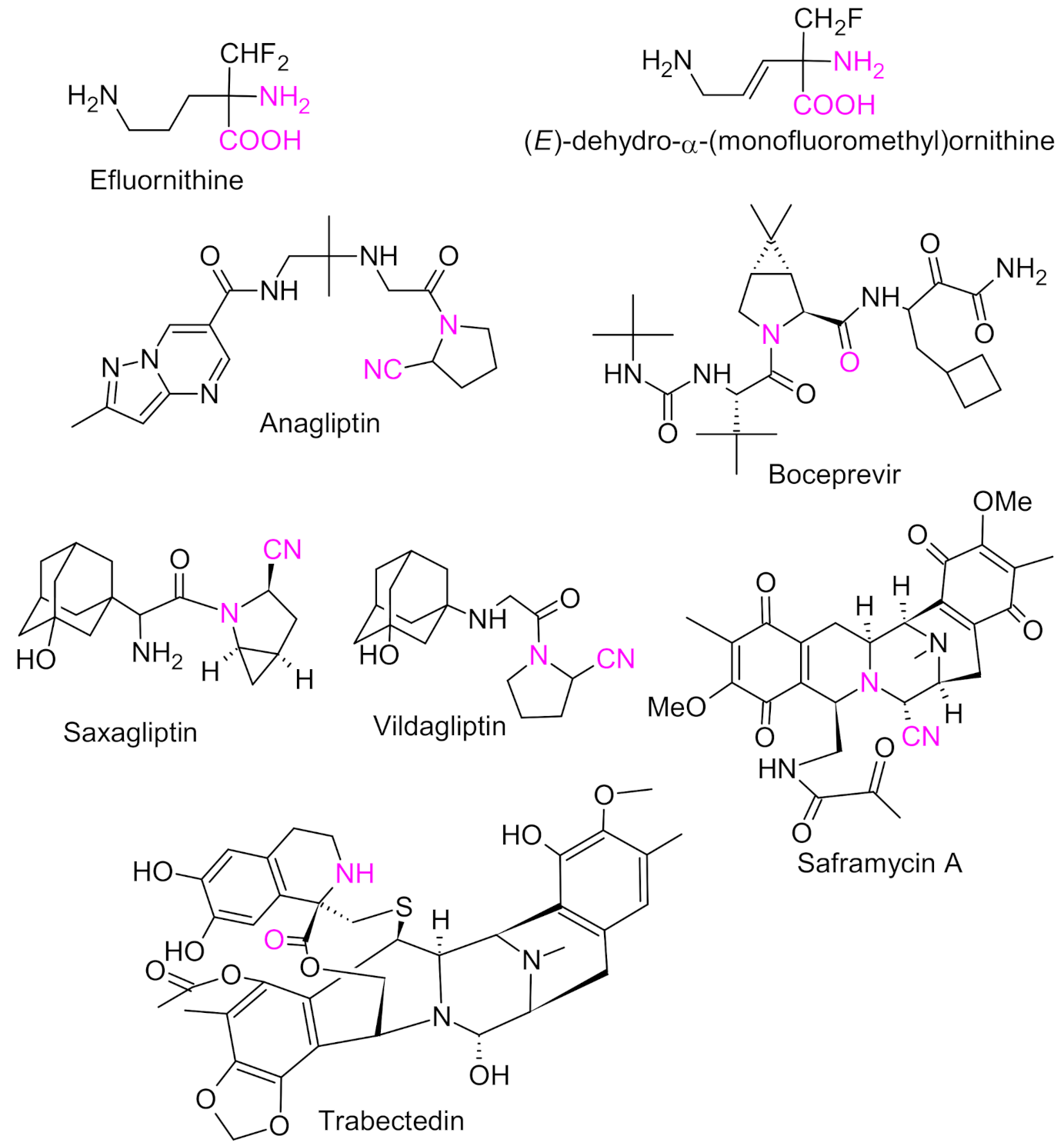
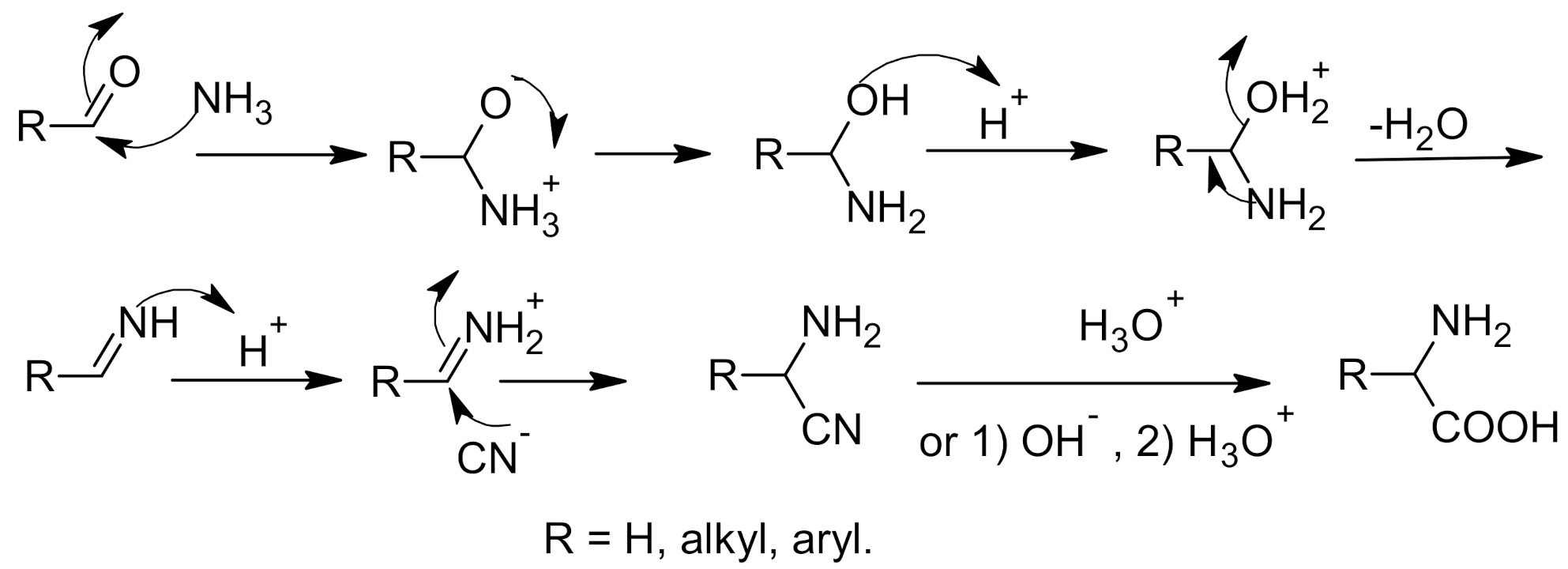
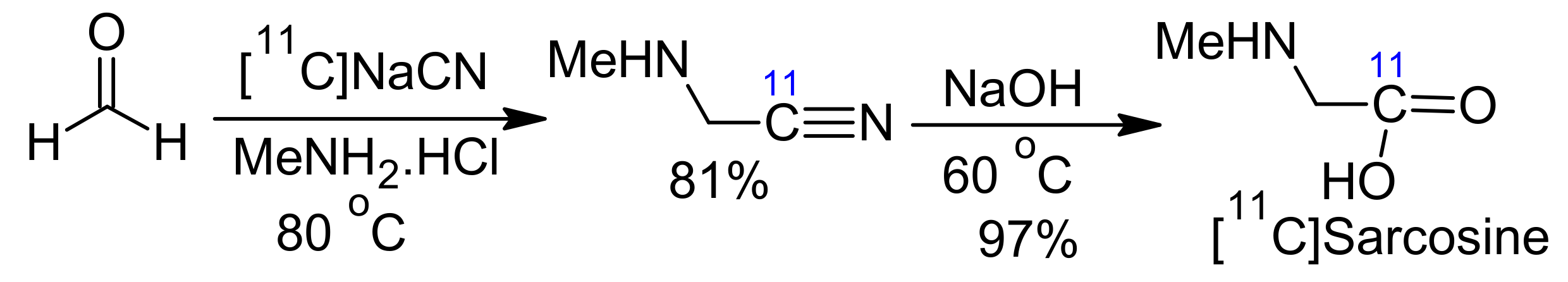
2. Strecker Reaction
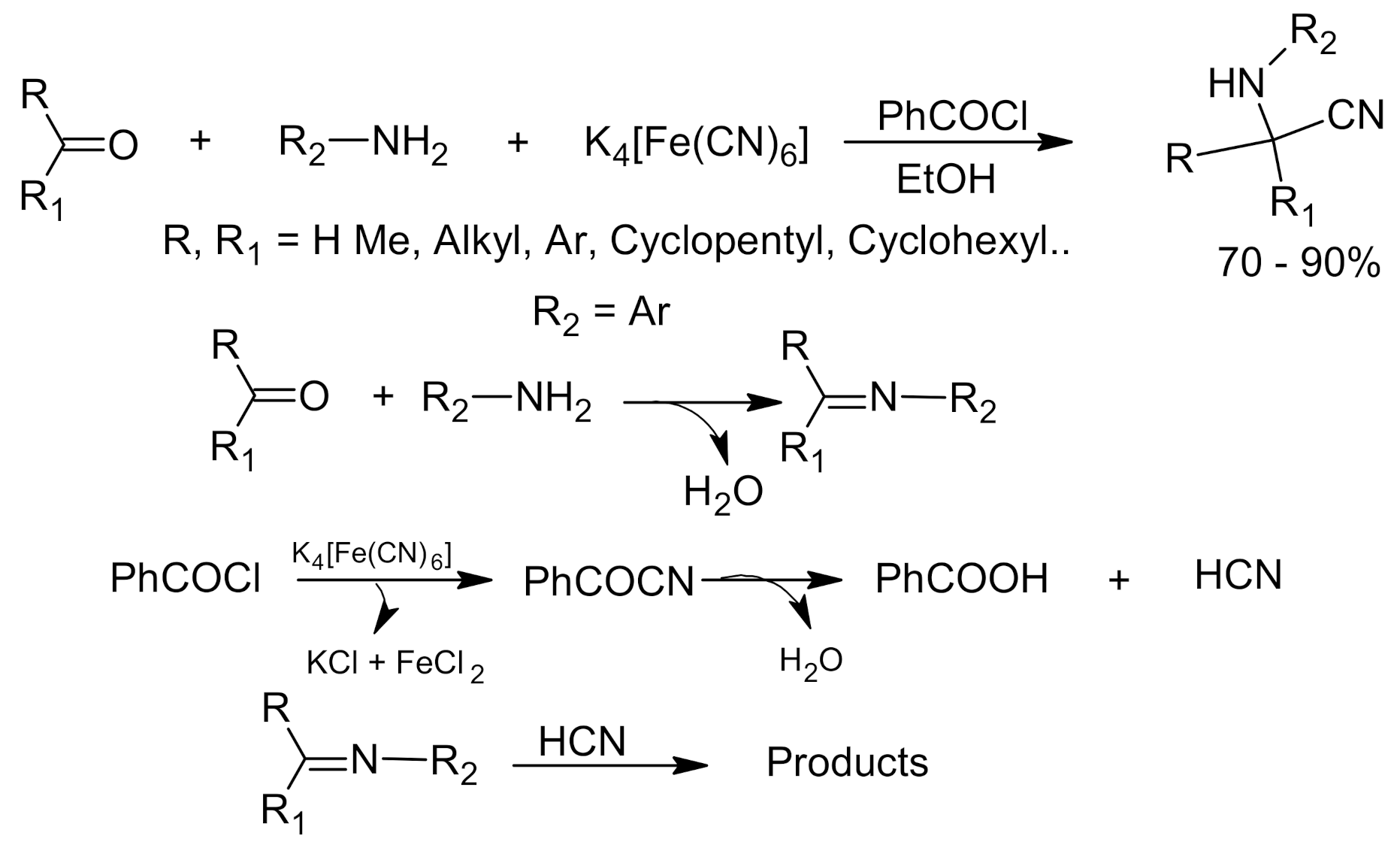
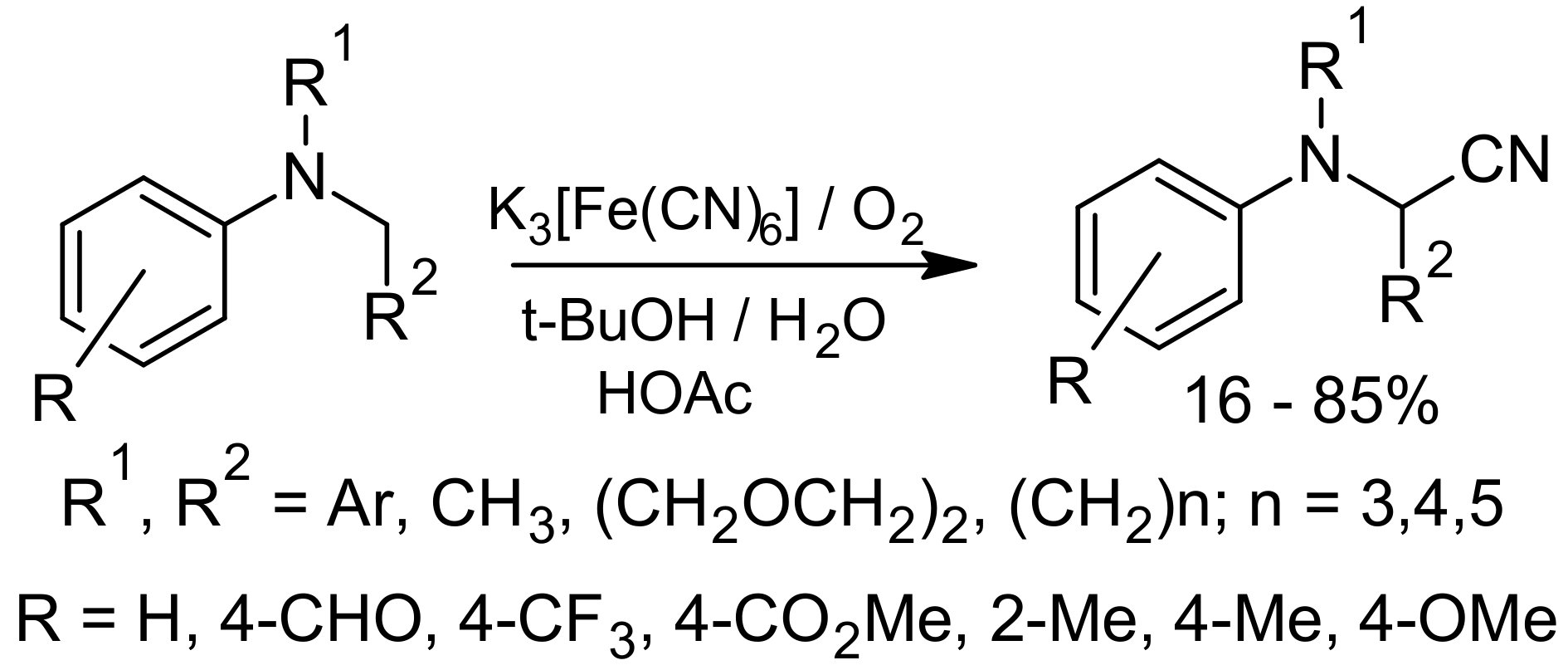
3. Green Strecker Reaction
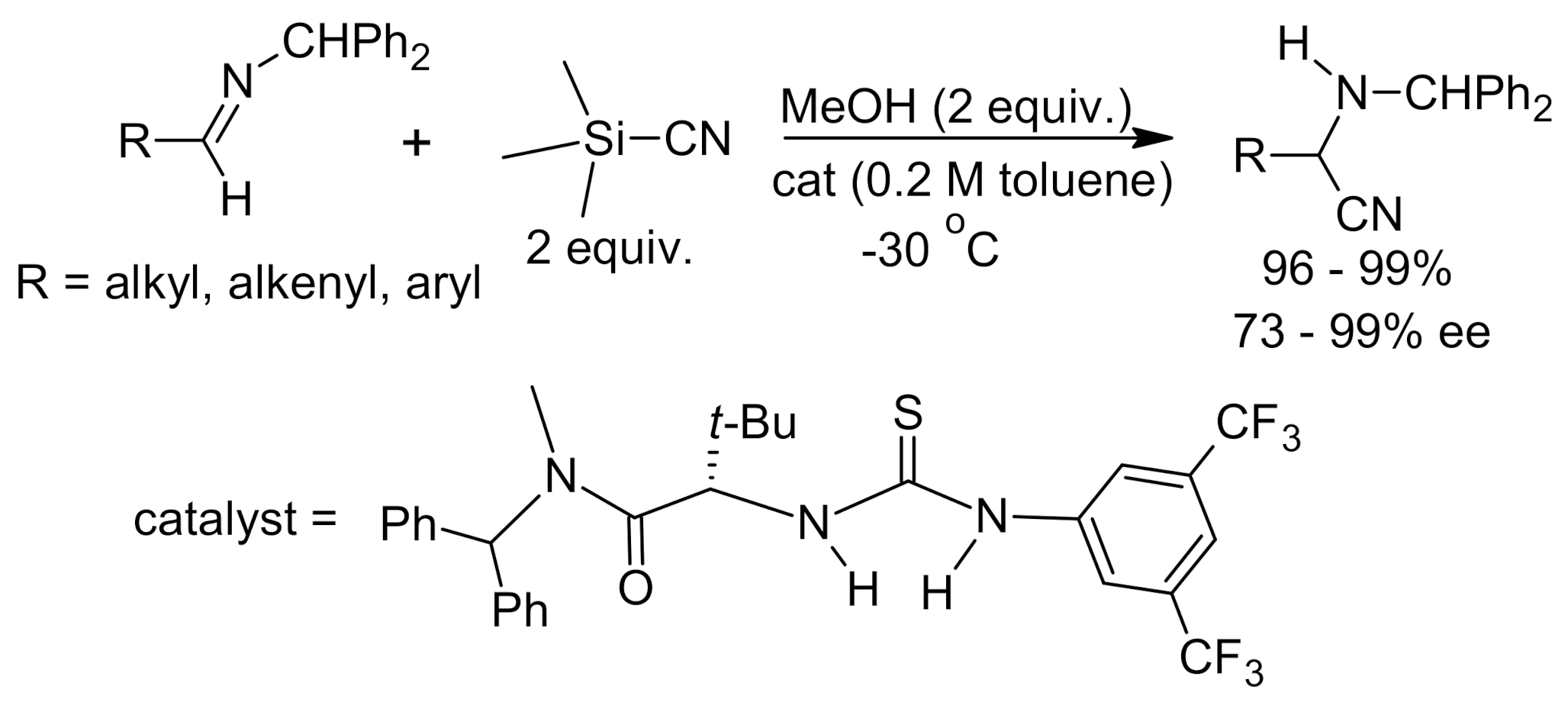
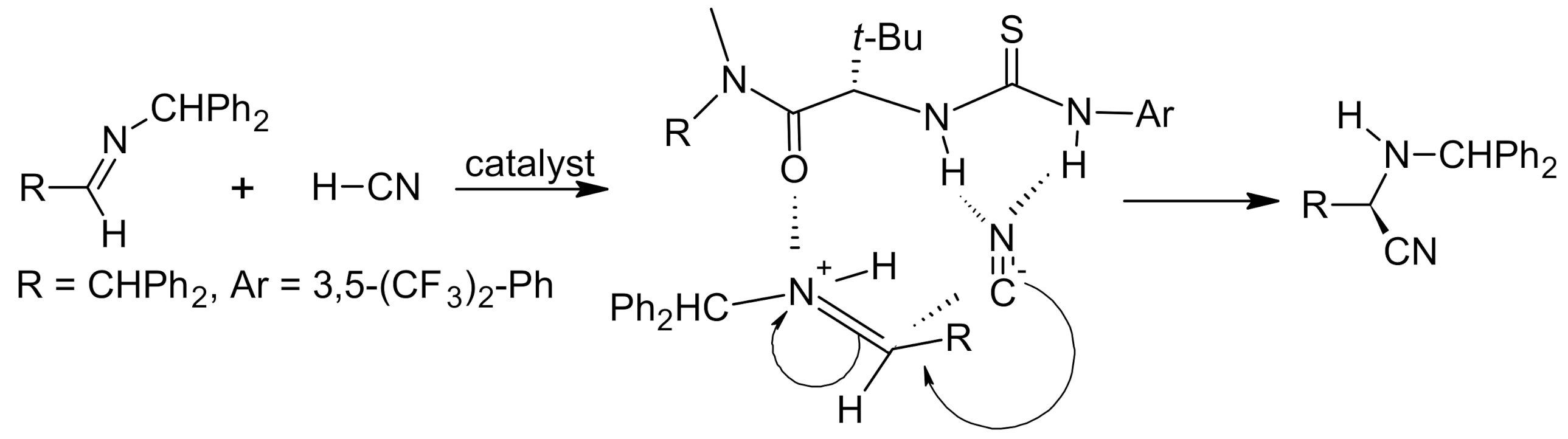
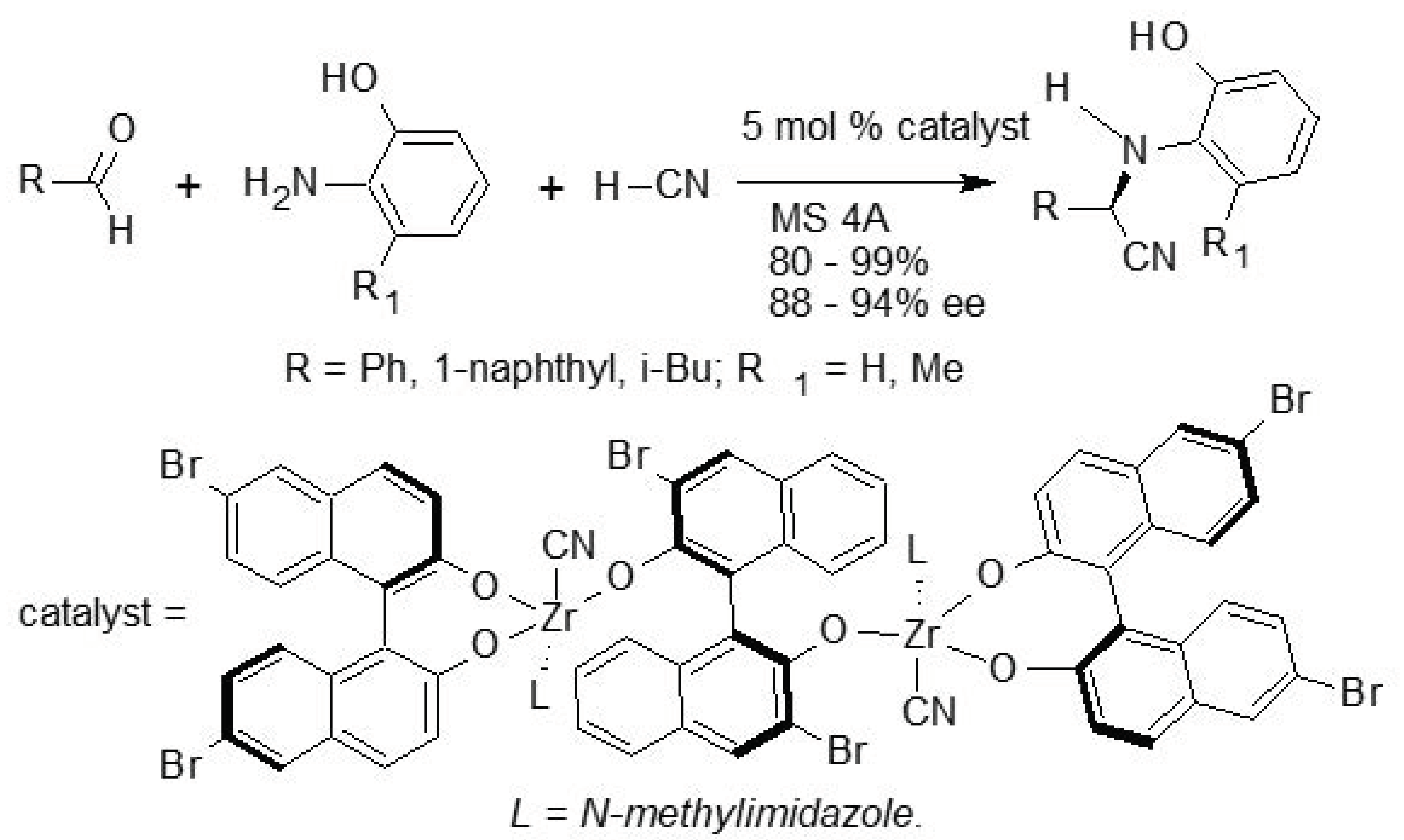
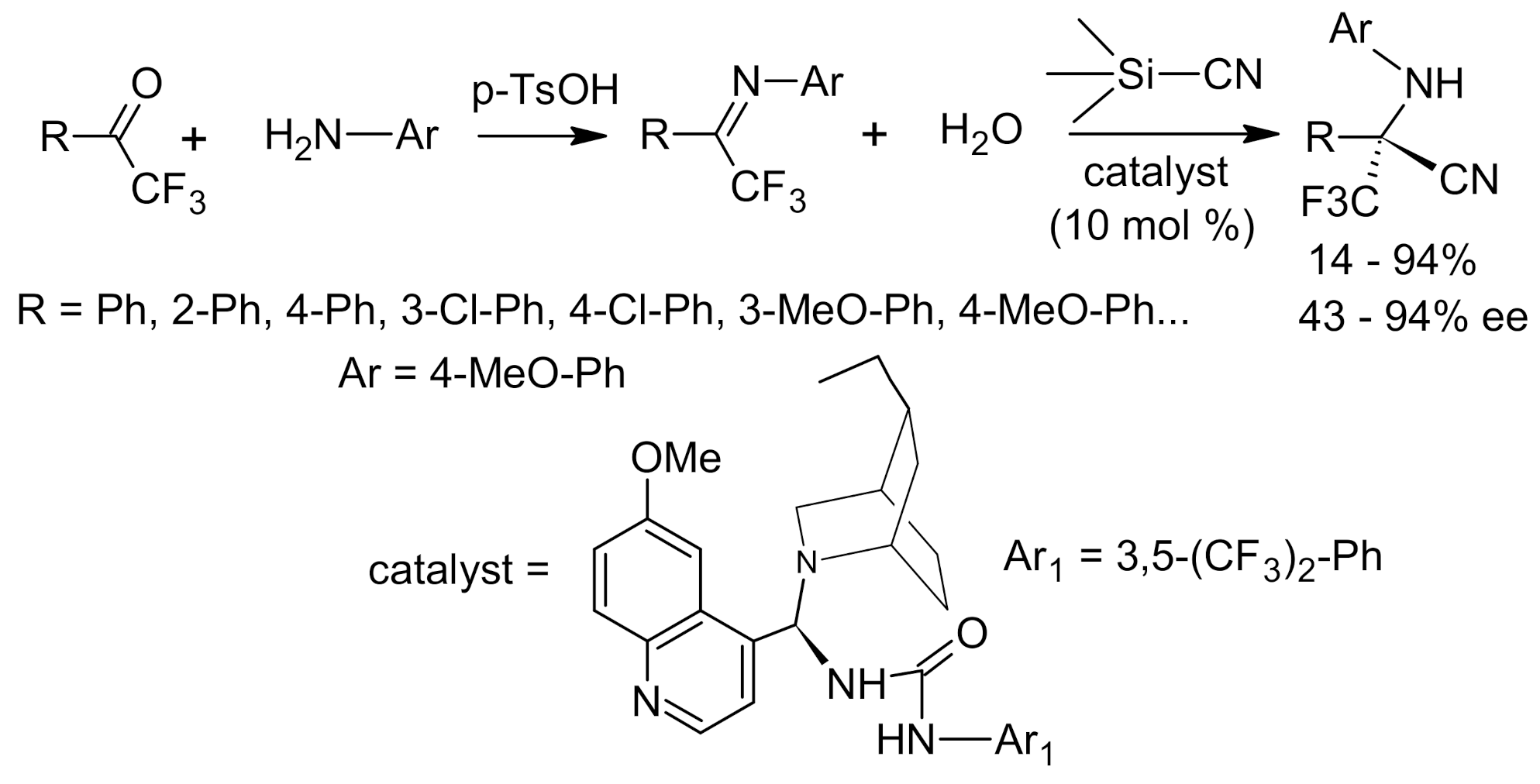
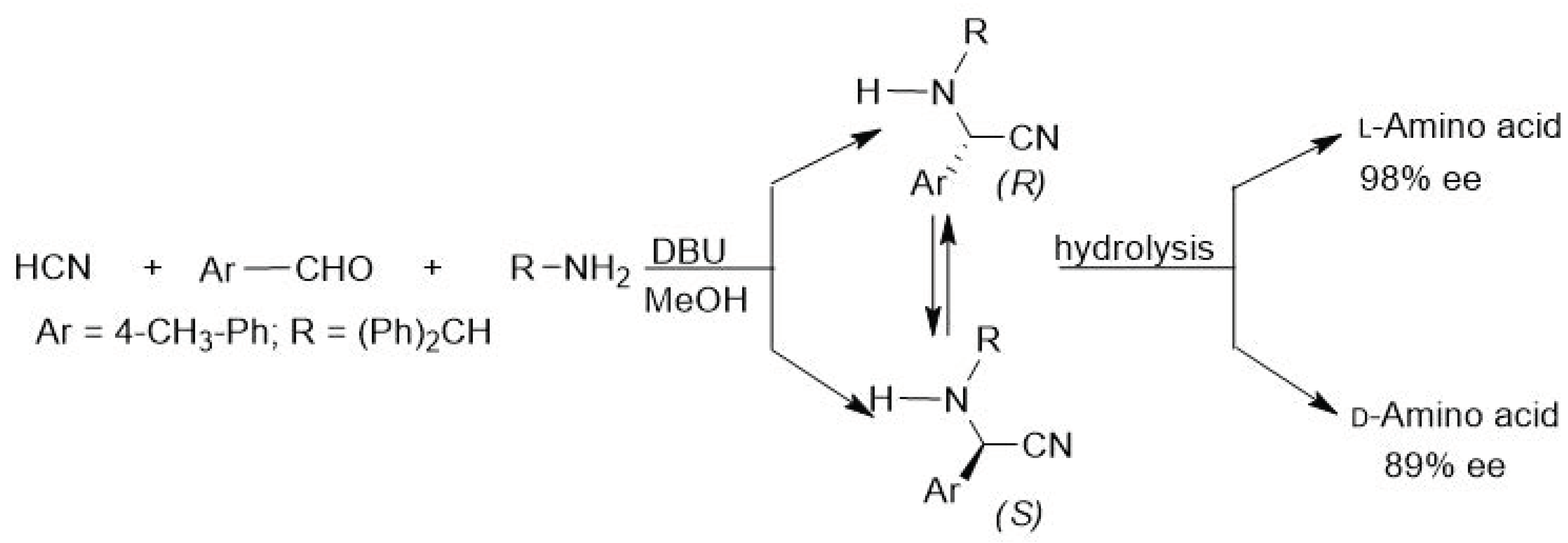
4. Asymmetric Strecker Reaction
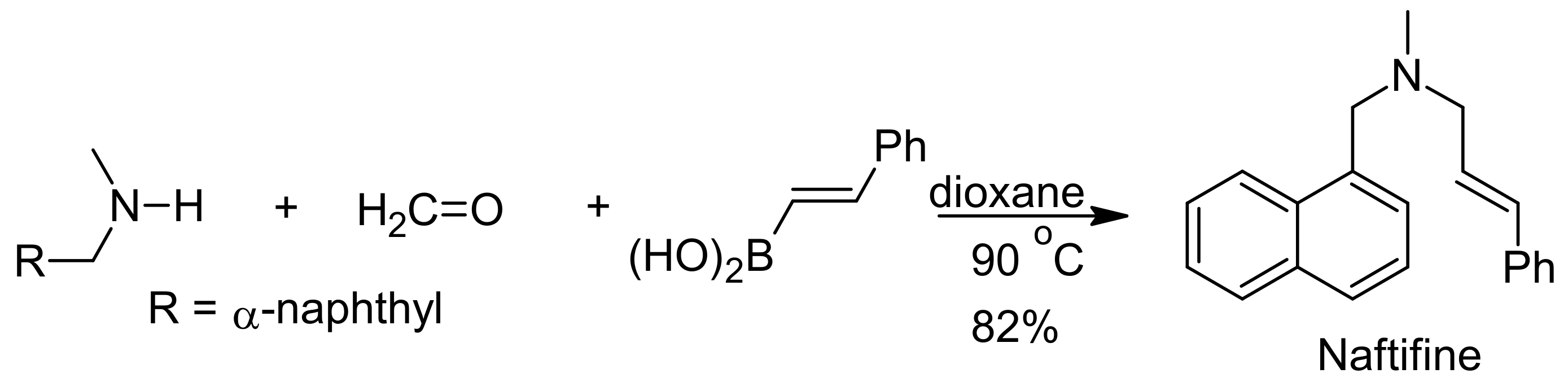
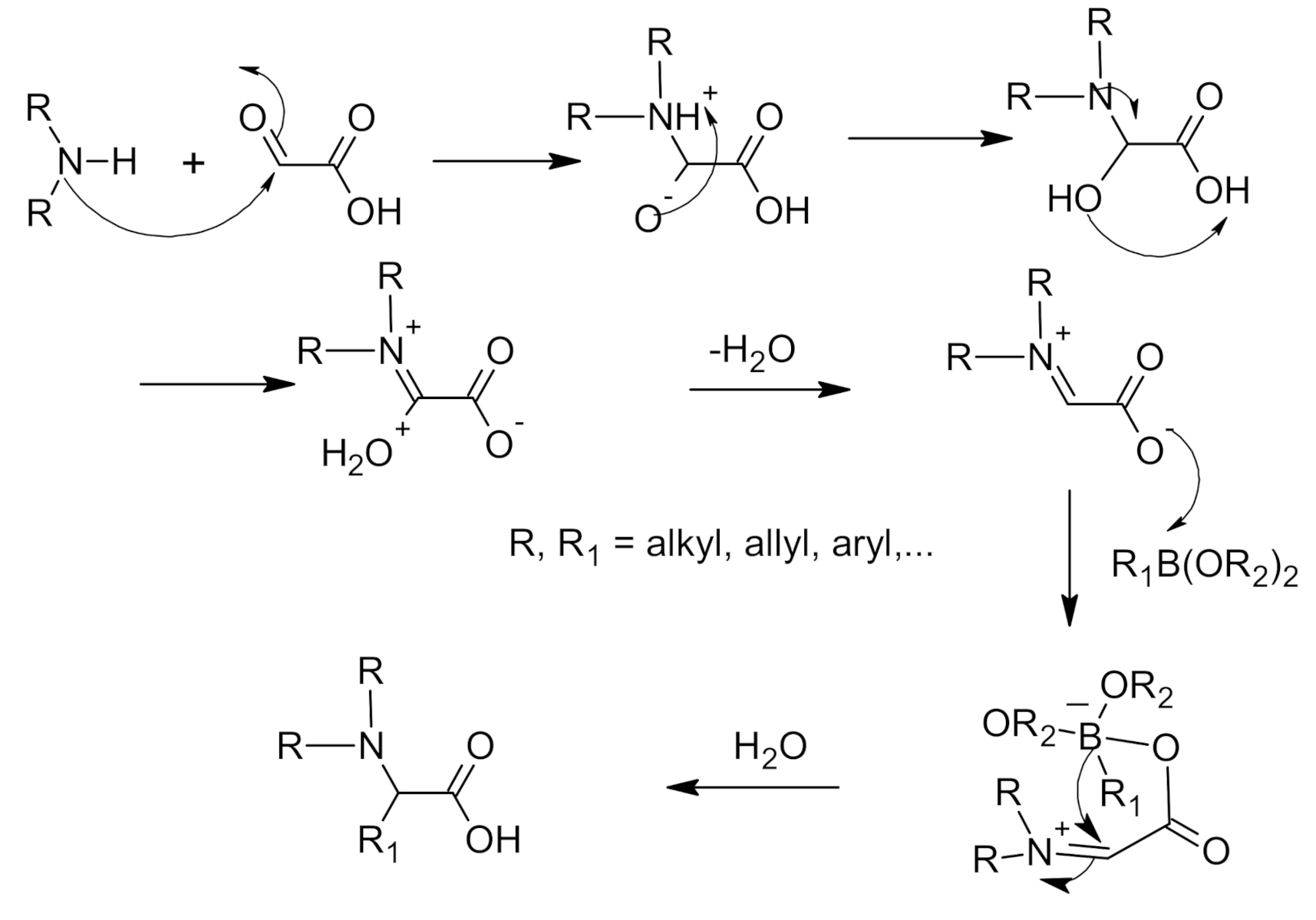
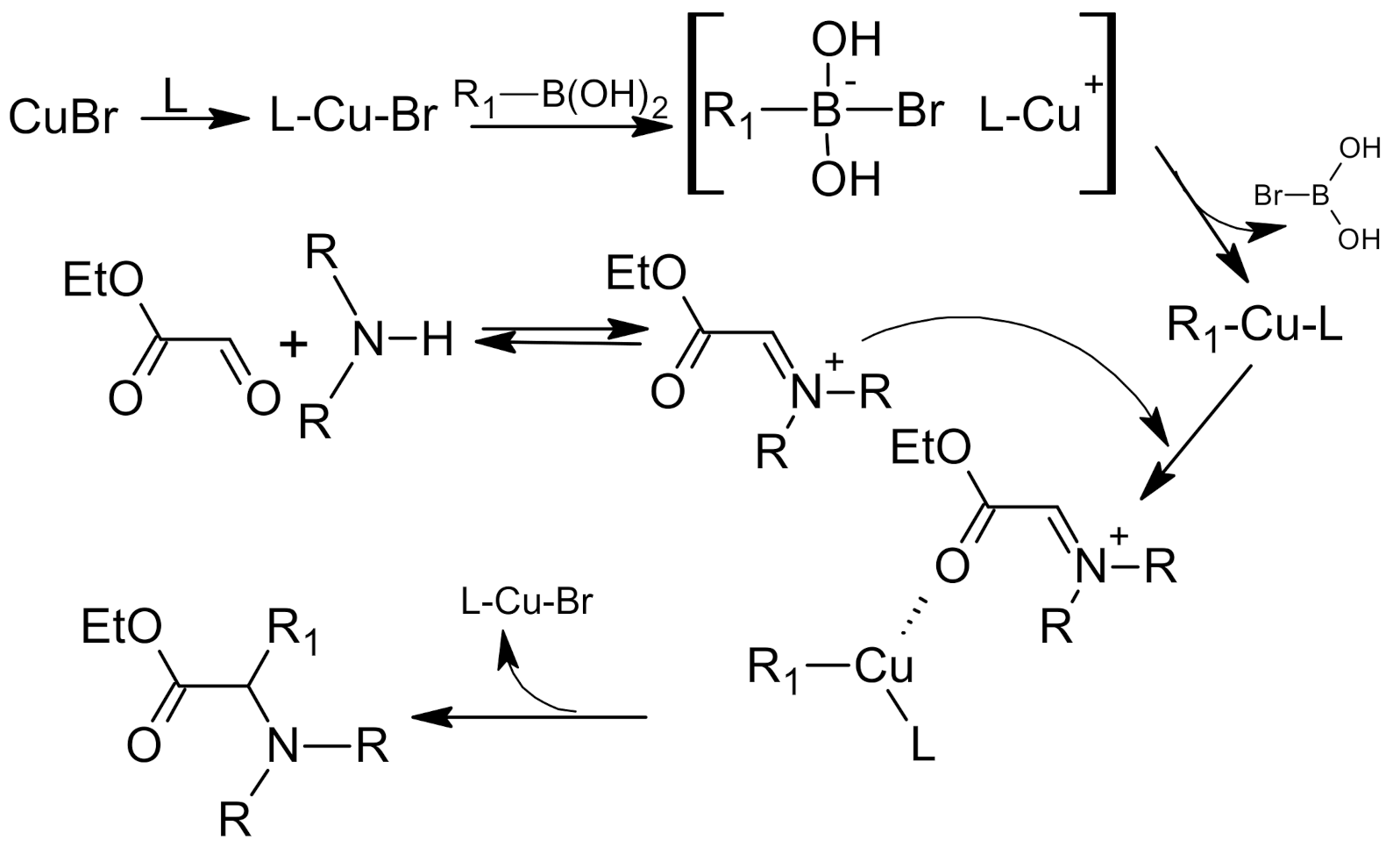
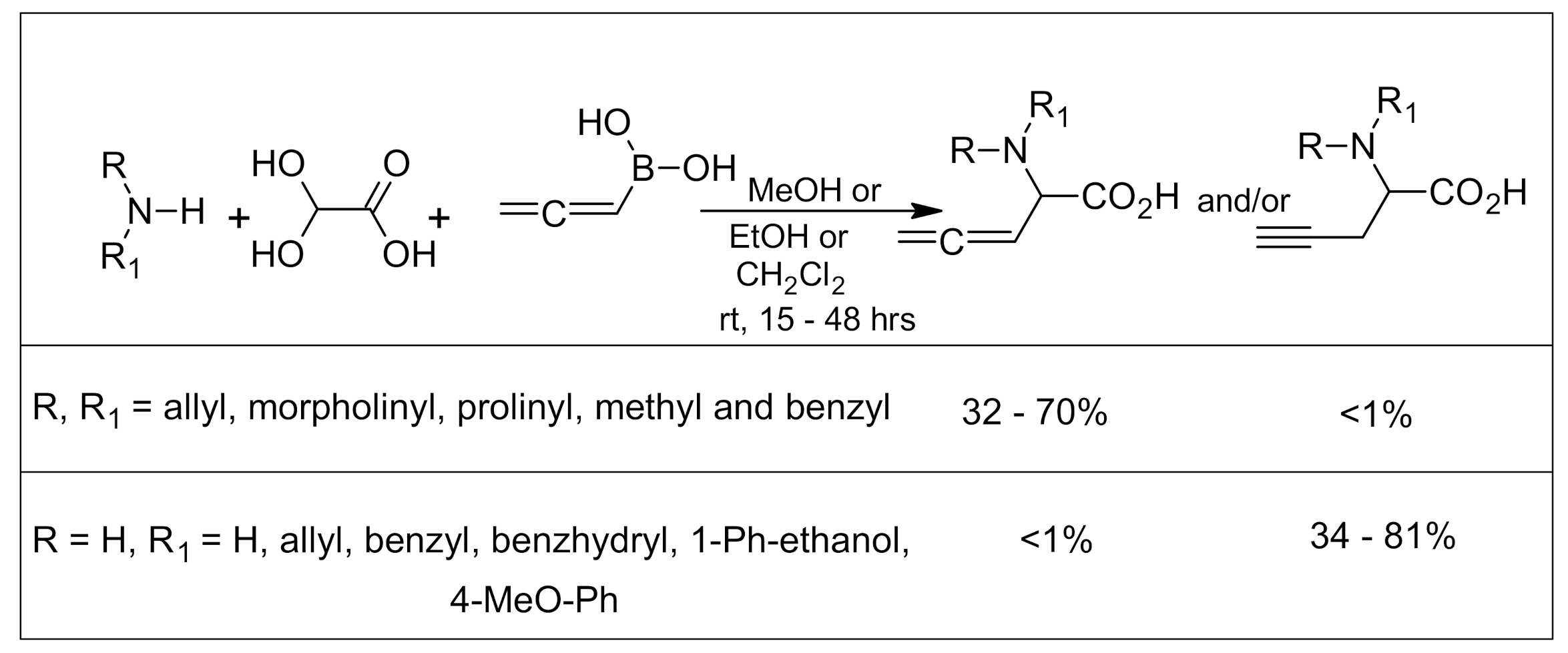
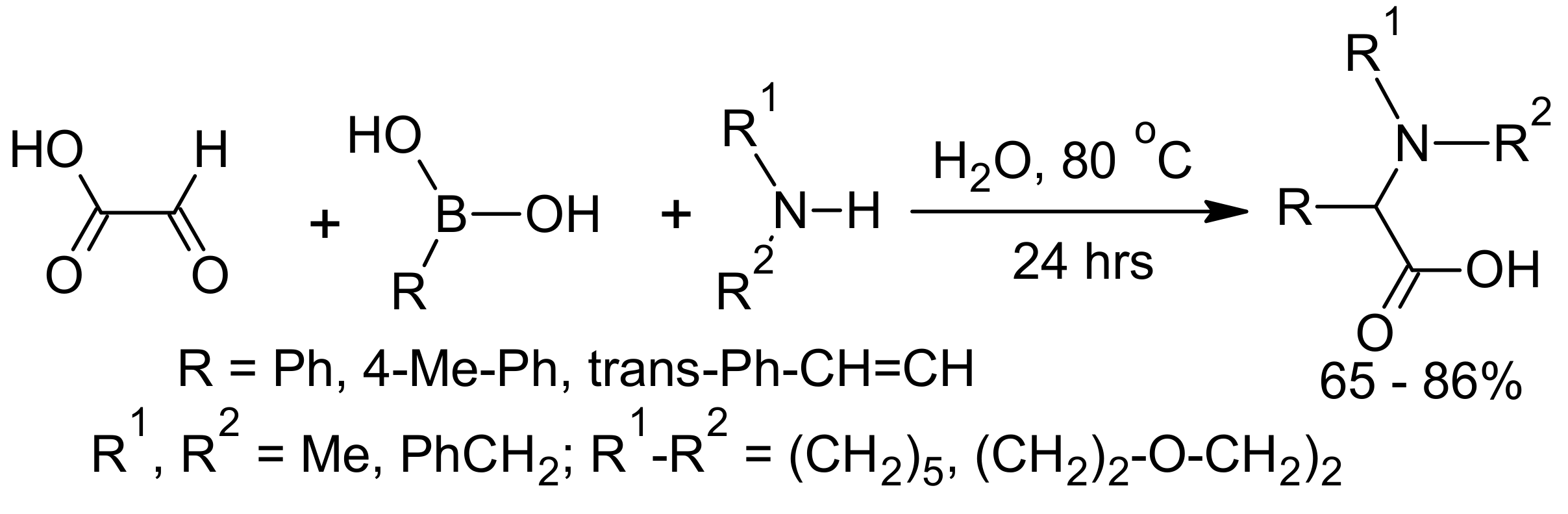
5. Petasis Reaction
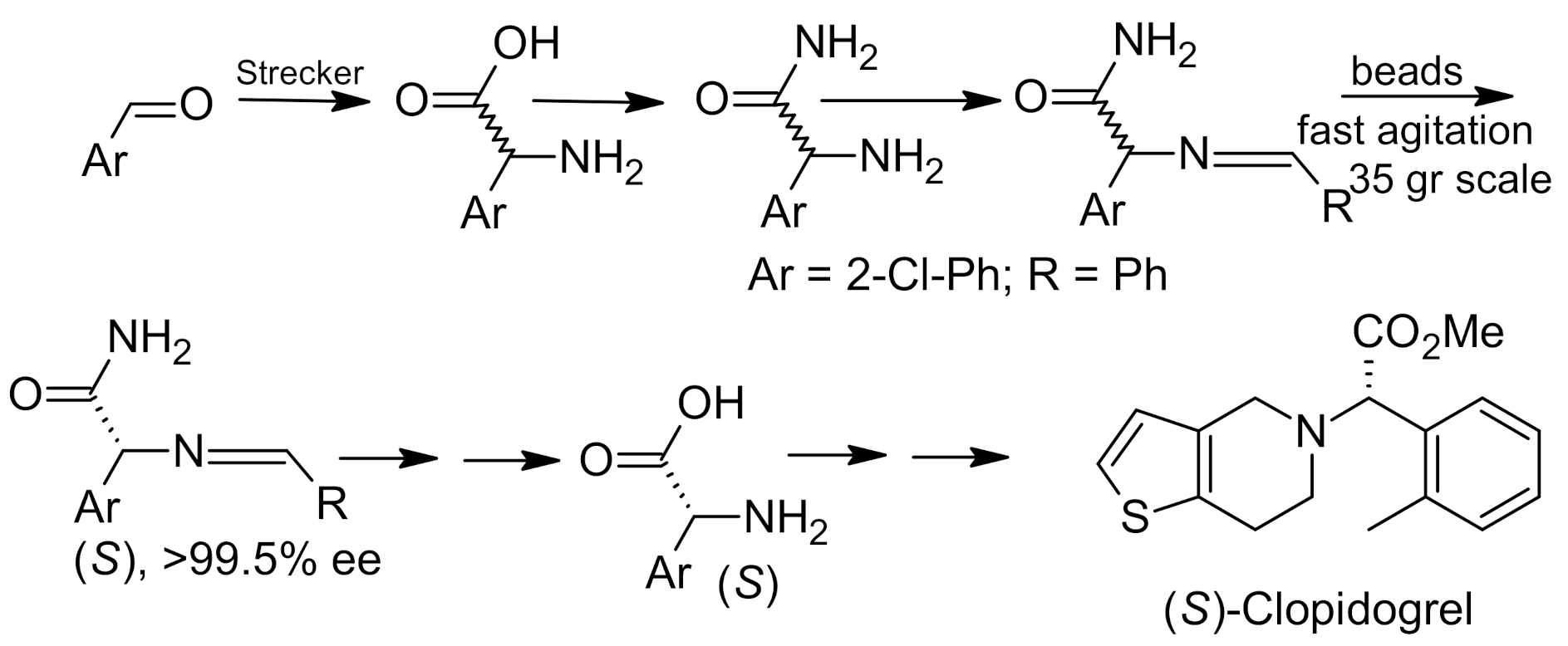
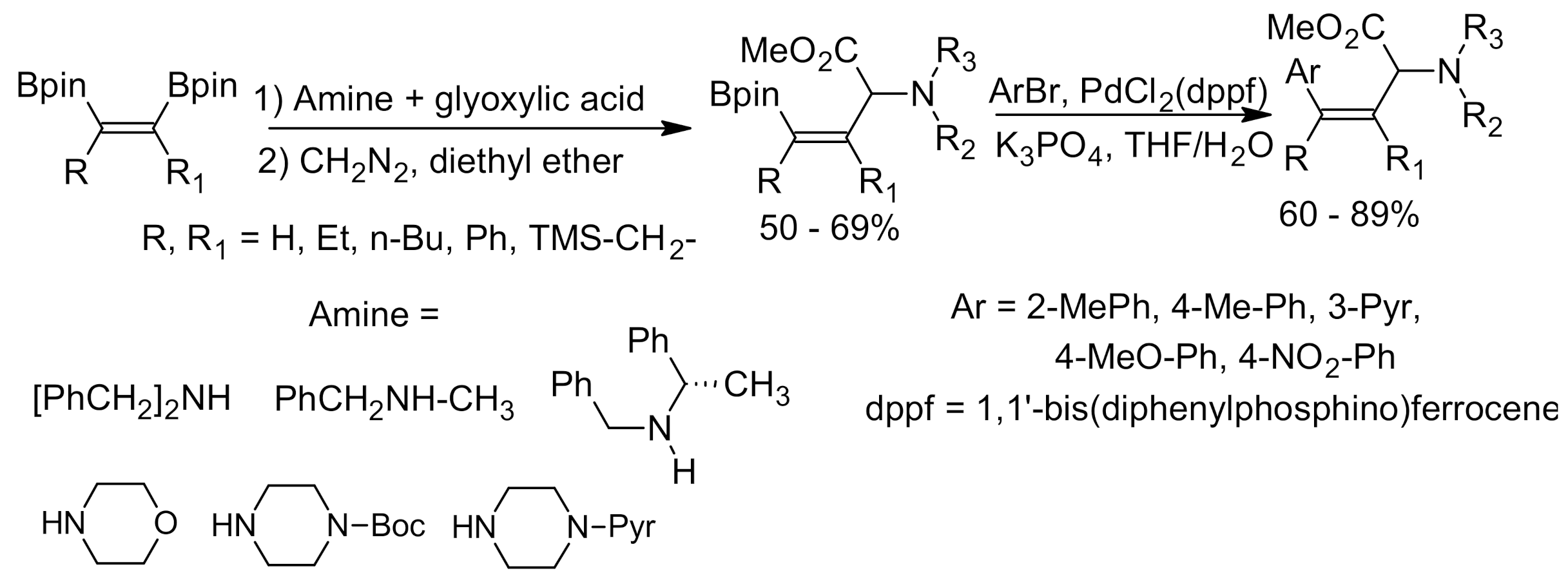
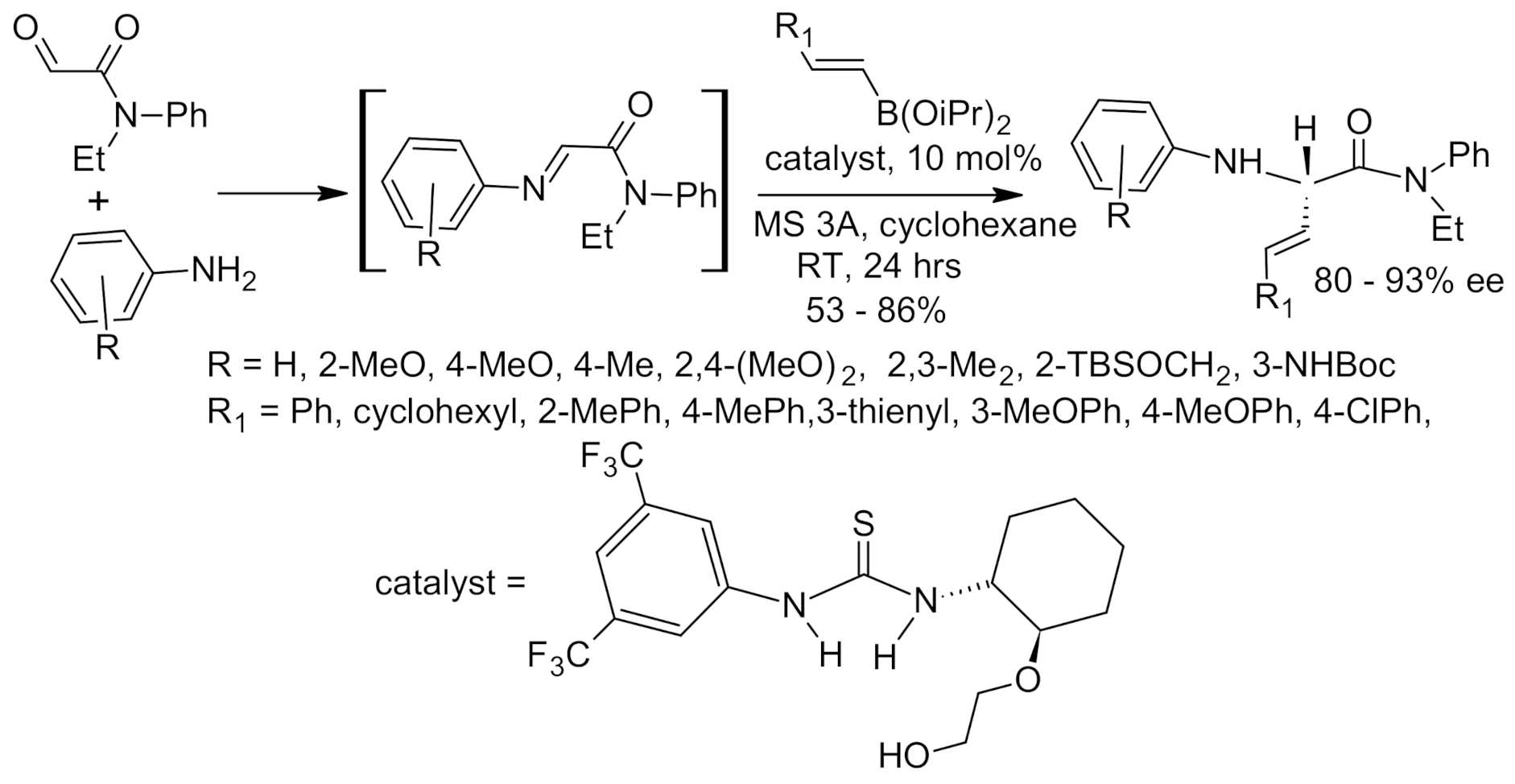
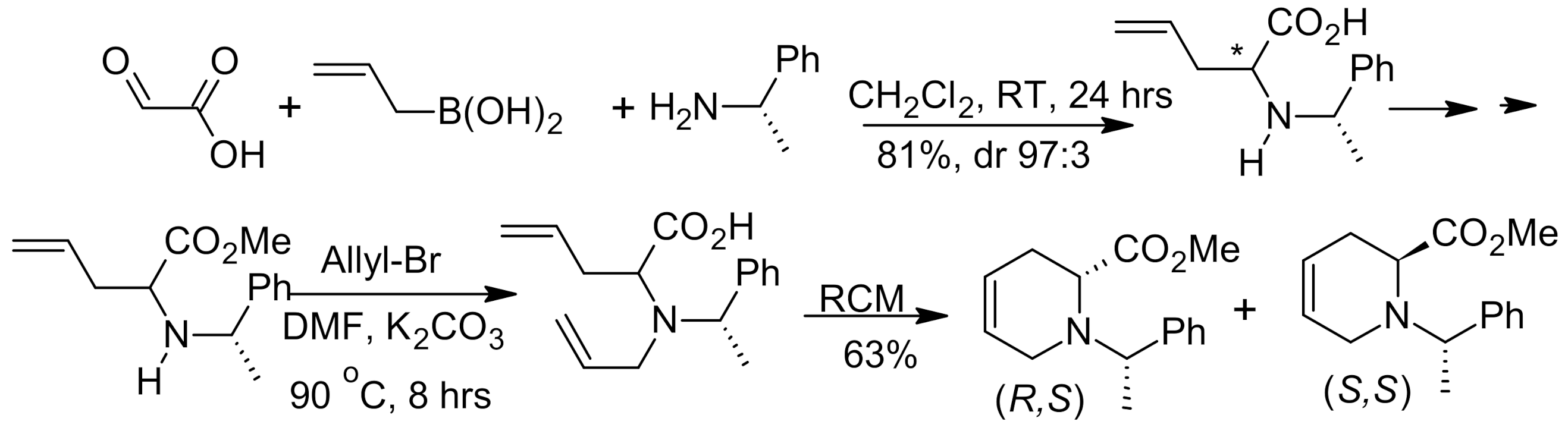
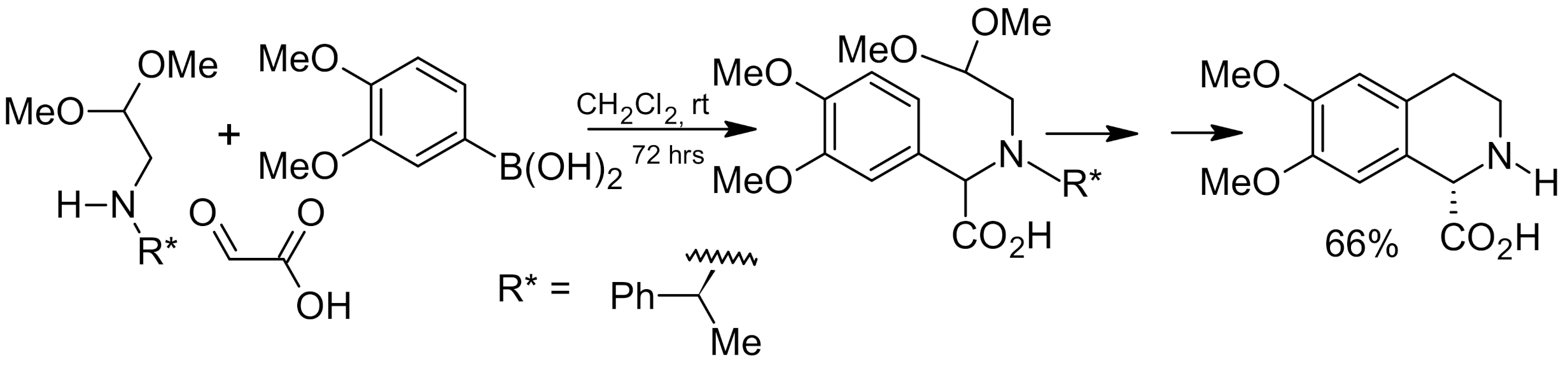
6. Asymmetric Petasis Reaction
7. Discussion
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Bada, J. Strecker Synthesis. In Encyclopedia of Astrobiology; Gargaud, M., Amils, R., Quintanilla, J.C., Cleaves, H.J., II, Irvine, W.M., Pinti, D.L., Viso, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; p. 1603. [Google Scholar] [CrossRef]
- Strecker, A. Ueber die künstliche Bildung der Milchsäure und einen neuen, dem Glycocoll homologen Körper. Justus Liebigs Ann. Der Chem. 1850, 75, 27–45. [Google Scholar] [CrossRef]
- Strecker, A. Ueber einen neuen aus Aldehyd-Ammoniak und Blausäure entstehenden Körper. Justus Liebigs Ann. Der Chem. 1854, 91, 349–351. [Google Scholar] [CrossRef]
- Pascal, R. Bücherer–Bergs Synthesis. In Encyclopedia of Astrobiology; Gargaud, M., Amils, R., Quintanilla, J.C., Cleaves, H.J., II, Irvine, W.M., Pinti, D.L., Viso, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 221–222. [Google Scholar] [CrossRef]
- Monteiro, J.L.; Pieber, B.; Corrêa, A.G.; Kappe, C.O. Continuous synthesis of hydantoins: Intensifying the Bucherer–Bergs reaction. Synlett 2016, 27, 83–87. [Google Scholar] [CrossRef]
- Ashe, K.; Fernández-García, C.; Corpinot, M.K.; Coggins, A.J.; Bučar, D.-K.; Powner, M.W. Selective prebiotic synthesis of phosphoroaminonitriles and aminothioamides in neutral water. Commun. Chem. 2019, 2, 23. [Google Scholar] [CrossRef]
- Williams, R.M.; Hendrix, J.A. Asymmetric synthesis of arylglycines. Chem. Rev. 1992, 92, 889–917. [Google Scholar] [CrossRef]
- Ivanov, K.; Ivanova, S.; Georgieva, M.; Atanasov, P. Production and regulatory analytical control of amino acids include in food additives. Pharmacia 2014, 61, 48–54. [Google Scholar]
- Yasufumi, O.; Tetsuro, S. Asymmetric Strecker Route toward the Synthesis of Biologically Active α,α-Disubstituted α-Amino Acids. Bull. Chem. Soc. Jpn. 2003, 76, 1115–1129. [Google Scholar] [CrossRef]
