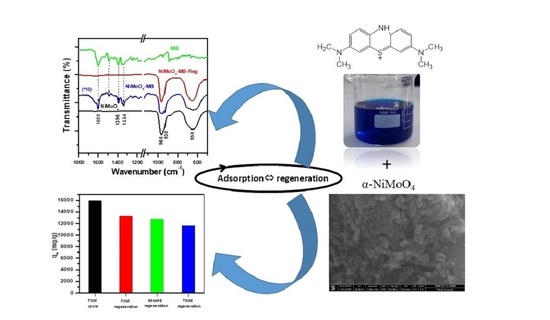
Highly Efficient Methylene Blue Dye Removal by Nickel Molybdate Nanosorbent
Abstract
1. Introduction
2. Results and Discussion
2.1. Removal of MB
2.1.1. pH Point of Zero Charge (pHpzc)
2.1.2. Effect of pH
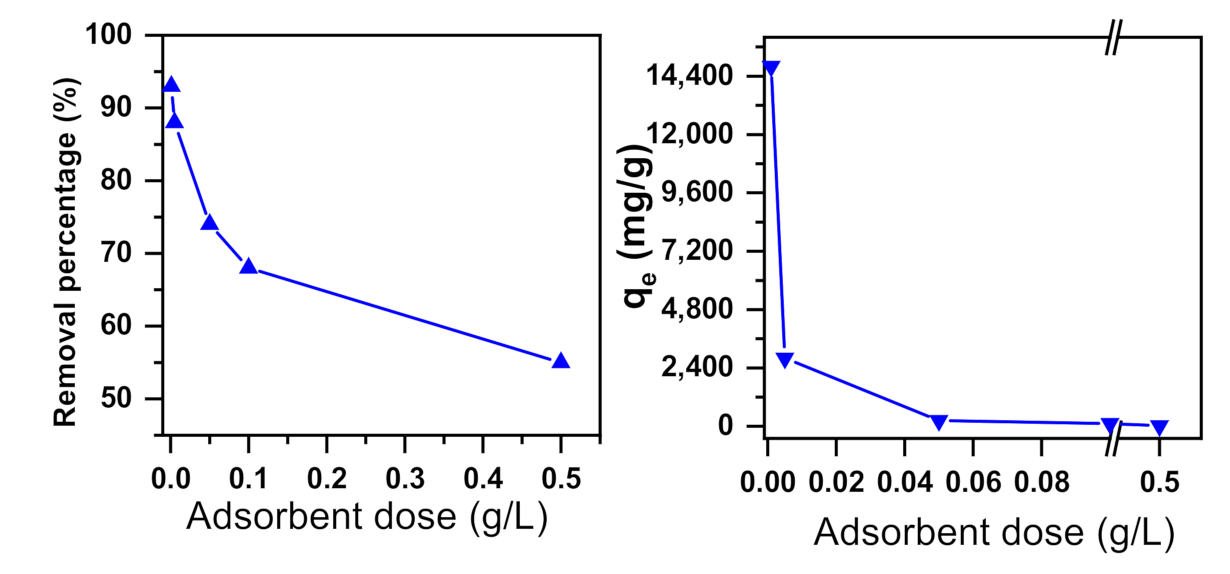
2.1.3. Effect of Adsorbent Dose
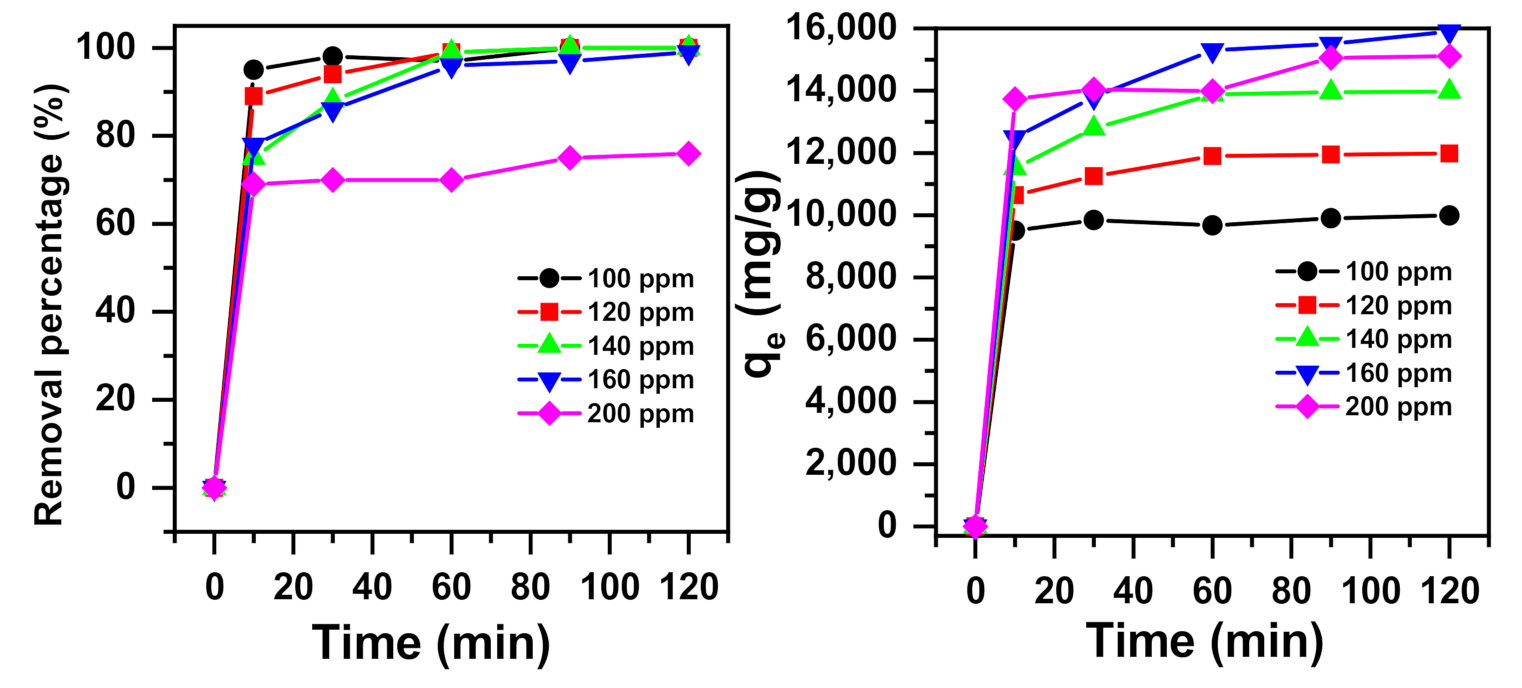
2.1.4. Effects of Initial Concentration and Contact Time
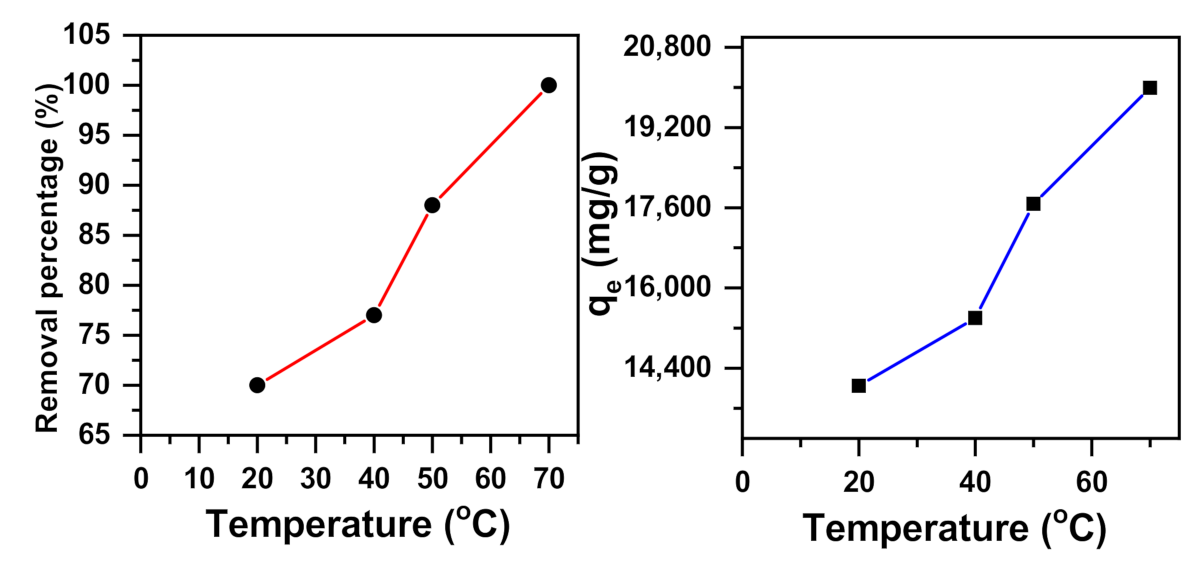
2.1.5. Temperature Effect
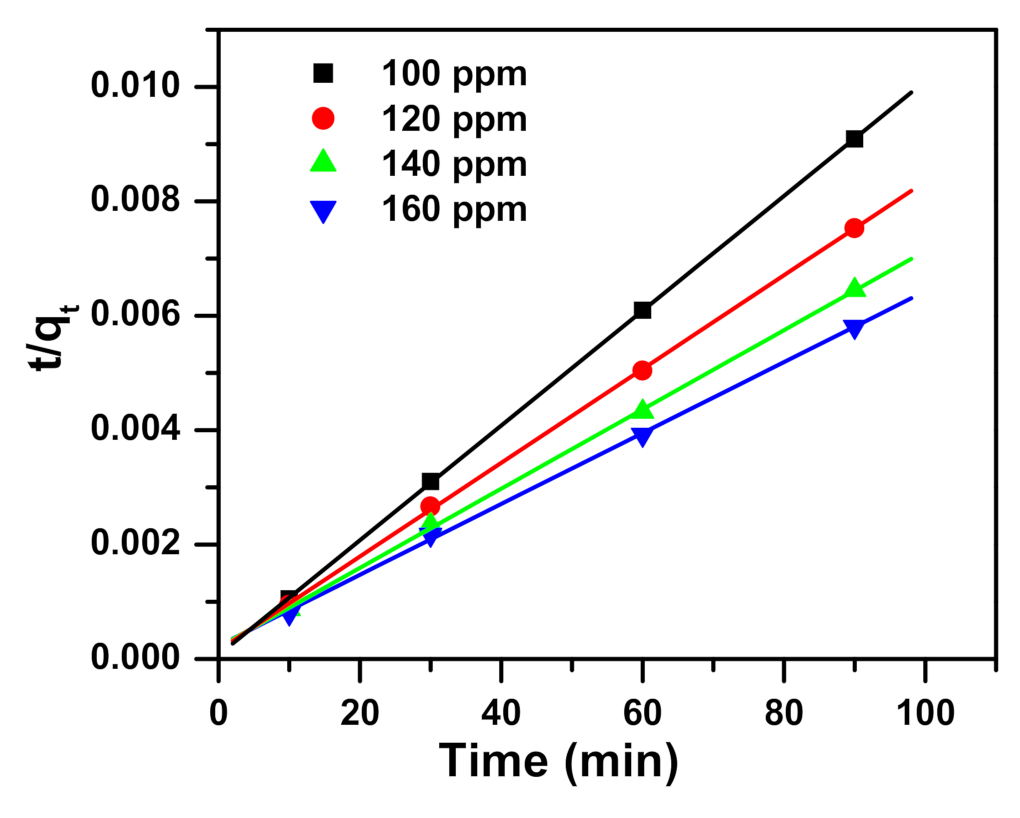
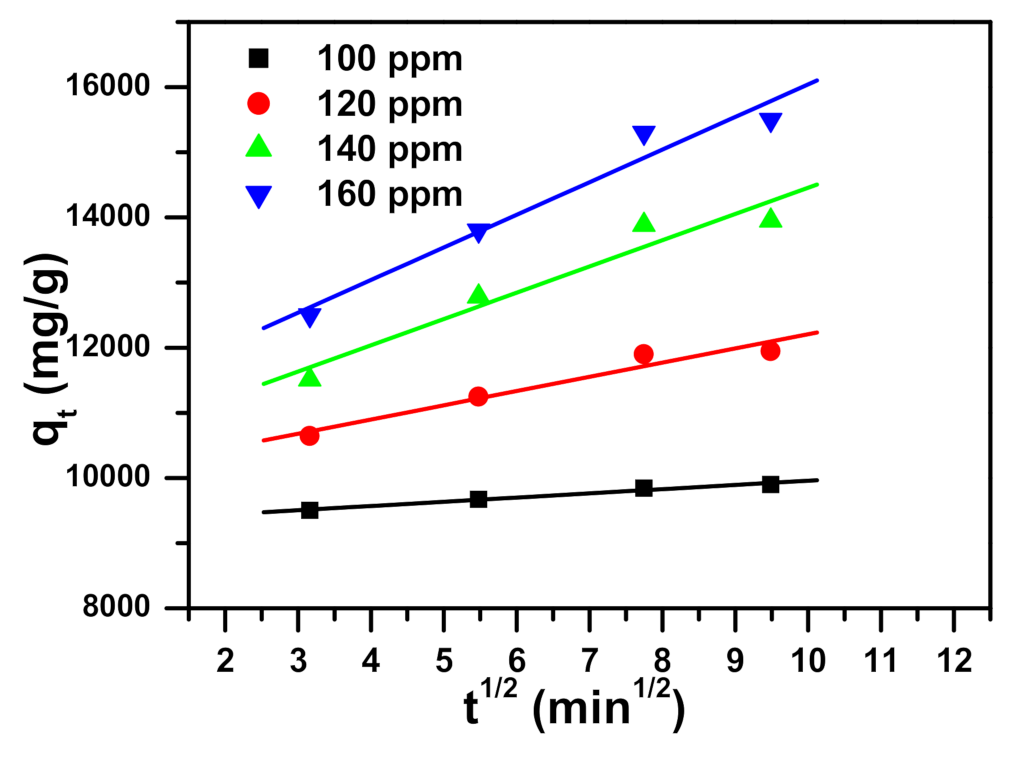
2.2. Kinetic Study
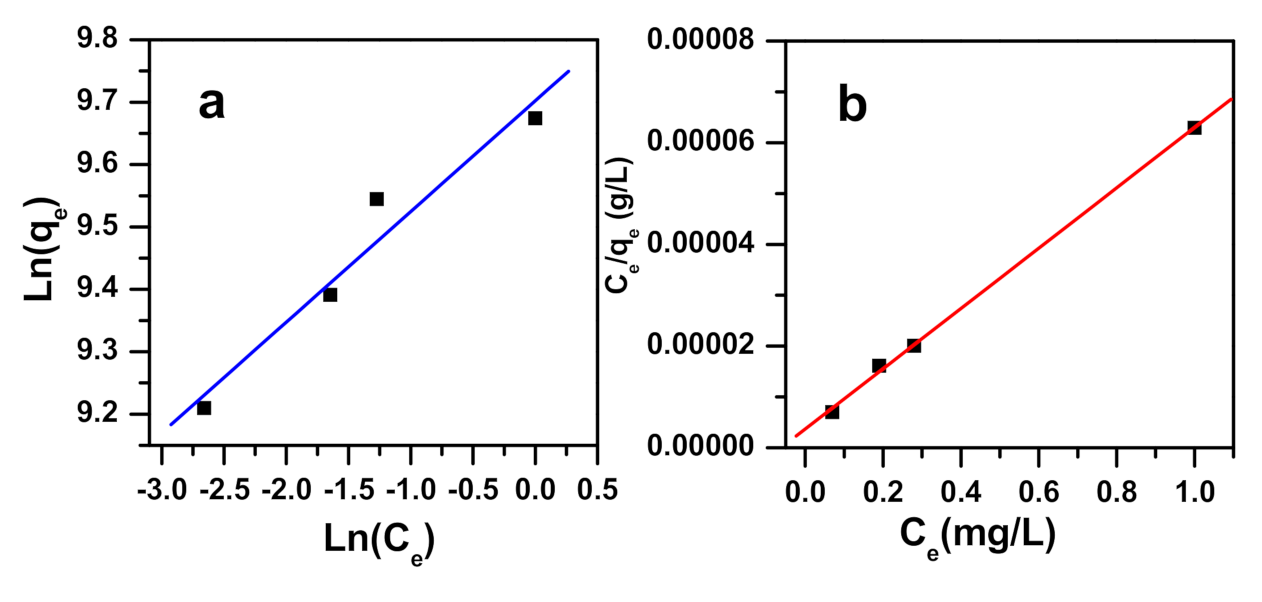
2.3. Adsorption Isotherms
2.4. Regeneration and Characterization of the α-NiMoO4 Nanosorbent
2.4.1. Regeneration Performance
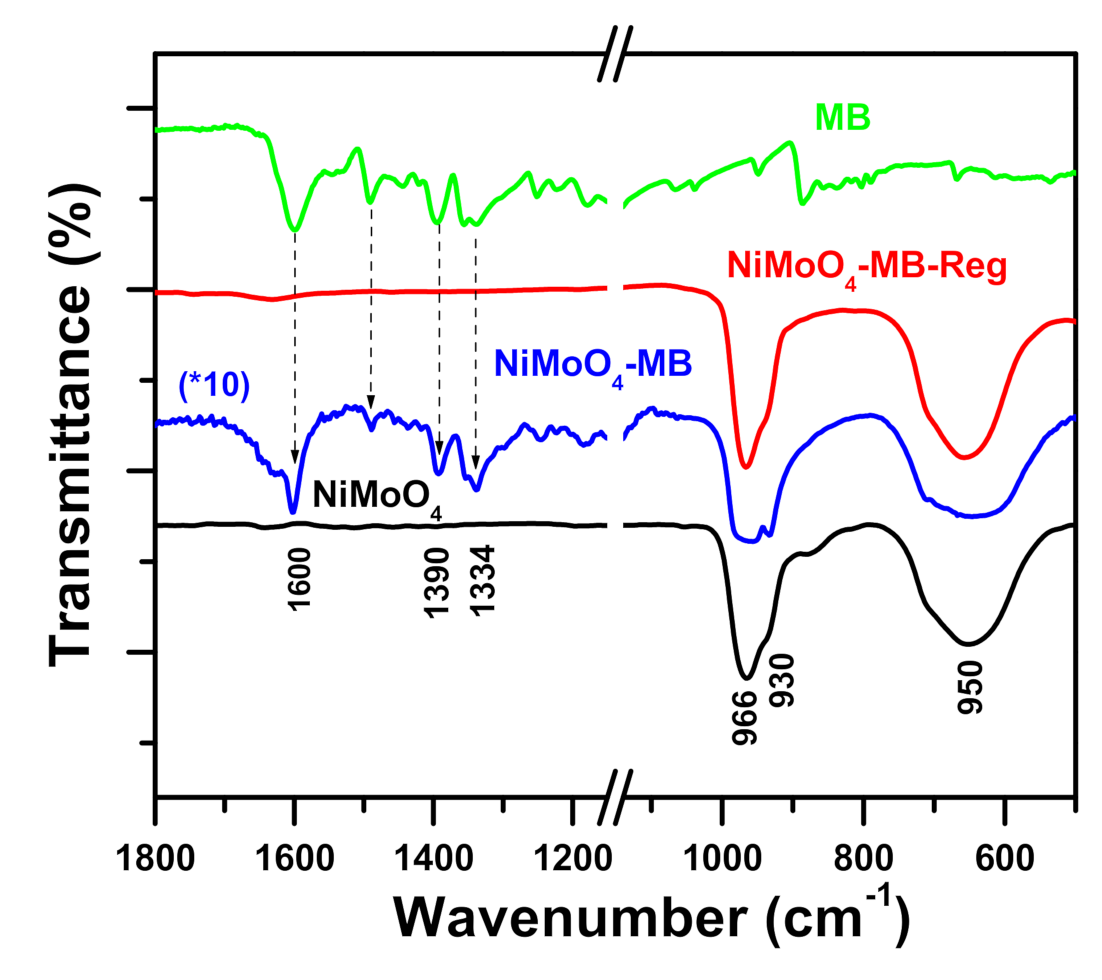
2.4.2. Fourier Transform Infrared Spectroscopy
2.5. Removal Mechanism of MB
3. Materials and Methods
3.1. Nickel Molybdate Nanosorbent Preparation
3.2. Adsorption Experiments
3.3. Adsorbent Regeneration Method
3.4. pH Point of Zero Charge (pHpzc) Measure
3.5. Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khin, M.M.; Nair, A.S.; Babu, V.J.; Murugana, R.; Ramakrishna, S. A review onnanomaterials for environmental remediation. Energy Environ. Sci. 2012, 5, 8075–8109. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Luque, R. Heterogeneous photocatalytic nanomaterials: Prospects and challenges in selective transformations of biomass-derived compounds. Chem. Soc. Rev. 2014, 43, 765–778. [Google Scholar] [CrossRef]
- Madrakian, T.; Afkhami, A.; Ahmadi, M.; Bagheri, H. Removal of some cationic dyes from aqueous solutions using magnetic modified multi-walled carbon nanotubes. J. Hazard. Mater. 2011, 196, 109–114. [Google Scholar] [CrossRef]
- Yang, N.; Zhu, S.; Zhang, D.; Xu, S. Synthesis and properties of magnetic Fe3O4-activated carbon nanocomposite particles for dye removal. Mater. Lett. 2008, 62, 645–647. [Google Scholar] [CrossRef]
- Sucharita, A. Textile Dyes: Its Impact on Environment and its Treatment. J. Bioremediat. Biodegrad. 2014, 5, 1. [Google Scholar]
- Solis, M.; Solis, A.; Perez, H.I.; Manjarrez, N.; Flores, M. Microbial decolouration of azo dyes: A review. Process Biochem. 2012, 47, 1723–1748. [Google Scholar] [CrossRef]
- Elemen, S.; Kumbasar, E.P.A.; Yapar, S. Modeling the adsorption of textile dye on organoclay using an artificial neural network. Dye. Pigment. 2012, 95, 102–111. [Google Scholar] [CrossRef]
- Greluk, M.; Hubicki, Z. Effect of basicity of anion exchangers and number and positions of sulfonic groups of acid dyes on dyes adsorption on macroporous anion exchangers with styrenic polymer matrix. Chem. Eng. J. 2013, 215–216, 731–739. [Google Scholar] [CrossRef]
- Turgay, O.; Ersoz, G.; Atalay, S.; Forss, J.; Welander, U. The treatment of azo dyes found in textile industry wastewater by anaerobic biological method and chemical oxidation. Sep. Purif. Technol. 2011, 79, 26–33. [Google Scholar] [CrossRef]
- Verma, A.K.; Dash, R.R.; Bhunia, P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manag. 2012, 93, 154–168. [Google Scholar] [CrossRef]
- Kanagaraj, J.; Senthilvelan, T.; Panda, R.C. Degradation of azo dyes by laccase: Biological method to reduce pollution load in dye wastewater. Clean Technol. Environ. Policy 2015, 17, 1443–1456. [Google Scholar] [CrossRef]
- Cornelia, P.; Oana, P.; Robert, I.; Simona, G.M. Effective removal of methylene blue from aqueous solution using a new magnetic iron oxide nanosorbent prepared by combustion synthesis. Clean Technol. Environ. Policy 2016, 18, 705–715. [Google Scholar]
