A Novel Method for Stabilizing Zein Gel Particles to Salt Ion-Induced Aggregation
Abstract
1. Introduction
2. Results and Discussion
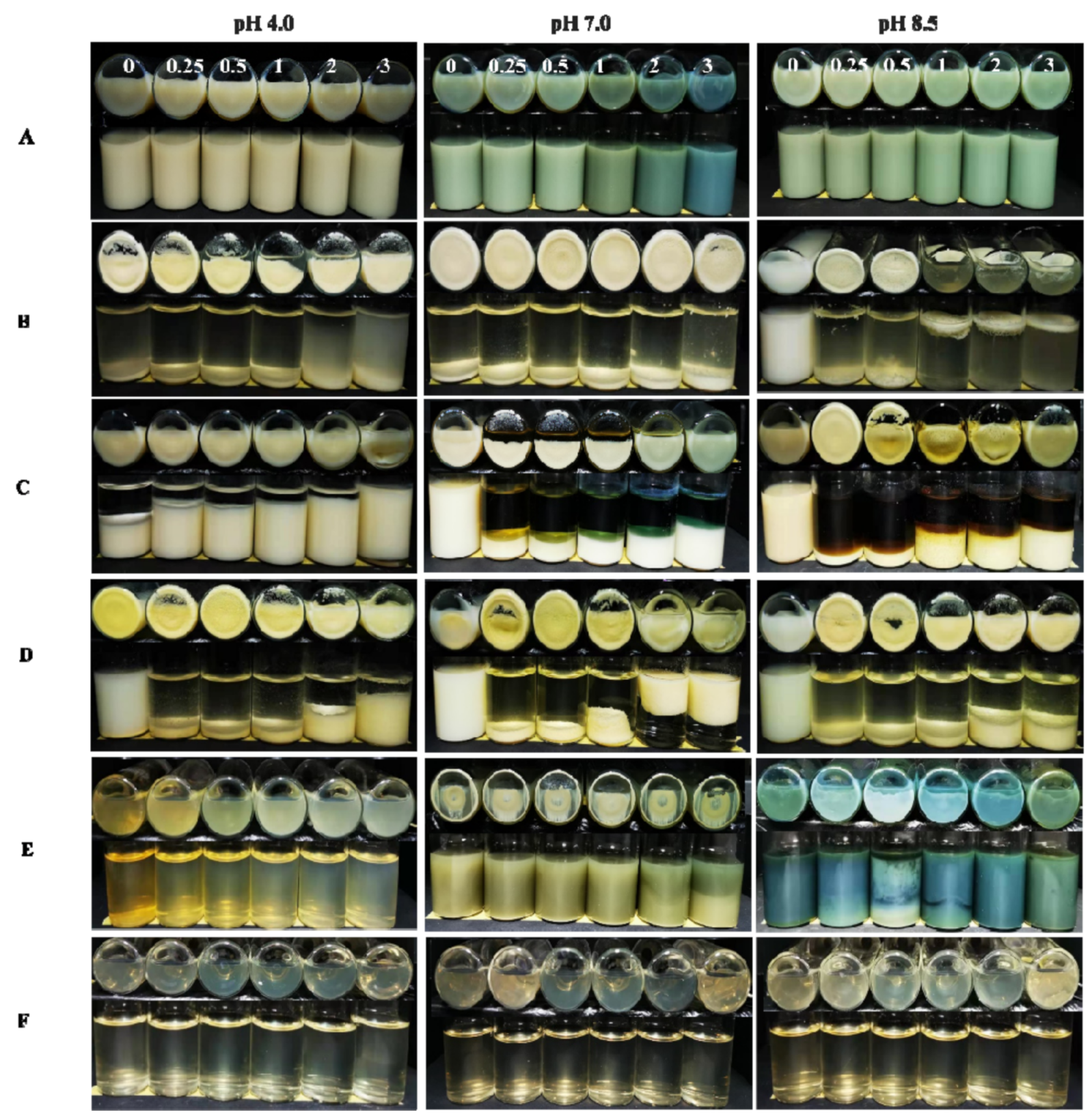
2.1. Appearance of the Dispersions
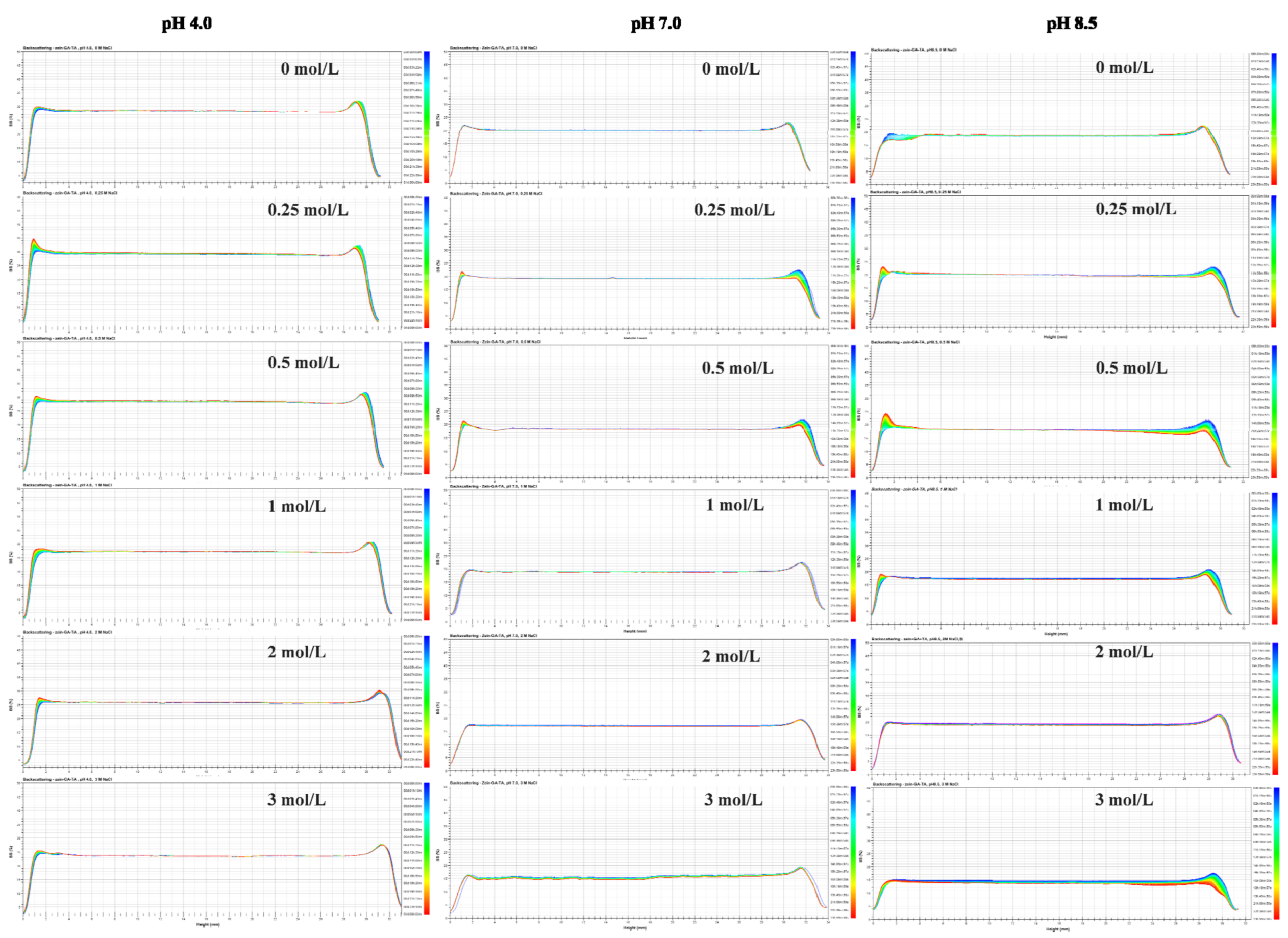
2.2. Backscattering Changes
2.3. Gel Particle Size, Polydispersity Index, and Zeta–Potential
