Encapsulation of Vitamin E in Yogurt-Based Beverage Emulsions: Influence of Bulk Pasteurization and Chilled Storage on Physicochemical Stability and Starter Culture Viability
Abstract
1. Introduction
2. Results and Discussion
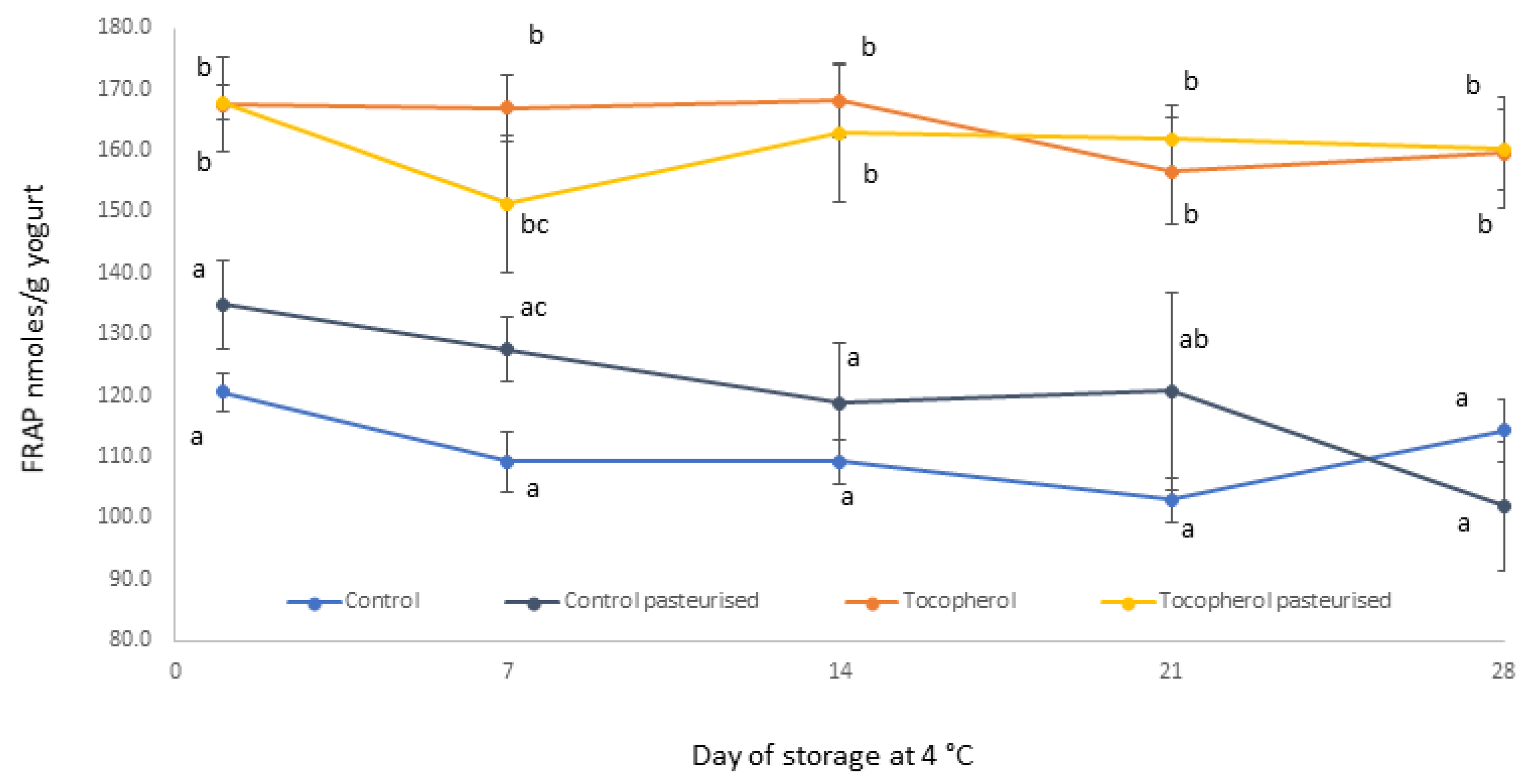
2.1. Processing and Storage Effects on Tocopherol Content and Antioxidant Capacity
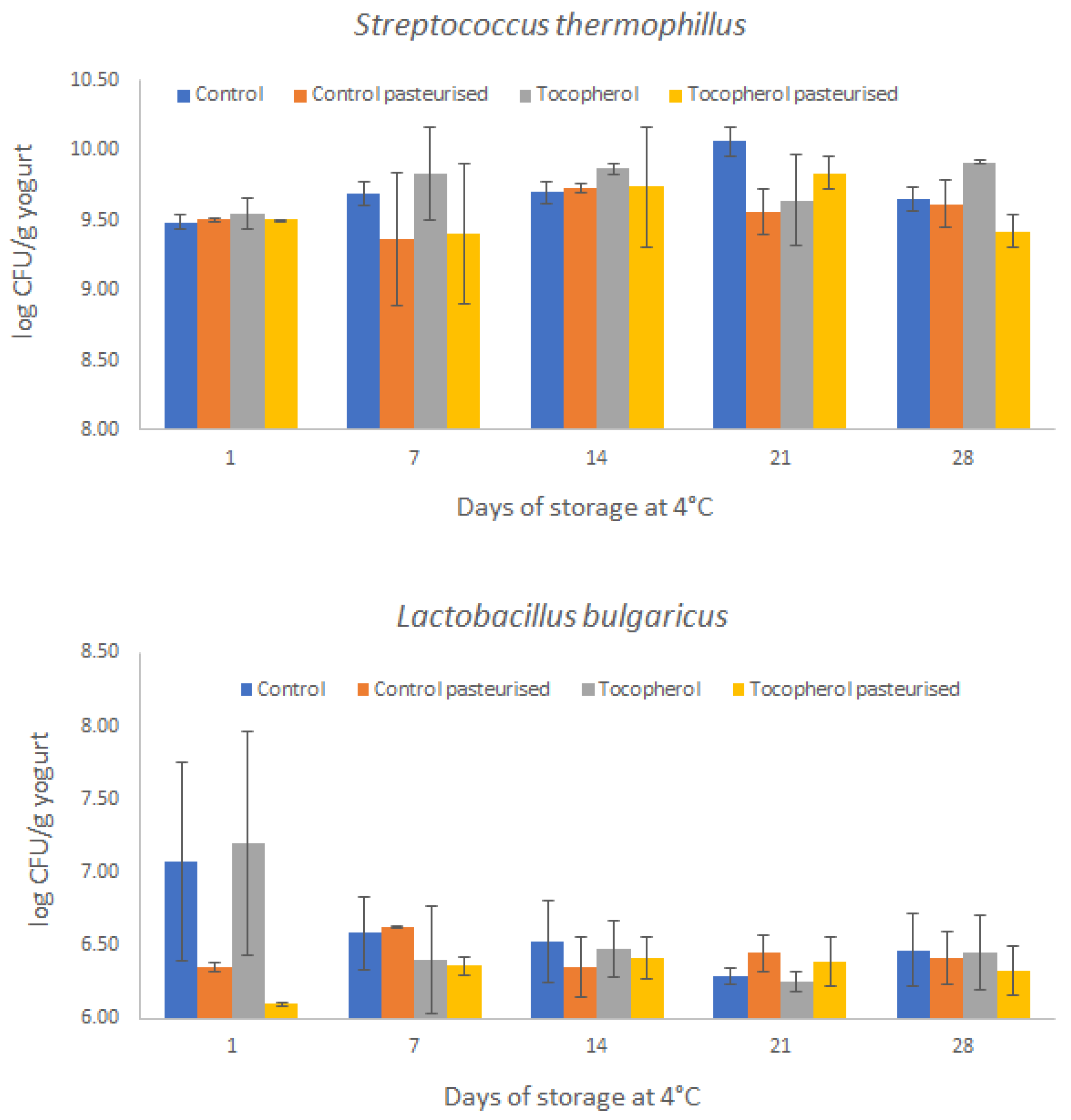
2.2. Processing and Storage Effects on Viability of Lactic Acid Bacteria
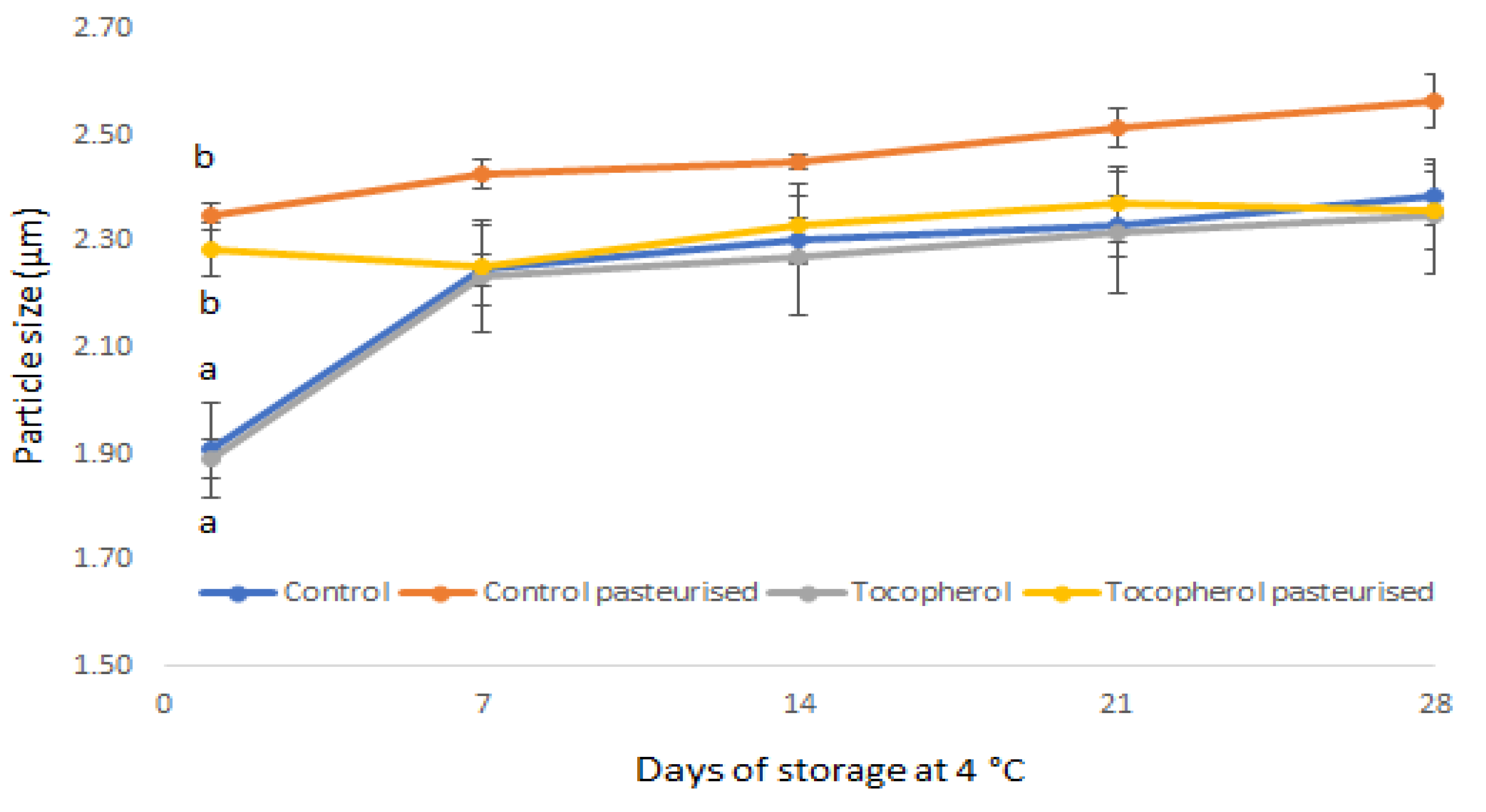
2.3. Processing and Storage Effects on Physical Stability
3. Materials and Methods
3.1. Materials/Microorganisms
3.2. Preparation, Processing and Storage of Yogurt-Type Beverage
3.3. Tocopherol Analysis
3.4. Particle Size Determination
3.5. Microbiological Analysis
3.6. Optical Microscopy
3.7. Antioxidant Activity (FRAP)
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gómez-Gallego, C.; Gueimonde, M.; Salminen, S. The role of yogurt in food-based dietary guidelines. Nutr. Rev. 2018, 76, 29–39. [Google Scholar] [CrossRef]
- Williams, E.B.; Hooper, B.; Spiro, A.; Stanner, S. The contribution of yogurt to nutrient intakes across the life course. Nutr. Bull. 2015, 40, 9–32. [Google Scholar] [CrossRef]
- Derrien, M.; van Hylckama Vlieg, J.E.T. Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol. 2015, 23, 354–366. [Google Scholar] [CrossRef] [PubMed]
- El-Abbadi, N.H.; Dao, M.C.; Meydani, S.N. Yogurt: Role in healthy and active aging. Am. J. Clin. Nutr. 2014, 99, 1263S–1270S. [Google Scholar] [CrossRef]
- Heaney, R.P. Functional indices of vitamin D status and ramifications of vitamin D deficiency. Am. J. Clin. Nutr. 2004, 80, 1706S–1709S. [Google Scholar] [CrossRef] [PubMed]
- Seppanen, C.M.; Song, Q.; Csallany, A.S. The antioxidant functions of tocopherol and tocotrienol homologues in oils, fats, and food systems. J. Am. Oil Chem. Soc. 2010, 87, 469–481. [Google Scholar] [CrossRef]