- Vanhulle, S.; Trovaslet, M.; Enaud, E.; Lucas, M.; Taghavi, S.; van der Lelie, D.; van Aken, B.; Foret, M.; Onderwater, R.C.A.; Wesenberg, D.; et al. Decolorization, cytotoxicity and genotoxicity reduction during a combined ozonation/fungal treatment of dye-contaminated wastewater. Environ. Sci. Technol. 2008, 42, 584–589. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserhati, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef]
- Filice, S.; Angelol, D.D.; Libertinol, S.; Kosma, V.; Nicotera, I.; Privitera, V.; Scalese, S. Graphene oxide and titania hybrid Nation membranes for efficient removal of methyl orange dye from water. Carbon 2015, 82, 489–499. [Google Scholar] [CrossRef]
- Kang, S.; Zhao, Y.; Wang, W.; Zhang, T.; Chen, T.; Yi, H.; Rao, F.; Song, S. Removal of methylene blue from water with montmorillonite nanosheets/chitosan hydrogels as adsorbent. Appl. Surf. Sci. 2018, 448, 203–211. [Google Scholar] [CrossRef]
- Miyah, Y.; Lahrichi, A.; Idrissi, M.; Khalil, A.; Zerrouq, F. Adsorption of methylene blue dye from aqueous solutions onto walnut shells powder: Equilibrium and kinetic studies. Surf. Interface 2018, 11, 74–81. [Google Scholar] [CrossRef]
- Ozdemir, U.; Ozbay, I.; Ozbay, B.; Veli, S. Application of economical models for dye removal from aqueous solutions: Cash flow, cost–benefit and alternative selection methods. Clean Technol. Environ. Policy 2014, 16, 423–429. [Google Scholar] [CrossRef]
- Chen, Y.H. Synthesis, characterization and dye adsorption of ilmenite nanoparticles. J. Non-Cryst. Solids 2011, 357, 136–139. [Google Scholar] [CrossRef]
- Sadhukhan, B.; Mondal, N.K.; Chattoraj, S. Biosorptive removal of cationic dye from aqueous system: A response surface methodological approach. Clean Technol. Environ. Policy 2014, 16, 1015–1025. [Google Scholar] [CrossRef]
- George, Z.; Kyzas, J.F.; Kostas, A.M. The Change from Past to Future for Adsorbent Materials in Treatment of Dyeing Wastewaters. Materials 2013, 6, 5131–5158. [Google Scholar]
- Mouni, L.; Belkhiri, L.; Bollinger, J.C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of methylene blue from aqueous solutions by adsorption on Kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Bentahar, S.; Dbik, A.; El Khomri, M.; El Messaoudi, N.; Lacherai, A. Removal of a cationic dye from aqueous solution by natural clay. Groundw. Sustain. Dev. 2018, 6, 255–262. [Google Scholar] [CrossRef]
- Low, S.K.; Tan, M.C. Dye adsorption characteristic of ultrasound pre-treated pomelo peel. J. Environ. Chem. Eng. 2018, 6, 3502–3509. [Google Scholar] [CrossRef]
- Rakass, S.; Mohmoud, A.; Oudghiri-Hassani, H.; Abboudi, M.; Kooli, F.; Al Wadaani, F. Modified Nigella Sativa Seeds as a Novel Efficient Natural Adsorbent for Removal of Methylene Blue Dye. Molecules 2018, 23, 1950. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Tavallali, H.; Sharifi, M.; NasiriKokhdan, S.; Asghari, A. Preparation of low cost activated carbon from Myrtuscommunis and pomegranate and their efficient application for removal of Congo red from aqueous solution. Spectrochim. Acta Part A 2012, 86, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, F.; Ghaedi, M.; Kamali, K.; Sharifpour, E.; Sahraie, R.; Purkait, M.K. Comparison of nickel and/or zinc selenide nanoparticle loaded on activated carbon as efficient adsorbents for kinetic and equilibrium study of removal of Arsenazo (ΙΙΙ) dye. Powder Technol. 2013, 245, 217–226. [Google Scholar] [CrossRef]