3. Materials and Methods
3.1. Materials
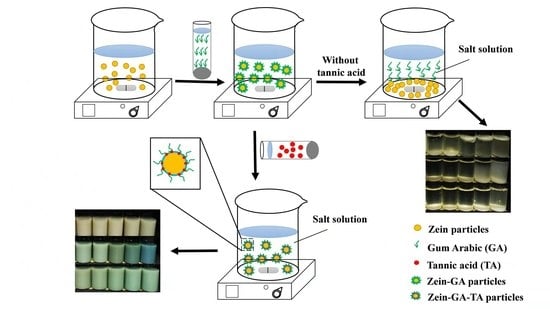
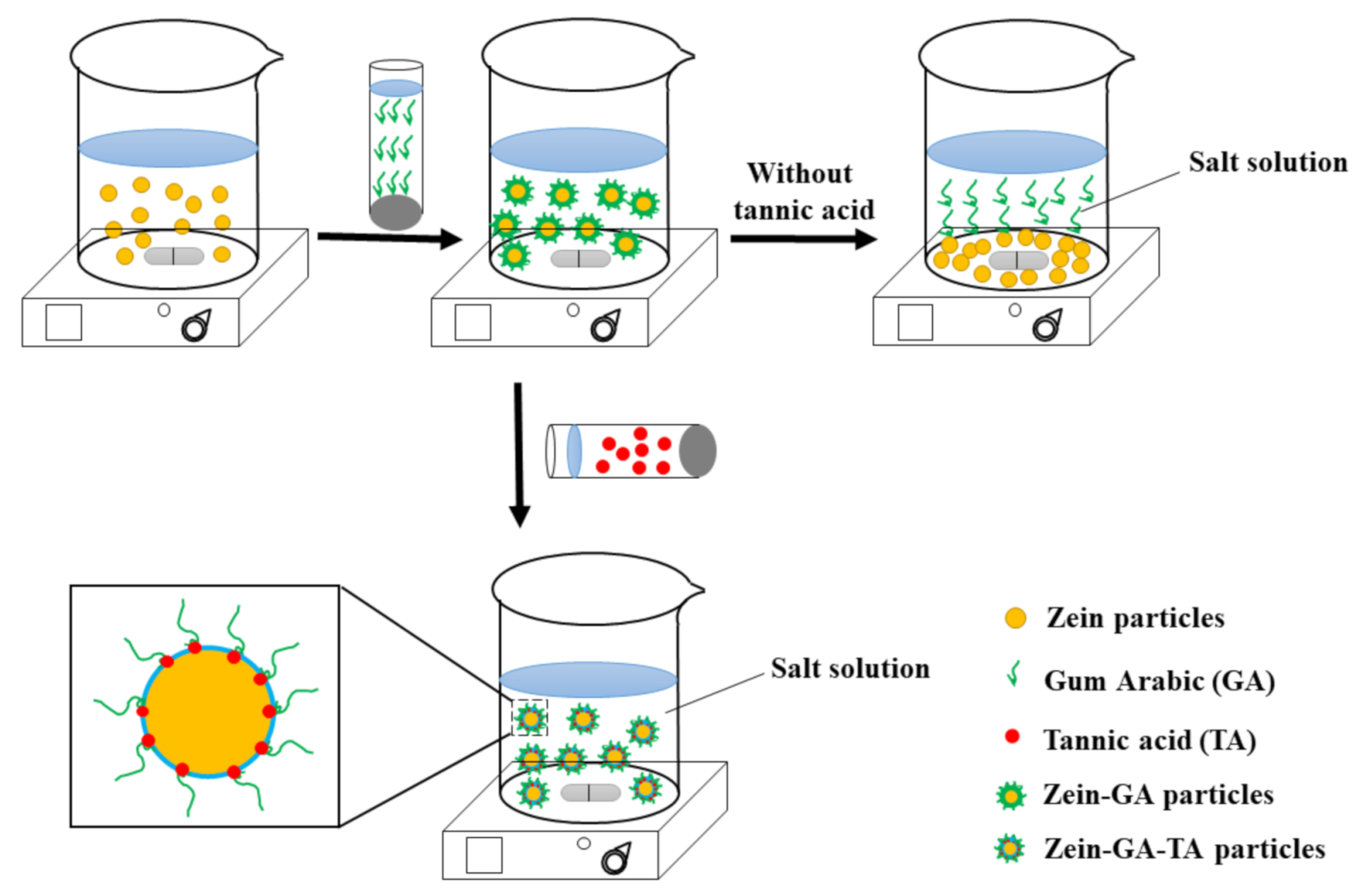
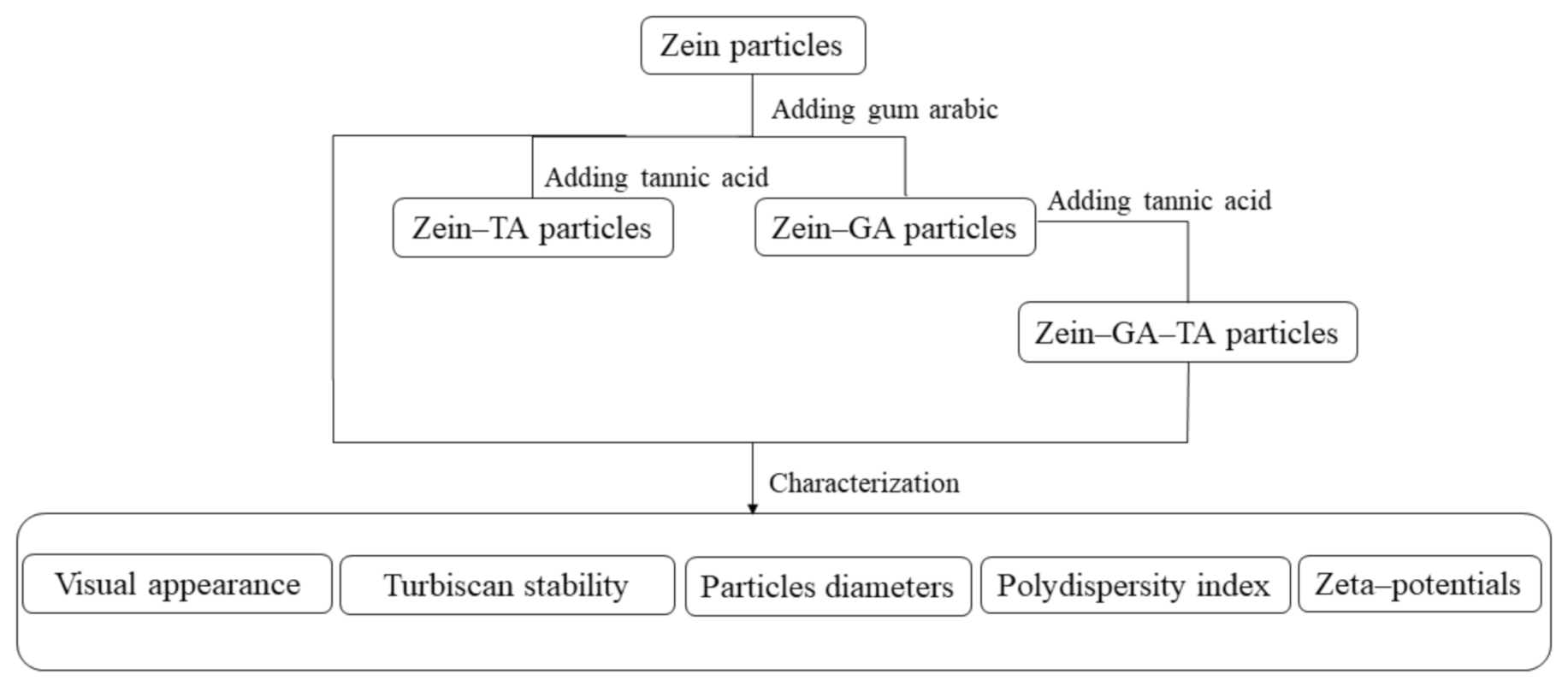
3.2. Preparation of Gel Particles
- (i)
- Zein gel particle dispersion (ZP). Zein powder (5 g) was added to 80% (v/v) ethanol/water (100 mL) with continuous stirring at room temperature (~25 °C) for 1 h. The pH of the solution was adjusted to 9.0 with 0.1 mol/L NaOH solution. The zein aqueous ethanol solution (20 mL, the dropping rate is 10 mL/min) was added to ultrapure water (100 mL) to prepare a zein gel particle dispersion. The dispersion was stirred at 600 rpm for 1 h and the pH was kept at 9.0 with 0.1 mol/L NaOH solution.
- (ii)
- GA dispersion. GA powder (10 g) was added to ultrapure water (240 mL, 50 °C) and stirred at 600 rpm for 1 h to prepare a GA dispersion. The pH was maintained at 9.0 with 0.1 mol/L NaOH solution and the dispersion was cooled to room temperature for further use.