- Gahruie, H.H.; Eskandari, M.; Mesbahi, G.; Hanifpour, M.A. Scientific and technical aspects of yogurt fortification: A review. Food Sci. Hum. Well. 2015, 4, 1–8. [Google Scholar] [CrossRef]
- Öztürk, B. Nanoemulsions for food fortification with lipophilic vitamins: Production challenges, stability, and bioavailability. Eur. J. Lipid Sci. Technol. 2017, 119, 1500539. [Google Scholar] [CrossRef]
- Jafari, S.M.; Vakili, S.; Dehnad, D. Production of a functional yogurt powder fortified with nanoliposomal vitamin D through spray drying. Food Bioproc. Technol. 2019, 12, 1220–1231. [Google Scholar] [CrossRef]
- Levinson, Y.; Ish-Shalom, S.; Segal, E.; Livney, Y.D. Bioavailability, rheology and sensory evaluation of fat-free yogurt enriched with VD3 encapsulated in re-assembled casein micelles. Food Funct. 2016, 7, 1477–1482. [Google Scholar] [CrossRef]
- Bouzgarrou, C.; Amara, K.; Reis, F.S.; Barreira, J.C.M.; Skhiri, F.; Chatti, N.; Martins, A.; Barros, L.; Ferreira, I.C.F.R. Incorporation of tocopherol-rich extracts from mushroom mycelia into yogurt. Food Funct. 2018, 9, 3166–3172. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Advances in edible nanoemulsions: Digestion, bioavailability, and potential toxicity. Prog. Lip. Res. 2021, 81, 101081. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Wagner, G. Vitamin E nanoparticle for beverage applications. Chem. Eng. Res. Des. 2004, 82, 1432–1437. [Google Scholar] [CrossRef]
- Hatanaka, J.; Chikamori, H.; Sato, H.; Uchida, S.; Debari, K.; Onoue, S.; Yamada, S. Physicochemical and pharmacological characterization of a-tocopherol-loaded nano-emulsion system. Int. J. Pharm. 2010, 396, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Raikos, V. Encapsulation of vitamin E in edible orange oil-in-water emulsion beverages: Influence of heating temperature on physicochemical stability during chilled storage. Food Hydrocolloid 2017, 72, 155–162. [Google Scholar] [CrossRef]