- Arasappan, A.; Venkatraman, S.; Padilla, A.I.; Wu, W.; Meng, T.; Jin, Y.; Wong, J.; Prongay, A.; Girijavallabhan, V.; George, N.F. Practical and efficient method for amino acid derivatives containing β-quaternary center: Application toward synthesis of hepatitis C virus NS3 serine protease inhibitors. Tetrahedron Lett. 2007, 48, 6343–6347. [Google Scholar] [CrossRef]
- Petasis, N.A.; Akritopoulou, I. The boronic acid mannich reaction: A new method for the synthesis of geometrically pure allylamines. Tetrahedron Lett. 1993, 34, 583–586. [Google Scholar] [CrossRef]
- Hu, X.; Ma, Y.; Li, Z. Eco-friendly synthesis of α-aminonitriles from ketones in PEG-400 medium using potassium Hexacyanoferrate(II) as cyanide source. J. Organomet. Chem. 2012, 705, 70–74. [Google Scholar] [CrossRef]
- Ivon, Y.M.; Tymtsunik, A.V.; Komarov, I.V.; Shishkin, O.V.; Grygorenko, O.O. Synthesis of a 2, 5-Diazabicyclo [2.2. 1] heptane-Derived α, β-Diamino Acid. Synth. Stuttg. 2015, 47, 1123–1130. [Google Scholar] [CrossRef]
- Van Hijfte, L.; Heydt, V.; Kolb, M. A versatile entry into the synthesis of α-(monofluoromethyl) amino acids: Preparation of α-(monofluoromethyl) serine and (E)-dehydro-α-(monofluoromethyl) ornithine. Tetrahedron Lett. 1993, 34, 4793–4796. [Google Scholar] [CrossRef]
- Razafindrabe, C.R.; Aubry, S.; Bourdon, B.; Andriantsiferana, M.; Pellet-Rostaing, S.; Lemaire, M. Synthesis of (±)-phthalascidin 650 analogue: New synthetic route to (±)-phthalascidin 622. Tetrahedron 2010, 66, 9061–9066. [Google Scholar] [CrossRef]
- Myers, A.G.; Kung, D.W. One-Step Construction of the Pentacyclic Skeleton of Saframycin A from a “Trimer” of α-Amino Aldehydes. Org. Lett. 2000, 2, 3019–3022. [Google Scholar] [CrossRef]
- Aoki, K.; Ijima, T.; Kamiyama, H.; Kamiko, K.; Terauchi, Y. Anagliptin decreases serum lathosterol level in patients with type 2 diabetes: A pilot study. Expert Opin. Pharmacother. 2015, 16, 1749–1754. [Google Scholar] [CrossRef] [PubMed]
- Augeri, D.J.; Robl, J.A.; Betebenner, D.A.; Magnin, D.R.; Khanna, A.; Robertson, J.G.; Wang, A.; Simpkins, L.M.; Taunk, P.; Huang, Q.; et al. Discovery and Preclinical Profile of Saxagliptin (BMS-477118): A Highly Potent, Long-Acting, Orally Active Dipeptidyl Peptidase IV Inhibitor for the Treatment of Type 2 Diabetes. J. Med. Chem. 2005, 48, 5025–5037. [Google Scholar] [CrossRef]
- Xing, J.; Brooks, A.F.; Fink, D.; Zhang, H.; Piert, M.R.; Scott, P.J.; Shao, X. High-yielding automated convergent synthesis of no-carrier-added [11C-carbonyl]-labeled amino acids using the Strecker Reaction. Synlett Acc. Rapid Commun. Synth. Org. Chem. 2017, 28, 371. [Google Scholar] [CrossRef][Green Version]
- Song, F.; Salter, R.; Weaner, L.E. A short synthesis of d-[1-14C]-serine of high enantiomeric purity. J. Label. Compd. Radiopharm. 2015, 58, 173–176. [Google Scholar] [CrossRef]
- Bandak, D.; Babii, O.; Vasiuta, R.; Komarov, I.V.; Mykhailiuk, P.K. Design and synthesis of novel 19F-amino acid: A promising 19F NMR label for peptide studies. Org. Lett. 2015, 17, 226–229. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Y.; Xu, J.; Shi, J.; Cai, H. One-pot three-component synthesis of α-aminonitriles using potassium hexacyanoferrate(II) as an eco-friendly cyanide source. Tetrahedron Lett. 2010, 51, 3922–3926. [Google Scholar] [CrossRef]