- (iii)
- Zein–GA gel particle dispersion. Zein powder (5 g) was added to 80% (v/v) ethanol/water (100 mL) with continuous stirring at room temperature (~25 °C) for 1 h. The pH of the solution was adjusted to 9.0 with 0.1 mol/L NaOH solution. The zein aqueous ethanol solution (20 mL) was added dropwise to ultrapure water (100 mL) to prepare a zein gel particle dispersion. The dispersion was stirred at 600 rpm for 1 h and the pH was kept at 9.0 with 0.1 mol/L NaOH solution. GA powder (10 g) was added to ultrapure water (240 mL, 50 °C) and stirred at 600 rpm for 1 h to prepare a GA dispersion. The pH was maintained at 9.0 with 0.1 mol/L NaOH solution and the dispersion was cooled to room temperature for later use. The GA dispersion was then added dropwise into the zein gel particle dispersion at a zein:GA mass ratio of 1:2 with magnetic stirring at 600 rpm for 1 h to obtain the zein–GA complex gel particle dispersion.
- (iv)
- Zein–TA gel particle dispersion. Zein powder (5 g) was added to 80% (v/v) ethanol/water (100 mL) with continuous stirring at room temperature (~25 °C) for 1 h. The pH of the solution was adjusted to 9.0 with 0.1 mol/L NaOH solution. The zein aqueous ethanol solution (20 mL) was added dropwise to ultrapure water (100 mL) to prepare a zein gel particle dispersion. The dispersion was stirred at 600 rpm for 1 h and the pH was kept at 9.0 with 0.1 mol/L NaOH solution. Tannic acid solution (5 mL, 0.04 g/mL) was then added to the zein gel particle dispersion (120 mL) while stirring (600 rpm) at room temperature for 30 min.
- (v)
- GA+TA dispersion. GA powder (10 g) was added to ultrapure water (240 mL, 50 °C) and stirred at 600 rpm for 1 h to prepare a GA dispersion. The pH was maintained at 9.0 with 0.1 mol/L NaOH solution and the dispersion was cooled to room temperature for later use. Tannic acid solution (5 mL, 0.04 g/mL) was added to the GA dispersion (48 mL) at a GA:TA mass ratio of 10:1 and stirred (600 rpm) at room temperature for 30 min.
3.3. Characterization of Gel Particle Stability to Salt Ions
3.3.1. Visual Appearance
3.3.2. Turbiscan Stability
3.3.3. Gel Particle Size, Polydispersity Index, Zeta–Potential and Morphology
3.3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liu, Z.; Cao, X.; Ren, S.; Wang, J.; Zhang, H. Physicochemical characterization of a zein prepared using a novel aqueous extraction technology and tensile properties of the zein film. Ind. Crop. Prod. 2019, 130, 57–62. [Google Scholar] [CrossRef]
- Yu, X.; Afreen, S.; Kong, Q.; Wang, J. Study on Self-Assembled Morphology and Structure Regulation of α-Zein in Ethanol–Water Mixtures. Langmuir 2020, 36, 11975. [Google Scholar] [CrossRef]