- Singh, K.P.; Gupta, S.; Singh, A.K.; Sinha, S. Optimizing adsorption of crystal violet dye from water by magnetic nanocomposite using response surface modeling approach. J. Hazard. Mater. 2011, 186, 1462–1473. [Google Scholar] [CrossRef]
- Yufei, Z.; Laiquan, L.; Haiquan, S.; Wei, H.; Xiaochen, D. Binary metal oxide: Advanced energy storage materials in supercapacitors. J. Mater. Chem. A 2015, 3, 43–59. [Google Scholar]
- Oudghiri-Hassani, H.; Rakass, S.; Abboudi, M.; Mohmoud, A.; Al Wadaani, F. Preparation and Characterization of α-Zinc Molybdate Catalyst: Efficient Sorbent for Methylene Blue and Reduction of 3-Nitrophenol. Molecules 2018, 23, 1462. [Google Scholar] [CrossRef]
- Oudghiri-Hassani, H. Synthesis, characterization and catalytic performance of iron molybdate Fe2 (MoO4)3 nanoparticles. Catal. Commun. 2015, 60, 19–22. [Google Scholar] [CrossRef]
- Oudghiri-Hassani, H.; Al Wadaani, F.T. Preparation, Characterization and Catalytic Activity of Nickel Molybdate (NiMoO4) Nanoparticles. Molecules 2018, 23, 273. [Google Scholar] [CrossRef]
- Al-Wadaani, F.; Omer, A.; Abboudi, M.; Oudghiri-Hassani, H.; Rakass, S.; Messali, M.; Benaissa, M. High Catalytic Efficiency of Nanostructured β-CoMoO4 in the Reduction of the Ortho-, Meta- and Para-Nitrophenol Isomers. Molecules 2018, 23, 364. [Google Scholar] [CrossRef] [PubMed]
- Madeley, R.A.; Wanke, S. Variation of the dispersion of active phases in commercial nickel—molybdenum/γ-alumina hydrotreating catalysts during oxidative regeneration. Appl. Catal. 1988, 39, 295–314. [Google Scholar] [CrossRef]
- Gates, B.C.; Katzer, J.R.; Schuit, G.C.A. Chemistry of Catalytic Processes; McGraw-Hill: New York, NY, USA, 1979; p. 390. [Google Scholar]
- Kaddouri, A.; Anouchinsky, R.; Mazzocchia, C.; Madeira, L.M.; Portela, M.F. Oxidative dehydrogenation of ethane on the α and β phases of NiMoO4. Catal. Today 1998, 40, 201–206. [Google Scholar] [CrossRef]
- Pillay, B.; Mathebula, M.R.; Friedrich, H.B. The oxidative dehydrogenation of n-hexane over Ni–Mo–O catalysts. Appl. Catal. A 2009, 361, 57–64. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Chaturvedi, S.; Hanson, J.C.; Brito, J.L. Reaction of H2 and H2S with CoMoO4 and NiMoO4: TPR, XANES, Time-Resolved XRD, and Molecular-Orbital Studies. J. Phys. Chem. 1999, 103, 770–781. [Google Scholar] [CrossRef]
- Sundaram, R.; Nagaraja, K.S. Solid state electrical conductivity and humidity sensing studies on metal molybdate–molybdenum trioxide composites (M = Ni2+, Cu2+ and Pb2+). Sens. Actuators B Chem. 2004, 101, 353–360. [Google Scholar] [CrossRef]
- Mi, Y.; Huang, Z.; Hu, F.; Jiang, J.; Li, Y. Controlled synthesis and growth mechanism of alpha nickel molybatemicrohombohedron. Mater. Lett. 2010, 64, 695–697. [Google Scholar] [CrossRef]
- Brito, J.L.; Barbosa, A.L.; Albornoz, A.; Severino, F. Nickel molybdate as precursor of HDS catalysts: Effect of phase composition. Catal. Lett. 1994, 26, 329–337. [Google Scholar] [CrossRef]
- Ryu, J.H.; Koo, S.M.; Yoon, J.W.; Lim, C.S.; Shim, K.B. Synthesis of nanocrystalline MMoO4 (M = Ni, Zn) phosphors via a citrate complex route assisted by microwave irradiation and their photoluminescence. Mater. Lett. 2006, 60, 1702–1705. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, F.; Ma, C.; Yang, Z.; Zhu, C.; Ouyang, Q.; Gao, P.; Li, J.; Sun, C. In situ diffusion growth of Fe2 (MoO4)3 nanocrystalson the surface of α-MoO3 nanorods with significantly enhanced ethanol sensing properties. J. Mater. Chem. 2012, 22, 12900–12906. [Google Scholar] [CrossRef]