- Poliakoff, M.; Licence, P. Green chemistry. Nature 2007, 450, 810–812. [Google Scholar] [CrossRef]
- Grundke, C.; Opatz, T. Strecker reactions with hexacyanoferrates as non-toxic cyanide sources. Green Chem. 2019, 21, 2362–2366. [Google Scholar] [CrossRef]
- Li, Z.; Li, R.; Zheng, H.; Wen, F.; Li, H.; Yin, J.; Yang, J. Hydrocyanation of sulfonylimines using potassium hexacyanoferrate(II) as an eco-friendly cyanide source. J. Braz. Chem. Soc. 2013, 24, 1739–1743. [Google Scholar] [CrossRef]
- Pechenyuk, S.I.; Domonov, D.P.; Shimkin, A.A.; Ivanov, Y.V. Thermal decomposition of iron cyano complexes in an inert atmosphere. Russ. Chem. Bull. 2015, 64, 322–328. [Google Scholar] [CrossRef]
- Kuhn, D.D.; Young, T.C. Photolytic degradation of hexacyanoferrate (II) in aqueous media: The determination of the degradation kinetics. Chemosphere 2005, 60, 1222–1230. [Google Scholar] [CrossRef]
- Bolm, C.; Mocci, R.; Schumacher, C.; Turberg, M.; Puccetti, F.; Hernández, J.G. Mechanochemical Activation of Iron Cyano Complexes: A Prebiotic Impact Scenario for the Synthesis of α-Amino Acid Derivatives. Angew. Chem. 2018, 130, 2447–2450. [Google Scholar] [CrossRef]
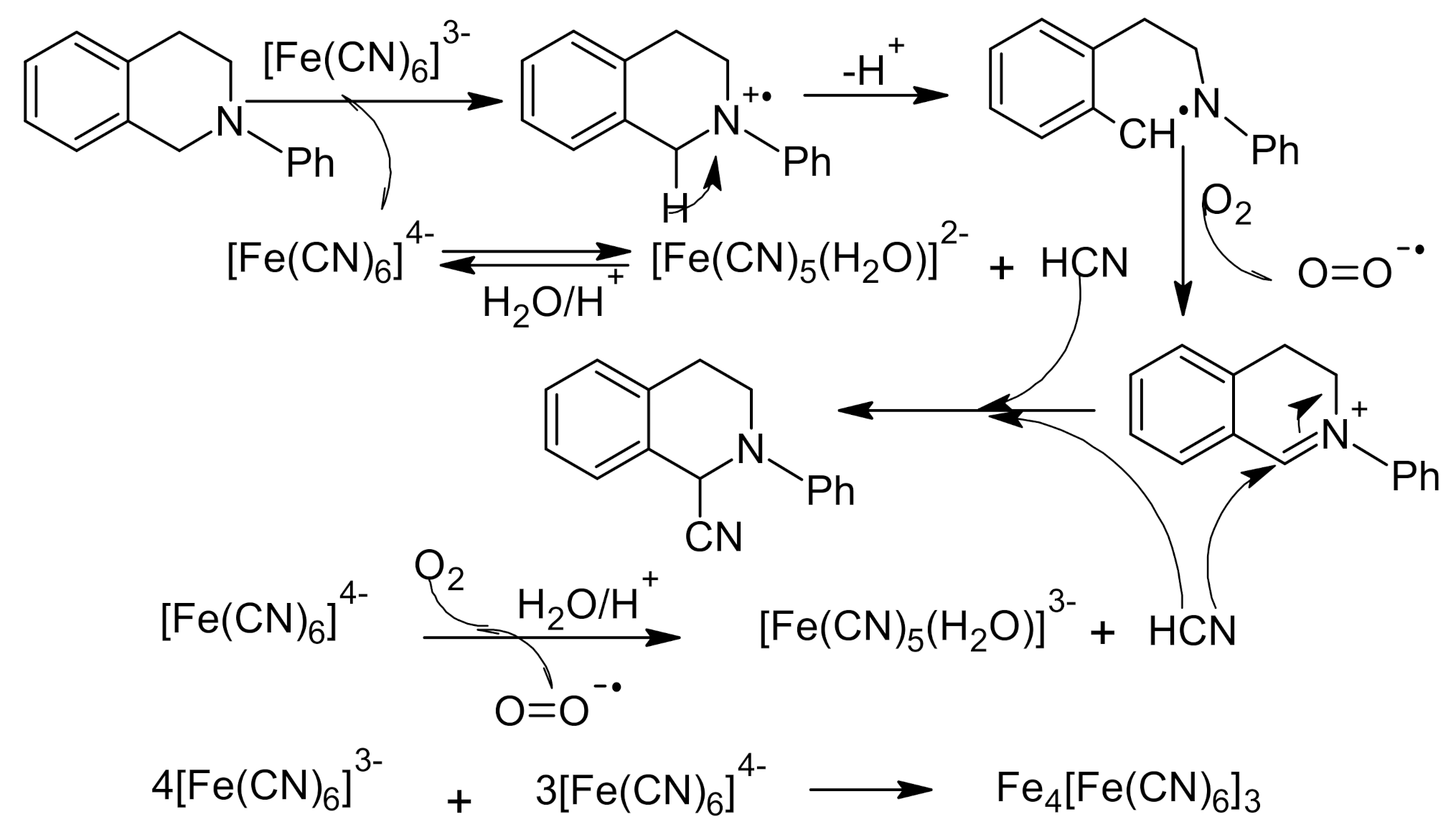
- Nauth, A.M.; Otto, N.; Opatz, T. α-Cyanation of Aromatic Tertiary Amines using Ferricyanide as a Non-Toxic Cyanide Source. Adv. Synth. Catal. 2015, 357, 3424–3428. [Google Scholar] [CrossRef]
- Li, Z.; Bohle, D.S.; Li, C.-J. Cu-catalyzed cross-dehydrogenative coupling: A versatile strategy for C–C bond formations via the oxidative activation of sp3 C–H bonds. Proc. Natl. Acad. Sci. USA 2006, 103, 8928–8933. [Google Scholar] [CrossRef]
- D’Este, M.; Alvarado-Morales, M.; Angelidaki, I. Amino acids production focusing on fermentation technologies–A review. Biotechnol. Adv. 2018, 36, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, S.; Yang, X.; Qiu, L.; Gao, B.; Li, R.; Chen, J. Reactive extraction of amino acids mixture in hydrolysate from cottonseed meal with di(2-ethylhexyl) phosphoric acid. J. Chem. Technol. Biotechnol. 2016, 91, 483–489. [Google Scholar] [CrossRef]
- Kim, Y.; Park, J.; Kim, M.-J. Dynamic Kinetic Resolution of Amines and Amino Acids by Enzyme–Metal Cocatalysis. ChemCatChem 2011, 3, 271–277. [Google Scholar] [CrossRef]
- Miyazawa, T. Enzymatic resolution of amino acids via ester hydrolysis. Amino Acids 1999, 16, 191–213. [Google Scholar] [CrossRef] [PubMed]
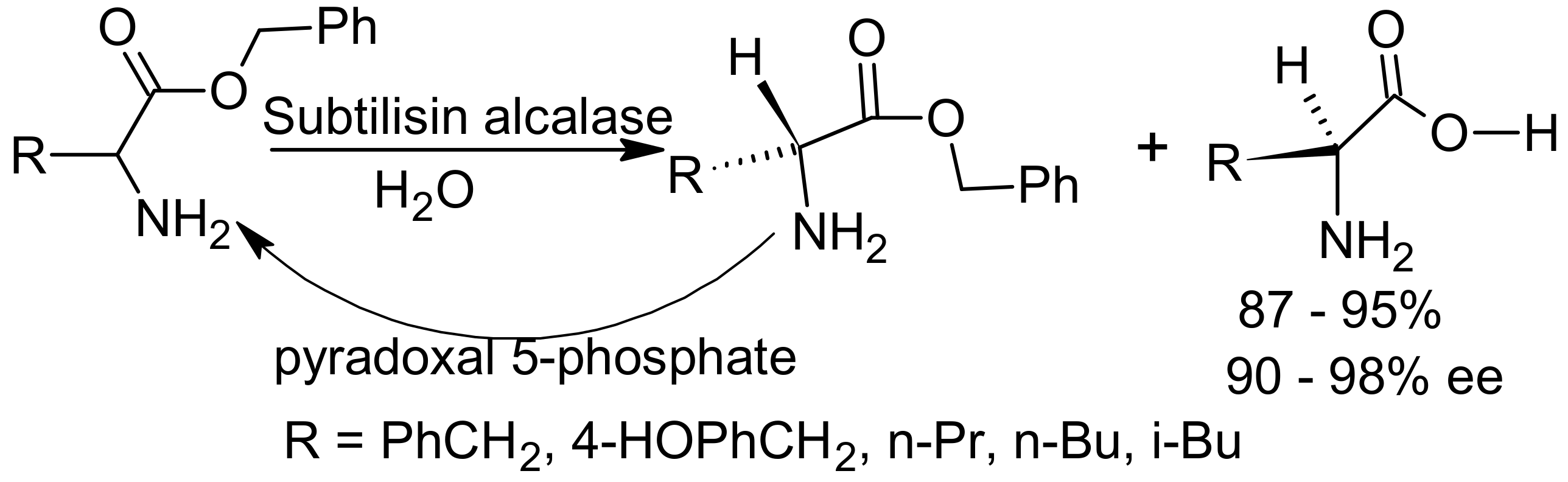
- Chen, S.-T.; Huang, W.-H.; Wang, K.-T. Resolution of Amino Acids in a Mixture of 2-Methyl-2-propanol/water (19:1) Catalyzed by Alcalase via in Situ Racemization of One Antipode Mediated by Pyridoxal 5-Phosphate. J. Org. Chem. 1994, 59, 7580–7581. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Feng, X. Asymmetric strecker reactions. Chem. Rev. 2011, 111, 6947–6983. [Google Scholar] [CrossRef]