- Ni, H.; Raikos, V. Lactic-acid bacteria fermentation-induced effects on microstructure and interfacial properties of oil-in-water emulsions stabilized by goat-milk proteins. LWT 2019, 109, 70–76. [Google Scholar] [CrossRef]
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes: Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academy Press: Washington, DC, USA, 2000. [Google Scholar]
- Sabliov, C.M.; Fronczek, C.; Astete, C.E.; Khachaturyan, M.; Khachatryan, L.; Leonardi, C. Effects of temperature and UV light on degradation of α-tocopherol in free and dissolved form. J. Am. Oil Chem. Soc. 2009, 86, 895–902. [Google Scholar] [CrossRef]
- Hategekimana, J.; Chamba, M.V.M.; Shoemaker, C.F.; Majeed, H.; Zhong, F. Vitamin E nanoemulsions by emulsion phase inversion: Effect of environmental stress and long-term storage on stability and degradation in different carrier oil types. Colloids Surf. A Physicochem. Eng. Asp. 2015, 483, 70–80. [Google Scholar] [CrossRef]
- Kristensen, D.; Hansen, E.; Arndal, A.; Trinderup, R.A.; Skibsted, L.H. Influence of light and temperature on the colour and oxidative stability of processed cheese. Int. Dairy J. 2001, 11, 837–843. [Google Scholar] [CrossRef]
- Mahrous, H.; Abd-El-Salam, R. Production of a functional frozen yoghurt fortified with omega-3 and vitamin E. Am. J. Food Nutr. 2014, 2, 77–84. [Google Scholar]
- Bramley, P.; Elmadfa, I.; Kafatos, A.; Kelly, F.; Manios, Y.; Roxborough, H.; Schuch, W.; Sheehy, P.J.A.; Wagner, K.-H. Vitamin E. J. Sci. Food Agric. 2000, 80, 913–938. [Google Scholar] [CrossRef]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Müller, L.; Theile, K.; Böhm, V. In vitro antioxidant activity of tocopherols and tocotrienols and comparison of vitamin E concentration and lipophilic antioxidant capacity in human plasma. Mol. Nutr. Food Res. 2010, 54, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Farvin, S.; Baron, C.; Skall, N.N.; Jacobsen, C. Antioxidant activity of yoghurt peptides: Part 1-in vitro assays and evaluation in omega-3 enriched milk. Food Chem. 2010, 123, 1081–1089. [Google Scholar] [CrossRef]