- Senthilkumar, B.; VijayaSankar, K.; Selvan, R.K.; Danielle, M.; Manickam, M. Nano α-NiMoO4 as a new electrode for electrochemical supercapacitors. RSC Adv. 2013, 3, 352–357. [Google Scholar] [CrossRef]
- Liu, M.; Kong, L.; Lu, C.; Li, X.; Luo, Y.; Kang, L. Waste paper based activated carbon monolith as electrode materials for high performance electric double-layer capacitors. RSC Adv. 2012, 2, 1890–1896. [Google Scholar] [CrossRef]
- Liu, P.; Deng, Y.; Zhang, Q.; Hu, Z.; Xu, Z.; Liu, Y.; Yao, M.; Ai, Z. Facile synthesis and characterization of high-performance NiMoO4·xH2O nanorods electrode material for supercapacitors. Ionics 2015, 21, 2797–2804. [Google Scholar] [CrossRef]
- Cherian, C.T.; Reddy, M.V.; Haur, S.C.; Chowdari, B.V.R. Interconnected Network of CoMoO4 Submicrometer Particles As High Capacity Anode Material for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2013, 5, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yu, S.H.; Liu, C.; Zang, Z.A. 3D Architectures of Iron Molybdate: Phase Selective Synthesis, Growth Mechanism, and Magnetic Properties. Chem. Eur. J. 2007, 13, 746–753. [Google Scholar] [CrossRef]
- Saberyan, K.; Soofivand, F.; Kianpour, G.; Salavati-Niasari, M.; Bagheri, S. Synthesis and characterization of NiMoO4 via ultrasonic route by a novel precursor. J. Mater. Sci. Mater. Electron. 2016, 27, 3765–3772. [Google Scholar] [CrossRef]
- Kianpour, G.; Salavati-Niasari, M.; Emadi, H. Sonochemical synthesis and characterization of NiMoO4nanorods. Ultrason. Sonochem. 2013, 20, 418–424. [Google Scholar] [CrossRef]
- Cai, D.; Wang, D.; Liu, B.; Wang, Y.; Liu, Y.; Wang, L.; Li, H.; Li, H.Q.; Wang, T. Comparison of the Electrochemical Performance of NiMoO4 Nanorods and Hierarchical Nanospheres for Supercapacitor Applications. ACS Appl. Mater. Interfaces 2013, 5, 12905–12910. [Google Scholar] [CrossRef]
- Ray, S.K.; Dipesh, D.; Yuwaraj, K.K.; Soo, W.L. Cu-α-NiMoO4photocatalyst for degradation of Methylene blue with pathways and antibacterial performance. J. Photochem. Photobiol. A 2017, 348, 18–32. [Google Scholar] [CrossRef]
- Kaddouri, A.; Tempesti, E.; Mazzocchia, C. Comparative study of β-nickel molybdate phase obtained by conventional precipitation and the sol-gel method. Mater. Res. Bull. 2004, 39, 695–706. [Google Scholar] [CrossRef]
- Mosleh, M. Facile approach to synthesize nanocristalline NiMoO4 in the presence of amino acids as capping agent. J. Mater. Sci. Mater. Electron. 2017, 28, 6788–6793. [Google Scholar] [CrossRef]
- Klissurski, D.; Mancheva, M.; Iordanova, R.; Kunev, B. Mechanochemical Synthesis of Nanocrystalline Nickel Molybdates. J. Alloys Compd. 2006, 422, 53–57. [Google Scholar] [CrossRef]
- Maione, A.; Devilers, M. Solid Solutions of Ni and Co Molybdates in Silica-Dispersed and Bulk Catalysts Prepared by Sol-Gel and Citrate Methods. J. Solid State Chem. 2004, 177, 2339–2349. [Google Scholar] [CrossRef]
- Saravanakumar, B.; Ramachandran, S.P.; Ravi, G.; Ganesh, V.; Sakunthala, A.; Yuvakkumar, R. Morphology dependent electrochemical capacitor performance of NiMoO4 nanoparticles. Mater. Lett. 2017, 209, 1–4. [Google Scholar] [CrossRef]
- Azadeh, T.; Raheleh, P.; Mina, I.; Samaneh, E.; Mohammad, S. A simplified microwave-assisted synthesis of NiMoO4 nanoparticles by using organic driving agent and study of photocatalytic activity. In Proceedings of the 18th International Electronic Conference on Synthetic Organic Chemistry Session Microwave Assisted Synthesis, 1–30 November 2014. [Google Scholar]
