5-Aryl-1-Arylideneamino-1H-Imidazole-2(3H)-Thiones: Synthesis and In Vitro Anticancer Evaluation
Abstract
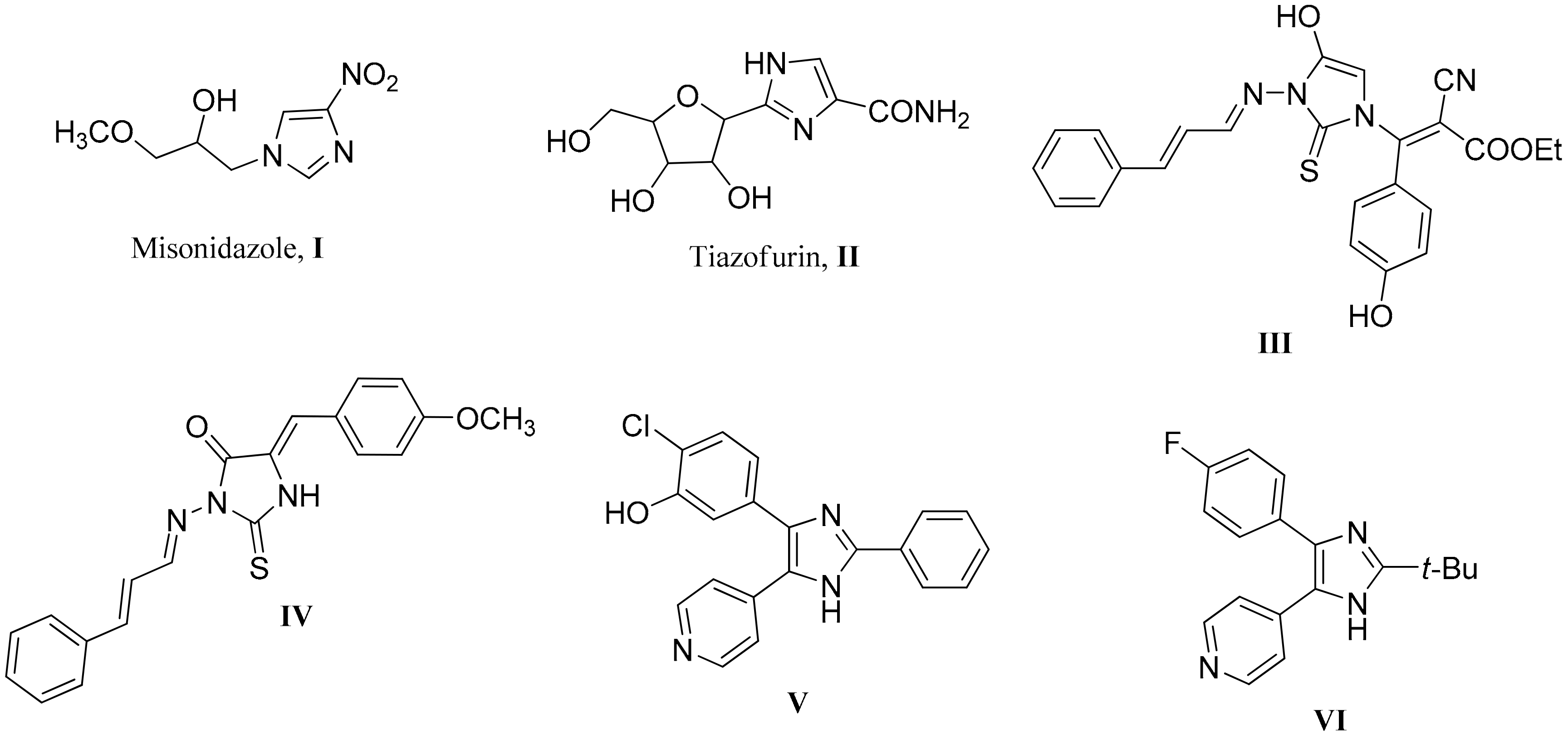
1. Introduction
2. Result and Discussion
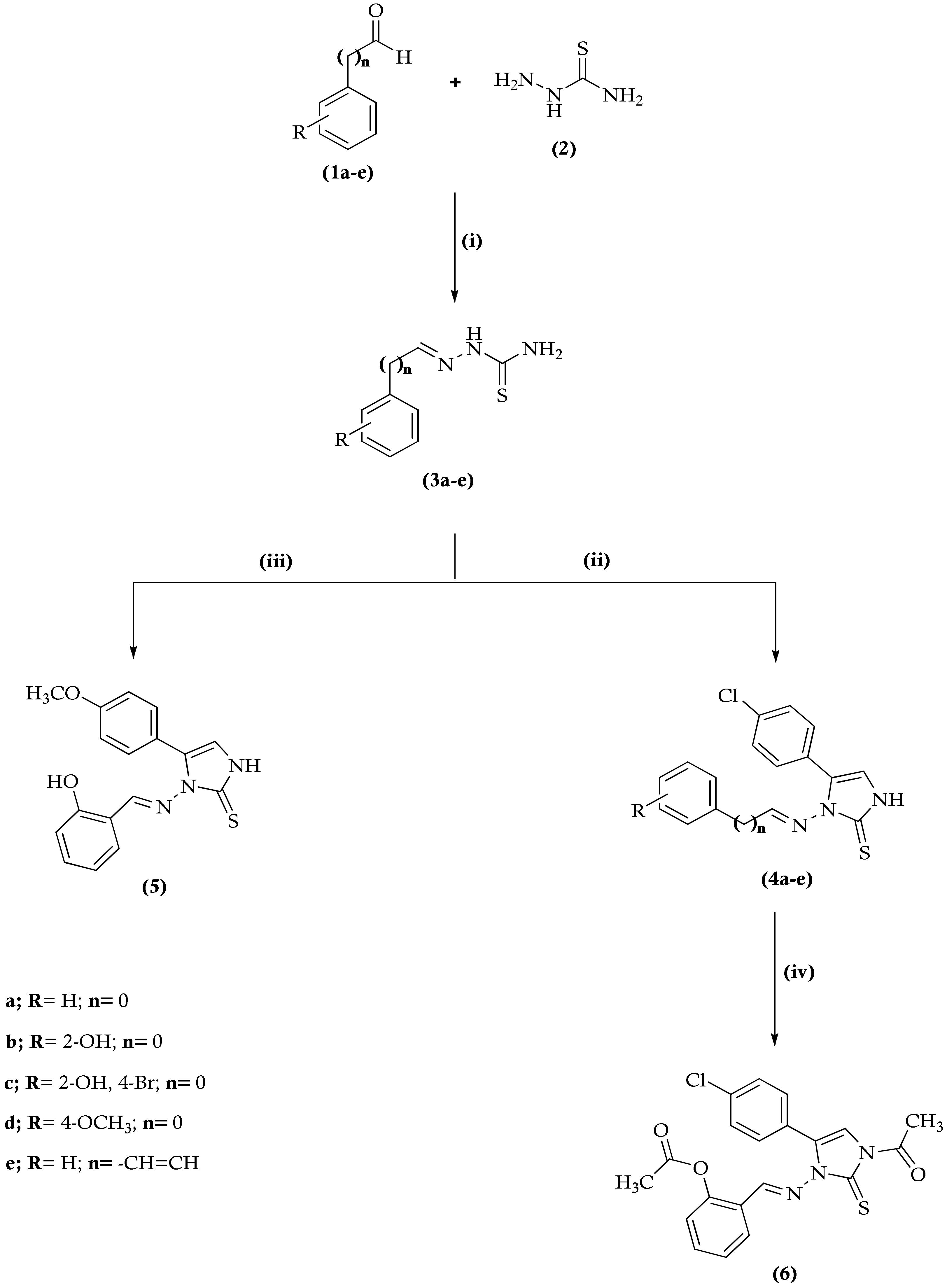
2.1. Chemistry
2.2. In Vitro Antitumor Evaluation
2.2.1. In Vitro Cytotoxic Activity against Three Cancer Cell Lines
2.2.2. Cell Cycle Analysis
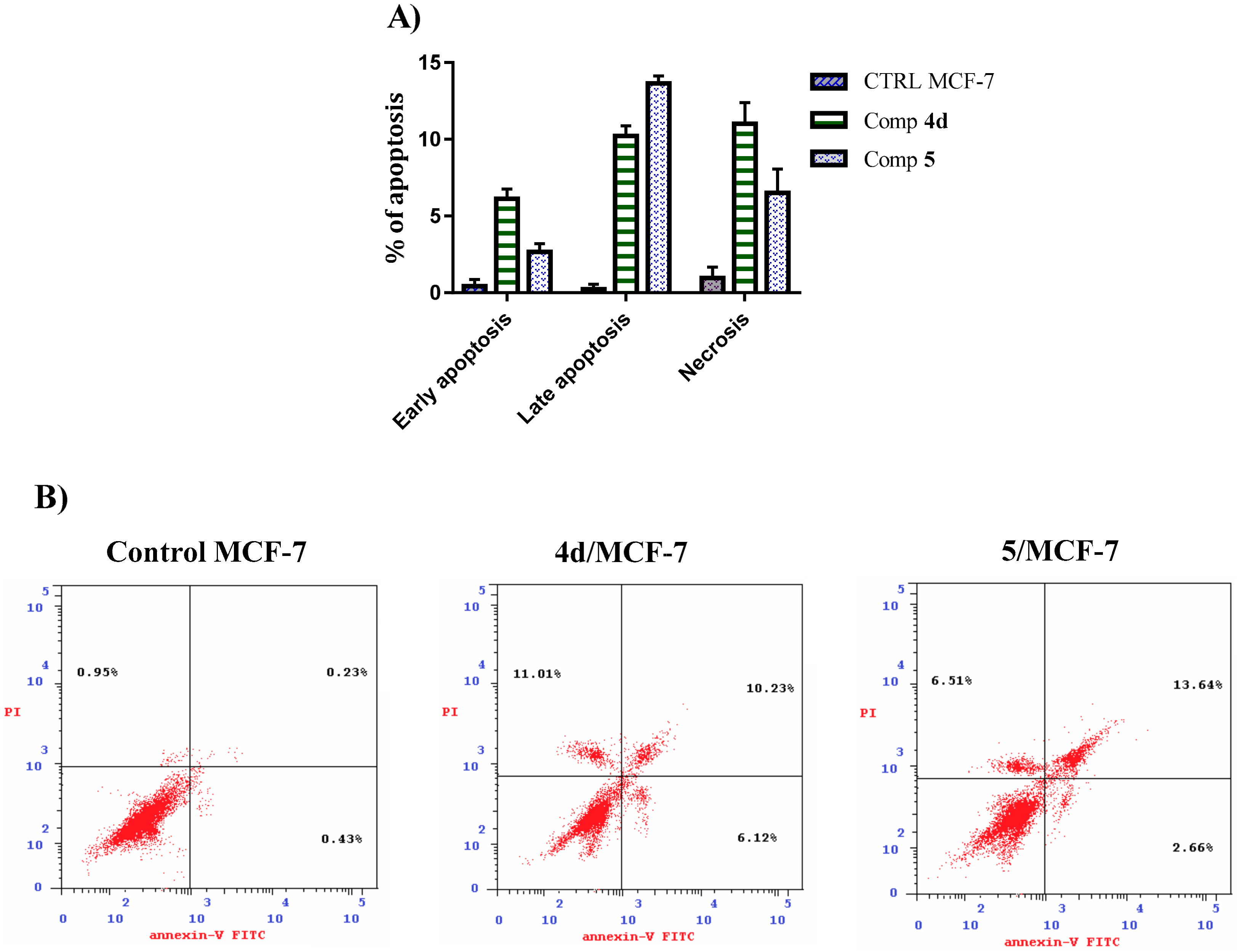
2.2.3. Annexin V-FITC/PI Apoptosis Induction Analysis
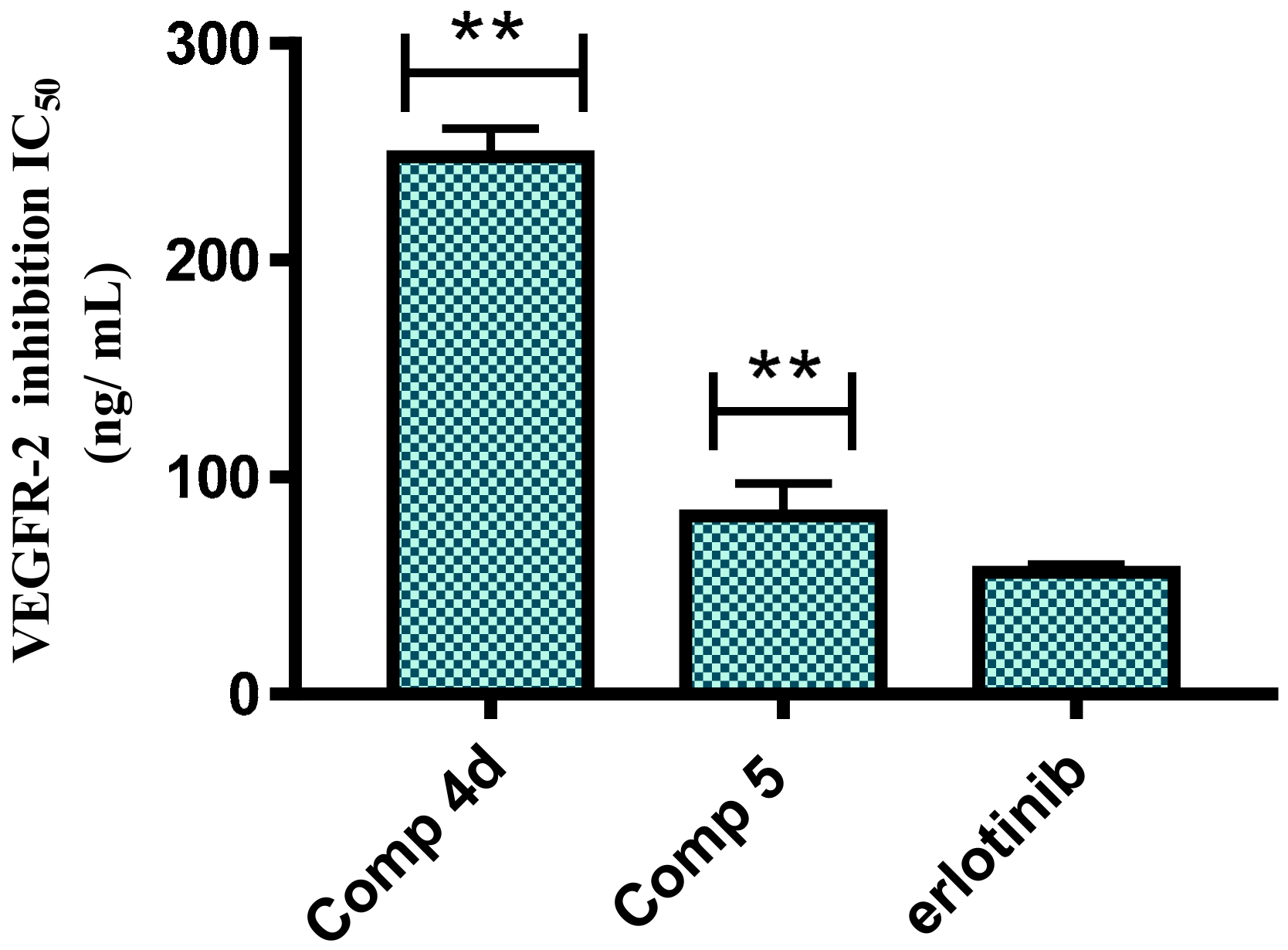
2.2.4. VEGFR-2 Kinase Inhibitory Activity
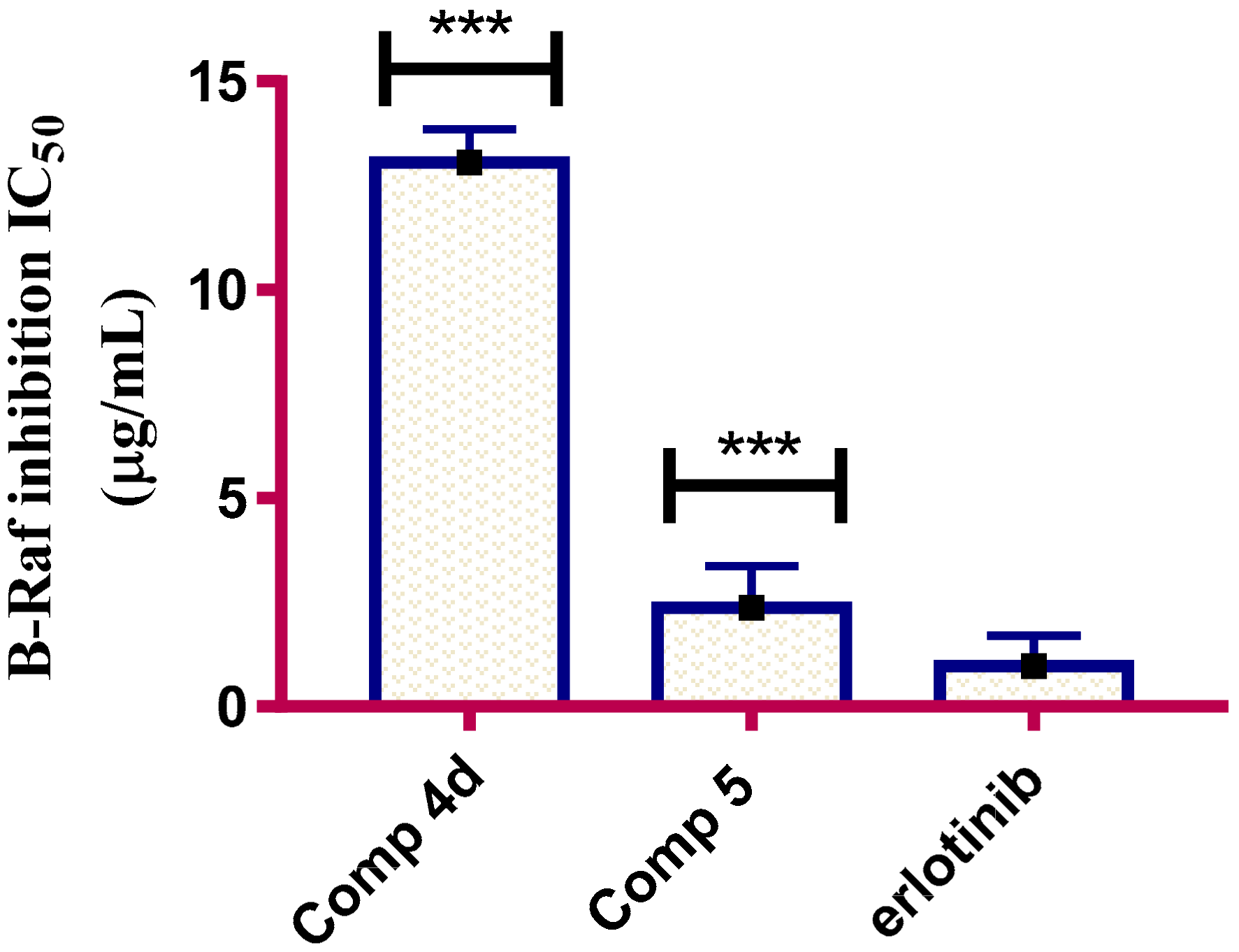
2.2.5. B-Raf Ihibitory Activity
2.2.6. Molecular Docking Study
3. Material and Methods
3.1. General
3.2. General Procedure for the Synthesis of 2-Arylhydrazinecarbothioamide (3a–d)
3.2.1. (E)-2-Benzylidenehydrazinecarbothioamide (3a)
3.2.2. (E)-2-(2-Hydroxybenzylidene)hydrazinecarbothioamide (3b)
3.2.3. (E)-2-(4-Methoxybenzylidene)hydrazinecarbothioamide (3c)
3.2.4. (E)-2-((E)-3-Phenylallylidene)hydrazinecarbothioamide (3d)
3.3. General Procedure for the Synthesis of Imidazole Derivatives 4a–e and 5
3.3.1. (E)-1-(Benzylideneamino)-5-(4-chlorophenyl)-1H-imidazole-2(3H)-thione (4a)