- Li, Q.; Gao, R.; Wang, L.; Xu, M.; Yuan, Y.; Ma, L.; Wan, Z.; Yang, X. Nanocomposites of Bacterial Cellulose Nanofibrils and Zein Nanoparticles for Food Packaging. ACS Appl. Nano Mater. 2020, 3, 2899–2910. [Google Scholar] [CrossRef]
- Yu, Y.-B.; Wu, M.-Y.; Wang, C.; Wang, Z.-W.; Chen, T.-T.; Yan, J.-K. Constructing biocompatible carboxylic curdlan-coated zein nanoparticles for curcumin encapsulation. Food Hydrocoll. 2020, 108, 106028. [Google Scholar] [CrossRef]
- Mahalakshmi, L.; Leena, M.M.; Moses, J.A.; Anandharamakrishnan, C. Micro- and nano-encapsulation of β-carotene in zein protein: Size-dependent release and absorption behavior. Food Funct. 2020, 11, 1647–1660. [Google Scholar] [CrossRef]
- Pauluk, D.; Padilha, A.K.; Khalil, N.M.; Mainardes, R.M. Chitosan-coated zein nanoparticles for oral delivery of resveratrol: Formation, characterization, stability, mucoadhesive properties and antioxidant activity. Food Hydrocoll. 2019, 94, 411–417. [Google Scholar] [CrossRef]
- Zhang, F.; Khan, M.A.; Cheng, H.; Liang, L. Co-encapsulation of α-tocopherol and resveratrol within zein nanoparticles: Impact on antioxidant activity and stability. J. Food Eng. 2019, 247, 9–18. [Google Scholar] [CrossRef]
- Chen, X.-W.; Fu, S.-Y.; Hou, J.-J.; Guo, J.; Wang, J.-M.; Yang, X.-Q. Zein based oil-in-glycerol emulgels enriched with β-carotene as margarine alternatives. Food Chem. 2016, 211, 836–844. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Chen, Z.; Wang, T.; Wang, L.; Zhong, Q. Biological macromolecule delivery system fabricated using zein and gum arabic to control the release rate of encapsulated tocopherol during in vitro digestion. Food Res. Int. 2018, 114, 251–257. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, C.; Yuan, J.; Wu, Y.; Li, F.; Li, D.; Huang, Q. Effects of pectin polydispersity on zein/pectin composite nanoparticles (ZAPs) as high internal-phase Pickering emulsion stabilizers. Carbohydr. Polym. 2019, 219, 77–86. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Wang, X.; Zhou, Y.; Gao, X.; Puligundla, P.; Wan, X. Encapsulation of epigallocatechin gallate in zein/chitosan nanoparticles for controlled applications in food systems. Food Chem. 2017, 231, 19–24. [Google Scholar] [CrossRef]
- Sun, C.; Dai, L.; Gao, Y. Interaction and formation mechanism of binary complex between zein and propylene glycol alginate. Carbohydr. Polym. 2017, 157, 1638–1649. [Google Scholar] [CrossRef]
- Dai, L.; Zhou, H.; Wei, Y.; Gao, Y.; McClements, D.J. Curcumin encapsulation in zein-rhamnolipid composite nanoparticles using a pH-driven method. Food Hydrocoll. 2019, 93, 342–350. [Google Scholar] [CrossRef]