- Edrissi, M.; Samadanian-Isfahani, S.; Soleymani, M. Preparation of cobalt molybdate nanoparticles; Taguchi optimization and photocatalytic oxidation of Reactive Black 8 dye. Powder Technol. 2013, 249, 378–385. [Google Scholar] [CrossRef]
- Ghoreishiana, S.M.; Raju, G.S.R.; Pavitra, E.; Kwak, C.H.; Han, Y.K.; Huh, Y.S. Controlled synthesis of hierarchical α-nickel molybdate with enhanced solar-light-responsive photocatalytic activity: A comprehensive study on the kinetics and effect of operational factors. Ceram. Int. 2019, 45, 12041–12052. [Google Scholar] [CrossRef]
- Ferreira, E.A.C.; Andrade Neto, N.F.; Bomio, M.R.D.; Motta, F.V. Influence of solution pH on forming silver molybdates obtained by sonochemical method and its application for methylene blue degradation. Ceram. Int. 2019, 45, 11448–11456. [Google Scholar] [CrossRef]
- Rashad, M.M.; Ibrahim, A.A.; Rayan, D.A.; Sanad, M.M.S.; Helmy, I.M. Photo-Fenton-like degradation of Rhodamine B dye from waste water using iron molybdate catalyst under visible light irradiation. Environ. Nanotechnol. Monit. Manag. 2017, 8, 175–186. [Google Scholar] [CrossRef]
- Oudghiri-Hassani, H. Synthesis, Characterization and Application of Chromium molybdate for Oxidation of Methylene Blue Dye. J. Mater. Environ. Sci. 2018, 9, 1051–1057. [Google Scholar]
- Kianpour, G.; Soofivand, F.; Badiei, M.; Salavati-Niasari, M.; Hamadanian, M. Facile synthesis and characterization of nickel molybdatenanorods as an effective photocatalyst by co-precipitation method. J. Mater. Sci. Mater. Electron. 2016, 27, 10244–10251. [Google Scholar] [CrossRef]
- Dhanasekar, M.; Ratha, S.; Rout, C.S.; Bhat, S.V. Efficient sono-photocatalytic degradation of methylene blue using nickel molybdate nanosheets under diffused sunlight. J. Environ. Chem. Eng. 2017, 5, 2997–3004. [Google Scholar] [CrossRef]
- Abia, A.A.; Asuquo, E.D. Lead (II) and nickel (II) adsorption kinetics from aqueous metal solutions using chemically modified and unmodified agricultural adsorbents. Afr. J. Biotechnol. 2006, 5, 1475–1482. [Google Scholar]
- Yogesh Kumar, K.; Archana, S.; Vinuth Raj, T.N.; Prasana, B.P.; Raghu, M.S.; Muralidhara, H.B. Superb Adsorption Capacity of Hydrothermally Synthesized Copper oxide and Nickel oxide nanoflakes towards Anionic and Cationic dyes. J. Sci. Adv. Mater. Dev. 2017, 2, 183–191. [Google Scholar] [CrossRef]
- Alver, E.; Metin, A.U. Anionic dye removal from aqueous solutions using modified zeolite: Adsorption kinetics and isotherm studies. Chem. Eng. J. 2012, 200–202, 59–67. [Google Scholar] [CrossRef]
- Jihyun, R.K.; Santiano, B.; Kim, H.; Kan, E. Heterogeneous Oxidation of Methylene Blue with Surface-Modified Iron-Amended Activated Carbon. Am. J. Anal. Chem. 2013, 4, 115–122. [Google Scholar]
- Kooli, F.; Liu, Y.; Abboudi, M.; Oudghiri Hassani, H.; Rakass, S.; Ibrahim, S.M.; Al-Wadaani, F. Waste bricks applied as removal agent of Basic Blue 41 from aqueous solution: Base treatment and their regeneration efficiency. Appl. Sci. 2019, 9, 1237. [Google Scholar] [CrossRef]
- Santhi, T.; Manonmani, S. Adsorption of methylene blue from aqueous solution onto a waste aquacultural shell powders (prawn waste). Sustain. Environ. Res. 2012, 22, 45–51. [Google Scholar]
- Kooli, F.; Liu, Y.; Hbaieb, K.; Ching, O.Y.; Al-Faze, R. Characterization of organo-kenyaites: Thermal stability and their effects on eosin removal characteristics. Clay Miner. 2018, 53, 91–104. [Google Scholar] [CrossRef]