- Harada, K. Asymmetric Synthesis of α-Amino-acids by the Strecker Synthesis. Nature 1963, 200, 1201. [Google Scholar] [CrossRef]
- Kouznetsov, V.V.; Galvis, C.E.P. Strecker reaction and α-amino nitriles: Recent advances in their chemistry, synthesis, and biological properties. Tetrahedron 2018, 74, 773–810. [Google Scholar] [CrossRef]
- Kunz, H.; Sager, W.; Pfrengle, W.; Schanzenbach, D. Reversal of asymmetric induction in stereoselective strecker synthesis on galactosyl amine as the chiral matrix. Tetrahedron Lett. 1988, 29, 4397–4400. [Google Scholar] [CrossRef]
- Ma, D.; Tian, H.; Zou, G. Asymmetric Strecker-Type Reaction of α-Aryl Ketones. Synthesis of (S)-αM4CPG, (S)-MPPG, (S)-AIDA, and (S)-APICA, the Antagonists of Metabotropic Glutamate Receptors. J. Org. Chem. 1999, 64, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Ding, K. Synthesis of Enantiopure α,α-Disubstituted Amino Acids from the Asymmetric Strecker Reaction Products of Aldehydes. Org. Lett. 2000, 2, 2515–2517. [Google Scholar] [CrossRef]
- Robak, M.T.; Herbage, M.A.; Ellman, J.A. Synthesis and Applications of tert-Butanesulfinamide. Chem. Rev. 2010, 110, 3600–3740. [Google Scholar] [CrossRef] [PubMed]
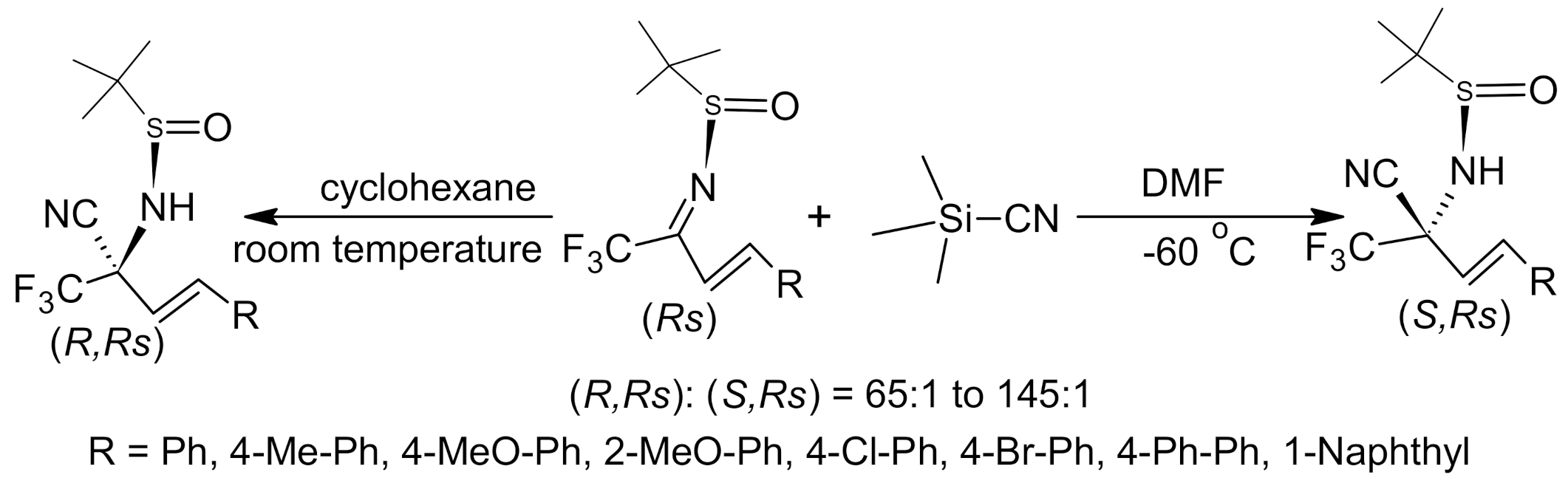
- Chen, D.; Xu, M.-H. Lewis acid promoted diastereoselective addition of TMSCN and TMSCF3 to isatin-derived N-sulfinyl ketimines: Synthesis of optically active tetrasubstituted 3-aminooxindoles. J. Org. Chem. 2014, 79, 7746–7751. [Google Scholar] [CrossRef]
- Cai, X.-H.; Xie, B. Recent advances in asymmetric Strecker reactions. Arkivoc 2014, 1, 205–248. [Google Scholar] [CrossRef]
- de Bruin, G.; Mock, E.D.; Hoogendoorn, S.; van den Nieuwendijk, A.M.; Mazurek, J.; van der Marel, G.A.; Florea, B.I.; Overkleeft, H.S. Enantioselective synthesis of adamantylalanine and carboranylalanine and their incorporation into the proteasome inhibitor bortezomib. Chem. Commun. 2016, 52, 4064–4067. [Google Scholar] [CrossRef] [PubMed]
- Iyer, M.S.; Gigstad, K.M.; Namdev, N.D.; Lipton, M. Asymmetric catalysis of the Strecker amino acid synthesis by a cyclic dipeptide. Amino Acids 1996, 11, 259–268. [Google Scholar] [CrossRef]
- Zuend, S.J.; Coughlin, M.P.; Lalonde, M.P.; Jacobsen, E.N. Scaleable catalytic asymmetric Strecker syntheses of unnatural α-amino acids. Nature, 2009; 461, 968–970. [Google Scholar] [CrossRef]
- Sigman, M.S.; Jacobsen, E.N. Enantioselective Addition of Hydrogen Cyanide to Imines Catalyzed by a Chiral (Salen)Al(III) Complex. J. Am. Chem. Soc. 1998, 120, 5315–5316. [Google Scholar] [CrossRef]
- Kobayashi, S.; Ishitani, H. Novel binuclear chiral zirconium catalysts used in enantioselective strecker reactions. Chirality Pharmacol. Biol. Chem. Conseq. Mol. Asymmetry 2000, 12, 540–543. [Google Scholar] [CrossRef]
- Sadhukhan, A.; Saravanan, S.; Khan, N.-u.H.; Kureshy, R.I.; Abdi, S.H.; Bajaj, H.C. Modified Asymmetric Strecker Reaction of Aldehyde with Secondary Amine: A Protocol for the Synthesis of S-Clopidogrel (An Antiplatelet Agent). J. Org. Chem. 2012, 77, 7076–7080. [Google Scholar] [CrossRef]