- Korhonen, H. Milk derived bioactive peptides: From science to applications. J. Funct. Foods 2009, 1, 177–187. [Google Scholar] [CrossRef]
- O’Connell, J.E.; Fox, P.F. Significance and applications of phenolic compounds in the production and quality of milk and dairy products: A review. Int. Dairy J. 2001, 11, 103–120. [Google Scholar] [CrossRef]
- Karmowski, J.; Hintze, V.; Kschonsek, J.; Killenberg, M.; Böhm, V. Antioxidant activities of tocopherols/tocotrienols and lip ophilic antioxidant capacity of wheat, vegetable oils, milk and milk cream by using photochemiluminescence. Food Chem. 2015, 175, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Gad, A.S.; El-Salam, M.H.A. The antioxidant properties of skim milk supplemented with rosemary and green tea extracts in response to pasteurisation, homogenisation and the addition of salts. Int. J. Dairy Technol. 2010, 63, 349–355. [Google Scholar] [CrossRef]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. ISRN Nutr. 2013, 2013, 481651. [Google Scholar] [CrossRef]
- Shah, N.P. Functional cultures and health benefits. Int. Dairy J. 2007, 17, 1262–1277. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Evans, C.A.; Adams, M.C.; Baines, S.K. Probiotic viability and physico-chemical and sensory properties of plain and stirred fruit yoghurts made from goat’s milk. Food Chem. 2012, 135, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Hamann, W.T.; Marth, E.H. Survival of Streptococcus thermophilus and Lactobacillus bulgaricus in commercial and experimental yogurts. J. Food Prot. 1984, 47, 781–786. [Google Scholar] [CrossRef]
- Coda, R.; Rizzello, C.G.; Trani, A.; Gobbetti, M. Manufacture and characterization of functional emmer beverages fermented by selected lactic acid bacteria. Food Microbiol. 2011, 28, 526–536. [Google Scholar] [CrossRef]