- Alzaydien, A.S. Adsorption Behavior of Methyl Orange onto Wheat Bran: Role of Surface and pH. Orient. J. Chem. 2015, 31, 643–651. [Google Scholar] [CrossRef]
- Garg, V.K.; Gupta, R.; Yadav, A.B.; Kumar, R. Dye removal from aqueous solution by adsorption on treated sawdust. Bioresour. Technol. 2003, 89, 121–124. [Google Scholar] [CrossRef]
- Mahmoud, D.K.; Salleh, M.A.M.; Karim, W.A.W.A.; Idris, A.; Abidin, Z.Z. Batch adsorption of basic dye using acid treated kenaf fibre char: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2012, 181–182, 449–457. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M.; Hubicki, Z. Removal of tartrazine from aqueous solutions by strongly basic polystyrene anion exchange resins. J. Hazard. Mater. 2009, 164, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, T.; Smith, J.M. Intraparticle mass transport in slurries by dynamic adsorption studies. AIChE J. 1974, 20, 88–93. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Ma, J.; Jia, Y.; Jing, Y.; Yao, Y.; Sun, J. Kinetics and thermodynamics of methylene blue adsorption by cobalt-hectorite composite. Dye. Pigment. 2012, 93, 1441–1446. [Google Scholar] [CrossRef]
- Chenglong, X.; Yan, J.; Yongzhong, J.; Duyuan, Y.; Jun, M.; Xiaojie, Y. Adsorption properties of congo red from aqueous solution on modified hectorite: Kinetic and thermodynamic studies. Desalination 2011, 265, 81–87. [Google Scholar]
- Vadivelan, V.; Kumar, K.V. Equilibrium, kinetics, mechanism and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef]
- Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y.; Indraswati, N.; Ismadji, S. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies. J. Hazard. Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Shahwan, T.; Erten, H.N. Temperature effects in barium sorption on natural kaolinite and chlorite-illite clays. J. Radioanal. Nucl. Chem. 2004, 260, 43–48. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Dada, A.O.; Olalekan, A.P.; Olatunya, A.M.; Dada, O. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich Isotherms Studies of Equilibrium Sorption of Zn2+ Unto Phosphoric Acid Modified Rice Husk. J. Appl. Chem. 2012, 3, 38–45. [Google Scholar]
- Mohmoud, A.; Rakass, S.; Oudghiri-Hassani, H.; Kooli, F.; Abboudi, M.; Ben Aouan, S. Iron Molybdate Fe2 (MoO4)3 Nanoparticles: Efficient Sorbent for Methylene Blue Dye Removal from Aqueous Solutions. Molecules 2020, 25, 5100. [Google Scholar] [CrossRef]
- Rakass, S.; Oudghiri-Hassani, H.; Abboudi, M.; Kooli, F.; Mohmoud, A.; Aljuhani, A.; Al Wadaani, F. Molybdenum Trioxide: Efficient Nanosorbent for Removal of Methylene Blue Dye from Aqueous Solutions. Molecules 2018, 23, 2295. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Cai, X.; Tan, S.; Li, H.; Liu, J.; Yang, W. Adsorption characteristics of acrylonitrile, p-toluenesulfonic acid, 1-naphthalenesulfonic acid and methyl blue on graphene in aqueous solutions. Chem. Eng. J. 2011, 173, 144–149. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, Y.; Luo, C.; Lu, F.; Qiu, H.; Sun, M. Synthesis and characterization of magnetic beta-cyclodextrin-chitosan nanoparticles as nano-adsorbents for removal of methyl blue. Int. J. Biol. Macromol. 2012, 50, 444–450. [Google Scholar] [CrossRef]
- Zhang, F.; Lan, J.; Yang, Y.; Wei, T.; Tan, R.; Song, W. Adsorption behavior and mechanism of methyl blue on zinc oxide nanoparticles. J. Nanopart. Res. 2013, 15, 2034–2043. [Google Scholar] [CrossRef]