- Dai, L.; Sun, C.; Wei, Y.; Mao, L.; Gao, Y. Characterization of Pickering emulsion gels stabilized by zein/gum arabic complex colloidal nanoparticles. Food Hydrocoll. 2018, 74, 239–248. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, Y.; Hu, Y.; Han, Y.; Xu, J.; Zhao, Y.; Chen, X.; Li, B. Tuning the molecular interactions between gliadin and tannic acid to prepare Pickering stabilizers with improved emulsifying properties. Food Hydrocoll. 2021, 111, 106179. [Google Scholar] [CrossRef]
- Patel, A.R.; Bouwens, E.C.M.; Velikov, K.P. Sodium Caseinate Stabilized Zein Colloidal Particles. J. Agric. Food Chem. 2010, 58, 12497–12503. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Huang, X.; Gao, Y.; Huang, X.; Xiao, H.; McClements, D.J. Core–shell biopolymer nanoparticle delivery systems: Synthesis and characterization of curcumin fortified zein–pectin nanoparticles. Food Chem. 2015, 182, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Aserin, A.; Ben Ishai, P.; Garti, N. Interactions between whey protein isolate and gum Arabic. Colloids Surf. B Biointerfaces 2010, 79, 377–383. [Google Scholar] [CrossRef]
- Chen, H.; Zhong, Q. A novel method of preparing stable zein nanoparticle dispersions for encapsulation of peppermint oil. Food Hydrocoll. 2015, 43, 593–602. [Google Scholar] [CrossRef]
- Zou, Y.; Guo, J.; Yin, S.-W.; Wang, J.-M.; Yang, X.-Q. Pickering Emulsion Gels Prepared by Hydrogen-Bonded Zein/Tannic Acid Complex Colloidal Particles. J. Agric. Food Chem. 2015, 63, 7405–7414. [Google Scholar] [CrossRef]
- Siebert, K.J.; Troukhanova, A.N.V.; Lynn, P.Y. Nature of Polyphenol−Protein Interactions. J. Agric. Food Chem. 1996, 44, 80–85. [Google Scholar] [CrossRef]
- Van Buren, J.P.; Robinson, W.B. Formation of complexes between protein and tannic acid. J. Agric. Food Chem. 1969, 17, 772–777. [Google Scholar] [CrossRef]
- Chang, C.; Wang, T.; Hu, Q.; Luo, Y. Zein/caseinate/pectin complex nanoparticles: Formation and characterization. Int. J. Biol. Macromol. 2017, 104, 117–124. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Z.; Xia, G.; Xue, F.; Chen, C.; Zhang, Y. Fabrication and characterization of zein/lactoferrin composite nanoparticles for encapsulating 7,8-dihydroxyflavone: Enhancement of stability, water solubility and bioaccessibility. Int. J. Biol. Macromol. 2020, 146, 179–192. [Google Scholar] [CrossRef]
- Santos, J.; Alcaide-González, M.; Trujillo-Cayado, L.; Carrillo, F.; Alfaro-Rodríguez, M. Development of food-grade Pickering emulsions stabilized by a biological macromolecule (xanthan gum) and zein. Int. J. Biol. Macromol. 2020, 153, 747–754. [Google Scholar] [CrossRef]
- Zou, L.; Zhang, Z.; Zhang, R.; Liu, W.; Liu, C.; Xiao, H.; McClements, D.J. Encapsulation of protein nanoparticles within alginate microparticles: Impact of pH and ionic strength on functional performance. J. Food Eng. 2016, 178, 81–89. [Google Scholar] [CrossRef]