- Luana, N.; Rossana, C.; Curiel, J.A.; Kaisa, P.; Marco, G.; Rizzello, C.G. Manufacture and characterization of a yogurt-like beverage made with oat flakes fermented by selected lactic acid bacteria. Int. J. Food Microbiol. 2014, 185, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Zamberlin, Š.; Samaržija, D. The effect of non-standard heat treatment of sheep’s milk on physicochemical properties, sensory characteristics, and the bacterial viability of classical and probiotic yogurt. Food Chem. 2017, 225, 62–88. [Google Scholar] [CrossRef] [PubMed]
- Sfakianakis, P.; Tzia, C. Conventional and innovative processing of milk for yogurt manufacture; development of texture and flavor: A review. Foods 2014, 3, 176–193. [Google Scholar] [CrossRef] [PubMed]
- Walstra, P.; Wouters, J.T.M.; Geurts, T.J. Chapter 22 Fermented milks. In Dairy Science and Technology; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2006; pp. 551–573. [Google Scholar]
- Walstra, P.; Wouters, J.T.M.; Geurts, T.J. Chapter 7 Heat treatment. In Dairy Science and Technology; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2006; pp. 225–272. [Google Scholar]
- Mulvihill, D.M.; Grufferty, M.B. Effect of thermal processing on the coagulability of milk by acid. In Heat Induced Changes in Milk; Special Issue No., 9501; Fox, P.F., Ed.; International Dairy Federation: Brussels, Belgium, 1995; pp. 188–205. [Google Scholar]
- Liu, Y.; Wei, Z.-C.; Deng, Y.-Y.; Dong, H.; Zhang, Y.; Tang, X.-J.; Li, P.; Liu, G.; Zhang, M.-W. Comparison of the Effects of Different Food-Grade Emulsifiers on the Properties and Stability of a Casein-Maltodextrin-Soybean Oil Compound Emulsion. Molecules 2020, 25, 458. [Google Scholar] [CrossRef] [PubMed]
- Kilara, A. Chapter 5 Basic dairy processing principles. In Manufacturing Yogurt and Fermented Milks; Chandan, R.C., Ed.; Blackwell Publishing: Ames, IA, USA, 2006; pp. 73–89. [Google Scholar]