- Li, L.H.; Xiao, J.; Liu, P.; Yang, G.W. Super adsorption capability from amorphousization of metal oxide nanoparticles for dye removal. Sci. Rep. 2015, 5, 9028. [Google Scholar] [CrossRef]
- Maisa El Gamal, M.; Mousa, H.A.; El-Naas, M.H.; Zacharia, R.; Judd, S. Bio-regeneration of activated carbon: A comprehensive review. Sep. Purif. Technol. 2018, 197, 345–359. [Google Scholar] [CrossRef]
- Yanlong Sun, Y.; Zhang, B.; Zheng, T.; Wang, P. Regeneration of activated carbon saturated with chloramphenicol by microwave and ultraviolet irradiation. Chem. Eng. J. 2017, 320, 264–270. [Google Scholar]
- Erfan Sadatshojaei, E.; Esmaeilzadeh, F.; Fathikaljahi, J.; Barzi, S.E.H.S.; Wood, D.A. Regeneration of the Midrex Reformer Catalysts Using Supercritical Carbon Dioxide. Chem. Eng. J. 2018, 343, 748–758. [Google Scholar] [CrossRef]
- Umapathy, V.; Neeraja, P.; Manikandan, A.; Ramu, P. Synthesis of NiMoO4 nanoparticles by sol–gel method and their structural, morphological, optical, magnetic and photocatlytic properties. Trans. Nonferrous Met. Soc. China 2017, 27, 1785–1793. [Google Scholar] [CrossRef]
- Ahmed, F.; Dewani, R.; Pervez, M.K.; Mahboob, S.J.; Soomro, S.A. Non-destructive FT-IR analysis of mono azo dyes. Bulg. Chem. Commun. 2016, 48, 71–77. [Google Scholar]
- Etman, A.S.; Abdelhamid, H.N.; Yuan, Y.; Wang, L.; Zou, X.; Sun, J. Facile Water-Based Strategy for Synthesizing MoO3–x Nanosheets: Efficient Visible Light Photocatalysts for Dye Degradation. ACS Omega 2018, 3, 2201–2209. [Google Scholar] [CrossRef]
- Aracena, A.; Sannino, A.; Jerez, O. Dissolution kinetics of molybdite in KOH media at different temperatures. Trans. Nonferrous Met. Soc. China 2018, 28, 177–185. [Google Scholar] [CrossRef]
- Altenor, S.; Carene, B.; Emmanuel, E.; Lambert, J.; Ehrhardt, J.J.; Gaspard, S. Adsorption studies of methylene blue and phenol onto vetiver roots activated carbon prepared by chemical activation. J. Hazard. Mater. 2009, 165, 1029–1039. [Google Scholar] [CrossRef] [PubMed]





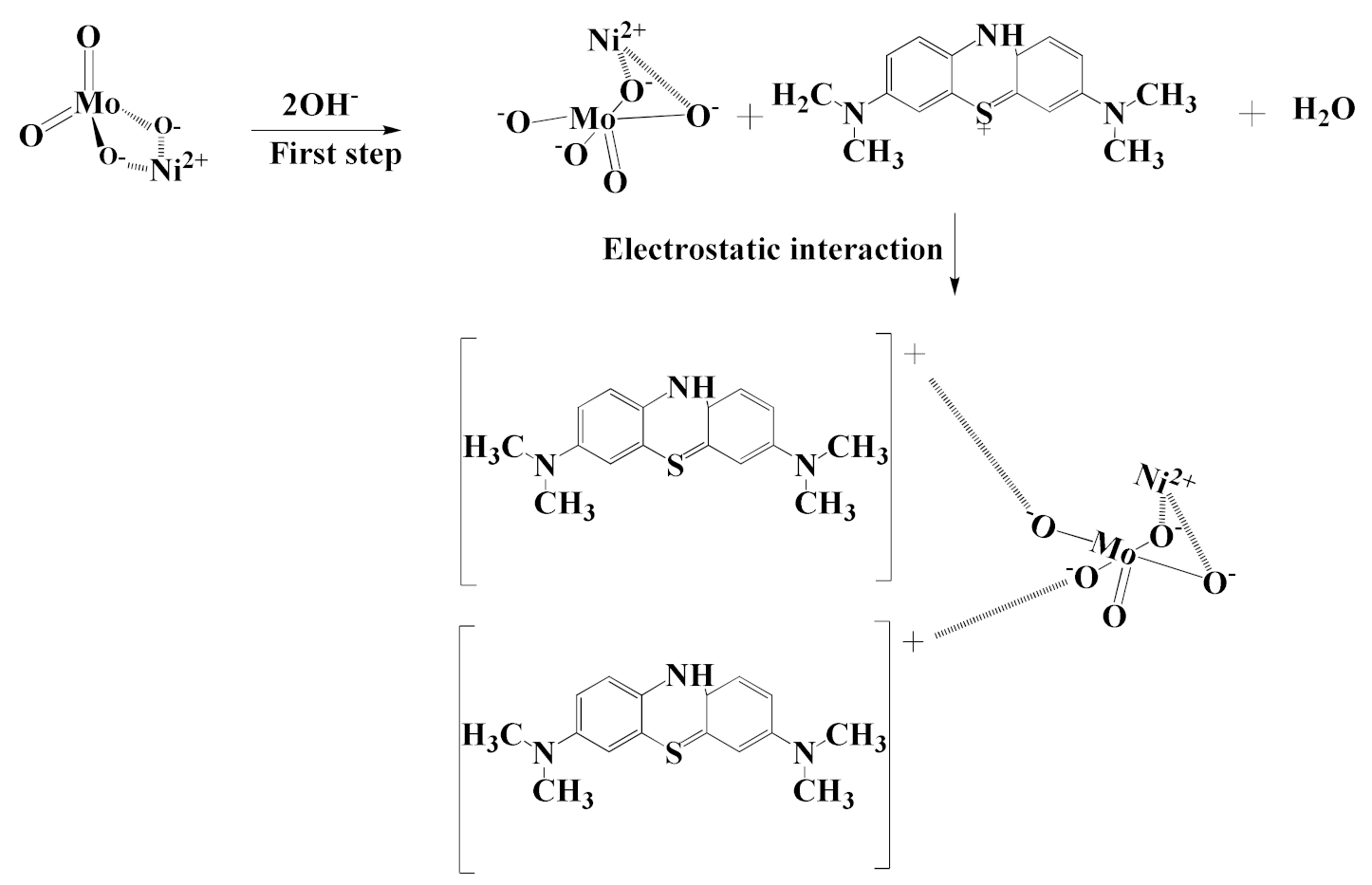
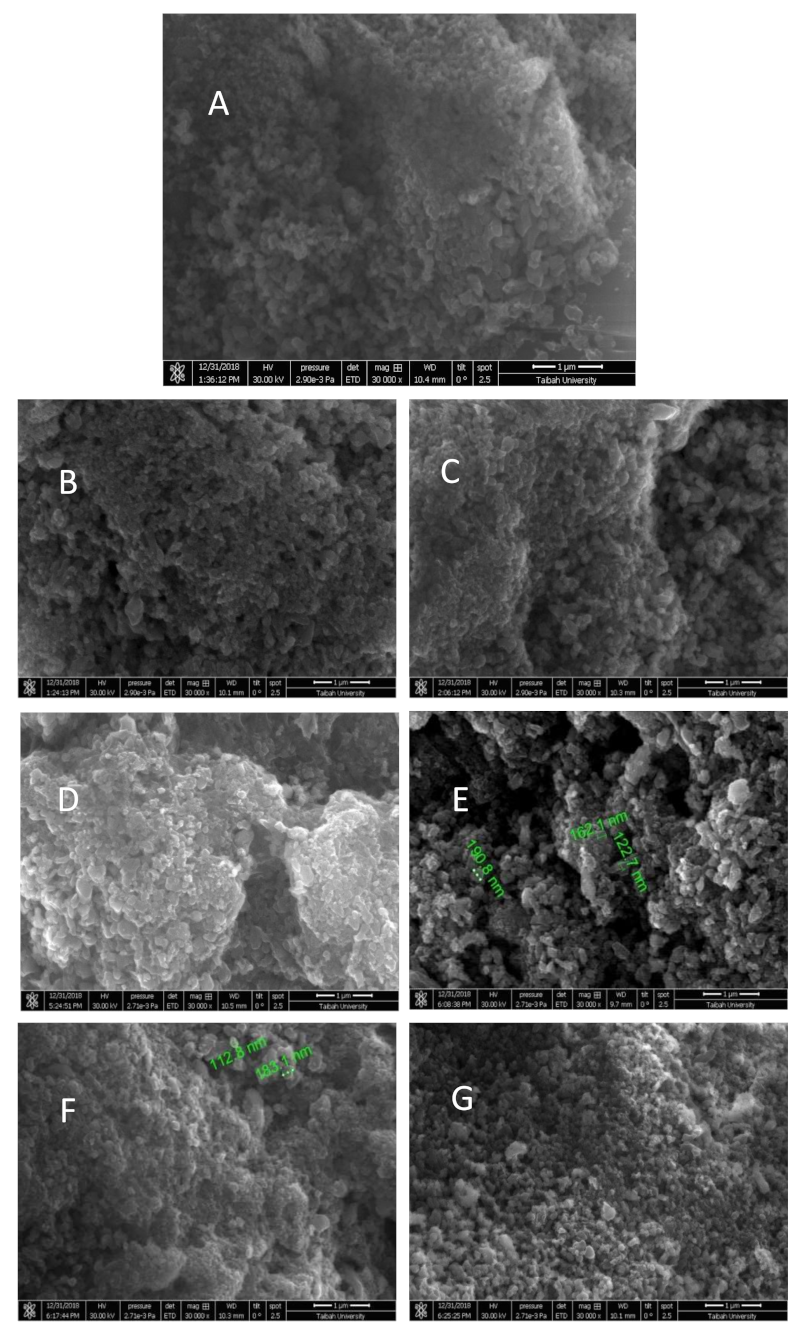
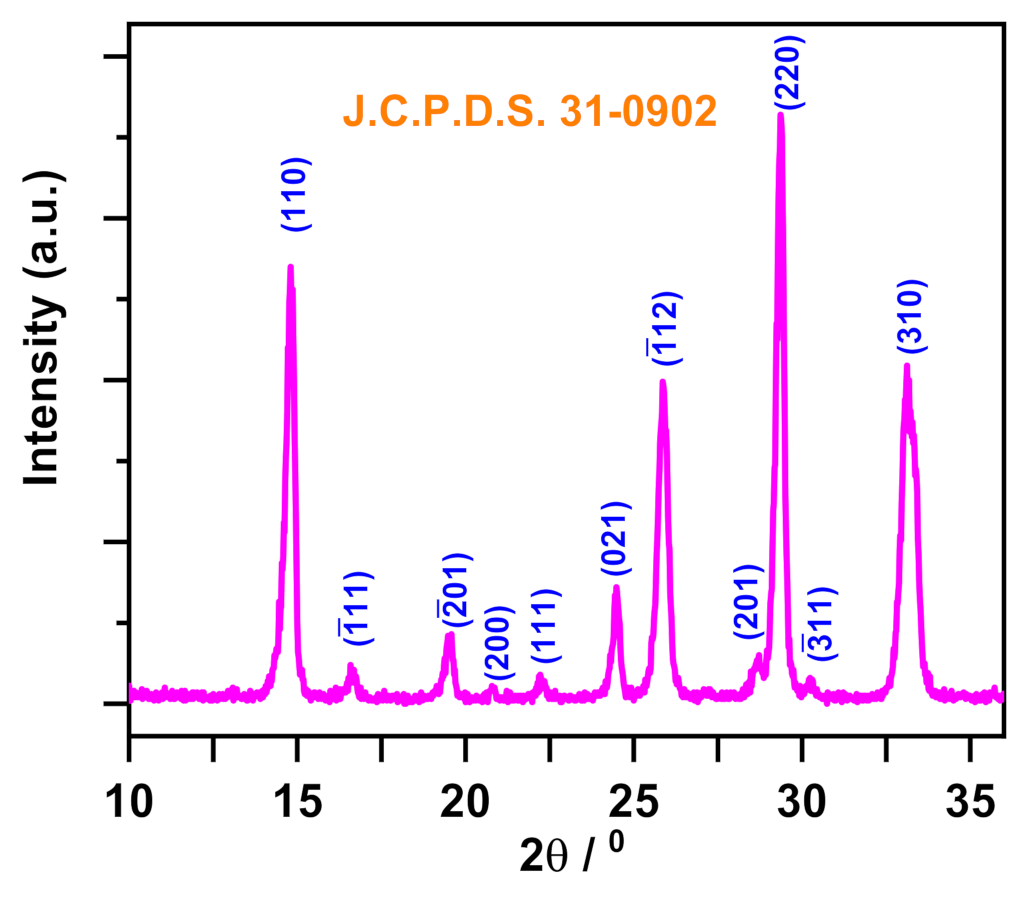
| Adsorbent | Adsorbate | Temperature (K) | Kd | ∆H° (kJ mol−1) | ∆S° (kJ mol−1 K) | ∆G° (kJ mol−1) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| α-NiMoO4 | MB | 293 | 2.401 | 35.12 | 0.126 | 293K | 313K | 323K | 333K | 343K |
| 313 | 3.348 | |||||||||
| 323 | 7.696 | |||||||||
| 333 | 23.390 | −2.134 | −3.144 | −5.480 | −8.727 | −14.822 | ||||
| 343 | 180.818 | |||||||||
| Model | Equation | Parameters |
|---|---|---|
| Pseudo-first-order (PFD) [83] | K1: the rate constant of pseudo-first-order adsorption (1/min) qe: the removal capacity at equilibrium (mg/g) qt: the removal capacity at time t (mg/g) | |
| Pseudo-second-order (PSD) [83] | K2: the pseudo-second-order rate constant (g·mg−1·min−1) qe: the removal capacity at equilibrium (mg/g) qt: the removal capacity at time t (mg/g) | |
| Intraparticle diffusion (IPD) [77] | qt: the removal capacity (mg/g) at time t t: the contact time (min) I (mg/g) and KI (mg/(g·min0.5)): the intraparticle diffusion constants |
| Cimg/L | Pseudo-First-Order | Pseudo-Second-Order | Intraparticle Diffusion Model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qexp (mg/g) | qe (mg/g) | k1 (1/min) | R12 | qe (mg/g) | k2 (g/mg min) | R22 | I (mg/g) | ki (mg/g min0.5) | R32 | |
| 100 | 10,000 | 584 | 0.020 | 0.988 | 9964 | 0.00015 | 1.000 | 9311 | 65 | 0.981 |