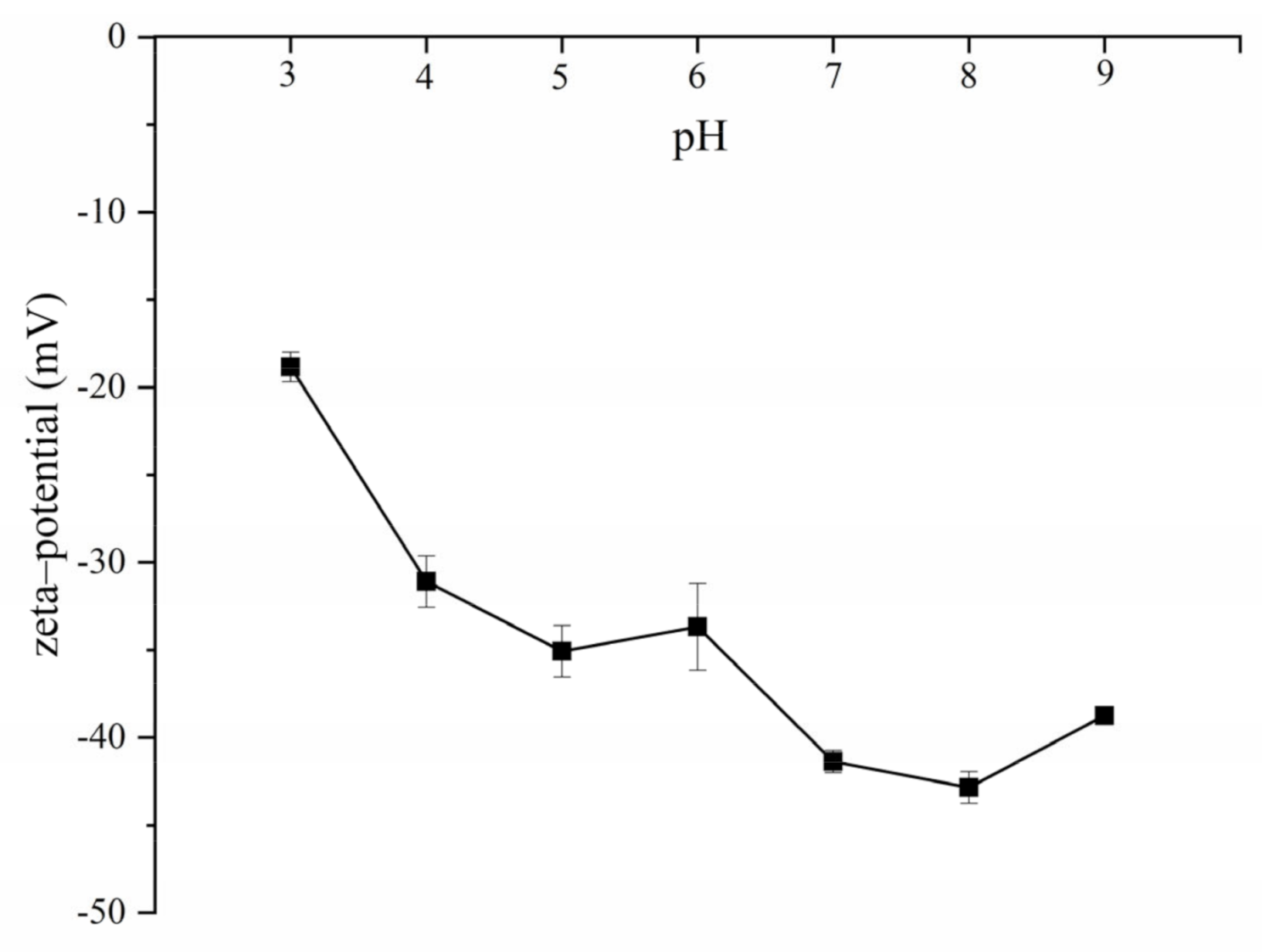
| Sample | Sodium Chloride (mol/L) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.25 | 0.5 | 1 | 2 | 3 | |||
| pH 4.0 | Zein–GA–TA | Diameters (nm) | 201.7 ± 9.7 c | 268.1 ± 2.8 a | 237.7 ± 12.9 b | 184.9 ± 6.9 d | 185.9 ± 5.4 d | 191.2 ± 5.4 c,d |
| PDI | 0.17 ± 0.01 b,c | 0.30 ± 0.08 a | 0.20 ± 0.03 b | 0.14 ± 0.01 b,c | 0.12 ± 0.01 c | 0.12 ± 0.01 c | ||
| Zein-GA | * | * | * | * | * | * | ||
| Zein-TA | * | * | * | * | * | * | ||
| ZP | * | * | * | * | * | * | ||
| GA+TA | – | – | – | – | – | – | ||
| GA | – | – | – | – | – | – | ||
| pH 7.0 | Zein–GA–TA | Diameters (nm) | 200.2 ± 13.6 b | 240.3 ± 17.8 a | 247.1 ± 16.6 a | 212.9 ± 10.0 b | 202.6 ± 5.1 b | 191.2 ± 4.8 b |
| PDI | 0.21 ± 0.07 b | 0.16 ± 0.01 b | 0.32 ± 0.08 a | 0.18 ± 0.03 b | 0.18 ± 0.08 b | 0.14 ± 0.00 b | ||
| Zein-GA | * | * | * | * | * | * | ||
| Zein-TA | * | * | * | * | * | * | ||
| ZP | * | * | * | * | * | * | ||
| GA+TA | – | – | – | – | – | – | ||
| GA | – | – | – | – | – | – | ||
| pH 8.5 | Zein–GA–TA | Diameters (nm) | 203.4 ± 9.1 b | 247.8 ± 8.7 a | 239.5 ± 17.3 a | 230.2 ± 4.4 a | 210.0 ± 6.9 b | 196.0 ± 10.5 b |
| PDI | 0.19 ± 0.01 c | 0.31 ± 0.05 a,b | 0.32 ± 0.03 a | 0.25 ± 0.01 a,b,c | 0.20 ± 0.06 c | 0.22 ± 0.09 b,c | ||
| Zein-GA | Diameters (nm) | 122.2 ± 1.2 | * | * | * | * | * | |
| PDI | 0.16 ± 0.01 | * | * | * | * | * | ||
| Zein-TA | Diameters (nm) | 77.9 ± 2.4 | * | * | * | * | * | |
| PDI | 0.22 ± 0.01 | * | * | * | * | * | ||
| ZP | Diameters (nm) | 88.0 ± 4.1 | * | * | * | * | * | |
| PDI | 0.19 ± 0.01 | * | * | * | * | * | ||
| GA+TA | – | – | – | – | – | – | ||
| GA | – | – | – | – | – | – | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Huang, J.; Ren, F.; Li, Y.; Tong, Y.; Wen, P.; Wang, P. A Novel Method for Stabilizing Zein Gel Particles to Salt Ion-Induced Aggregation. Molecules 2021, 26, 1458. https://doi.org/10.3390/molecules26051458
Zhang Y, Huang J, Ren F, Li Y, Tong Y, Wen P, Wang P. A Novel Method for Stabilizing Zein Gel Particles to Salt Ion-Induced Aggregation. Molecules. 2021; 26(5):1458. https://doi.org/10.3390/molecules26051458
Chicago/Turabian StyleZhang, Yiquan, Jiaqiang Huang, Fazheng Ren, Yi Li, Yi Tong, Pengcheng Wen, and Pengjie Wang. 2021. "A Novel Method for Stabilizing Zein Gel Particles to Salt Ion-Induced Aggregation" Molecules 26, no. 5: 1458. https://doi.org/10.3390/molecules26051458
APA StyleZhang, Y., Huang, J., Ren, F., Li, Y., Tong, Y., Wen, P., & Wang, P. (2021). A Novel Method for Stabilizing Zein Gel Particles to Salt Ion-Induced Aggregation. Molecules, 26(5), 1458. https://doi.org/10.3390/molecules26051458