3.3.2. (E)-5-(4-Chlorophenyl)-1-(2-hydroxybenzylideneamino)-1H-imidazole-2(3H)-thione (4b)
3.3.3. (E)-1-(5-Bromo-2-hydroxybenzylideneamino)-5-(4-chlorophenyl)-1H-imidazole-2(3H)-thione (4c)
3.3.4. (E)-5-(4-Chlorophenyl)-1-(4-methoxybenzylideneamino)-1H-imidazole-2(3H)-thione (4d)
3.3.5. 5-(4-Chlorophenyl)-1-((E)-((E)-3-phenylallylidene)amino)-1H-imidazole-2(3H)-thione (4e)
3.3.6. (E)-1-(2-Hydroxybenzylideneamino)-5-(4-methoxyphenyl)-1H-imidazole-2(3H)-thione (5)
3.4. General Procedure for the Synthesis (E)-2-((3-Acetyl-5-(4-chlorophenyl)-2-thioxo-2,3-dihydro-1H-imidazol-1-ylimino)methyl)phenyl Acetate (6)
3.5. Biological Studies
3.5.1. Antitumor Activity against Three Cancer Cell Lines
3.5.2. Cell Cycle Analysis of Compounds 4d and 5
3.5.3. Annexin V FITC/PI Apoptosis Detection Staining Assay
3.5.4. In Vitro VEGFR-2 Kinase Assay
3.5.5. In Vitro B-Raf Kinase Assay
3.6. Molecular Docking Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Li, Q.; Zhang, Z.; Fan, Y.; Zhang, Q. Epigenetic Alterations in Renal Cell Cancer with TKIs Resistance: From Mechanisms to Clinical Applications. Front. Genet. 2021, 11, 562868. [Google Scholar] [CrossRef] [PubMed]