| 120 | 11,981 | 2101 | 0.048 | 0.966 | 12,200 | 0.00004 | 1.000 | 10,027 | 218 | 0.946 |
| 140 | 13,970 | 4770 | 0.062 | 0.982 | 14,436 | 0.00002 | 1.000 | 10,426 | 403 | 0.934 |
| 160 | 15,900 | 4218 | 0.028 | 0.952 | 16,136 | 0.00002 | 0.999 | 11,036 | 501 | 0.959 |
| Model | Equation | Parameters |
|---|---|---|
| Freundlich [78] | Ce: concentration of MB at equilibrium (ppm) n: the heterogeneity factor (g/L) qF: the Freundlich constant (mg(1−1/n) L1/n g−1) qe: the methylene blue dye quantity adsorbed by α-NiMoO4 at equilibrium (mg/g) | |
| Langmuir [85] | qe: the methylene blue dye quantity adsorbed by α-NiMoO4 at equilibrium (mg/g) Ce: concentration of MB at equilibrium (ppm) qm: the maximum quantity of methylene blue dye removed by α-NiMoO4 (mg/g) KL: Langmuir constant of adsorption (L/mg) | |
| Ci: the initial concentration of methylene blue KL: the Langmuir constant RL: values indicate that the removal of methylene blue dye could be linear (RL = 1), irreversible (RL = 0), favorable (0 < RL< 1), or unfavorable (RL > 1) | ||
| Dubinin–Radushkevich (D-R) [84] | K: constant for the sorption energy (mol2/kJ2) ε: the Polanyi potential T: the temperature (K) R: the universal gas constant (8.314 J.mol−1 K−1) qm: the theoretical saturation capacity Ce: the equilibrium concentration of the methylene blue dye left in the solution (ppm) | |
| Temkin [86] | bT: the Temkin constant related to heat of sorption (J/mol) BT = RT/bT R: the gas constant (8.314 J/mol K) AT: the Temkin isotherm constant (L/g) T: the absolute temperature (K) |
| Langmuir | Freundlich | Temkin | Dubinin–Radushkevich | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/ mg) | R2 | Range RL | qF(mg(1−1/n)L1/ng−1) | 1/n | R2 | AT (L/g) | BT | R2 | qm (mg/g) | R2 | E (Kj/ mol) |
| 16,863 | 16 | 0.999 | 0.0004–0.0006 | 16,318 | 6 | 0.948 | 1220 | 2269 | 0.960 | 14,429 | 0.782 | 43 |
| Nanosorbent | Qmax (mg/g) | Reference |
|---|---|---|
| ZnMoO4 nanoparticles | 217.86 | [30] |
| Fe2(MoO4)3 nanoparticles | 6173.00 | [87] |
| α-MoO3 nanoparticles | 152.00 | [88] |
| Chemically reduced graphene oxide | 1519.60 | [89] |
| Magnetic β-cyclodextrin–chitosan nanoparticles | 2783.30 | [90] |
| ZnO | 7918.02–9197.70 | [91] |
| Fe2O3 | 1124.70 | [92] |
| CoO | 5501.93 | [92] |
| NiO | 10,585.00 | [92] |
| Nickel molybdate (α-NiMoO4) | 16,863.00 | This work |
Sample Availability: Samples of the compounds NiMoO4 are available from the corresponding author. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakass, S.; Oudghiri Hassani, H.; Mohmoud, A.; Kooli, F.; Abboudi, M.; Assirey, E.; Al Wadaani, F. Highly Efficient Methylene Blue Dye Removal by Nickel Molybdate Nanosorbent. Molecules 2021, 26, 1378. https://doi.org/10.3390/molecules26051378
Rakass S, Oudghiri Hassani H, Mohmoud A, Kooli F, Abboudi M, Assirey E, Al Wadaani F. Highly Efficient Methylene Blue Dye Removal by Nickel Molybdate Nanosorbent. Molecules. 2021; 26(5):1378. https://doi.org/10.3390/molecules26051378
Chicago/Turabian StyleRakass, Souad, Hicham Oudghiri Hassani, Ahmed Mohmoud, Fethi Kooli, Mostafa Abboudi, Eman Assirey, and Fahd Al Wadaani. 2021. "Highly Efficient Methylene Blue Dye Removal by Nickel Molybdate Nanosorbent" Molecules 26, no. 5: 1378. https://doi.org/10.3390/molecules26051378
APA StyleRakass, S., Oudghiri Hassani, H., Mohmoud, A., Kooli, F., Abboudi, M., Assirey, E., & Al Wadaani, F. (2021). Highly Efficient Methylene Blue Dye Removal by Nickel Molybdate Nanosorbent. Molecules, 26(5), 1378. https://doi.org/10.3390/molecules26051378