- Qiu, X.-L.; Qing, F.-L. Recent Advances in the Synthesis of Fluorinated Amino Acids. Eur. J. Org. Chem. 2011, 2011, 3261–3278. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Yin, X.-P.; Zhou, J. Internally Reuse Waste: Catalytic Asymmetric One-Pot Strecker Reaction of Fluoroalkyl Ketones, Anilines and TMSCN by Sequential Catalysis. Chin. J. Chem. 2018, 36, 321–328. [Google Scholar] [CrossRef]
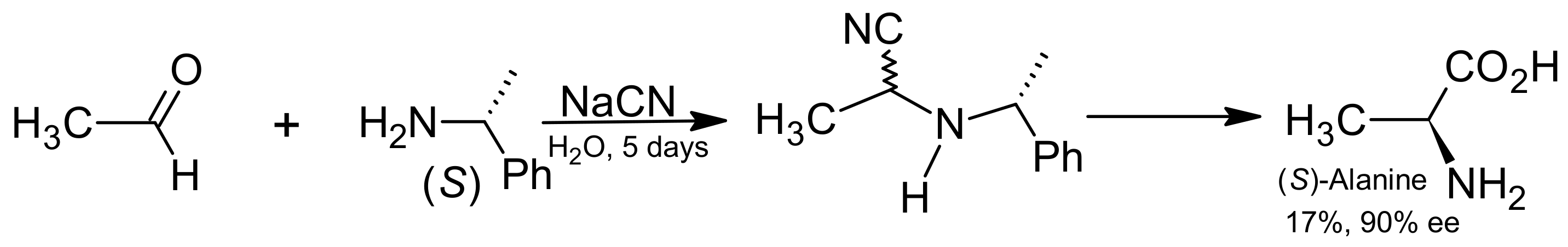
- Kawasaki, T.; Takamatsu, N.; Aiba, S.; Tokunaga, Y. Spontaneous formation and amplification of an enantioenriched α-amino nitrile: A chiral precursor for Strecker amino acid synthesis. Chem. Commun. 2015, 51, 14377–14380. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, S.; Yoshimura, K.; Yamazaki, Y.; Takamatsu, N.; Kuraishi, T.; Aiba, S.; Tokunaga, Y.; Kawasaki, T. Asymmetric Strecker Reaction Arising from the Molecular Orientation of an Achiral Imine at the Single-Crystal Face: Enantioenriched l-and d-Amino Acids. Angew. Chem. Int. Ed. 2017, 56, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Sögütoglu, L.-C.; Steendam, R.R.; Meekes, H.; Vlieg, E.; Rutjes, F.P. Viedma ripening: A reliable crystallisation method to reach single chirality. Chem. Soc. Rev. 2015, 44, 6723–6732. [Google Scholar] [CrossRef] [PubMed]
- Viedma, C. Chiral symmetry breaking during crystallization: Complete chiral purity induced by nonlinear autocatalysis and recycling. Phys. Rev. Lett. 2005, 94, 065504. [Google Scholar] [CrossRef] [PubMed]
- Baglai, I.; Leeman, M.; Wurst, K.; Kaptein, B.; Kellogg, R.M.; Noorduin, W.L. The Strecker reaction coupled to Viedma ripening: A simple route to highly hindered enantiomerically pure amino acids. Chem. Commun. 2018, 54, 10832–10834. [Google Scholar] [CrossRef]
- van der Meijden, M.W.; Leeman, M.; Gelens, E.; Noorduin, W.L.; Meekes, H.; van Enckevort, W.J.P.; Kaptein, B.; Vlieg, E. Kellogg, R.M. Attrition-Enhanced Deracemization in the Synthesis of Clopidogrel-A Practical Application of a New Discovery. Org. Process. Res. Dev. 2009, 13, 1195–1198. [Google Scholar] [CrossRef]
- Jumbam, N.D.; Masamba, W. Bio-Catalysis in Multicomponent Reactions. Molecules 2020, 25, 5935. [Google Scholar] [CrossRef]
- Vongvilai, P.; Ramström, O. Dynamic Asymmetric Multicomponent Resolution: Lipase-Mediated Amidation of a Double Dynamic Covalent System. J. Am. Chem. Soc. 2009, 131, 14419–14425. [Google Scholar] [CrossRef]
- Chrzanowska, M.; Grajewska, A.; Meissner, Z.; Rozwadowska, M.; Wiatrowska, I. A concise synthesis of tetrahydroisoquinoline-1-carboxylic acids using a Petasis reaction and Pomeranz–Fritsch–Bobbitt cyclization sequence. Tetrahedron 2012, 68, 3092–3097. [Google Scholar] [CrossRef]
- Wu, P.; Givskov, M.; Nielsen, T.E. Reactivity and Synthetic Applications of Multicomponent Petasis Reactions. Chem. Rev. 2019, 119, 11245–11290. [Google Scholar] [CrossRef] [PubMed]