- Fane, M.; Weeraratna, A.T. How the ageing microenvironment influences tumour progression. Nat. Rev. Cancer 2020, 20, 89–106. [Google Scholar] [CrossRef]
- Bianchi, J.J.; Zhao, X.; Mays, J.C.; Davoli, T. Not all cancers are created equal: Tissue specificity in cancer genes and pathways. Curr. Opin. Cell Biol. 2020, 63, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Shang, C.; Wang, H.; Yun, J. Isatin–azole hybrids and their anticancer activities. Arch. Pharm. 2019, 353, 1900272. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Diao, Q.-P. 1, 3, 5-Triazine-azole Hybrids and their Anticancer Activity. Curr. Top. Med. Chem. 2020, 20, 1481–1492. [Google Scholar] [CrossRef]
- Ali, E.M.H.; Abdel-Maksoud, M.S.; Ammar, U.M.; Mersal, K.I.; Yoo, K.H.; Jooryeong, P.; Oh, C.-H. Design, synthesis, and biological evaluation of novel imidazole derivatives possessing terminal sulphonamides as potential BRAFV600Einhibitors. Bioorg. Chem. 2021, 106, 104508. [Google Scholar] [CrossRef]
- Yang, Y.-Q.; Chen, H.; Liu, Q.-S.; Sun, Y.; Gu, W. Synthesis and anticancer evaluation of novel 1H-benzo[d]imidazole derivatives of dehydroabietic acid as PI3Kα inhibitors. Bioorg. Chem. 2020, 100, 103845. [Google Scholar] [CrossRef]
- Singh, I.; Luxami, V.; Paul, K. Synthesis of naphthalimide-phenanthro[9,10-d]imidazole derivatives: In vitro evaluation, binding interaction with DNA and topoisomerase inhibition. Bioorg. Chem. 2020, 96, 103631. [Google Scholar] [CrossRef] [PubMed]
- Biskupiak, J.E.; Grierson, J.R.; Rasey, J.S.; Martin, G.V.; Krohn, K.A. Synthesis of an (iodovinyl)misonidazole derivative for hypoxia imaging. J. Med. Chem. 1991, 34, 2165–2168. [Google Scholar] [CrossRef] [PubMed]
- Franchetti, P.; Marchetti, S.; Cappellacci, L.; Yalowitz, J.A.; Jayaram, H.N.; Goldstein, B.M.; Grifantini, M. ChemInform Abstract: A New C-Nucleoside Analogue of Tiazofurin: Synthesis and Biological Evaluation of 2-β-D-Ribofuranosylimidazole-4-carboxamide (Imidazofurin). Bioorg. Med. Chem. Lett. 2001, 32, 67–69. [Google Scholar] [CrossRef]
- Cui, B.; Zheng, B.L.; He, K.; Zheng, Q.Y. Imidazole Alkaloids from Lepidium meyenii. J. Nat. Prod. 2013, 66, 1101–1103. [Google Scholar] [CrossRef]