- Monahan, F.J.; McClements, D.J.; German, J.B. Disulfide-mediated polymerization reactions and physical properties of heated WPI-stabilised emulsions. J. Food Sci. 1996, 61, 504–509. [Google Scholar] [CrossRef]
- Liang, Y.; Gillies, G.; Matia-Merino, L.; Ye, A.; Patel, H.; Golding, M. Structure and stability of sodium-caseinate-stabilized oil-in-water emulsions as influenced by heat treatment. Food Hydrocolloid 2017, 66, 307–317. [Google Scholar] [CrossRef]
- Tribst, A.A.L.; Falcade, L.T.P.; Carvalho, N.S.; De Castro Leite Junior, B.R.; Meirelles de Oliveira, M. Using stirring and homogenization to improve the fermentation profile and physicochemical characteristics of set yogurt from fresh, refrigerated and frozen/thawed sheep milk. LWT 2020, 130, 109557. [Google Scholar] [CrossRef]
- Srinivasan, M.; Singh, H.; Munro, P.A. Formation and stability of sodium caseinate emulsions: Influence of retorting (121 °C for 15 min) before or after emulsification. Food Hydrocolloid 2002, 16, 153–160. [Google Scholar] [CrossRef]
- Hess, D.; Keller, H.E.; Oberlin, B.; Bonfanti, R.; Schüep, W. Simultaneous determination of retinol, tocopherols, carotenes and lycopene in plasma by means of high-performance liquid chromatography on reversed phase. Int. J. Vitam. Nutr. Res. 1991, 61, 232–238. [Google Scholar] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]

| Days of Storage at 4 °C | 1 | 7 | 14 | 21 | 28 | |
|---|---|---|---|---|---|---|
| α-tocopherol | Control | ND | ND | ND | ND | ND |
| Control-P | ND | ND | ND | ND | ND | |
| Tocopherol | 16.98 ± 2.9 a | 18.37 ± 0.6 a | 12.40 ± 1.7 a | 13.27 ± 2.9 a | 13.35 ± 3.5 a | |
| Tocopherol-P | 14.99 ± 1.3 a | 16.95 ± 3.0 a | 13.13 ± 1.0 a | 14.01 ± 1.1 a | 12.87 ± 1.3 a | |
| γ-tocopherol | Control | 0.17 ± 0.0 a | 0.21 ± 0.0 a | 0.15 ± 0.0 a | 0.16 ± 0.0 a | 0.17 ± 0.0 a |