- Boguszewski, P.A.; Davies, J.W.; Marsh, P.A.; Williamson, M. MEDI 309-Polymer assisted, high throughput methods for Petasis and Ugi reactions. In Abstracts of Papers of the American Chemical Society; The American Chemical Society: Washington, DC, USA, 2008. [Google Scholar]
- Zhang, J.; Yun, F.; Xie, R.; Cheng, C.; Chen, G.; Li, J.; Tang, P.; Yuan, Q. Petasis three-component reaction accelerated by trifluoroacetic acid: Synthesis of indoline-derived glycines. Tetrahedron Lett. 2016, 57, 3916–3919. [Google Scholar] [CrossRef]
- Frauenlob, R.; García, C.; Bradshaw, G.A.; Burke, H.M.; Bergin, E. A Copper-Catalyzed Petasis Reaction for the Synthesis of Tertiary Amines and Amino Esters. J. Org. Chem. 2012, 77, 4445–4449. [Google Scholar] [CrossRef]
- Cornier, P.G.; Delpiccolo, C.M.L.; Boggián, D.B.; Mata, E.G. Solid-phase Petasis multicomponent reaction for the generation of β-lactams 3-substituted with non-proteinogenic α-amino acids. Tetrahedron Lett. 2013, 54, 4742–4745. [Google Scholar] [CrossRef]
- Potowski, M.; Esken, R.; Brunschweiger, A. Translation of the copper/bipyridine-promoted Petasis reaction to solid phase-coupled DNA for encoded library synthesis. Biorg. Med. Chem. 2020, 28, 115441. [Google Scholar] [CrossRef] [PubMed]
- Petasis, N.A.; Goodman, A.; Zavialov, I.A. A new synthesis of α-arylglycines from aryl boronic acids. Tetrahedron 1997, 53, 16463–16470. [Google Scholar] [CrossRef]
- Liepouri, F.; Bernasconi, G.; Petasis, N.A. Component-Selective and Stereocontrolled One-Step Three-Component Reaction among Aldehydes, Amines, and Allenyl Boronic Acids or Allenyl Pinacolboronates. Org. Lett. 2015, 17, 1628–1631. [Google Scholar] [CrossRef]
- Murafuji, T.; Tasaki, Y.; Fujinaga, M.; Tao, K.; Kamijo, S.; Ishiguro, K. Blue Amino Acids Derived from Azulen-1-ylboronic Acid Pinacol Ester via the Petasis Reaction. Synthesis 2017, 49, 1037–1042. [Google Scholar] [CrossRef]
- Petasis, N.A.; Zavialov, I.A. A New and Practical Synthesis of α-Amino Acids from Alkenyl Boronic Acids. J. Am. Chem. Soc. 1997, 119, 445–446. [Google Scholar] [CrossRef]
- Tao, C.-Z.; Zhang, Z.-T.; Wu, J.-W.; Li, R.-H.; Cao, Z.-L. Synthesis of unnatural N-glycosyl α-amino acids via Petasis reaction. Chin. Chem. Lett. 2014, 25, 532–534. [Google Scholar] [CrossRef]
- Naskar, D.; Roy, A.; Seibel, W.L.; Portlock, D.E. Hydroxylamines and sulfinamide as amine components in the Petasis boronic acid–Mannich reaction: Synthesis of N-hydroxy or alkoxy-α-aminocarboxylicacids and N-(tert-butyl sulfinyl)-α-amino carboxylicacids. Tetrahedron Lett. 2003, 44, 8865–8868. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Smith, G.P.; Begtrup, M.; Kristensen, J.L. Synthesis of N-alkylated amino acids using fluorous-tagged hydroxylamines. Tetrahedro 2011, 67, 5261–5267. [Google Scholar] [CrossRef]
- Dhudshia, B.; Tiburcio, J.; Thadani, A.N. Diastereoselective allylation and crotylation of N-unsubstituted imines derived from ketones. Chem. Commun. 2005, 5551–5553. [Google Scholar] [CrossRef]