- Zeng, L.; Wu, Q.; Wang, T.; Li, L.-P.; Zhao, X.; Chen, K.; Qian, J.; Yuan, L.; Xu, H.; Mei, W.-J. Selective stabilization of multiple promoter G-quadruplex DNA by using 2-phenyl-1H-imidazole-based tanshinone IIA derivatives and their potential suppressing function in the metastatic breast cancer. Bioorg. Chem. 2021, 106, 104433. [Google Scholar] [CrossRef] [PubMed]
- Chavda, J.; Bhatt, H. Systemic review on B-RafV600E mutation as potential therapeutic target for the treatment of cancer. Eur. J. Med. Chem. 2020, 206, 112675. [Google Scholar] [CrossRef]
- Johnson, J.C.; Martinez, O.; Honko, A.N.; Hensley, L.E.; Olinger, G.G.; Basler, C.F. Pyridinyl imidazole inhibitors of p38 MAP kinase impair viral entry and reduce cytokine induction by Zaire ebolavirus in human dendritic cells. Antivir. Res. 2014, 107, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Zaki, I.; Ramadan, H.M.; El-Sayed, E.S.; Abd El-Moneim, M. Design, synthesis, and cytotoxicity screening of new synthesized imidazolidine-2-thiones as VEGFR-2 enzyme inhibitors. Arch. Pharm. 2020, 353, 2000121. [Google Scholar] [CrossRef] [PubMed]