| Control-P | 0.15 ± 0.0 a | 0.12 ± 0.0 a | 0.10 ± 0.0 a | 0.07 ± 0.0 a | 0.12 ± 0.0 a | |
| Tocopherol | 169.52 ± 27.0 b | 177.15 ± 7.4 b | 132.95 ± 19.5 b | 143.90 ± 29.4 b | 148.33 ± 33.8 b | |
| Tocopherol-P | 156.75 ± 12.4 b | 165.78 ± 32.5 b | 139.43 ± 14.6 b | 146.77 ± 13.7 b | 144.54 ± 15.2 b | |
| δ-tocopherol | Control | 0.02 ± 0.0 a | 0.02 ± 0.0 a | 0.02 ± 0.0 a | 0.01 ± 0.0 a | 0.02 ± 0.0 a |
| Control-P | 0.02 ± 0.0 a | 0.02 ± 0.0 a | 0.01 ± 0.0 a | 0.01 ± 0.0 a | ND | |
| Tocopherol | 39.79 ± 4.7 b | 41.99 ± 2.0 b | 33.86 ± 4.4 b | 35.98 ± 7.0 b | 36.25 ± 8.0 b | |
| Tocopherol-P | 38.81 ± 2.7 b | 39.40 ± 7.6 b | 35.05 ± 3.5 b | 36.86 ± 3.2 b | 36.92 ± 3.5 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raikos, V.; Pirie, L.P.; Gürel, S.; Hayes, H.E. Encapsulation of Vitamin E in Yogurt-Based Beverage Emulsions: Influence of Bulk Pasteurization and Chilled Storage on Physicochemical Stability and Starter Culture Viability. Molecules 2021, 26, 1504. https://doi.org/10.3390/molecules26061504
Raikos V, Pirie LP, Gürel S, Hayes HE. Encapsulation of Vitamin E in Yogurt-Based Beverage Emulsions: Influence of Bulk Pasteurization and Chilled Storage on Physicochemical Stability and Starter Culture Viability. Molecules. 2021; 26(6):1504. https://doi.org/10.3390/molecules26061504
Chicago/Turabian StyleRaikos, Vassilios, Lynn P. Pirie, Sati Gürel, and Helen E. Hayes. 2021. "Encapsulation of Vitamin E in Yogurt-Based Beverage Emulsions: Influence of Bulk Pasteurization and Chilled Storage on Physicochemical Stability and Starter Culture Viability" Molecules 26, no. 6: 1504. https://doi.org/10.3390/molecules26061504
APA StyleRaikos, V., Pirie, L. P., Gürel, S., & Hayes, H. E. (2021). Encapsulation of Vitamin E in Yogurt-Based Beverage Emulsions: Influence of Bulk Pasteurization and Chilled Storage on Physicochemical Stability and Starter Culture Viability. Molecules, 26(6), 1504. https://doi.org/10.3390/molecules26061504