- Diehl, A.M.; Ouadoudi, O.; Andreadou, E.; Manolikakes, G. Sulfonamides as Amine Component in the Petasis-Borono Mannich Reaction: A Concise Synthesis of α-Aryl-and α-Alkenylglycine Derivatives. Synthesis 2018, 50, 3936–3946. [Google Scholar] [CrossRef]
- Candeias, N.R.; Cal, P.M.S.D.; André, V.; Duarte, M.T.; Veiros, L.F.; Gois, P.M.P. Water as the reaction medium for multicomponent reactions based on boronic acids. Tetrahedron 2010, 66, 2736–2745. [Google Scholar] [CrossRef]
- Ishiyama, T.; Matsuda, N.; Murata, M.; Ozawa, F.; Suzuki, A.; Miyaura, N. Platinum(0)-Catalyzed Diboration of Alkynes with Tetrakis(alkoxo)diborons: An Efficient and Convenient Approach to cis-Bis(boryl)alkenes. Organometallics 1996, 15, 713–720. [Google Scholar] [CrossRef]
- Sridhar, T.; Berrée, F.; Sharma, G.V.M.; Carboni, B. Regio- and Stereocontrolled Access to γ-Boronated Unsaturated Amino Esters and Derivatives from (Z)-Alkenyl 1,2-Bis(boronates). J. Org. Chem. 2014, 79, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, Y.; Miyabe, H.; Takemoto, Y. Catalytic Enantioselective Petasis-Type Reaction of Quinolines Catalyzed by a Newly Designed Thiourea Catalyst. J. Am. Chem. Soc. 2007, 129, 6686–6687. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.; Schaus, S.E. Asymmetric Petasis Reactions Catalyzed by Chiral Biphenols. J. Am. Chem. Soc. 2008, 130, 6922–6923. [Google Scholar] [CrossRef] [PubMed]
- Churches, Q.I.; Stewart, H.E.; Cohen, S.B.; Shröder, A.; Turner, P.; Hutton, C.A. Stereoselectivity of the Petasis reaction with various chiral amines and styrenylboronic acids. Pure Appl. Chem. 2008, 80, 687–694. [Google Scholar] [CrossRef]
- Churches, Q.I.; White, J.M.; Hutton, C.A. Synthesis of β,γ-Dihydroxyhomotyrosines by a Tandem Petasis–Asymmetric Dihydroxylation Approach. Org. Lett. 2011, 13, 2900–2903. [Google Scholar] [CrossRef]
- Li, Y.; Xu, M.-H. Lewis Acid Promoted Highly Diastereoselective Petasis Borono-Mannich Reaction: Efficient Synthesis of Optically Active β,γ-Unsaturated α-Amino Acids. Org. Lett. 2012, 14, 2062–2065. [Google Scholar] [CrossRef]
- Inokuma, T.; Suzuki, Y.; Sakaeda, T.; Takemoto, Y. Synthesis of Optically Active N-Aryl Amino Acid Derivatives through the Asymmetric Petasis Reaction Catalyzed by a Novel Hydroxy–Thiourea Catalyst. Chem. An Asian J. 2011, 6, 2902–2906. [Google Scholar] [CrossRef]
- Morozova, V.A.; Beletskaya, I.P.; Titanyuk, I.D. Synthesis of enantiopure cyclic amino acid derivatives via a sequential diastereoselective Petasis reaction/ring closing olefin metathesis process. Tetrahedron: Asymmetry 2017, 28, 349–354. [Google Scholar] [CrossRef]
- Bułyszko, I.; Chrzanowska, M.; Grajewska, A. Rozwadowska, M.D. Synthesis of (+)-6,7-Dimethoxy-1,2,3,4-tetrahydroisoquinoline-1-carboxylic Acid, a Diastereoselective Approach. Eur. J. Org. Chem. 2015, 2015, 383–388. [Google Scholar] [CrossRef]