- Bellina, F.; Cauteruccio, S.; Rossi, R. Synthesis and biological activity of vicinal diaryl-substituted 1H-imidazoles. Tetrahedron 2007, 63, 4571–4624. [Google Scholar] [CrossRef]
- Inman, G.J.; Nicolás, F.J.; Callahan, J.F.; Harling, J.D.; Gaster, L.M.; Reith, A.D.; Laping, N.J.; Hill, C.S. SB-431542 Is a Potent and Specific Inhibitor of Transforming Growth Factor-β Superfamily Type I Activin Receptor-Like Kinase (ALK) Receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 2002, 62, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.K.; Singh, L.; Sharma, D.K. Synthesis, Spectral, and Biological Properties of Copper(II) Complexes of Thiosemicarbazones of Schiff Bases Derived from 4-Aminoantipyrine and Aromatic Aldehydes. Bioinorg. Chem. Appl. 2006, 2006, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Ghaffari, R.; Sardari, S.; Farahani, Y.F.; Mohebbi, S. Discovery of novel isatin-based thiosemicarbazones: Synthesis, antibacterial, antifungal, and antimycobacterial screening. Res. Pharm. Sci. 2020, 15, 281–290. [Google Scholar] [CrossRef]
- Abdelhameid, M.K.; Zaki, I.; Mohammed, M.R.; Mohamed, K.O. Design, synthesis, and cytotoxic screening of novel azole derivatives on hepatocellular carcinoma (HepG2 Cells). Bioinorg. Chem. 2020, 101, 103995. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. Properties of FDA-approved small molecule protein kinase inhibitors: A 2021 update. Pharmacol. Res. 2019, 144, 19–50. [Google Scholar] [CrossRef] [PubMed]
- Norman, M.H.; Liu, L.; Lee, M.; Xi, N.; Fellows, I.; D’Angelo, N.D.; Dominguez, C.; Rex, K.; Bellon, S.F.; Kim, T.S. Structure-Based Design of Novel Class II c-Met Inhibitors: 1. Identification of Pyrazolone-Based Derivatives. J. Med. Chem. 2012, 55, 1858–1867. [Google Scholar] [CrossRef] [PubMed]


| Comp No. | IC50 (μM) | |||
|---|---|---|---|---|
| MCF-7 | HepG2 | HCT-116 | MCF-10A | |
| 4a | >50 | 61.81 ± 4.1 | 20.56 ± 1.4 | NT |
| 4b | 24.94 ± 1.6 | >50 | 23.53 ± 1.6 | NT |
| 4c | 43.95 ± 2.9 | 11.36 ± 0.8 | 9.962 ± 0.7 | NT |
| 4d | 7.967 ± 0.5 | 4.086 ± 0.3 | 28.95 ± 1.9 | 44.06 ± 0.23 |
| 4e | 13.15 ± 0.9 | 17.01 ± 1.1 | 3.988 ± 0.3 | NT |
| 5 | 1.071 ± 0.1 | 1.301 ± 0.1 | 3.99 ± 0.3 | 27.81 ± 0.31 |
| DOC | 11.09 ± 0.7 | 8.128 ± 0.5 | 13.96 ± 0.9 | 33.17 ± 0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu Almaaty, A.H.; Toson, E.E.M.; El-Sayed, E.-S.H.; Tantawy, M.A.M.; Fayad, E.; Abu Ali, O.A.; Zaki, I. 5-Aryl-1-Arylideneamino-1H-Imidazole-2(3H)-Thiones: Synthesis and In Vitro Anticancer Evaluation. Molecules 2021, 26, 1706. https://doi.org/10.3390/molecules26061706
Abu Almaaty AH, Toson EEM, El-Sayed E-SH, Tantawy MAM, Fayad E, Abu Ali OA, Zaki I. 5-Aryl-1-Arylideneamino-1H-Imidazole-2(3H)-Thiones: Synthesis and In Vitro Anticancer Evaluation. Molecules. 2021; 26(6):1706. https://doi.org/10.3390/molecules26061706
Chicago/Turabian StyleAbu Almaaty, Ali H., Eslam E. M. Toson, El-Sherbiny H. El-Sayed, Mohamed A. M. Tantawy, Eman Fayad, Ola A. Abu Ali, and Islam Zaki. 2021. "5-Aryl-1-Arylideneamino-1H-Imidazole-2(3H)-Thiones: Synthesis and In Vitro Anticancer Evaluation" Molecules 26, no. 6: 1706. https://doi.org/10.3390/molecules26061706
APA StyleAbu Almaaty, A. H., Toson, E. E. M., El-Sayed, E.-S. H., Tantawy, M. A. M., Fayad, E., Abu Ali, O. A., & Zaki, I. (2021). 5-Aryl-1-Arylideneamino-1H-Imidazole-2(3H)-Thiones: Synthesis and In Vitro Anticancer Evaluation. Molecules, 26(6), 1706. https://doi.org/10.3390/molecules26061706