- Koolmeister, T.; Södergren, M.; Scobie, M. The first example of chiral induction using homochiral boronic esters in the Petasis reaction. Tetrahedron Lett. 2002, 43, 5969–5970. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, R.; Sivaguru, P.; Cong, X.; Bi, X. Silver-Catalyzed anti-Markovnikov Hydroboration of C–C Multiple Bonds. Org. Lett. 2019, 21, 4035–4038. [Google Scholar] [CrossRef]
- Agahi, R.; Challinor, A.J.; Carter, N.B.; Thomas, S.P. Earth-abundant metal catalysis enabled by counterion activation. Org. Lett. 2019, 21, 993–997. [Google Scholar] [CrossRef]
- Jang, W.J.; Song, S.M.; Moon, J.H.; Lee, J.Y.; Yun, J. Copper-catalyzed enantioselective hydroboration of unactivated 1, 1-disubstituted alkenes. J. Am. Chem. Soc. 2017, 139, 13660–13663. [Google Scholar] [CrossRef] [PubMed]
- Joshi-Pangu, A.; Ma, X.; Diane, M.; Iqbal, S.; Kribs, R.J.; Huang, R.; Wang, C.-Y.; Biscoe, M.R. Palladium-catalyzed borylation of primary alkyl bromides. J. Org. Chem. 2012, 77, 6629–6633. [Google Scholar] [CrossRef] [PubMed]
- Dudnik, A.S.; Fu, G.C. Nickel-catalyzed coupling reactions of alkyl electrophiles, including unactivated tertiary halides, to generate carbon–boron bonds. J. Am. Chem. Soc. 2012, 134, 10693–10697. [Google Scholar] [CrossRef]
- Muncipinto, G.; Moquist, P.N.; Schreiber, S.L.; Schaus, S.E. Catalytic Diastereoselective Petasis Reactions. Angew. Chem. Int. Ed. 2011, 50, 8172–8175. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.-C.; Wang, Y.; Li, E.-Q.; Cui, H.; Duan, Z. Enantio- and Diastereoselective Synthesis of β-Aryl-β-pyrazolyl α-Amino Acid Esters via Copper-Catalyzed Reaction of Azomethine Ylides with Benzylidenepyrazolones. Adv. Synth. Catal. 2019, 361, 1389–1393. [Google Scholar] [CrossRef]
- Menor-Salván, C. From the dawn of organic chemistry to astrobiology: Urea as a foundational component in the origin of nucleobases and nucleotides. In Prebiotic Chemistry and Chemical Evolution of Nucleic Acids; Springer: Berlin/Heidelberg, Germany, 2018; pp. 85–142. [Google Scholar] [CrossRef]
- Hall, D.G. Structure, properties, and preparation of boronic acid derivatives. Overview of their reactions and applications. Boronic Acids 2005, 1. [Google Scholar] [CrossRef]
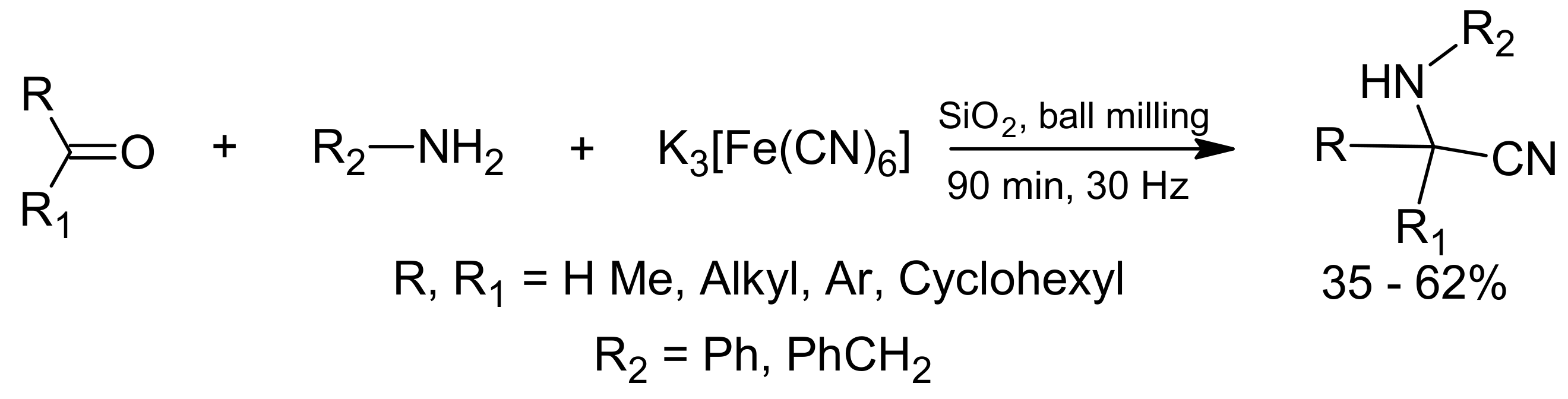


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masamba, W. Petasis vs. Strecker Amino Acid Synthesis: Convergence, Divergence and Opportunities in Organic Synthesis. Molecules 2021, 26, 1707. https://doi.org/10.3390/molecules26061707
Masamba W. Petasis vs. Strecker Amino Acid Synthesis: Convergence, Divergence and Opportunities in Organic Synthesis. Molecules. 2021; 26(6):1707. https://doi.org/10.3390/molecules26061707
Chicago/Turabian StyleMasamba, Wayiza. 2021. "Petasis vs. Strecker Amino Acid Synthesis: Convergence, Divergence and Opportunities in Organic Synthesis" Molecules 26, no. 6: 1707. https://doi.org/10.3390/molecules26061707
APA StyleMasamba, W. (2021). Petasis vs. Strecker Amino Acid Synthesis: Convergence, Divergence and Opportunities in Organic Synthesis. Molecules, 26(6), 1707. https://doi.org/10.3390/molecules26061707